Abstract
Previous study indicates that long noncoding RNA NORAD could serve as a competing endogenous RNA to pancreatic cancer metastasis. However, its role in colorectal cancer (CRC) needs to be investigated. In the present study, we found that the expression of NORAD was significantly upregulated in CRC tissues. Furthermore, the expression of NORAD was positively related with CRC metastasis and patients’ poor prognosis. Knockdown of NORAD markedly inhibited CRC cell proliferation, migration, and invasion but induced cell apoptosis in vitro. In vivo experiments also indicated an inhibitory effect of NORAD on tumor growth. Mechanistically, we found that NORAD served as a competing endogenous RNA for miR-202-5p. We found that there was an inverse relationship between the expression of NORAD and miR-202-5p in CRC tissues. Moreover, overexpression of miR-202-5p in SW480 and HCT116 cells significantly inhibited cellular proliferation, migration, and invasion. Taken together, our study demonstrated that the NORAD/miR-202-5p axis plays a pivotal function on CRC progression.
Key words: NORAD, Colorectal cancer (CRC), Proliferation, Metastasis, miR-202-5p
INTRODUCTION
Colorectal cancer (CRC) is one of the most common cancers and gives rise to a large number of cancer-related deaths worldwide1. In China, the rates of CRC incidence and mortality have been quickly increasing in the past years due to late tumor presentation and rapid progression2,3. In recent decades, great efforts have been made to develop effective methods for CRC diagnosis and treatment. Although some advance has been achieved, the 5-year survival rate of CRC remains rather low, especially in China and India4. Therefore, there is an urgent requirement for the novel and effective therapeutic strategies to CRC treatment. The underlying molecular mechanism regulating CRC development and progression needs to be fully understood.
Long noncoding RNAs (lncRNAs) belong to the noncoding RNA family that makes up over 90% of the genomic transcripts5. lncRNAs have a length of longer than 200 nucleotides and no protein-coding ablitiy6. An increasing number of studies have demonstrated that lncRNAs are involved in many kinds of biological processes, such as cellular proliferation, apoptosis, and differentiation7–10. Following the development of sequencing technology, more and more lncRNAs have been identified in different cells or tissues. Accumulating evidence indicates that dysregulation of lncRNAs is closely related with human diseases, including human cancers11. lncRNAs are demonstrated to be new important regulators in the human tumor paradigm12. For instance, downregulation of cancer susceptibility 2 (CASC2) in thyroid carcinoma indicates a poor prognosis and promotes tumorigenesis13. He et al. reported that lncRNA CASC15 is overexpressed in hepatocellular carcinoma and regulates hepatocarcinogenesis14. Luo et al. demonstrated that downregulation of lncRNA-RP11-317J10.2 contributes to cell proliferation and invasion and predicts a poor prognosis in CRC15. In addition, Wang and colleagues reported that lncRNA RP11-436H11.5 sponges miR-335-5p to upregulate BCL-W expression and promotes proliferation and invasion in renal cell carcinoma16. Recently, Li et al.’s study indicated that lncRNA NORAD enhances epithelial–mesenchymal transition (EMT) to promote metastasis in pancreatic cancer17. However, the function of NORAD is still largely unknown in other cancers such as CRC. Our aim was to investigate the expression and molecular mechanism of NORAD in CRC.
In this study, we found that the expression of NORAD was upregulated in CRC tissues. The expression of NORAD was positively correlated with CRC metastasis and poor prognosis. Furthermore, we found that knockdown of NORAD significantly inhibited CRC cell proliferation, migration, and invasion but induced cell apoptosis. In mechanism, we found that NORAD could bind to miR-202-5p, whose expression was downregulated in CRC tissues. Moreover, we found that overexpression markedly inhibited CRC cell proliferation, migration, and invasion. Collectively, our data demonstrated that NORAD promoted CRC cell proliferation, migration, and invasion via sponging miR-202-5p, which provides a new insight into the design of therapeutic targets for CRC.
MATERIALS AND METHODS
Patient Samples
Forty-seven pairs of CRC tissues and adjacent normal tissues involved in our study were collected from Linyi Central Hospital (Shandong Province, P.R. China). Written consent to approve our use of these tissues in the research was obtained from all patients. The protocol was approved by Linyi Central Hospital. All methods involving human patients were performed in accordance with the relevant guidelines and regulations of Linyi Central Hospital.
Cell Lines and Cell Culture
Human CRC cell lines (SW480 and HCT116) and a normal colorectal mucosa cell line (FHC) were obtained from the ATCC (Manassas, VA, USA) and maintained in DMEM supplemented with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA). Cells were incubated at 37°C in a humidified atmosphere with 5% CO2.
Cell Transfection
siRNA against NORAD and miR-202-5p mimic were synthetized by Invitrogen and transduced into HCT116 and SW480 cells with Lipofectamine 3000 (Invitrogen).
Apoptosis Analysis
Apoptosis analysis was performed through Annexin-V-FITC/PI apoptosis detection kit (eBiosciences, USA) by FACS.
Xenograft Tumor Formation
We purchased 6-week-old male BALB/c nude mice from HFK Biosciences (Beijing, P.R. China), and the mice were maintained under pathogen-free conditions with the approval of Linyi Central Hospital. For tumor propagation analysis, 2 × 106 NORAD-silenced cells or control cells were subcutaneously injected into BALB/c nude mice. Six mice were used for each group. Tumor weight was measured on day 30 postinjection. Animal experiments were performed in accordance with the relevant guidelines and regulations of the Institutional Animal Care and Use Committees at Linyi Central Hospital, and protocols were approved by the Institutional Animal Care and Use Committees at Linyi Central Hospital.
Cell Proliferation
Each well of a 96-well plate contained about 5,000 transfected cells. Cell viability was assessed using a cell counting kit-8 (CCK-8) assay (7 Sea Biotech, Shanghai, P.R. China). Following incubation for 24, 48, and 72 h, 10 μl of CCK-8 was added to each well and incubated for 2 h. The absorbance value was determined using a multimode microplate reader (Berthold Technologies GmbH & Co.KG, Bad Wildbad, Germany) at 450 nm.
Transwell Assay
SW480 and HCT-116 cells were suspended in serum-free medium, and 3 × 104 cells (100 μl) were plated into the upper chambers of Transwell inserts (8.0-μm pore size; Corning Incorporated, Corning, NY, USA) for invasion (with Matrigel) or migration (without Matrigel) assays. The inserts were placed in 24-well plates containing 600 μl of media with 10% FBS. After incubation for 24 h for migration and 48 h for invasion, cells were fixed for 20 min and stained with 1% crystal violet. The cell numbers were calculated and imaged under a microscope from five random fields.
Real-Time Quantitative PCR
Total RNAs were extracted with TRIzol according to the manufacturer’s protocol. Then cDNA was synthesized with the M-MLV reverse transcriptase (Promega, Madison, WI, USA). Then mRNA transcripts were analyzed with the ABI 7300 qPCR system using specific primer pairs. Relative expressions were calculated and normalized to endogenous Actb or U6. The primer sequence information is available if requested.
Western Blot
After transfection, CRC total protein was extracted by lysis buffer. All the protein lysates were separated using 10% SDS-PAGE and transferred onto a polyvinylidene fluoride membrane. Then the membrane was incubated with specific primary anti-human antibodies, followed by their respective appropriate secondary antibodies. GAPDH was used as a control.
Luciferase Reporter Assay
The sequence of NORAD containing the binding sites [wild type (WT) or all sites mutated] for miR-202-5p was synthesized by Sangon Biotech (Beijing, P.R. China) and cloned into pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega). Cells were cotransfected with miR-202-5p mimics or NC and reporter vector using Lipofectamine 2000 (Invitrogen). After incubation for 24 h, cells were lysed and the luciferase activity was measured using a Dual-Luciferase Assay System (Promega).
Statistical Analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences version 20.0 software (SPSS Inc., Chicago, IL, USA). Survival curves were calculated using the Kaplan–Meier method and were analyzed using the log-rank test. For comparisons, one-way analyses of variance and two-tailed Student’s t-tests were performed, as appropriate. A value of p < 0.05 was considered statistically significant.
RESULTS
NORAD Was Overexpressed in Colorectal Cancer Tissues
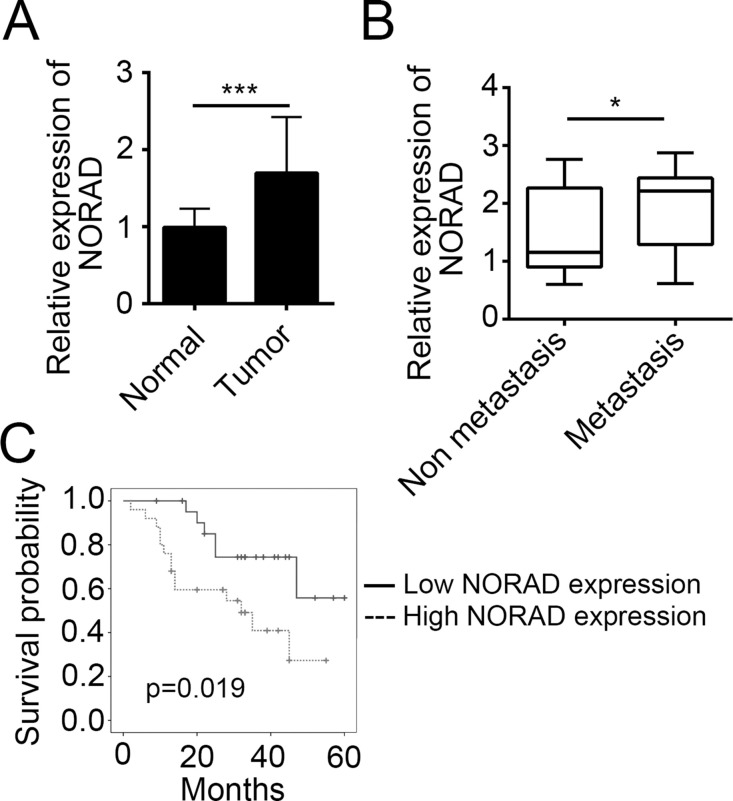
To explore the role of NORAD in CRC, we determined the expression level of NORAD in CRC tissues. By RT-qPCR, we found that the expression of NORAD was upregulated in tumor tissues compared to adjacent normal tissues (Fig. 1A). A previous study indicates that NORAD enhances EMT in pancreatic cancer17. To determine whether there is also a relationship between NORAD expression and CRC metastasis, we grouped CRC tissues into nonmetastasis and metastasis subgroups based on clinical characteristics. Then we analyzed the expression of NORAD and found a higher expression of NORAD in metastatic tissues (Fig. 1B). Then according to the expression level of NORAD (the median value was used as a cutoff), we divided these 47 CRC tissues into high- and low-expression groups, followed by Kaplan–Meier survival analysis. Results indicated that higher expression of NORAD in CRC patients suggested poorer prognosis (Fig. 1C).
Figure 1.
NORAD was overexpressed in colorectal cancer (CRC) tissues. (A) Real-time quantitative PCR (RT-qPCR) was used to determine the expression level of NORAD in CRC tissues (n = 47) and adjacent normal tissues (n = 47). (B) The CRC tissues were classified into two groups based on clinical characteristics, and the expression level of NORAD was determined by RT-qPCR. (C) The CRC tissues were divided into two groups according to their expression level of NORAD. Kaplan–Meier survival analysis was performed. *p < 0.05 and ***p < 0.001 by two-tailed Student’s t-test. All data presented are shown as means ± SD collected from three independent experiments.
Knockdown of NORAD Suppressed Cell Proliferation but Increased Cellular Apoptosis
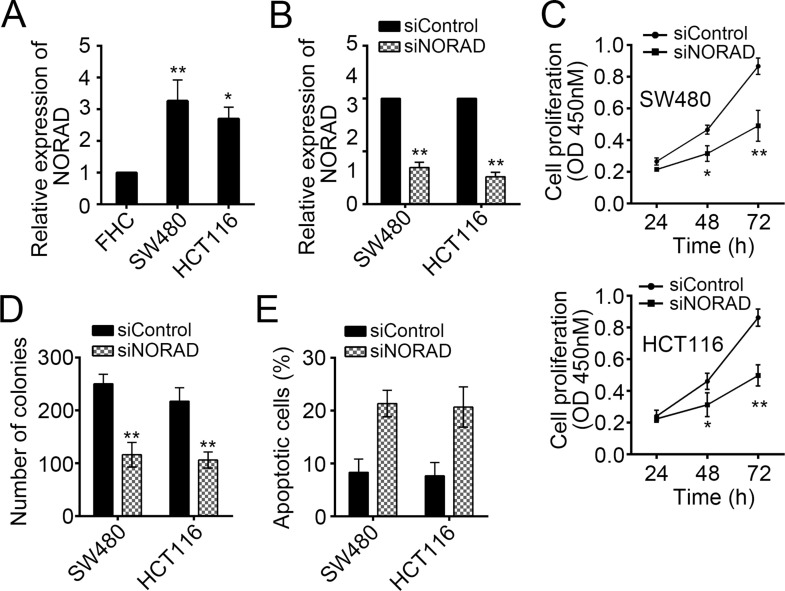
To determine the physiological function of NORAD, we examined its expression pattern in CRC cell lines (SW480 and HCT116 cells). By RT-qPCR, we found that NORAD was upregulated in SW480 and HCT116 cells compared with FHC cells (Fig. 2A). Then we knocked down NORAD by specific siRNA-mediated intervention in SW480 and HCT116 cells (Fig. 2B). To evaluate the effect of NORAD on cell proliferation, we performed CCK-8 and colony formation assays. As shown, NORAD knockdown significantly inhibited cell growth and colony formation (Fig. 2C and D). Then we analyzed the change in cell apoptosis after NORAD knockdown. We found that NORAD silencing remarkably promoted cell death in SW480 and HCT116 cells (Fig. 2E). Taken together, NORAD knockdown resulted in reduced cell proliferation but increased apoptosis.
Figure 2.
Knockdown of NORAD suppressed cell proliferation but increased cellular apoptosis. (A) RT-qPCR was used to examine the expression of NORAD in CRC cell lines. (B) NORAD was silenced in SW480 and HCT116 cells. (C) Cell counting kit-8 (CCK-8) and (D) colony formation assays were performed to determine the effect of NORAD on cell proliferation. (E) SW480 and HCT116 cells were stained with annexin-V/PI and analyzed by FACS for apoptosis. *p < 0.05 and **p < 0.01 by two-tailed Student’s t-test. All data were collected from three independent experiments.
Knockdown of NORAD Inhibited Tumor Growth In Vivo
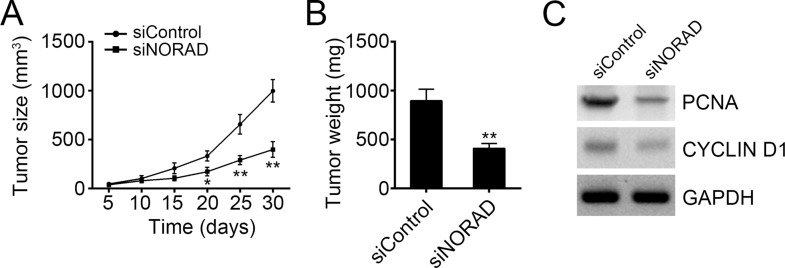
To further verify the effect of NORAD on tumor cell growth, we conducted a xenograft experiment. We injected control or NORAD-silenced SW480 cells into recipient nude mice subcutaneously. At indicative time points, we measured the tumor sizes and found that NORAD knockdown significantly inhibited tumor growth in vivo (Fig. 3A). Moreover, at the endpoint of this experiment, we analyzed the tumor weights and found that tumor tissues derived from NORAD-silenced SW480 cells were smaller (Fig. 3B). Finally, we used Western blot to check cell proliferation in the tumor tissues. Results indicated a lower expression of PCNA and cyclin D1 in tumor tissues derived from NORAD-silenced SW480 cells (Fig. 3C), which suggests less proliferation.
Figure 3.
Knockdown of NORAD inhibited tumor growth in vivo. (A) Wild-type (WT) and NORAD-silenced SW480 cells were injected into recipient nude mice. The tumor sizes were measured every 5 days. N = 6 for each group. (B) At the endpoint of the experiment, tumor weights were measured. N = 6 for each group. (C) The cellular proliferation was examined by Western blot in the tumor tissues. The protein levels of PCNA and cyclin D1 were determined. *p < 0.05 and **p < 0.01 by two-tailed Student’s t-test. All data presented are shown as means ± SD collected from three independent experiments.
NORAD Knockdown Reduced the Migration and Invasion of CRC Cells
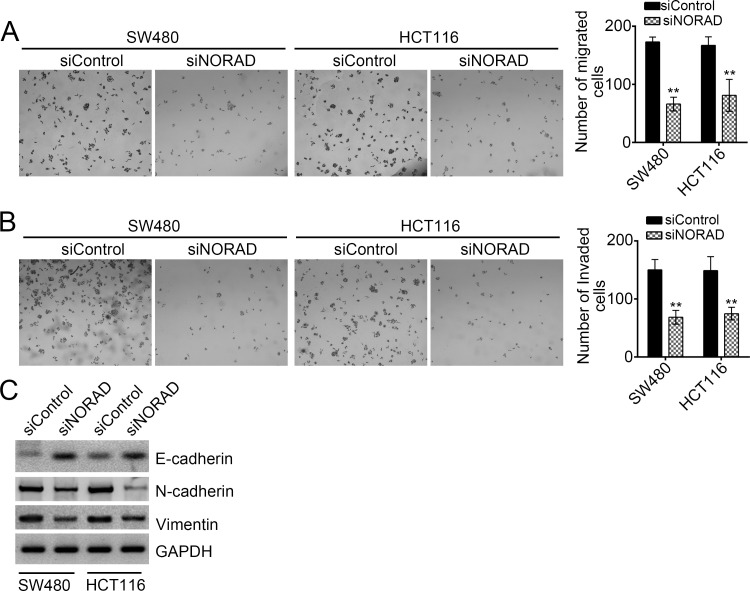
We found a positive relationship between the expression of NORAD and CRC metastasis (Fig. 1B). To further verify this conclusion, we performed Transwell assays. We found that NORAD knockdown significantly inhibited the numbers of migrated and invaded SW480 or HCT116 cells (Fig. 4A and B). Moreover, after knockdown of NORAD in SW480 and HCT116 cells, the expression of the epithelial cell marker E-cadherin was upregulated, while that of mesenchymal cell markers (N-cadherin and vimentin) were downregulated (Fig. 4C), which suggested that NORAD also promoted the EMT in CRC.
Figure 4.
NORAD knockdown reduced the migration and invasion of CRC cells. (A, B) Transwell assay was conducted with WT or NORAD-depleted SW480 and HCT116 cells. NORAD knockdown significantly decreased the numbers of migrated and invaded cells. (C) NORAD knockdown promoted E-cadherin expression but inhibited the expression of N-cadherin and vimentin in SW480 and HCT116 cells. **p < 0.01 by two-tailed Student’s t-test. All data presented are shown as means ± SD collected from three independent experiments.
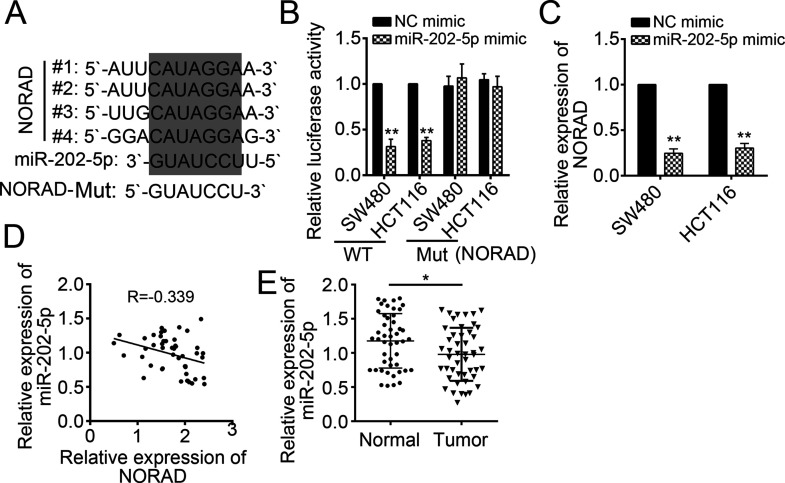
NORAD Served as a Sponge for miR-202-5p
To further determine the downstream mechanism mediated by NORAD, we analyzed its potential binding miRNAs using the miRDB tool (http://mirdb.org/miRDB/index.html). Among them, miR-202-5p ranked top. There were four potential binding sites in NORAD for miR-202-5p (Fig. 5A). To demonstrate this prediction, we constructed a luciferase report plasmid containing these WT or mutant binding sites. Through luciferase report assay, we found that overexpression of miR-202-5p significantly suppressed the luciferase activity in SW480 and HCT116 cells transduced with WT NORAD-reporter plasmid (Fig. 5B). Notably, after the binding site mutation, miR-202-5p could not inhibit luciferase activity (Fig. 5B). Furthermore, by RT-qPCR, we found that overexpression of miR-202-5p markedly downregulated the expression of NORAD in SW480 and HCT116 cells (Fig. 5C). We also found that there was an inverse correlation between the expression of NORAD and miR-202-5p in CRC tissues (Fig. 5D). We then determined the expression patterns of miR-202-5p in CRC tissues by RT-qPCR. We found that the expression of miR-202-5p was dramatically downregulated in tumor tissues compared with normal tissues (Fig. 5E).
Figure 5.
NORAD served as a sponge for miR-202-5p. (A) There were four potential binding sites in NORAD for miR-202-5p. (B) Luciferase activity reporter assay indicated that miR-202-5p mimic significantly reduced the luciferase activity, while mutation of binding sites in NORAD abolished this effect in SW480 and HCT116 cells. Mut, mutant. (C) Overexpression of miR-202-5p inhibited the expression of NORAD in SW480 and HCT116 cells. (D) RT-qPCR analysis indicated that the expression of NORAD was negatively correlated with that of miR-202-5p in CRC tissues. (E) RT-qPCR analysis demonstrated that the expression of miR-202-5p was downregulated in CRC tissues (n = 47). *p < 0.05 and **p < 0.01 by two-tailed Student’s t-test. All data presented are shown as means ± SD collected from three independent experiments.
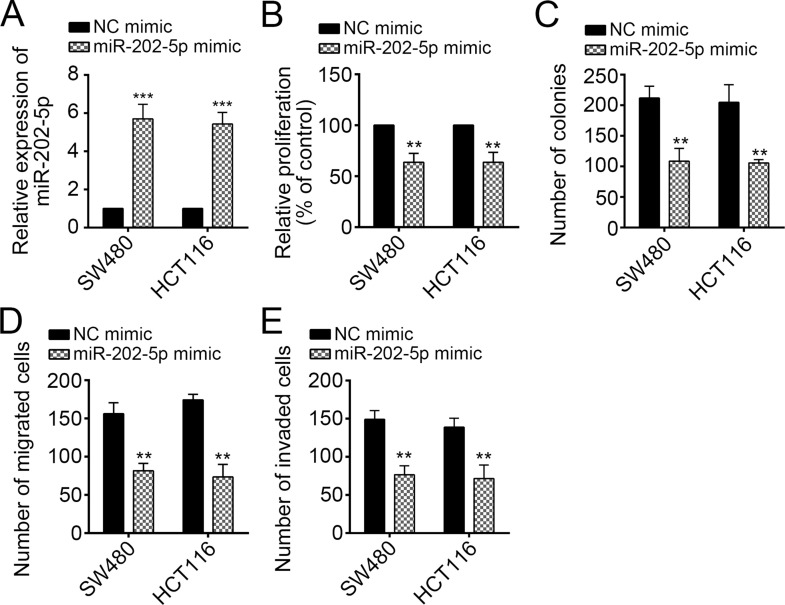
Overexpression of miR-202-5p Inhibited CRC Cell Proliferation, Migration, and Invasion
Finally, we explored the function of miR-202-5p in CRC cells. We overexpressed miR-202-5p in SW480 and HCT116 cells (Fig. 6A). Then CCK-8 and colony formation assays were conducted, and the results indicated that overexpression of miR-202-5p significantly suppressed cell proliferation (Fig. 6B and C). Overexpression of miR-202-5p reduced the number of migrated and invaded SW480 and HCT116 cells as shown by Transwell assays (Fig. 6D and E). Taken together, miR-202-5p was a tumor suppressor and inhibited by NORAD in CRC.
Figure 6.
Overexpression of miR-202-5p inhibited CRC cell proliferation, migration, and invasion. (A) RT-qPCR analysis indicated that miR-202-5p was overexpressed in SW480 and HCT116 cells. (B) CCK-8 and (C) colony formation assays were used to assess the function of miR-202-5p on cell proliferation. (D, E) Transwell assay was used to determine the effect of miR-202-5p on cell migration and invasion. **p < 0.01 and ***p < 0.001 by two-tailed Student’s t-test. All data presented are shown as means ± SD collected from three independent experiments.
DISCUSSION
CRC is one of the main public health problems around the world nowadays. Effective novel therapeutic strategies are urgently required for CRC patients. Therefore, defining the molecular mechanism in CRC development and progression is particularly important. Following the rapid advances in sequencing technology, more and more important lncRNAs are being identified in human tissues. A previous report indicated a close relationship between lncRNA expression and human cancers18. Thus, it is necessary to screen out important lncRNAs and determine their roles and mechanism in cancers, including CRC. In the present study, we found that lncRNA NORAD was involved in the development and progression of CRC.
Recently, lncRNAs have attracted wide attention for their unexpected important functions. lncRNAs are involved in nearly all kinds of human cancers. For example, Li et al. reported that overexpression of metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) indicates a poor prognosis in bladder cancer19. Lei and colleagues showed that lncRNA TUG1 regulates cell proliferation, migration, and invasion in papillary thyroid cancer20. In CRC, long intergenic non-protein-coding RNA, regulator of reprogramming (lincRNA-ROR) is reported to promote colon cancer progression21. In our study, we found that NORAD was highly expressed in CRC tissues. Moreover, the expression of NORAD was positively related with CRC metastasis and patients’ prognosis. By CCK-8 and Transwell assays, we demonstrated that knockdown of NORAD significantly inhibited cell proliferation, migration, and invasion. Furthermore, we found that NORAD silencing markedly promoted cell apoptosis.
lncRNAs could serve as endogenous sponges via binding to miRNAs and regulating their function22. For instance, lncRNA small nucleolar RNA host gene 3 (SNHG3) promotes aggressive development of CRC by functioning as a competing endogenous RNA23. NORAD is also reported to act as a competing endogenous RNA and promote pancreatic cancer metastasis17. Thus, we searched for potential miRNAs sponged by NORAD in CRC. We found that miR-202-5p was the most potential target miRNA of NORAD. A previous study reported that miR-202 attenuates TGF-β1-induced EMT in pancreatic cancer24. miR-202 also could inhibit tumor cell proliferation in endometrial adenocarcinoma25. Another report indicated that miR-202 inhibits cell proliferation, migration, and invasion in glioma26. Additionally, miR-202 was found to promote cell apoptosis in esophageal squamous cell carcinoma27. However, the role of miR-202-5p in CRC remains to be further investigated. In our study, we demonstrated that NORAD directly bound to miR-202-5p in CRC cells. Moreover, we found that miR-202-5p was downregulated in CRC tissues. By functional experiments, we found that overexpression of miR-202-5p significantly inhibited CRC cell proliferation, migration, and invasion, which suggested that NORAD regulated CRC progression via inhibiting miR-202-5p. However, the downstream mechanism of miR-202-5p requires further exploration in CRC.
In summary, our study showed that NORAD was overexpressed in CRC tissues. Moreover, highly expressed NORAD could promote cell proliferation, migration, and invasion by inhibition of miR-202-5p, which suggests that NORAD/miR-202-5p may be a potential therapeutic target.
ACKNOWLEDGMENTS
The authors thank all the patients who participated in this study.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136(5):E359–86. [DOI] [PubMed] [Google Scholar]
- 3. Chen W, Zheng R, Zhang S, Zhao P, Zeng H, Zou X. Report of cancer incidence and mortality in China, 2010. Ann Transl Med. 2014;2(7):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee YC, Lee YL, Chuang JP, Lee JC. Differences in survival between colon and rectal cancer from SEER data. PLoS One 2013;8(11):e78709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Su J, Zhang E, Han L, Yin D, Liu Z, He X, Zhang Y, Lin F, Lin Q, Mao P, Mao W, Shen D. Long noncoding RNA BLACAT1 indicates a poor prognosis of colorectal cancer and affects cell proliferation by epigenetically silencing of p15. Cell Death Dis. 2017;8(3):e2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu BY, Ye BQ, Yang LL, Zhu XX, Huang GL, Zhu PP, Du Y, Wu JY, Qin XW, Chen RS, Tian Y, Fan ZS. Long noncoding RNA lncKdm2b is required for ILC3 maintenance by initiation of Zfp292 expression. Nat Immunol. 2017;18(5):499–508. [DOI] [PubMed] [Google Scholar]
- 7. Wang JD, Cao L, Wu JH, Wang QG. Long non-coding RNA SNHG1 regulates NOB1 expression by sponging miR-326 and promotes tumorigenesis in osteosarcoma. Int J Oncol. 2018;52(1):77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luo JH, Chen J, Li H, Yang Y, Yun HC, Yang SQ, Mao XM. LncRNA UCA1 promotes the invasion and EMT of bladder cancer cells by regulating the miR-143/HMGB1 pathway. Oncol Lett. 2017;14(5):5556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhan Y, Li Y, Guan B, Wang Z, Peng D, Chen Z, He A, He S, Gong Y, Li X, Zhou L. Long non-coding RNA HNF1A-AS1 promotes proliferation and suppresses apoptosis of bladder cancer cells through upregulating Bcl-2. Oncotarget 2017;8(44):76656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stephanova DI, Kossev A. Theoretical predication of temperature effects on accommodative processes in simulated amyotrophic lateral sclerosis during hypothermia and hyperthermia. J Integr Neurosci. 2016;15(4):553–69. [DOI] [PubMed] [Google Scholar]
- 11. Nagano T, Fraser P. No-nonsense functions for long noncoding RNAs. Cell 2011;145(2):178–81. [DOI] [PubMed] [Google Scholar]
- 12. Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21(11):1253–61. [DOI] [PubMed] [Google Scholar]
- 13. Xiong X, Zhu H, Chen X. Low expression of long noncoding RNA CASC2 indicates a poor prognosis and promotes tumorigenesis in thyroid carcinoma. Biomed Pharmacother. 2017;93:391–7. [DOI] [PubMed] [Google Scholar]
- 14. He T, Zhang L, Kong Y, Huang Y, Zhang Y, Zhou D, Zhou X, Yan Y, Zhang L, Lu S, Zhou J, Wang W. Long non-coding RNA CASC15 is upregulated in hepatocellular carcinoma and facilitates hepatocarcinogenesis. Int J Oncol. 2017;51(6):1722–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luo J, Xu LN, Zhang SJ, Jiang YG, Zhuo DX, Wu LH, Jiang X, Huang Y. Downregulation of LncRNA-RP11-317J10.2 promotes cell proliferation and invasion and predicts poor prognosis in colorectal cancer. Scand J Gastroenterol. 2018;53(1):38–45. [DOI] [PubMed] [Google Scholar]
- 16. Wang K, Jin W, Song Y, Fei X. LncRNA RP11-436H11.5, functioning as a competitive endogenous RNA, upregulates BCL-W expression by sponging miR-335-5p and promotes proliferation and invasion in renal cell carcinoma. Mol Cancer 2017;16(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li H, Wang X, Wen C, Huo Z, Wang W, Zhan Q, Cheng D, Chen H, Deng X, Peng C, Shen B. Long noncoding RNA NORAD, a novel competing endogenous RNA, enhances the hypoxia-induced epithelial-mesenchymal transition to promote metastasis in pancreatic cancer. Mol Cancer 2017;16(1):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu JH, Chang WH, Fu HW, Shu WQ, Yuan T, Chen P. Upregulated long non-coding RNA LOC90784 promotes cell proliferation and invasion and is associated with poor clinical features in HCC. Biochem Biophys Res Commun. 2017;490(3):920–6. [DOI] [PubMed] [Google Scholar]
- 19. Li C, Cui Y, Liu LF, Ren WB, Li QQ, Zhou X, Li YL, Li Y, Bai XY, Zu XB. High expression of long noncoding RNA MALAT1 indicates a poor prognosis and promotes clinical progression and metastasis in bladder cancer. Clin Genitourin Cancer 2017;15(5):570–6. [DOI] [PubMed] [Google Scholar]
- 20. Lei HW, Gao Y, Xu XY. LncRNA TUG1 influences papillary thyroid cancer cell proliferation, migration and EMT formation through targeting miR-145. Acta Biochim Biophys Sin. 2017;49(7):588–97. [DOI] [PubMed] [Google Scholar]
- 21. Zhou P, Sun LX, Liu DF, Liu CK, Sun L. Long non-coding RNA ROR promotes the progression of colon cancer and holds prognostic value by associating with miR-145. Pathol Oncol Res. 2016;22(4):733–40. [DOI] [PubMed] [Google Scholar]
- 22. Han YS, Lee JH, Lee SH. Fucoidan inhibits the migration and proliferation of HT-29 human colon cancer cells via the phosphoinositide-3 kinase/Akt/mechanistic target of rapamycin pathways. Mol Med Rep. 2015;12(3):3446–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang WZ, Tian YM, Dong ST, Cha YL, Li J, Guo XH, Yuan X. The long non-coding RNA SNHG3 functions as a competing endogenous RNA to promote malignant development of colorectal cancer. Oncol Rep. 2017;38(3):1402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mody HR, Hung SW, Pathak RK, Griffin J, Cruz-Monserrate Z, Govindarajan R. miR-202 diminishes TGF beta receptors and attenuates TGF beta 1-induced EMT in pancreatic cancer. Mol Cancer Res. 2017;15(8):1029–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deng X, Hou C, Liang Z, Wang H, Zhu L, Xu H. miR-202 suppresses cell proliferation by targeting FOXR2 in endometrial adenocarcinoma. Dis Markers 2017;2017:2827435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang J, Fan B, Zhao Y, Fang J. MicroRNA-202 inhibits cell proliferation, migration and invasion of glioma by directly targeting metadherin. Oncol Rep. 2017;38(3):1670–8. [DOI] [PubMed] [Google Scholar]
- 27. Meng X, Chen X, Lu P, Ma W, Yue D, Song L, Fan Q. miR-202 promotes cell apoptosis in esophageal squamous cell carcinoma by targeting HSF2. Oncol Res. 2017;25(2):215–23. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]