Abstract
The dysregulation of microRNA (miRNA) expression is closely related with tumorigenesis and tumor development in glioblastoma (GBM). In this study, we found that miRNA-598 (miR-598) expression was significantly downregulated in GBM tissues and cell lines. Restoring miR-598 expression inhibited cell proliferation and invasion in GBM. Moreover, we validated that metastasis associated in colon cancer-1 (MACC1) is a novel target of miR-598 in GBM. Restoring MACC1 expression reversed the inhibitory effects of miR-598 overexpression on GBM cells. In addition, miR-598 overexpression suppressed Met/AKT pathway activation in GBM. Our results provided compelling evidence that miR-598 serves tumor-suppressive roles in GBM and that its antioncogenic effects are mediated chiefly through the direct suppression of MACC1 expression and regulation of the Met/AKT signaling pathway. Therefore, miR-598 is a potential target in the treatment of GBM.
Key words: Glioblastoma, MicroRNA-598, Metastasis associated in colon cancer-1 (MACC1), Proliferation, Invasion
INTRODUCTION
Gliomas, the most common type of primary cerebral tumors, originate from neural mesenchymal cells1. Gliomas could be classified into four histopathologic grades (I–IV) on the basis of their degree of malignancy2. Glioblastoma (GBM), a grade IV glioma, is the most prevalent and aggressive histological subtype of glioma in the adult population3. Despite recent improvements in combined approaches, including surgery, radiotherapy, and chemotherapy, for the treatment of GBM, patients with this malignancy exhibit poor therapeutic outcome with a median survival time of only 9–12 months4. Distant metastasis occurs frequently, and the local infiltration of GBM to other parts of the brain appears as tentacle-like projections, which hinder the treatment of this malignancy through complete surgical excision5. Moreover, our insufficient understanding of the malignant phenotype of GBM is also responsible for the poor prognosis of patients with this disease6. Hence, studying the molecular mechanisms that underlie the pathogenesis of GBM should aid in the identification of novel and effective targets for the treatment of this life-threatening disease.
microRNAs (miRNA) are a series of endogenous, noncoding small RNA molecules that act as gene regulators7. miRNAs modulate gene expression by binding to partially complementary sequences in the 3′-untranslated regions (3′-UTRs) of their target genes and then inhibiting mRNA translation or inducing mRNA degradation8. Over 2,500 miRNAs have been identified in the human genome, and these miRNAs may regulate the expression of >50% of all human protein-coding genes9. In recent years, emerging evidence has shown that the dysregulation of miRNAs is a common characteristic of human malignancies, including GBM10. Aberrantly expressed miRNAs are involved in the regulation of important pathological processes, such as cell proliferation, apoptosis, angiogenesis, epithelial–mesenchymal transition, and metastasis11–13. Numerous miRNAs have been implicated in the occurrence and development of GBM and may serve as new markers for the diagnosis and treatment of this malignancy14–16. Therefore, in-depth research on the role of miRNAs in GBM may aid the development of novel therapeutic strategies for this disease.
miR-598 was downregulated in colorectal cancer17 and osteosarcoma18 and may play key roles in multiple malignancy-associated processes. However, the expression, detailed biological roles, and associated molecular mechanisms of miR-598 in GBM remain unclear. Therefore, we aimed to detect miR-598 expression, as well as examine its biological roles and underlying mechanisms, in GBM.
MATERIALS AND METHODS
Ethics Statement and Tissue Samples
This study was approved by the Ethics Committee of Xiangyang No. 1 People’s Hospital (Hubei, P.R. China). All patients provided written informed consent before participating in this study. A total of 21 paired GBM tissues and matched adjacent normal brain tissues were collected from patients who were diagnosed with GBM and underwent surgical resection at Xiangyang No. 1 People’s Hospital. No recruited patients received radiotherapy or chemotherapy before surgical resection. All tissues were quickly frozen in liquid nitrogen and maintained in liquid nitrogen until analysis.
Cell Culture
Four human GBM cell lines (T98G, LN229, U87, and U251) were obtained from the Shanghai Cell Bank of the Chinese Academy of Sciences (Shanghai, P.R. China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS), 100 U of penicillin/ml, and 100 ng of streptomycin/ml (all from Gibco, Invitrogen, Carlsbad, CA, USA). Normal human astrocytes (NHAs) were ordered from Sciencell Research Laboratories (Carlsbad, CA, USA) and grown in astrocyte medium (Sciencell Research Laboratories) according to the supplier’s instructions. All cell lines were maintained at 37°C in a fully humidified atmosphere containing 5% CO2.
Cell Transfection
miR-598 mimic and miRNA negative control mimic (miR-NC) were purchased from GenePharma (Shanghai, P.R. China). Metastasis associated in colon cancer-1 (MACC1) overexpression plasmid pCMV-MACC1 and empty pCMV plasmid were produced by Amspring Biological Technology Co., Ltd. (Changsha, P.R. China). Cells were inoculated into six-well cell culture plates at a density of 50%–60% confluence. miRNA mimic or plasmids were transfected into cells using Lipofectamine 2000 Transfection Reagent (Invitrogen) in accordance with the manufacturer’s instructions.
RNA Isolation and Reverse Transcription Quantitative Polymerase Chain Reaction
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Waltham, MA, USA) was employed to isolate total RNA from tissues or cells in accordance with the manufacturer’s instructions. The purity and concentration of total RNA were detected using NanoDrop 2000 (NanoDrop Technologies; Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA). To quantify miR-598 expression, total RNA was reverse transcribed into complementary DNA (cDNA) using a TaqMan MicroRNA Reverse Transcription Kit and then amplified with a TaqMan MicroRNA PCR Kit (all from Applied Biosystems, Foster City, CA, USA). To analyze MACC1 mRNA expression, cDNA was prepared from total RNA using a PrimeScript RT Reagent Kit (Takara, Dalian, P.R. China). Quantitative polymerase chain reaction (qPCR) analysis was performed with a SYBR Premix Ex Taq™ II Kit (Takara) with ABI PRISM7900 Sequence Detection System (Thermo Fisher Scientific). miR-598 and MACC1 mRNA expression was normalized to U6 snRNA and GAPDH mRNA expression, respectively. Data were analyzed using the 2−ΔΔCt method19.
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
At 24 h posttransfection, cells were trypsinized, collected, and inoculated into 96-well culture plates at a density of 3,000 cells per well. The plates were incubated at 37°C with 5% CO2 for 0, 1, 2, and 3 days. At every time point, cell proliferation was determined through MTT assay. Briefly, 10 μl of MTT solution (5 mg/ml; Sigma-Aldrich, St. Louis, MO, USA) was added to each well, and cells were incubated at 37°C for an additional 4 h. Thereafter, the medium was discarded, and 150 μl of dimethyl sulfoxide (DMSO) was added to each well to dissolve the formazan crystals. The absorbance was measured at a wavelength of 490 nm with SpectraMax M3 microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA). Three independent experiments were carried out with five replicate wells in each group.
Matrigel Invasion Assay
To detect cell invasion capacity, 24-well Transwell plates (Corning Inc., Corning, NY, USA) precoated with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) were utilized. At 48 h posttransfection, cells were trypsinized, collected, and suspended in FBS-free DMEM. A total of 5 × 104 cells were placed into the upper chamber of Transwell plates, whereas the lower chambers were filled with 500 μl of DMEM containing 10% FBS. Cells were allowed to invade for 24 h at 37°C under 5% CO2. Noninvasive cells were removed from the upper chambers with cotton swabs. Invasive cells were fixed with 4% paraformaldehyde at room temperature for 15 min and stained with 0.1% crystal violet (all from Beyotime, Haimen, P.R. China) at room temperature for 15 min. Invasive cells were counted under an inverted microscope (CKX41; Olympus, Tokyo, Japan), and the average values of five randomly selected fields were used.
Bioinformatics Prediction and Dual-Luciferase Reporter Assay
The 3′-UTRs of human MACC1 gene containing the wild-type or mutant binding sequences for miR-598 were amplified by GenePharma and inserted into pGL3 vectors (Promega Corp., Madison, WI, USA). The constructs were designated as pGL3-MACC1-3′-UTR Wt and pGL3-MACC1-3′-UTR Mut. One night prior to transfection, cells were inoculated into 24-well cell culture plates. Then miR-598 mimics or miR-NC, together with pGL3-MACC1-3′-UTR Wt or pGL3-MACC1-3′-UTR Mut, was introduced into cells using Lipofectamine 2000 Transfection Reagent in accordance with the protocol provided by the manufacturer. After 48 h of transfection, cell lysates were collected and then subjected to luciferase activity analysis using the Dual-Luciferase Reporter Assay System (Promega Corp.) in accordance with the manufacturer’s instructions. Firefly luciferase activity was normalized to Renilla luciferase activity.
Western Blot Analysis
Total Protein Extraction Kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing, P.R. China) was used to extract total protein from cells. Total protein concentration was evaluated using the Pierce™ BCA Protein Assay Kit (Pierce; Thermo Fisher Scientific, Inc.). Equal amounts of protein were separated by 10% SDS-PAGE, electrotransferred onto polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA, USA), and blocked at room temperature with 5% skimmed milk dissolved in TBS-Tween 20 (TBST) for 2 h. After washing with TBST three times, membranes were incubated with primary antibodies at 4°C overnight. Primary antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA) and included rabbit anti-human monoclonal MACC1 (#86290; 1:1,000 dilution) and rabbit anti-human GAPDH monoclonal (#5174; 1:1,000 dilution) antibodies. Membranes were washed three times for 10 min with TBST and further incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (#7074; 1:2,000 dilution; Cell Signaling Technology, Inc.). After three cycles of washing with TBST, protein bands were visualized using an enhanced chemiluminescence reagent (EMD Millipore). Band density was analyzed with ImageJ software (version 1.48; National Institutes of Health, Bethesda, MD, USA).
Statistical Analysis
Data were expressed as mean ± standard deviation and analyzed with SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Differences between groups were analyzed with Student’s t-test and one-way ANOVA with Student–Newman–Keuls post hoc test. A value of p < 0.05 was considered statistically significant.
RESULTS
miR-598 Is Downregulated in GBM Tissues and Cell Lines
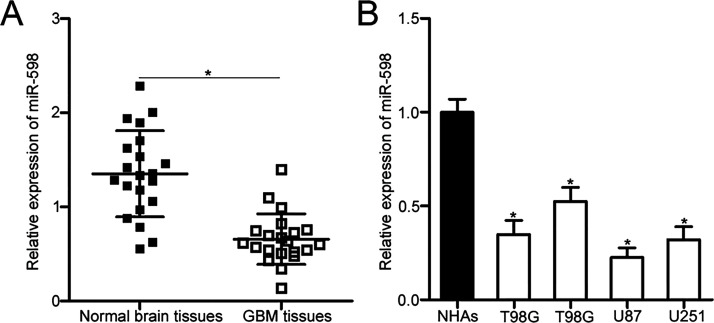
To quantify miR-598 expression in GBM, we first detected miR-598 expression in 21 paired GBM tissues and matched adjacent normal brain tissues through reverse transcription quantitative polymerase chain reaction (RT-qPCR). The data revealed that miR-598 expression in GBM tissues was downregulated relative to that in adjacent normal brain tissues (p < 0.05) (Fig. 1A). Consistent with the result from GBM tissues, miR-598 expression in all four tested GBM cell lines was lower than that in NHAs (p < 0.05) (Fig. 1B). These results suggested that miR-598 may play important roles in GBM development.
Figure 1.
MicroRNA (miR)-598 underexpressed in glioblastoma (GBM) tissue and cell lines. (A) Reverse transcription quantitative polymerase chain reaction (RT-qPCR) analysis of miR-598 expression in 21 paired GBM tissues and matched adjacent normal brain tissues. *p < 0.05 compared with normal brain tissues. (B) miR-598 expression levels in four human GBM cell lines (T98G, LN229, U87, and U251) and normal human astrocytes (NHAs) were examined through RT-qPCR. *p < 0.05 compared with NHAs.
Upregulated miR-598 Expression Restricts Cell Proliferation and Invasion in GBM
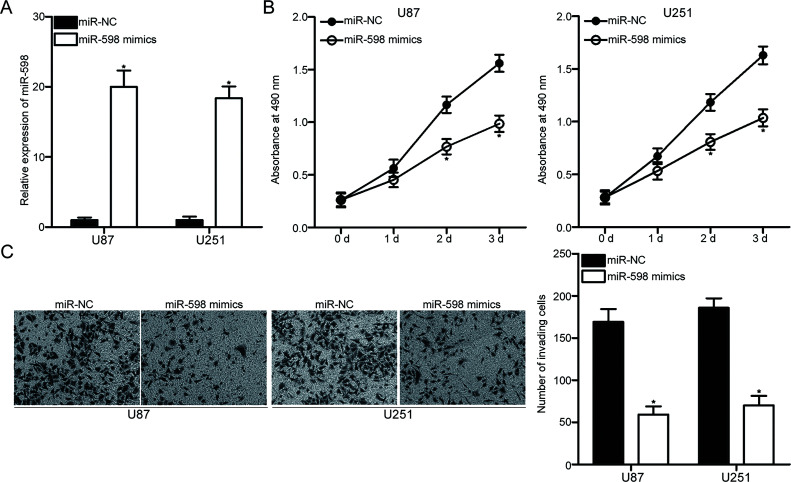
To explore the effects of miR-598 on the progression of GBM, we transfected miR-598 mimic or miR-NC into U87 and U251 cells, which express relatively lower levels of miR-598 among the four GBM cell lines. RT-qPCR analysis confirmed that miR-598 expression markedly increased in U87 and U251 cells transfected with miR-598 mimic compared with that in cells transfected with miR-NC (p < 0.05) (Fig. 2A). The effect of miR-598 overexpression on GBM cell proliferation was determined through MTT assay. The restored expression of miR-598 attenuated the proliferation of U87 and U251 cells (p < 0.05) (Fig. 2B). Additionally, we performed Matrigel invasion assay to examine the invasion ability of U87 and U251 cells transfected with miR-598 mimics or miR-NC. Figure 2C shows that the enforced expression of miR-598 drastically reduced the invasion capacities of U87 and U251 cells (p < 0.05) (Fig. 2C). Therefore, these results suggested that miR-598 may play tumor-suppressive roles in GBM.
Figure 2.
miR-598 overexpression suppresses the proliferation and invasion abilities of U87 and U251 cells. U87 and U251 cells were transfected with miR-598 mimic or miR-negative control (miR-NC) and used in the following experiments. (A) Total RNA was isolated at 48 h posttransfection, and miR-598 expression was detected through RT-qPCR. *p < 0.05 compared with miR-NC. (B) MTT assays were employed to determine the effect of miR-598 overexpression on U87 and U251 cell proliferation. *p < 0.05 compared with miR-NC. (C) Matrigel invasion assay was performed to evaluate the invasion ability of cells at 48 h posttransfection. *p < 0.05 compared with miR-NC.
miR-598 Directly Targets MACC1 in GBM
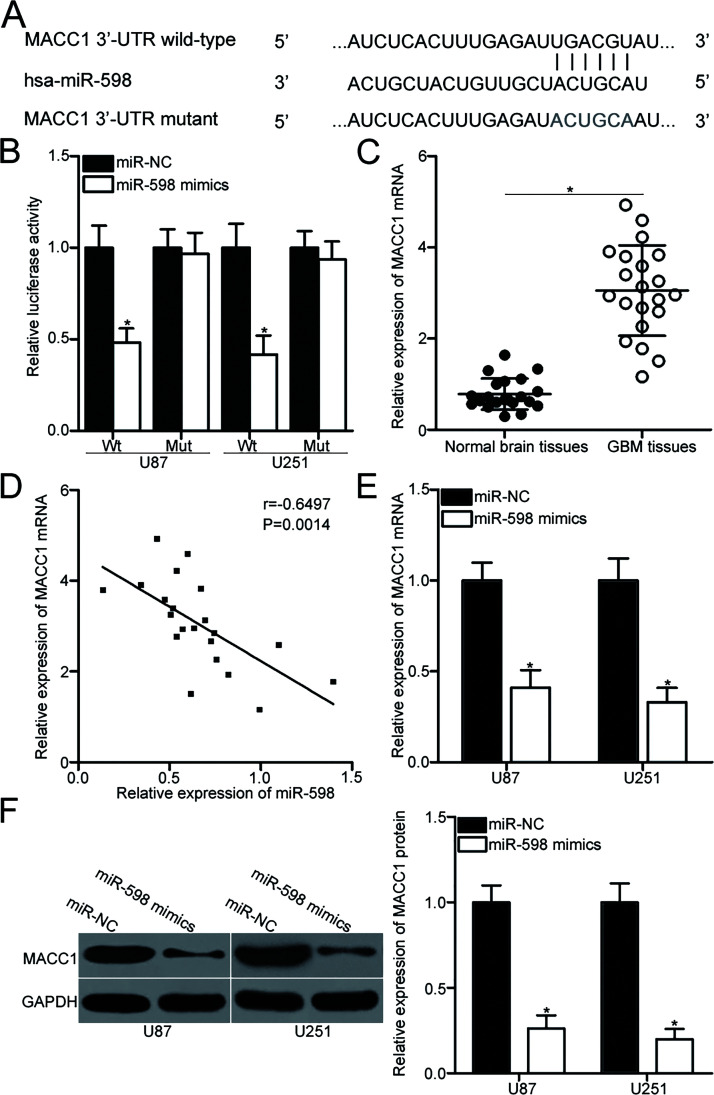
To identify the molecular mechanism that underlies the action of miR-598 in GBM, bioinformatics analysis was performed to predict the potential targets of miR-598. MACC1, which contains a 3′-UTR sequence complementary to the seed sequence of miR-598 (Fig. 3A), attracted our attention because MACC1 is overexpressed in GBM and contributes to the onset and progression of this malignancy20–23. Therefore, we conducted the dual-luciferase reporter assay to determine if the 3′-UTR of MACC1 is a direct target of miR-598. The results showed that compared with introducing miR-NC, introducing miR-598 mimic to U87 and U251 cells with the wild-type reporter plasmid significantly inhibited luciferase activities (p < 0.05). However, the luciferase activities of the mutant reporter plasmid were unaffected in U87 and U251 cells cotransfected with miR-598 mimics (Fig. 3B). To further investigate the association between miR-598 and MACC1 in GBM, we detected MACC1 expression in 21 paired GBM tissues and matched adjacent normal brain tissues. Data from RT-qPCR analysis indicated that GBM tissues exhibited higher MACC1 mRNA expression than adjacent normal brain tissues (p < 0.05) (Fig. 3C). Spearman’s correlation analysis revealed that miR-598 and MACC1 mRNA levels in GBM tissues were inversely correlated (r = −0.6497, p = 0.0014) (Fig. 3D). Furthermore, RT-qPCR and Western blot analysis demonstrated that miR-598 overexpression downregulated MACC1 expression on the mRNA (p < 0.05) (Fig. 3E) and protein (p < 0.05) (Fig. 3F) levels in U87 and U251 cells. The above results suggested that MACC1 is a direct target gene of miR-598 in GBM.
Figure 3.
Metastasis associated in colon cancer-1 (MACC1) is a direct target of miR-598. (A) Predicted wild-type (Wt) and mutant (Mut) miR-598 binding sequence in the 3′-untranslated region (3′-UTR) of the MACC1 gene. (B) U87 and U251 cells were cotransfected with miR-598 mimics or miR-NC and a luciferase reporter plasmid containing either Wt or Mut miR-598 binding sites in the 3′-UTR of the MACC1 gene. Luciferase activity was measured at 48 h posttransfection using a dual-luciferase reporter assay system. *p < 0.05 compared with miR-NC. (C) RT-qPCR was performed to detect MACC1 mRNA expression in 21 paired GBM tissues and matched adjacent normal brain tissues. *p < 0.05 compared with normal brain tissues. (D) Spearman’s correlation analysis was performed to identify the association between miR-598 and MACC1 mRNA expression in GBM tissues. r = −0.6497, p = 0.0014. (E, F) U87 and U251 cells were transfected with miR-598 mimics or miR-NC. Total RNA and protein were extracted to quantify MACC1 mRNA and protein expression through RT-qPCR and Western blot analysis. *p < 0.05 compared with miR-NC.
MACC1 Reintroduction Partially Reverses the Suppressive Effects of miR-598 in GBM
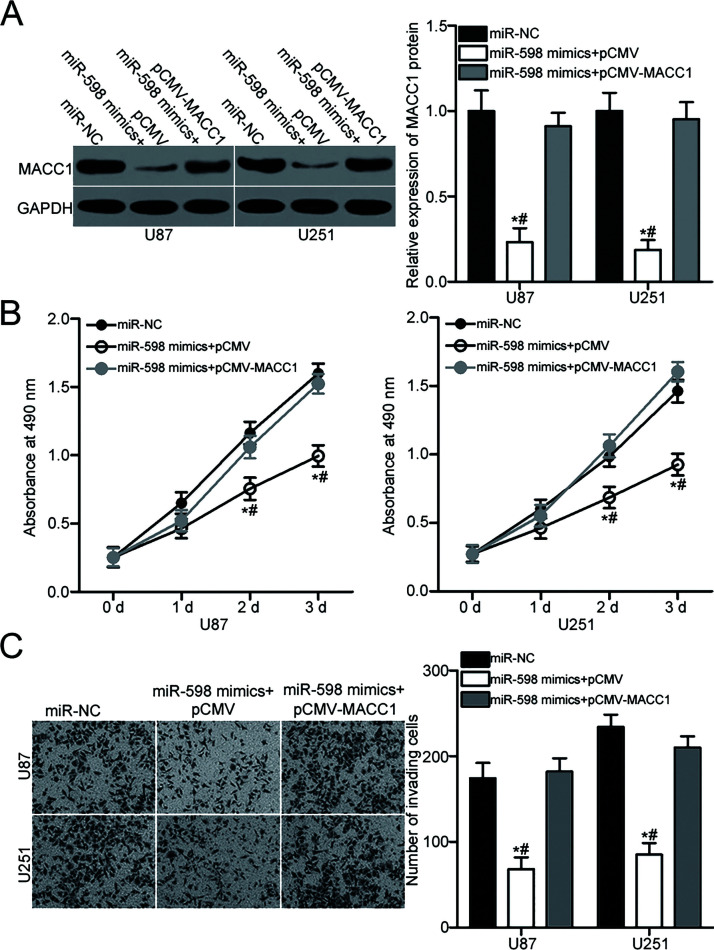
The above results showed that upregulating miR-598 expression suppresses GBM cell proliferation and invasion and validated that MACC1 is a direct target of miR-598. Therefore, we addressed whether the inhibitory effects of miR-598 on GBM cell proliferation and invasion are directly mediated by MACC1 suppression. We cotransfected U87 and U251 cells with miR-598 mimics and the empty pCMV plasmid or pCMV-MACC1. After transfection, Western blot analysis proved that cotransfection with pCMV-MACC1 restored MACC1 protein levels in miR-598-overexpressing U87 and U251 (p < 0.05) (Fig. 4A). Subsequent MTT and Matrigel invasion assays indicated that restored MACC1 expression antagonizes the inhibition of proliferation (p < 0.05) (Fig. 4B) and invasion (p < 0.05) (Fig. 4C) of U87 and U251cells induced by miR-598 overexpression. These results demonstrated that miR-598 exerts tumor-suppressive roles in GBM, at least in part, by downregulating MACC1 expression.
Figure 4.
Restored MACC1 expression counteracts the suppressive effects of miR-598 overexpression on U87 and U251 cells. pCMV-MACC1 or pCMV was introduced into U87 and U251 cells in the presence of ectopic miR-598 expression. (A) Western blot analysis was performed at 72 h posttransfection to measure MACC1 protein level. *p < 0.05 compared with miR-NC. #p < 0.05 compared with miR-598 mimics + pCMV-MACC1. (B) MTT and (C) Matrigel invasion assays were applied to detect cell proliferation and invasion. *p < 0.05 compared with miR-NC. #p < 0.05 compared with miR-598 mimics + pCMV-MACC1.
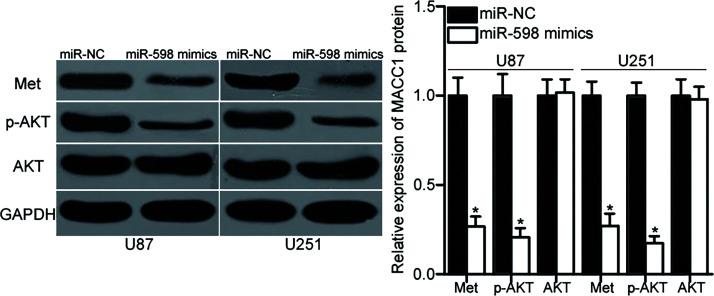
miR-598 Inhibits the Met/AKT Signaling Pathway in GBM
MACC1 participates in the Met/AKT pathway regulation24,25. Hence, we explored the effect of miR-598 on the Met/AKT pathway in GBM. U87 and U251 cells were transfected with miR-598 mimic or miR-NC. After 72 h of transfection, total protein was isolated from the cells and then subjected to Western blot analysis for the detection of Met, p-AKT, and AKT protein levels. The results showed that the ectopic expression of miR-598 decreased Met and p-AKT expression without altering total AKT expression in U87 and U251 cells (p < 0.05) (Fig. 5). These results suggested that miR-598 inhibits the Met/AKT pathway activation in GBM.
Figure 5.
Resumed miR-598 expression suppresses the Met/AKT signaling pathway in GBM. Western blot analysis was used to detect Met, p-AKT, and AKT expression in U87 and U251 cells transfected with miR-598 mimics or miR-NC. GAPDH was used as the loading control. *p < 0.05 compared with miR-NC.
DISCUSSION
A growing body of evidence has demonstrated that miRNAs act as oncogenes or tumor suppressor genes in GBM by regulating their target genes; thus, the dysregulation of miRNA expression is closely related with tumorigenesis and tumor development in GBM26–28. Therefore, investigating the mechanisms that underlie GBM formation and progression may improve the diagnosis and therapy of this aggressive disease. Our current study demonstrated that miR-598 was significantly underexpressed in GBM tissues and cell lines. Upregulated miR-598 expression suppressed cell proliferation and invasion in GBM. Additionally, MACC1 was confirmed as a novel direct target of miR-598 in GBM. Restored MACC1 expression partially abolished the tumor-suppressive effects of miR-598 in GBM. Moreover, the upregulation of miR-598 expression suppressed the Met/AKT signaling pathway in GBM. Overall, miR-598 may act as a tumor suppressor in GBM by directly targeting MACC1 and suppressing the Met/AKT signaling pathway.
The expression and functions of miR-598 in several types of human cancer have been well studied. For example, a previous study showed that miR-598 was overexpressed in bile duct cancer and that the expression level of miR-598 could serve as prognostic and predictive markers for survival of patients with bile duct cancer29. However, Chen et al. found that miR-598 was significantly underexpressed in colorectal cancer tissues and cell lines. Restoring miR-598 expression decreased metastasis and epithelial–mesenchymal transition in colorectal cancer because it inhibited the jagged 1 (JAG1)/Notch2 pathway17. Liu et al. also found that miR-598 was underexpressed in osteosarcoma tissues and cell lines, as well as plasmids. Upregulated miR-598 expression directly targeted platelet-derived growth factor B (PDGFB) and MET to attenuate the proliferation, migration, and invasion of osteosarcoma in vitro and to decrease tumor growth in vivo18. These findings indicated that the expression and roles of miR-598 are tissue specific and suggested that miR-598 is a potential target in the therapy of patients with different malignancies.
miRNAs act as either tumor suppressors or oncogenes in human cancers. The actions of miRNAs are mainly dependent on the functional characteristics of their target genes. Thus, identifying the targets of miR-598 is essential to understand its biological functions in GBM and may help develop effective targeted therapies for patients with this fatal disease. In our current study, we identified MACC1 as a novel direct target of miR-598 in GBM. MACC1 was overexpressed in multiple types of human malignancies, such as gastric cancer30, colorectal cancer31, and hepatocellular carcinoma32. The aberrant expression of MACC1 was closely correlated with the clinicopathological characteristics and prognosis of patients with different malignancies. For example, upregulated MACC1 expression in lung squamous cell carcinoma was strongly associated with tumor grade, lymph node metastasis, and TNM stages. High MACC1 expression levels were associated with the low overall survival rate of patients with lung squamous cell carcinoma. Multivariate analysis had validated that MACC1 was an independent prognostic factor for the overall survival of patients with lung squamous cell carcinoma33. MACC1 deregulation had crucial roles in the biological behaviors, including proliferation, cell cycle, apoptosis, migration, invasion, epithelial–mesenchymal transition, autophagy, angiogenesis, and chemoresistance, of various malignancies34–36. These findings suggested that MACC1 is a potential prognostic indicator and oncotherapy target.
MACC1 was highly expressed in GBM, and its upregulated expression was associated with advanced pathological grade. GBM patients with high MACC1 expression had poorer prognosis than those with low MACC1 expression20,21. Additionally, MACC1 was an independent prognostic factor of the overall survival of patients with GBM21. In GBM, inhibiting MACC1 expression restricted cell proliferation, migration, and invasion, as well as promoted apoptosis and increased chemosensitivity22,23. Hence, targeting MACC1 may be an effective method for GBM treatment. In GBM, MACC1 has been previously reported to be regulated by miR-33837. Furthermore, miR-94438, miR-43339, and miR-200a40 directly targeted MACC1 and therefore inhibited progression of gastric cancer, colorectal cancer, and hepatocellular carcinoma, respectively. In combination with the results of this study, miRNA-based therapy targeting MACC1 could be investigated as an effective therapeutic strategy for GBM patients in the future.
In conclusion, miR-598 was downregulated in GBM and exerted tumor-suppressive effects by regulating the MACC1/Met/AKT signaling pathway. Therefore, this research proposes that miR-598 can be targeted for the development of novel treatment for GBM patients.
REFERENCES
- 1. Buckner JC, Brown PD, O’Neill BP, Meyer FB, Wetmore CJ, Uhm JH. Central nervous system tumors. Mayo Clin Proc. 2007;82:1271–86. [DOI] [PubMed] [Google Scholar]
- 2. Wang Y, Jiang T. Understanding high grade glioma: Molecular mechanism, therapy and comprehensive management. Cancer Lett. 2013;331:139–46. [DOI] [PubMed] [Google Scholar]
- 3. Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol. 2012;14(Suppl 5):v1–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups, National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. [DOI] [PubMed] [Google Scholar]
- 5. Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen AJ, Perry SR, Tonon G, Chu GC, Ding Z, Stommel JM, Dunn KL, Wiedemeyer R, You MJ, Brennan C, Wang YA, Ligon KL, Wong WH, Chin L, dePinho RA. PTEN and p53 converge on c-Myc to control differentiation, self-renewal, and transformation of normal and neoplastic stem cells in glioblastoma. Cold Spring Harb Symp Quant Biol. 2008;73:427–37. [DOI] [PubMed] [Google Scholar]
- 6. Holland EC. Gliomagenesis: Genetic alterations and mouse models. Nat Rev Genet. 2001;2:120–9. [DOI] [PubMed] [Google Scholar]
- 7. Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell 2009;136:215–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Esquela-Kerscher A, Slack FJ. Oncomirs—MicroRNAs with a role in cancer. Nat Rev Cancer 2006;6:259–69. [DOI] [PubMed] [Google Scholar]
- 9. Kozomara A, Griffiths-Jones S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42:D68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu X, Cai N, Zhi T, Bao Z, Wang D, Liu Y, Jiang K, Fan L, Ji J, Liu N. MicroRNA-1179 inhibits glioblastoma cell proliferation and cell cycle progression via directly targeting E2F transcription factor 5. Am J Cancer Res. 2017;7:1680–92. [PMC free article] [PubMed] [Google Scholar]
- 11. Macfarlane LA, Murphy PR. MicroRNA: Biogenesis, function and role in cancer. Curr Genomics 2010;11:537–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dogini DB, Pascoal VD, Avansini SH, Vieira AS, Pereira TC, Lopes-Cendes I. The new world of RNAs. Genet Mol Biol. 2014;37:285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stahlhut Espinosa CE, Slack FJ. The role of microRNAs in cancer. Yale J Biol Med. 2006;79:131–40. [PMC free article] [PubMed] [Google Scholar]
- 14. Ahir BK, Ozer H, Engelhard HH, Lakka SS. MicroRNAs in glioblastoma pathogenesis and therapy: A comprehensive review. Crit Rev Oncol Hematol. 2017;120:22–33. [DOI] [PubMed] [Google Scholar]
- 15. Mao K, Lei D, Zhang H, You C. MicroRNA-485 inhibits malignant biological behaviour of glioblastoma cells by directly targeting PAK4. Int J Oncol. 2017;51:1521–32. [DOI] [PubMed] [Google Scholar]
- 16. Cheng ZX, Song YX, Wang ZY, Wang Y, Dong Y. miR-144-3p serves as a tumor suppressor by targeting FZD7 and predicts the prognosis of human glioblastoma. Eur Rev Med Pharmacol Sci. 2017;21:4079–86. [PubMed] [Google Scholar]
- 17. Chen J, Zhang H, Chen Y, Qiao G, Jiang W, Ni P, Liu X, Ma L. miR-598 inhibits metastasis in colorectal cancer by suppressing JAG1/Notch2 pathway stimulating EMT. Exp Cell Res. 2017;352:104–12. [DOI] [PubMed] [Google Scholar]
- 18. Liu K, Sun X, Zhang Y, Liu L, Yuan Q. MiR-598: A tumor suppressor with biomarker significance in osteosarcoma. Life Sci. 2017;188:141–8. [DOI] [PubMed] [Google Scholar]
- 19. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 20. Hagemann C, Fuchs S, Monoranu CM, Herrmann P, Smith J, Hohmann T, Grabiec U, Kessler AF, Dehghani F, Lohr M, Ernestus RI, Vince GH, Stein U. Impact of MACC1 on human malignant glioma progression and patients’ unfavorable prognosis. Neuro Oncol. 2013;15:1696–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang T, Kong B, Kuang YQ, Cheng L, Gu JW, Zhang JH, Shu HF, Yu SX, He WQ, Xing XM, Huang HD. Overexpression of MACC1 protein and its clinical implications in patients with glioma. Tumour Biol. 2014;35:815–9. [DOI] [PubMed] [Google Scholar]
- 22. Sun L, Li G, Dai B, Tan W, Zhao H, Li X, Wang A. Silence of MACC1 expression by RNA interference inhibits proliferation, invasion and metastasis, and promotes apoptosis in U251 human malignant glioma cells. Mol Med Rep. 2015;12:3423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shang C, Hong Y, Guo Y, Liu YH, Xue YX. Influence of the MACC1 gene on sensitivity to chemotherapy in human U251 glioblastoma cells. Asian Pac J Cancer Prev. 2015;16:195–9. [DOI] [PubMed] [Google Scholar]
- 24. Huang N, Wu Z, Lin L, Zhou M, Wang L, Ma H, Xia J, Bin J, Liao Y, Liao W. MiR-338-3p inhibits epithelial-mesenchymal transition in gastric cancer cells by targeting ZEB2 and MACC1/Met/Akt signaling. Oncotarget 2015;6:15222–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yao Y, Dou C, Lu Z, Zheng X, Liu Q. MACC1 suppresses cell apoptosis in hepatocellular carcinoma by targeting the HGF/c-MET/AKT pathway. Cell Physiol Biochem. 2015;35:983–96. [DOI] [PubMed] [Google Scholar]
- 26. Tivnan A, McDonald KL. Current progress for the use of miRNAs in glioblastoma treatment. Mol Neurobiol. 2013;48:757–68. [DOI] [PubMed] [Google Scholar]
- 27. Karsy M, Arslan E, Moy F. Current progress on understanding microRNAs in glioblastoma multiforme. Genes Cancer 2012;3:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peng T, Zhou L, Zuo L, Luan Y. MiR-506 functions as a tumor suppressor in glioma by targeting STAT3. Oncol Rep. 2016;35:1057–64. [DOI] [PubMed] [Google Scholar]
- 29. Wang M, Wen TF, He LH, Li C, Zhu WJ, Trishul NM. A six-microRNA set as prognostic indicators for bile duct cancer. Int J Clin Exp Med. 2015;8:17261–70. [PMC free article] [PubMed] [Google Scholar]
- 30. Koh YW, Hur H, Lee D. Increased MACC1 expression indicates a poor prognosis independent of MET expression in gastric adenocarcinoma. Pathol Res Pract. 2016;212:93–100. [DOI] [PubMed] [Google Scholar]
- 31. Tang J, Chen JX, Chen L, Tang JY, Cui Z, Liu CH, Wang Z. Metastasis associated in colon cancer 1 (MACC1) promotes growth and metastasis processes of colon cancer cells. Eur Rev Med Pharmacol Sci. 2016;20:2825–34. [PubMed] [Google Scholar]
- 32. Sun DW, Zhang YY, Qi Y, Liu GQ, Chen YG, Ma J, Lv GY. Prognostic and clinicopathological significance of MACC1 expression in hepatocellular carcinoma patients: A meta-analysis. Int J Clin Exp Med. 2015;8:4769–77. [PMC free article] [PubMed] [Google Scholar]
- 33. Wu SW, Wang YC, Ci HF, Tao YS. [Expression of vasohibin-1 and MACC1 in lung squamous cell carcinoma and their clinicopathological significance]. Nan Fang Yi Ke Da Xue Xue Bao 2017;37:952–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Duan J, Chen L, Zhou M, Zhang J, Sun L, Huang N, Bin J, Liao Y, Liao W. MACC1 decreases the chemosensitivity of gastric cancer cells to oxaliplatin by regulating FASN expression. Oncol Rep. 2017;37:2583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu J, Zhang D, Li J, Deng X, Liang G, Long Y, He X, Dai T, Ren D. MACC1 induces autophagy to regulate proliferation, apoptosis, migration and invasion of squamous cell carcinoma. Oncol Rep. 2017;38:2369–77. [DOI] [PubMed] [Google Scholar]
- 36. Chen S, Zong ZH, Wu DD, Sun KX, Liu BL, Zhao Y. The role of metastasis-associated in colon cancer 1 (MACC1) in endometrial carcinoma tumorigenesis and progression. Mol Carcinog. 2017;56:1361–71. [DOI] [PubMed] [Google Scholar]
- 37. Shang C, Hong Y, Guo Y and Xue YX. Mir-338-3p inhibits malignant biological behaviors of glioma cells by targeting MACC1 gene. Med Sci Monit. 2016;22:710–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pan T, Chen W, Yuan X, Shen J, Qin C, Wang L. miR-944 inhibits metastasis of gastric cancer by preventing the epithelial-mesenchymal transition via MACC1/Met/AKT signaling. FEBS Open Bio 2017;7:905–14. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39. Li J, Mao X, Wang X, Miao G, Li J. miR-433 reduces cell viability and promotes cell apoptosis by regulating MACC1 in colorectal cancer. Oncol Lett. 2017;13:81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Feng J, Wang J, Chen M, Chen G, Wu Z, Ying L, Zhuo Q, Zhang J, Wang W. miR-200a suppresses cell growth and migration by targeting MACC1 and predicts prognosis in hepatocellular carcinoma. Oncol Rep. 2015;33:713–20. [DOI] [PubMed] [Google Scholar]