Abstract
Resistance to bortezomib (BZ) is the major problem that largely limits its clinical application in multiple myeloma treatment. In the current study, we investigated whether ClC5, a member of the chloride channel family, is involved in this process. The MTT assay showed that BZ treatment decreased cell viability in three multiple myeloma cell lines (ARH77, U266, and SKO-007), with IC50 values of 2.83, 4.37, and 1.91 nM, respectively. Moreover, BZ increased the conversion of LC3B-I to LC3B-II and expressions of beclin-1 and ATG5, concomitantly with a decreased p62 expression. Pharmacological inhibition of autophagy with 3-MA facilitated cell death in response to BZ treatment. Additionally, BZ increased ClC5 protein expression in ARH77, U266, and SKO-007 cells. Knockdown of ClC5 with small interfering RNA sensitized cells to BZ treatment, and upregulation of ClC5 induced chemoresistance to BZ. Furthermore, ClC5 downregulation promoted BZ-induced LC3B-I to LC3B-II conversion and beclin-1 expression, whereas overexpression of ClC5 showed the opposite results in ARH77 cells. Finally, BZ induced dephosphorylation of AKT and mTOR, which was significantly attenuated by ClC5 inhibition. However, ClC5 upregulation further enhanced AKT and mTOR dephosphorylation induced by BZ. Our study demonstrates that ClC5 induces chemoresistance of multiple myeloma cells to BZ via increasing prosurvival autophagy by inhibiting the AKT–mTOR pathway. These data suggest that ClC5 may play a critical role in future multiple myeloma treatment strategies.
Key words: Bortezomib (BZ), Multiple myeloma, Chemoresistance, Autophagy, ClC5, AKT–mTOR pathway
INTRODUCTION
Multiple myeloma is a frequent malignancy of plasma cells that is characterized by bone destruction, anemia, hypercalcemia, renal disease, and immunodeficiency1–3. Its incidence varies globally from 1/100,000 people in China4. Patients with multiple myeloma often succumb to recurrence or increased susceptibility to viral, bacterial, and fungal infection, which are the major underlying cause of death5. For the last decades, extensive research efforts have sought to characterize the molecular mechanisms or targets that contribute to the development of multiple myeloma6. Although the survival rates could now exceed 10 years due to the use of various novel treatment options including bortezomib (BZ), thalidomide, lenalidomide, and hematopoietic stem cell transplantation, multiple myeloma is still an incurable hematological malignancy7,8. The major problem in multiple myeloma treatment is drug resistance that is produced by high-dose chemotherapy4,5. Therefore, there is an unmet clinical need for developing more effective treatments to facilitate the sensitivity of multiple myeloma cells to chemotherapy.
Increasing lines of evidence indicate that the proteasome is necessary for tumor cell angiogenesis, growth, and apoptosis9–11. Proteasome inhibition has been implicated as an important therapeutic strategy for the treatment of multiple myeloma12. BZ is the first proteasome inhibitor to be introduced for clinical treatment and is predominantly used in combination with prednisolone or thalidomide in multiple myeloma patients13,14. BZ inhibits nuclear factor κ light chain enhancer of activated B cell (NF-κB) activation and antiapoptotic B-cell lymphoma 2 (Bcl-2) expression and disrupts the balance between protein biosynthesis and protein degradation, leading to cell death by apoptosis11,14. However, emerging studies have suggested that increasing administration of BZ can eventually develop drug resistance12,15,16. While there are several possible explanations involved in the resistance to BZ in multiple myeloma cells, such as insulin growth factor-1, proteasome subunit β5 mutation, and the differentiation status of myeloma cells17–19, the exact mechanisms have not yet been fully understood.
Autophagy is a highly conserved catabolic process that could digest and recycle cellular proteins and organelles to maintain cellular homeostasis20. Multiple research studies have shown that autophagy serves as a potential mechanism for the development of chemoresistance in cancer cells11,12,21. Moreover, it should be noted that autophagy exerts different roles in regulating chemoresistance toward various antineoplastic therapies20,21. Recent findings support the theory that the protective role of autophagy may promote the survival of tumor cells to overcome the stress caused by chemotherapy22,23. On the contrary, autophagy-activated cell death is also the predominant mechanism that enhances chemotherapy sensitivity24,25.
Growing evidence has shown that chloride channels are critical for growth control in a number of cell types, including rat aortic smooth muscle cells, glioma cells, and mouse liver cells26–28. Chloride channel 5 (ClC5), a member of the chloride channel family, is primarily found to be expressed in the kidney29. It has been demonstrated that ClC5 is expressed in glioma cells, native leukocytes, and leukemic cells30–32. Several microRNAs (miRNAs) hosted in the ClC5 locus could increase the resistance of chronic lymphocytic leukemia cells to chemotherapy33. Moreover, the expression of ClC5 was cell cycle dependent in myeloid cells29. In the present study, we set out to examine the role of autophagy in BZ resistance and the crosstalk between autophagy and ClC5 in multiple myeloma cells under BZ exposure. Our findings suggest, through different multiple myeloma cell lines, that ClC5 may contribute to the evasion of multiple myeloma cell death in response to BZ through enhancing prosurvival autophagy.
MATERIALS AND METHODS
Materials and Reagents
Roswell Park Memorial Institute (RPMI)-1640 medium, fetal bovine serum (FBS), streptomycin, penicillin, and glutamine were purchased from Gibco (Grand Island, NY, USA). BZ was obtained from Selleckchem (Houston, TX, USA) and dissolved in 10 mM ethanol. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and 3-methyladenine (3-MA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The 3-MA was dissolved in dimethyl sulfoxide (DMSO) to make stock solutions of 1 mM. Antibodies targeting microtubule-associated protein 1A/1B light chain 3-I/II (LC3B-I/II), autophagy protein 5 (ATG5), phosphotyrosine-independent ligand for the Lck SH2 domain of 62 kDa (p62), phosphorylated AKT (p-AKT), AKT, phosphorylated mechanistic target of rapamycin (p-mTOR), and mTOR were obtained from Cell Signaling Technology (Boston, MA, USA). Antibodies targeting beclin-1 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were from Santa Cruz Biotech (Santa Cruz, CA, USA). Antibody of ClC5 was purchased from Abcam (Cambridge, MA, USA). All secondary horseradish peroxidase (HRP)-linked antibodies were from Thermo (Wilmette, IL, USA).
Cell Culture
The human multiple myeloma cell lines ARH77, U266, and SKO-007 were acquired from the American Type Culture Collection (Manassas, VA, USA) and cultured in RPMI-1640 medium supplemented with 10% FBS, 100 μg/ml streptomycin, 100 U/ml penicillin, and 4 mM glutamine in a humidified atmosphere with 5% CO2 at 37°C.
Cell Viability Assay
The cell viability was detected using the MTT assay. ARH77, U266, or SKO-007 cells were seeded in 96-well plates at a density of 104 cells per well. After the indicated treatments, the cells were incubated with MTT at a final concentration of 10 μg/ml for 4 h (5% CO2, 37°C). The medium was removed, and the cells were dissolved in DMSO. The absorbance was measured at a wavelength of 490 nm using a microplate reader (Bio-Tek, Winooski, VT, USA). The value of IC50 was calculated with the SPSS 17.0 statistical software (SPSS Inc., Chicago, IL, USA).
Western Blotting Analysis
The cells were lysed with radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime, Jiangsu, P.R. China) containing protease and phosphatase inhibitors (Roche Applied Science, Indianapolis, IN, USA) at 4°C. The lysates were centrifuged at 12,000 × g for 15 min at 4°C, and the supernatants were harvested. The protein content was quantified by the Enhanced BCA Protein Assay Kit (Beyotime). Equal amounts of protein were supplemented with concentrated sodium dodecyl sulfate (SDS) sample buffer and denatured at 100°C for 5 min. Forty micrograms of protein was separated on a 10%–12% polyacrylamide gel and then transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad, Hercules, CA, USA). The membranes were blocked with 5% nonfat milk at room temperature for 1 h and then probed with primary antibodies including anti-LC3B-I/II (1:1,000), anti-beclin-1 (1:500), anti-ATG5 (1:2,000), anti-p62 (1:500), anti-ClC5 (1:500), anti-p-AKT (1:1,000), anti-AKT (1:1,000), anti-p-mTOR (1:1,000), anti-mTOR (1:1,000), and anti-GAPDH (1:4,000). The immunoreactive proteins were detected with HRP-linked secondary antibodies (1:1,000) and an enhanced chemiluminescence reagent (Beyotime). Densitometry of bands was quantified using a Molecular Imager, ChemiDoc XRS System (Bio-Rad).
Knockdown of ClC5 by siRNA
The stealth small interfering RNA (siRNA) targeting ClC5 (5′-GCACTTCCATCATTCATTT-3′) was synthesized and purchased from Invitrogen (Carlsbad, CA, USA). ARH77, U266, or SKO-007 cells were electroporated with either ClC5 siRNA (si-ClC5; 40 nM) or negative siRNA (NS; 40 nM) using the Nucleofector Kit (Lonza, Basel, Switzerland). The cells were then replaced with RPMI-1640 medium containing 10% FBS and incubated for 48 h at 37°C.
ClC5 Transfection
Full-length cDNA for human ClC5 was purchased from OpenBioSystems (Huntsville, AL, USA) and then was amplified and cloned into pCMV-Tag2 (Invitrogen). ClC5 plasmid DNA (2 μg) was transfected using Lipofetamine Plus reagent diluted in Opti-Minimum Essential Medium (Opti-MEM; Invitrogen) for 48 h according to the supplier’s instructions.
Statistical Analysis
All data were given as mean ± SEM. Statistical analysis of the data between the control and treatment groups was performed using one-way analysis of variance (ANOVA) or the unpaired two-tailed Student’s t-test. Statistical analysis was performed by the SPSS 17.0 statistical software. A value of p < 0.05 indicated statistical significance.
RESULTS
BZ Induced Cell Growth Inhibition and Autophagy in Multiple Myeloma Cell Lines
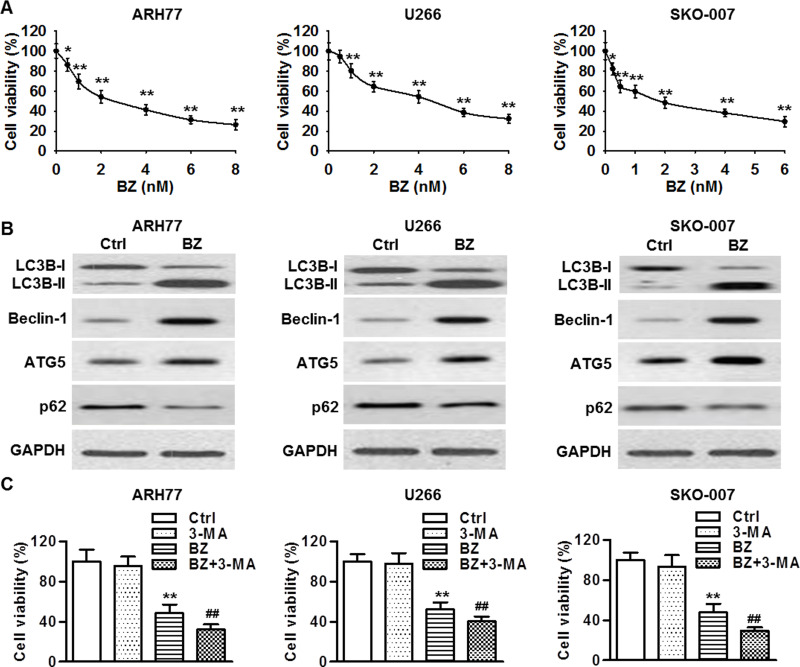
The MTT assay showed that BZ inhibited the cell viability of ARH77, U266, and SKO-007 cells in a dose-dependent manner. After 48 h of BZ treatment, the IC50 value of BZ was 2.83 ± 0.15, 4.37 ± 0.21, and 1.91 ± 0.11 nM in ARH77, U266, and SKO-007 cells, respectively (Fig. 1A). A 3, 4.5, and 2 nM dose of BZ was therefore used for ARH77, U266, and SKO-007 cells, respectively. Autophagy has been demonstrated to play an important role in the development of chemoresistance in multiple myeloma34. LC3B-I conversion to LC3B-II is a hallmark of autophagic activity, and Western blotting showed that BZ treatment significantly promoted the conversion of LC3B-I to LC3B-II in ARH77, U266, and SKO-007 cells. Furthermore, BZ treatment also increased the expression of beclin-1 and ATG5, along with decreased expression of p62, suggesting BZ induces autophagy in multiple myeloma cells (Fig. 1B). To further explore whether autophagy serves as a survival or cell death mechanism that facilitates or attenuates chemoresistance of multiple myeloma cells to BZ treatment, all three multiple myeloma cell lines were treated with BZ in the presence or absence of 3-MA (1 μM) for 48 h, and cell viability was measured. The 3-MA treatment alone had little influence on the viability of ARH77, U266, and SKO-007 cells at a dose of 1 μM. However, combined treatment with BZ and 3-MA further decreased cell viability, compared with cells treated with BZ alone (Fig. 1C). These data suggest that autophagy is likely to be a prosurvival mechanism that attenuates the chemosensitivity of multiple myeloma cells to BZ treatment.
Figure 1.
Bortezomib (BZ) treatment induced cell growth inhibition and autophagy in multiple myeloma cell lines. (A) ARH77, U266, and SKO-007 cells were treated with various concentrations of BZ for 48 h. The viability of multiple myeloma cells was decreased in a dose-dependent manner. *p < 0.05, **p < 0.01 versus control (0 nM), n = 8. (B) The protein expressions of microtubule-associated protein 1A/1B light chain 3-I/II (LC3B-I/II), beclin-1, autophagy protein 5 (ATG5), and phosphotyrosine-independent ligand for the Lck SH2 domain of 62 kDa (p62) were determined by Western blotting. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was the loading control. (C) All three multiple myeloma cell lines were treated with BZ in the presence or absence of 3-methyladenine (3-MA; 1 μM) for 48 h, and cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. **p < 0.01 versus control; ##p < 0.01 versus BZ, n = 6.
BZ Increased ClC5 Expression in Multiple Myeloma Cells
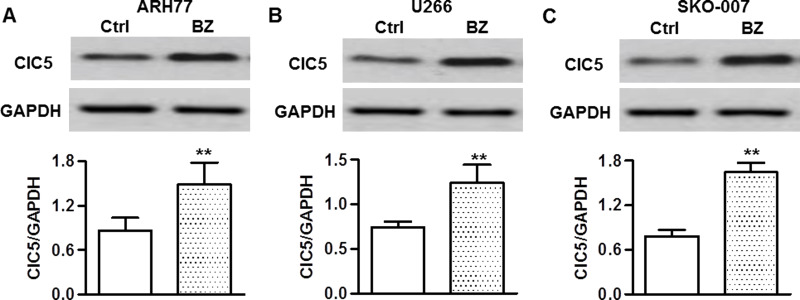
ClC5 has been found to be expressed in several cancer cell types, and its expression has been demonstrated to be associated with proliferation of myeloid cells27,29,33. To investigate whether ClC5 is involved in BZ resistance of multiple myeloma cells, the effect of BZ on ClC5 expression was examined. Western blotting analysis showed that BZ treatment significantly increased ClC5 expression in ARH77 cells (Fig. 2A). In addition, we confirmed these phenomena in two other multiple myeloma cell lines, U266 (Fig. 2B) and SKO-007 (Fig. 2C). These results indicate that the increased ClC5 expression may be associated with BZ resistance of multiple myeloma cells.
Figure 2.
BZ increased chloride channel 5 (ClC5) expression in multiple myeloma cells. ARH77 (A), U266 (B), and SKO-007 (C) cells were treated with BZ for 48 h. The protein expression of ClC5 was analyzed by Western blotting. GAPDH was used as a loading control. **p < 0.01 versus control, n = 6.
Knockdown of ClC5 Facilitated Multiple Myeloma Cell Sensitivity to BZ
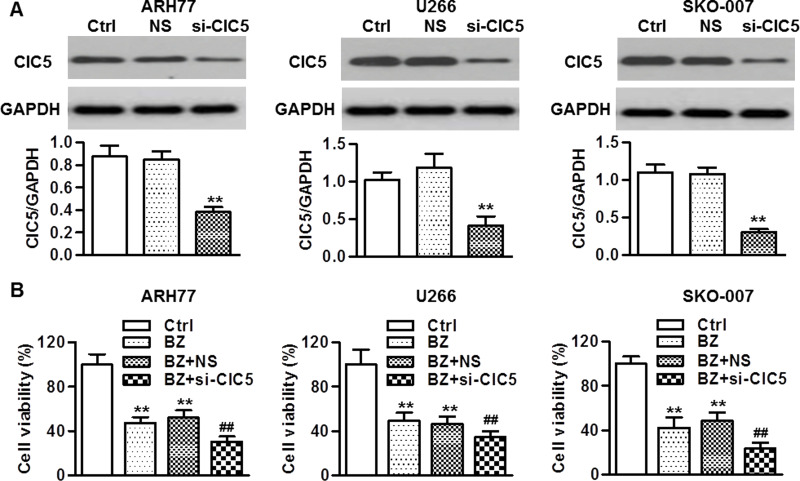
To clarify the function of ClC5 in BZ resistance, ClC5 siRNA was transfected into ARH77, U266, and SKO-007 cells, respectively. The data revealed that ClC5 protein expression was markedly decreased by more than 65% in ClC5 siRNA-transfected cells (Fig. 3A). Furthermore, all three multiple myeloma cell lines were transfected with ClC5 siRNA, followed by incubation with BZ. Inhibition of ClC5 had no effect on the viability of multiple myeloma cells under the basal level (data not shown). Nevertheless, ClC5 knockdown further decreased the cell viability of multiple myeloma cells with BZ treatment (Fig. 3B).
Figure 3.
Knockdown of ClC5 enhanced BZ-induced cell growth inhibition. (A) Transfection efficiencies of ClC5 small interfering RNA (siRNA) were determined. Multiple myeloma cells were transfected with 40 nM ClC5 siRNA (si-ClC5) or negative siRNA (NS) for 48 h. ClC5 expression was examined by Western blotting. GAPDH was used as the loading control. **p < 0.01 versus control, n = 6. (B) After 48 h of siRNA transfection, the cells were then incubated with BZ for another 48 h. MTT assay was used to test cell viability. **p < 0.01 versus control; ##p < 0.01 versus BZ, n = 8.
Upregulation of ClC5 Attenuated Chemosensitivity of Multiple Myeloma Cells to BZ
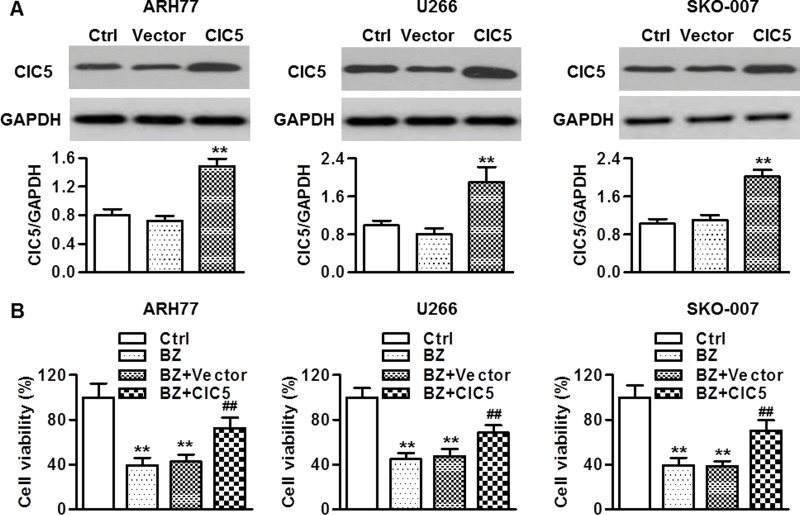
To further evaluate the role of ClC5 in regulating BZ resistance in multiple myeloma cells, we overexpressed ClC5 in ARH77, U266, and SKO-007 cells. Expression of the ClC5 plasmid showed increased ClC5 expression, whereas no increase in the empty vector group was observed (Fig. 4A). Moreover, overexpression of ClC5 had little influence on the viability of multiple myeloma cells under the basal level (data not shown). However, cell survival was restored in cells that were treated with ClC5 plasmid and BZ (Fig. 4B), suggesting that ClC5 overexpression induces BZ resistance.
Figure 4.
ClC5 upregulation decreased the sensitivity of multiple myeloma cells to BZ treatment. (A) The cells were transfected with ClC5 plasmid for 48 h. Overexpressing efficiencies were detected by Western blotting. GAPDH was used as a loading control. **p < 0.01 versus control, n = 6. (B) After transfection with the ClC5 plasmid for 48 h, the cells were then treated with BZ for 48 h. The viability of multiple myeloma cells was examined by MTT assay. **p < 0.01 versus control; ##p < 0.01 versus BZ, n = 6.
ClC5 Enhanced BZ-Induced Autophagy via Inhibition of the AKT–mTOR Pathway
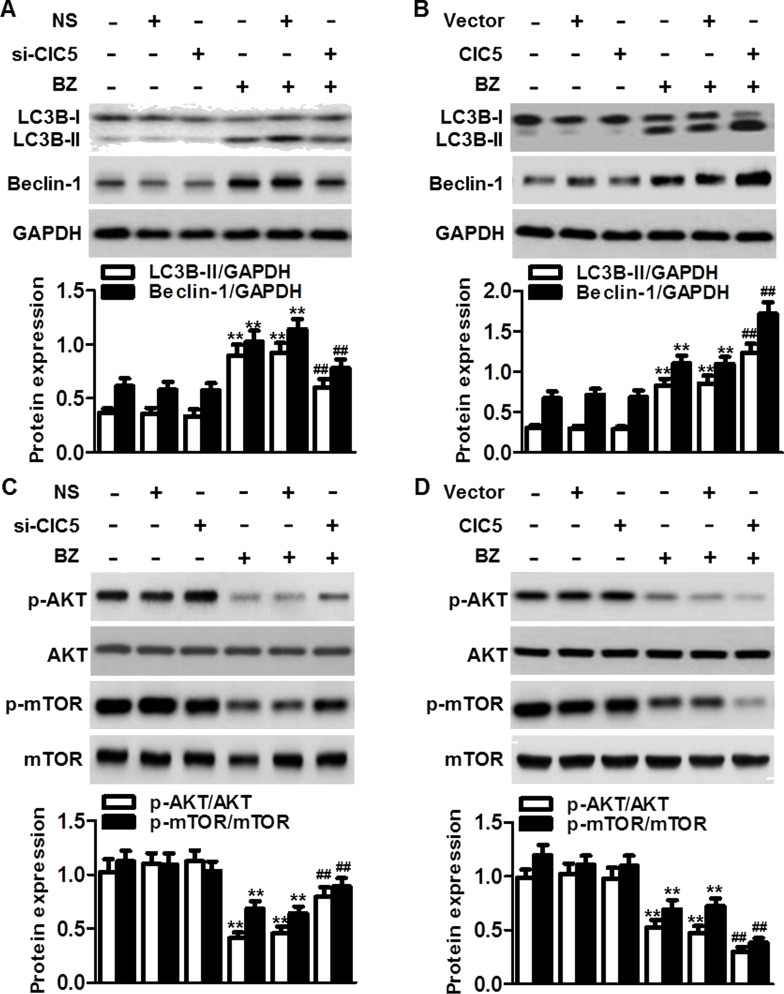
Our above results showed that ClC5 knockdown or inhibition of autophagy further augmented the BZ-induced decrease in multiple myeloma cell viability. To explore the association between ClC5 and autophagy in regulating multiple myeloma cell sensitivity to BZ, we first analyzed the effect of ClC5 on autophagic markers in ARH-77 cells. Although the conversion of LC3B-I to LC3B-II and beclin-1 expression remained unchanged in cells transfected with ClC5 siRNA, blockade of ClC5 markedly inhibited the BZ-induced conversion and beclin-1 expression (Fig. 5A). On the contrary, overexpression of ClC5 significantly enhanced the conversion of LC3B-I to LC3B-II and beclin-1 expression induced by BZ (Fig. 5B). The data indicate that ClC5 downregulation facilitates multiple myeloma cell sensitivity to BZ via suppression of autophagy. AKT–mTOR is an important signaling pathway in the regulation of autophagic activity22. We next investigated whether AKT–mTOR signaling was also involved in the mechanism by which ClC5 inhibition suppresses autophagy in multiple myeloma cells. Western blotting showed that the phosphorylation of AKT and mTOR in ARH77 cells was markedly inhibited after BZ treatment. However, ClC5 downregulation significantly attenuated the dephosphorylation of AKT and mTOR (Fig. 5C). On the other hand, upregulation of ClC5 further enhanced the inhibitory effect of BZ on AKT–mTOR signaling (Fig. 5D). Collectively, the results demonstrate the involvement of AKT–mTOR signaling in ClC5-mediated autophagy.
Figure 5.
ClC5 facilitated BZ-induced autophagy by inactivating the AKT–mTOR signaling pathway. ARH77 cells were transfected with ClC5 siRNA (A) or plasmid (B) for 48 h and then treated with or without BZ for another 48 h. The protein expressions of LC3B-I/II and beclin-1 were analyzed by Western blotting. GAPDH was the loading control. **p < 0.01 versus control; ##p < 0.01 versus BZ, n = 6. (C) Knockdown of ClC5 downregulation attenuated BZ-induced dephosphorylation of AKT and mechanistic target of rapamycin (mTOR). Ratios of phosphorylated to unphosphorylated AKT and mTOR, respectively, are depicted. (D) ClC5 upregulation further enhanced the inactivation of AKT–mTOR signaling induced by BZ. **p < 0.01 versus control; ##p < 0.01 versus BZ, n = 4.
DISCUSSION
In the present study, we explored the relationship between ClC5 and BZ resistance in multiple myeloma cells. Our results are the first to demonstrate that ClC5 is involved in multiple myeloma cell chemoresistance to BZ. The findings can be summarized as follows: 1) BZ treatment induced autophagy, and inhibition of autophagy augmented the cytotoxic activity of BZ against multiple myeloma cells. 2) ClC5 expression was increased after treatment with BZ. Knockdown of ClC5 enhanced BZ chemosensitivity in multiple myeloma cells, whereas ClC5 upregulation inhibited the BZ-induced decrease in cell viability. 3) The inhibitory effect of ClC5 on BZ sensitivity may be associated with increased autophagy by inactivating the AKT–mTOR signaling pathway.
Multiple studies have shown that BZ is able to induce autophagy in different cancer cell types. For example, BZ could induce autophagy via endoplasmic reticulum stress in breast cancer cells16,35. Moreover, BZ has the ability to induce autophagy through a mitochondria–caspase-dependent pathway in chondrosarcoma cells36. Our results showed that BZ was capable of increasing autophagy in multiple myeloma cells, which was supported by the increased expression of LC3B-II, beclin-1, and ATG5. In fact, this was in line with an earlier study12. Furthermore, the function of autophagy is controversial in cancer; likely this could be a survival or cell death mechanism. On the one hand, autophagy appears to function as a tumor suppressor24,25. On the other hand, it has been proposed as a prosurvival mechanism that resists cell death22,23. Despite the confusing role of autophagy, there is increasing evidence showing that autophagy is associated with the resistance of cancer cells to BZ therapy. For instance, the development of BZ-resistant breast cancer cells was accompanied with increased autophagy, and increased autophagy could attenuate apoptotic death35,37. Moreover, inhibition of autophagy sensitized cervical cancer cells to BZ therapy10. Our data in this study revealed that inhibition of autophagy with 3-MA further augmented the effect of BZ on cell death in multiple myeloma cells. This was consistent with a previous study showing that blockade of autophagy with another autophagy inhibitor, bafilomycin A1, enhanced BZ-induced apoptosis in multiple myeloma cells34.
On the basis of the above observations, targeting protective autophagy may be an alternative approach to enhance the efficacy of BZ therapy. Our findings showed that BZ exposure markedly increased ClC5 expression in different multiple myeloma cell lines. The expression of ClC5 was positively correlated with the increased level of autophagy, indicating that ClC5 may have a role in autophagy-mediated chemoresistance under BZ treatment. To the best of our knowledge, there have been only a few reports showing expression of ClC5 in several cancer cells30,32,33. The function of ClC5 in cancer cell growth or chemosensitivity is still unknown. In the current study, we investigated the non-ion channel function of ClC5 in mediating BZ sensitivity in multiple myeloma cells. Our results showed that the BZ-induced decrease in cell viability was suppressed by ClC5 overexpression, whereas ClC5 knockdown exhibited opposite effects. Moreover, ClC5 further promoted autophagy that in part attenuated drug-induced cell death. These data clearly support the notion that ClC5 plays a critical role in the chemosensitivity of BZ by mediating autophagy.
It is well known that the AKT pathway has an important role in regulating human cancer cell survival38. Dysregulation of the AKT pathway can be observed in cancers, which may be involved in the resistance mechanisms of tumor cells15,38. Phosphorylation of AKT can activate the serine/threonine kinase mTOR that acts as an important upstream regulator of autophagy24. Related studies have demonstrated that inhibition of the AKT–mTOR pathway results in autophagic activation22,39. In our study, the results showed that BZ dephosphorylated AKT as well as mTOR in multiple myeloma cells, which was further enhanced by ClC5 overexpression. The results strongly indicate that the AKT–mTOR signaling pathway may contribute to BZ-induced autophagy, and ClC5 is required for the process involved.
In conclusion, our study demonstrates that BZ induces protective autophagy, at least in part, through inhibiting the AKT–mTOR signaling pathway. ClC5 attenuates BZ sensitivity in multiple myeloma cells through enhancing autophagy. These findings suggest that inhibition of ClC5 may be a potential strategy to overcome the chemoresistance of BZ for the treatment of multiple myeloma.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Glavey SV, Gertz MA, Dispenzieri A, Kumar S, Buadi F, Lacy M, Hayman SR, Kapoor P, Dingli D, McCurdy A, Hogan WJ, Gastineau DA, Leung N. Long-term outcome of patients with multiple [corrected] myeloma-related advanced renal failure following auto-SCT. Bone Marrow Transplant. 2013;48:1543–7. [DOI] [PubMed] [Google Scholar]
- 2. Di Martino MT, Gulla A, Cantafio ME, Lionetti M, Leone E, Amodio N, Guzzi PH, Foresta U, Conforti F, Cannataro M, Neri A, Giordano A, Tagliaferri P, Tassone P. In vitro and in vivo anti-tumor activity of miR-221/222 inhibitors in multiple myeloma. Oncotarget 2013;4:242–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sezer O. Myeloma bone disease: Recent advances in biology, diagnosis, and treatment. Oncologist 2009;14:276–83. [DOI] [PubMed] [Google Scholar]
- 4. Bauer K, Rancea M, Schmidtke B, Kluge S, Monsef I, Hubel K, Engert A, Skoetz N. Thirteenth biannual report of the Cochrane Haematological Malignancies Group—Focus on multiple myeloma. J Natl Cancer Inst. 2011;103:E1–19. [DOI] [PubMed] [Google Scholar]
- 5. Kyle RA, Rajkumar SV. Multiple myeloma. Blood 2008;111:2962–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Glasmacher A, Hahn C, Hoffmann F, Naumann R, Goldschmidt H, von Lilienfeld-Toal M, Orlopp K, Schmidt-Wolf I, Gorschluter M. A systematic review of phase-II trials of thalidomide monotherapy in patients with relapsed or refractory multiple myeloma. Br J Haematol. 2006;132:584–93. [DOI] [PubMed] [Google Scholar]
- 7. Richardson PG, Mitsiades C, Schlossman R, Ghobrial I, Hideshima T, Munshi N, Anderson KC. Bortezomib in the front-line treatment of multiple myeloma. Expert Rev Anticancer Ther. 2008;8:1053–72. [DOI] [PubMed] [Google Scholar]
- 8. Perrotta C, Staines A, Codd M, Kleefeld S, Crowley D, A TM, Becker N, Brennan P, De Sanjose S, Foretova L, Maynadie M, Nieters A, Boffetta P, Cocco P. Multiple myeloma and lifetime occupation: Results from the EPILYMPH study. J Occup Med Toxicol. 2012;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–79. [DOI] [PubMed] [Google Scholar]
- 10. Zhang Y, Bai C, Lu D, Wu X, Gao L, Zhang W. Endoplasmic reticulum stress and autophagy participate in apoptosis induced by bortezomib in cervical cancer cells. Biotechnol Lett. 2016;38:357–65. [DOI] [PubMed] [Google Scholar]
- 11. Hamouda MA, Belhacene N, Puissant A, Colosetti P, Robert G, Jacquel A, Mari B, Auberger P, Luciano F. The small heat shock protein B8 (HSPB8) confers resistance to bortezomib by promoting autophagic removal of misfolded proteins in multiple myeloma cells. Oncotarget 2014;5:6252–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moriya S, Che XF, Komatsu S, Abe A, Kawaguchi T, Gotoh A, Inazu M, Tomoda A, Miyazawa K. Macrolide antibiotics block autophagy flux and sensitize to bortezomib via endoplasmic reticulum stress-mediated CHOP induction in myeloma cells. Int J Oncol. 2013;42:1541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anderson KC. New insights into therapeutic targets in myeloma. Hematology Am Soc Hematol Educ Program 2011;2011:184–90. [DOI] [PubMed] [Google Scholar]
- 14. Chen D, Frezza M, Schmitt S, Kanwar J, Dou QP. Bortezomib as the first proteasome inhibitor anticancer drug: Current status and future perspectives. Curr Cancer Drug Targets 2011;11:239–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ding JH, Yuan LY, Chen GA. Aspirin enhances the cytotoxic activity of bortezomib against myeloma cells via suppression of Bcl-2, survivin and phosphorylation of AKT. Oncol Lett. 2017;13:647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Komatsu S, Miyazawa K, Moriya S, Takase A, Naito M, Inazu M, Kohno N, Itoh M, Tomoda A. Clarithromycin enhances bortezomib-induced cytotoxicity via endoplasmic reticulum stress-mediated CHOP (GADD153) induction and autophagy in breast cancer cells. Int J Oncol. 2012;40:1029–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oerlemans R, Franke NE, Assaraf YG, Cloos J, van Zantwijk I, Berkers CR, Scheffer GL, Debipersad K, Vojtekova K, Lemos C, van der Heijden JW, Ylstra B, Peters GJ, Kaspers GL, Dijkmans BA, Scheper RJ, Jansen G. Molecular basis of bortezomib resistance: Proteasome subunit beta5 (PSMB5) gene mutation and overexpression of PSMB5 protein. Blood 2008;112:2489–99. [DOI] [PubMed] [Google Scholar]
- 18. Kuhn DJ, Berkova Z, Jones RJ, Woessner R, Bjorklund CC, Ma W, Davis RE, Lin P, Wang H, Madden TL, Wei C, Baladandayuthapani V, Wang M, Thomas SK, Shah JJ, Weber DM, Orlowski RZ. Targeting the insulin-like growth factor-1 receptor to overcome bortezomib resistance in preclinical models of multiple myeloma. Blood 2012;120:3260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gu JL, Li J, Zhou ZH, Liu JR, Huang BH, Zheng D, Su C. Differentiation induction enhances bortezomib efficacy and overcomes drug resistance in multiple myeloma. Biochem Biophys Res Commun. 2012;420:644–50. [DOI] [PubMed] [Google Scholar]
- 20. Corcelle EA, Puustinen P, Jaattela M. Apoptosis and autophagy: Targeting autophagy signalling in cancer cells—‘Trick or treats’? FEBS J. 2009;276:6084–96. [DOI] [PubMed] [Google Scholar]
- 21. Fong MY, Jin S, Rane M, Singh RK, Gupta R, Kakar SS. Withaferin A synergizes the therapeutic effect of doxorubicin through ROS-mediated autophagy in ovarian cancer. PLoS One 2012;7:e42265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang Z, Klionsky DJ. An overview of the molecular mechanism of autophagy. Curr Top Microbiol Immunol. 2009;335:1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Song YJ, Zhang SS, Guo XL, Sun K, Han ZP, Li R, Zhao QD, Deng WJ, Xie XQ, Zhang JW, Wu MC, Wei LX. Autophagy contributes to the survival of CD133+ liver cancer stem cells in the hypoxic and nutrient-deprived tumor microenvironment. Cancer Lett. 2013;339:70–81. [DOI] [PubMed] [Google Scholar]
- 24. Yang ZJ, Chee CE, Huang S, Sinicrope FA. The role of autophagy in cancer: Therapeutic implications. Mol Cancer Ther. 2011;10:1533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer 2012;12:401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang GL, Wang XR, Lin MJ, He H, Lan XJ, Guan YY. Deficiency in ClC-3 chloride channels prevents rat aortic smooth muscle cell proliferation. Circ Res. 2002;91:E28–32. [DOI] [PubMed] [Google Scholar]
- 27. Rouzaire-Dubois B, Milandri JB, Bostel S, Dubois JM. Control of cell proliferation by cell volume alterations in rat C6 glioma cells. Pflugers Arch. 2000;440:881–8. [DOI] [PubMed] [Google Scholar]
- 28. Wondergem R, Gong W, Monen SH, Dooley SN, Gonce JL, Conner TD, Houser M, Ecay TW, Ferslew KE. Blocking swelling-activated chloride current inhibits mouse liver cell proliferation. J Physiol. 2001;532:661–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jiang B, Hattori N, Liu B, Nakayama Y, Kitagawa K, Sumita K, Inagaki C. Expression and roles of Cl- channel ClC-5 in cell cycles of myeloid cells. Biochem Biophys Res Commun. 2004;317:192–7. [DOI] [PubMed] [Google Scholar]
- 30. Ernest NJ, Weaver AK, Van Duyn LB, Sontheimer HW. Relative contribution of chloride channels and transporters to regulatory volume decrease in human glioma cells. Am J Physiol Cell Physiol. 2005;288:C1451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duffy SM, Leyland ML, Conley EC, Bradding P. Voltage-dependent and calcium-activated ion channels in the human mast cell line HMC-1. J Leukoc Biol. 2001;70:233–40. [PubMed] [Google Scholar]
- 32. Jiang B, Hattori N, Liu B, Kitagawa K, Inagaki C. Expression of swelling- and/or pH-regulated chloride channels (ClC-2, 3, 4 and 5) in human leukemic and normal immune cells. Life Sci. 2002;70:1383–94. [DOI] [PubMed] [Google Scholar]
- 33. Ruiz-Lafuente N, Alcaraz-Garcia MJ, Sebastian-Ruiz S, Garcia-Serna AM, Gomez-Espuch J, Moraleda JM, Minguela A, Garcia-Alonso AM, Parrado A. IL-4 up-regulates miR-21 and the miRNAs hosted in the CLCN5 gene in chronic lymphocytic leukemia. PLoS One 2015;10:e0124936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kawaguchi T, Miyazawa K, Moriya S, Ohtomo T, Che XF, Naito M, Itoh M, Tomoda A. Combined treatment with bortezomib plus bafilomycin A1 enhances the cytocidal effect and induces endoplasmic reticulum stress in U266 myeloma cells: Crosstalk among proteasome, autophagy-lysosome and ER stress. Int J Oncol. 2011;38:643–54. [DOI] [PubMed] [Google Scholar]
- 35. Miyahara K, Kazama H, Kokuba H, Komatsu S, Hirota A, Takemura J, Hirasawa K, Moriya S, Abe A, Hiramoto M, Ishikawa T, Miyazawa K. Targeting bortezomib-induced aggresome formation using vinorelbine enhances the cytotoxic effect along with ER stress loading in breast cancer cell lines. Int J Oncol. 2016;49:1848–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lohberger B, Steinecker-Frohnwieser B, Stuendl N, Kaltenegger H, Leithner A, Rinner B. The proteasome inhibitor bortezomib affects chondrosarcoma cells via the mitochondria-caspase dependent pathway and enhances death receptor expression and autophagy. PLoS One 2016;11:e0168193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chang I, Wang CY. Inhibition of HDAC6 protein enhances bortezomib-induced apoptosis in head and neck squamous cell carcinoma (HNSCC) by reducing autophagy. J Biol Chem. 2016;291:18199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. [DOI] [PubMed] [Google Scholar]
- 39. Gallagher LE, Williamson LE, Chan EY. Advances in autophagy regulatory mechanisms. Cells 2016;5(2):E24. [DOI] [PMC free article] [PubMed] [Google Scholar]