Abstract
Numerous studies have suggested that microRNAs (miRNAs) are dysregulated in osteosarcoma (OS), implicating miRNAs in OS initiation and progression. Therefore, knowledge of aberrantly expressed miRNAs in OS may provide novel mechanistic insights into the tumorigenesis and tumor development of OS and facilitate therapeutic methods for patients with this aggressive bone neoplasm. In this study, data obtained from reverse transcription quantitative polymerase chain reaction (RT-qPCR) revealed that miR-935 was significantly decreased in OS tissues and cell lines. Restoration expression of miR-935 obviously restricted proliferation and invasion of OS cells. In addition, high-mobility group box 1 (HMGB1) was predicted to be a putative target of miR-935. Subsequent dual-luciferase reporter assay, RT-qPCR, and Western blot analysis confirmed that miR-935 could directly target the 3′-untranslated region of HMGB1 and negatively regulated HMGB1 expression in OS cells. Furthermore, a significant negative association was found between miR-935 and HMGB1 mRNA expression in OS tissues. Rescue experiments showed that recovery of HMGB1 expression partially rescued miR-935-induced suppression of cell proliferation and invasion in OS. These results provide the first evidence for the suppressive roles of miR-935 in OS by directly targeting HMGB1, suggesting that miR-935 may be a potential candidate for the treatment of patients with this disease.
Key words: MicroRNA-935, Osteosarcoma (OS), Proliferation, Invasion, High-mobility group box 1 (HMGB1)
INTRODUCTION
Osteosarcoma (OS) is the most common type of malignant bone neoplasm and is mainly distributed in the metaphysis of long bones of the limbs1. Moreover, 60% of OS occurs within 2 years of age2. OS patients often possess extensive complicated pathologies and frequent distal metastasis, which leads to death in adolescents and young adults3. Several risk factors contributing to OS oncogenesis and progression have been identified, including ionizing radiation, alkylating agents, Paget’s disease, the Li–Fraumeni familial cancer syndrome, and other chromosomal abnormalities4. To date, surgical resection remains the basic and major therapy for patients with OS, whereas chemotherapy and radiotherapy can be administered before or after surgery5. Despite the recent advancements in diagnosis and therapy, therapeutic outcomes of OS patients who are diagnosed at an advanced stage remain poor, with a median survival time of only 23 months6. Hence, identifying the underlying molecular mechanisms of OS occurrence and development is urgently required for the development of novel therapeutic strategies of patients with this fatal disease.
MicroRNAs (miRNAs) are a cluster of single-stranded, noncoding, small RNA molecules with highly conserved sequences in plants, animals, and DNA viruses7. miRNAs act as critical controllers of gene expression by directly interacting with complementary 3′-untranslated region (3′-UTR) sequences of their target genes, which leads to mRNA degradation and/or translation inhibition8. By negatively modulating their targets, miRNAs are known to regulate various key cellular processes, such as cell proliferation, survival, differentiation, apoptosis, autophagy, motility, angiogenesis, and tumorigenesis8. Emerging evidence shows that a large number of miRNAs are dysregulated in human cancers, such as miR-212 in OS9, miR-181a in thyroid cancer10, and miR-363 in ovarian cancer11. Dysregulated miRNAs are closely related with the clinical characteristics and prognosis of patients with OS12. Additionally, the dysregulation of miRNAs, which serve as oncogenes or tumor suppressors via negative regulation of their target genes, is involved in the formation and progression of OS13,14. Therefore, further investigation into miRNAs in OS may provide new prognostic biomarkers and therapeutic potential targets.
miR-935 has been found to be dysregulated in several types of cancer, such as gastric cancer15, hepatocellular carcinoma16, and pancreatic carcinoma17. However, the expression, functional roles, and underlying regulatory mechanism of miR-935 in OS remain to be elucidated. The results of this study may provide a better understanding of OS pathogenesis.
MATERIALS AND METHODS
Tissue Collection
This research was approved by the Ethics Committee of Daqing Long Nan Hospital (Heilongjiang, P.R. China). Written informed consent was also provided by all patients prior to their participation in this research. A total of 34 primary human OS tissues and corresponding adjacent normal tissues were collected from patients who underwent surgery at Daqing Long Nan Hospital. No chemotherapy or radiotherapy was administered before surgical resection. All tissue specimens were immediately snap frozen in liquid nitrogen and kept in −80°C cryogenic refrigerator until RNA extraction.
Cell Lines and Culture Conditions
A normal human osteoblast hFOB1.19 and three human OS cell lines (MG-63, Saos-2, and U2OS) were acquired from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, P.R. China). All cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Invitrogen, Carlsbad, CA, USA) containing 10% (v/v) fetal bovine serum (FBS; Gibco, Invitrogen), 100 U/ml penicillin, and 100 U/ml streptomycin (Gibco, Invitrogen), and grown at 37°C in the presence of 5% CO2 air atmosphere.
Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA was isolated using TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in accordance with the manufacturer’s instructions. All-in-One™ miRNA qRT-PCR Detection Kit (GeneCopoeia, Inc., Rockville, MD, USA) was employed to detect miR-935 expression levels, with U6 snRNA as the internal control. For the detection of high-mobility group box 1 (HMGB1) mRNA, complementary DNA was synthesized from total RNA using PrimeScript RT Reagent Kit (Takara Bio, Dalian, P.R. China). Afterward, qPCR was carried out on an ABI 7500 thermocycler (Applied Biosystems; Thermo Fisher Scientific, Inc.) using an SYBR Premix Ex Taq™ Kit (Takara Bio). GAPDH served as the internal reference for HMGB1 mRNA. Relative expression was calculated according to the 2−ΔΔCt method18.
Cell Transfection
The miR-935 mimic and negative control miRNA mimic (miR-NC) were purchased from GenePharma Co., Ltd. (Shanghai, P.R. China). HMGB1 overexpression plasmid pCMV-HMGB1 and empty plasmid pCMV were designed and synthesized by Integrated Biotech Solutions (Shanghai, P.R. China). For cell transfection, cells were plated into six-well plates with a density of 50%–60% confluence and incubated at 37°C with 5% CO2 overnight. Cells were transfected with miRNA mimics or plasmid using Lipofectamine™ 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer’s protocol. After incubation for 6–8 h, the culture medium was discarded, and transfected cells were further cultured in DMEM supplemented with 10% FBS.
3-(4,5-Dimethyl-2-Thiazolyl)-2,5-Diphenyl-2-H-Tetrazolium Bromide (MTT) Assay
Following 24 h of transfection, the cells were harvested and then inoculated in 96-well plates with a density of 3,000 cells in each well. Cell proliferation was determined using MTT assay at four time points: 0, 24, 48, and 72 h. A total of 20 μl of MTT solution (5 mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added into each well, followed by incubation at 37°C in a humidified 5% CO2 atmosphere for 4 h. Subsequently, the culture supernatant was gently discarded. To dissolve the formazan product, a total of 150 μl DMSO (Sigma-Aldrich; Merck KGaA) was added into each well. Finally, SpectraMax Microplate® Spectrophotometer (Molecular Devices LLC, Sunnyvale, CA, USA) was used to detect the absorbance at a wavelength of 490 nm.
Transwell Invasion Assay
Twenty-four-well 8-μm Transwell plates (Corning; Costar, Cambridge, MA, USA) coated with Matrigel (BD Biosciences, San Jose, CA, USA) were employed to evaluate cell invasion ability. After 48 h of transfection, the cells were collected and suspended in DMEM without FBS. A total of 1 × 105 cells were added into each upper chamber. The lower chambers were covered in 500 μl of DMEM with 10% FBS. Twenty-four hours later, a cotton swab was applied to gently wipe away the noninvaded cells remaining on the top of the chamber. The invaded cells were fixed with 4% paraformaldehyde (Beyotime Institute of Biotechnology, Inc., Shanghai, P.R. China) and stained with 0.05% crystal violet (Beyotime). The invaded cells were photographed and counted under an inverted microscope (CKX41; Olympus Corporation, Tokyo, Japan) in five randomly selected fields per chamber.
Bioinformatics Analysis and Dual-Luciferase Reporter Assay
The potential targets of miR-935 were predicted using TargetScan7.1 (http://www.targetscan.org/) and miRanda (http://www.microrna.org/). HMGB1 was predicted as a major target of miR-935. The 3′-UTRs of HMGB1 containing the wild-type (WT) or mutant (MUT) miR-935 binding sites were designed and synthesized by Shanghai GenePharma Co., Ltd., inserted into the pMIR-GLOTM luciferase vector (Promega Corporation, Madison, WI, USA), and named as pMIR-HMGB1-3′-UTR WT and pMIR-HMGB1-3′-UTR MUT, respectively. For the reporter assay, cells were seeded into 24-well plates and incubated at 37°C overnight. miR-935 mimic or miR-NC, in combination with pMIR-HMGB1-3′-UTR WT or pMIR-HMGB1-3′-UTR MUT, was transfected into cells using Lipofectamine™ 2000 reagent according to the manufacturer’s instructions. At 48 h posttransfection, the cells were harvested, and luciferase activity was measured with a dual-luciferase reporter assay system (Promega Corporation). The firefly luciferase activity was normalized to Renilla luciferase activity.
Western Blot Analysis
Radioimmunoprecipitation assay lysis buffer (Invitrogen; Thermo Fisher Scientific, Inc.) was utilized to extract total protein from tissues or cells. The concentration of total protein was detected using a bicinchoninic acid protein assay kit (Beyotime). Equal amounts of protein were separated using 10% SDS-PAGE and transferred onto polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). The membranes were then blocked with 5% fat-free milk in Tris-buffered saline containing 0.1% Tween 20 (TBST) at room temperature for 2 h and incubated overnight at 4°C with the following primary antibodies: mouse anti-human monoclonal HMGB1 antibody (ab77302; 1:1,000 dilution; Abcam, Cambridge, UK) and mouse anti-human GAPDH antibody (sc-365062; 1:1,000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After being washed with TBST for three times, the membranes were incubated with goat anti-mouse horseradish peroxidase-conjugated secondary antibody (sc-516102; 1:1,000 dilution; Santa Cruz Biotechnology) for 2 h at room temperature. After three washes with TBST, ECL Western Blotting kit (Pierce; Thermo Fisher Scientific, Inc.) was used to visualize the protein bands according to the manufacturer’s instructions. Relative protein expression was represented as density ratio versus GAPDH.
Statistical Analysis
Data were expressed as the mean ± standard deviation. Statistical analysis was carried out with SPSS version 19.0 (IBM SPSS, Inc., Armonk, NY, USA). Differences between groups were analyzed using Student’s t-test or one-way analysis of variance with Tukey’s post hoc test. Spearman’s correlation analysis was utilized to investigate the relationship between miR-935 and HMGB1 mRNA expression in OS tissues. A value of p < 0.05 was considered statistically significant.
RESULTS
miR-935 Is Downregulated in OS Tissues and Cell Lines
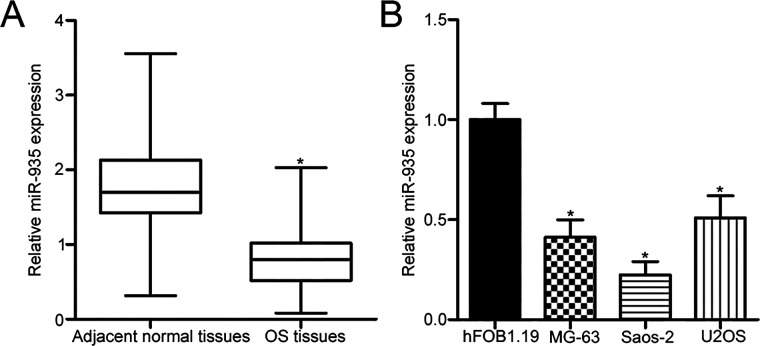
To investigate the intrinsic levels of miR-935 in OS, we first measured miR-935 expression in 34 primary human OS tissues and corresponding adjacent normal tissues using RT-qPCR. miR-935 expression was obviously underexpressed in OS tissues compared with that in corresponding adjacent normal tissues (p < 0.05) (Fig. 1A). We next determined miR-935 expression in three human OS cell lines, including MG-63, Saos-2, and U2OS, as well as in normal human osteoblast hFOB1.19. The data of the RT-qPCR analysis showed that the expression level of miR-935 was lower in all examined OS cell lines than that in hFOB1.19 (p < 0.05) (Fig. 1B). The MG-63 and Saos-2 cell lines possessed relatively lower miR-935 levels among the three OS cell lines. Hence, these two cell lines were chosen for subsequent experiments.
Figure 1.
MicroRNA-935 (miR-935) expression is downregulated in osteosarcoma (OS) tissues and cell lines. (A) Reverse transcription quantitative polymerase chain reaction (RT-qPCR) was used to detect miR-935 expression in 34 primary human OS tissues and corresponding adjacent normal tissues. (B) Relative miR-935 expression in three OS cell lines (MG-63, Saos-2, and U2OS) and a normal human osteoblast hFOB1.19 was examined using RT-qPCR. *p < 0.05.
miR-935 Overexpression Decreases Cell Proliferation and Invasion of OS
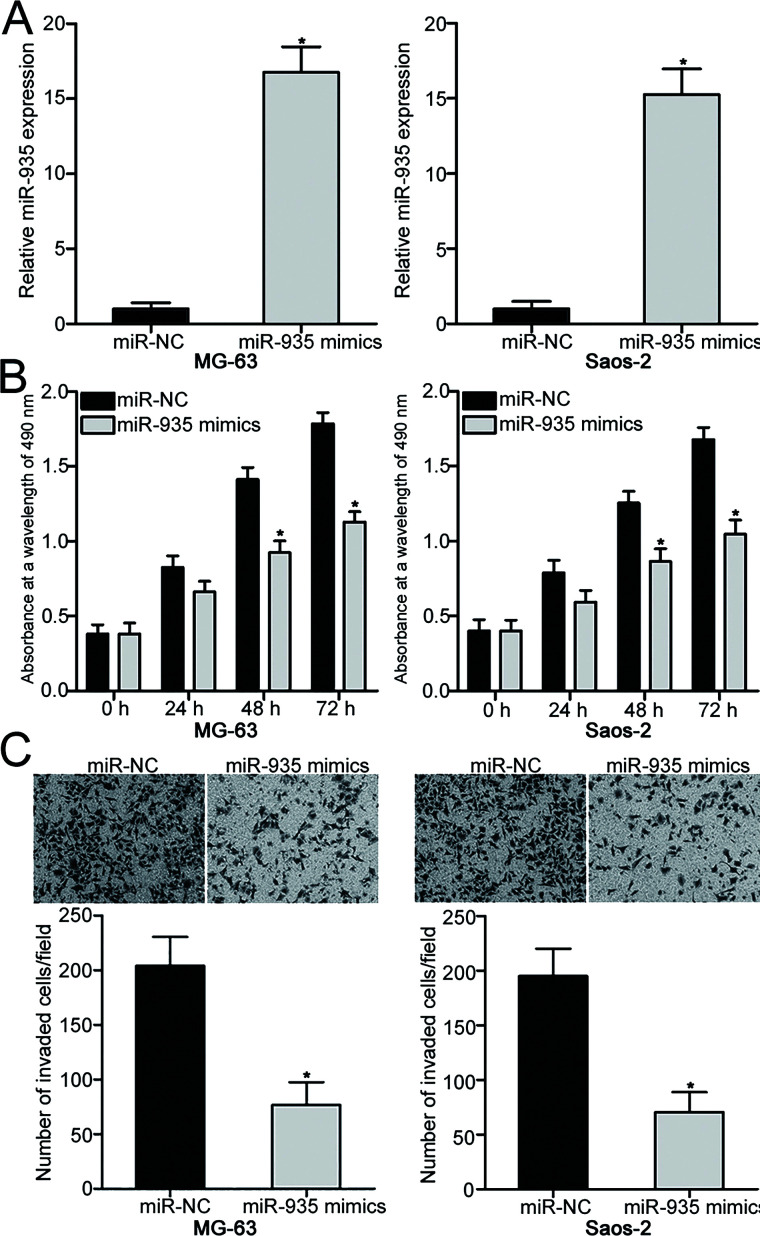
To reveal the biological roles of miR-935 in OS, MG-63 and Saos-2 cells were transfected with miR-935 mimic to increase its endogenous level. Transfection with miR-935 mimic resulted in a marked upregulation of miR-935 in MG-63 and Saos-2 cells in comparison with those cells transfected with miR-NC (p < 0.05) (Fig. 2A). MTT assay was carried out to determine the effects of miR-935 overexpression in OS cell proliferation. As shown in Figure 2B, restoration expression of miR-935 suppressed the proliferative ability of MG-63 and Saos-2 cells compared with the miR-NC group (p < 0.05). Transwell invasion assay was conducted to examine the invasion capacities of MG-63 and Saos-2 cells transfected with miR-935 mimic or miR-NC. The invasion abilities of MG-63 and Saos-2 cells overexpressing miR-935 were obviously reduced compared with those of the miR-NC group (p < 0.05) (Fig. 2C). These findings suggest that miR-935 may play tumor-suppressive roles in OS progression.
Figure 2.
miR-935 upregulation inhibits MG-63 and Saos-2 cell proliferation and invasion. (A) MG-63 and Saos-2 cells were transfected with miR-935 mimic or miR-negative control (NC). RT-qPCR was performed at 48 h posttransfection to determine the transfection efficiency. (B) MTT assay was conducted to evaluate the proliferation of MG-63 and Saos-2 cells following transfection with miR-935 mimic or miR-NC. (C) Transwell invasion assay was used to detect invasion ability of MG-63 and Saos-2 cells transfected with miR-935 mimic or miR-NC. *p < 0.05.
HMGB1 Is a Direct Target of miR-935 in OS
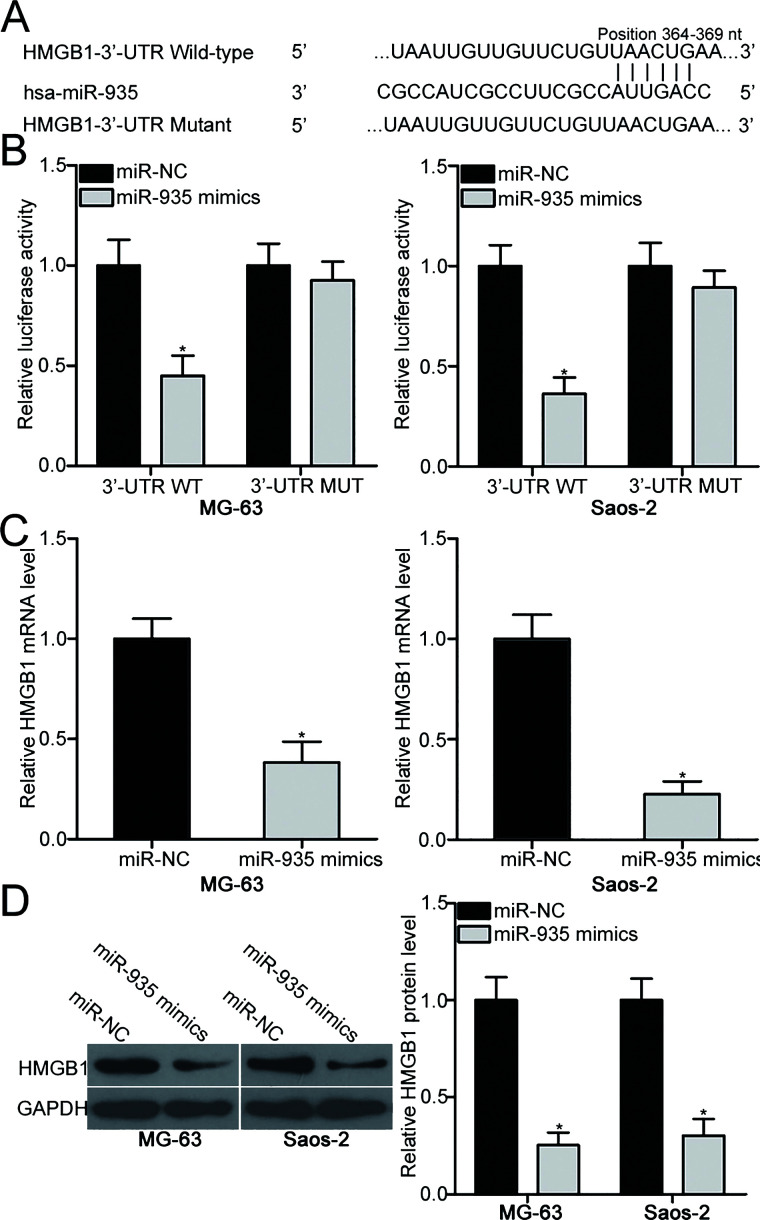
To illustrate the molecular mechanisms underlying the action of miR-935 in OS, bioinformatics analysis was performed to predict the potential targets of miR-935. HMGB1 was predicted as a major target of miR-935 with the predicted binding sites between base positions 364 and 369 (Fig. 3A). Dual-luciferase reporter assay was applied to validate whether the 3′-UTR of HMGB1 could be directly targeted by miR-935 in OS cells. MG-63 and Saos-2 cells were cotransfected with miR-935 mimic or miR-NC and pMIR-HMGB1-3′-UTR WT and pMIR-HMGB1-3′-UTR MUT. The results revealed that upregulation of miR-935 significantly reduced the luciferase activity of the reporter plasmid carrying the wild-type miR-935 binding sites (p < 0.05), but not the reporter plasmid with mutant 3′-UTR of HMGB1 in MG-63 and Saos-2 cells (Fig. 3B). Additionally, RT-qPCR and Western blot analysis were further employed to determine whether the endogenous HMGB1 expression in OS cells could be regulated by miR-935. Ectopic expression of miR-935 resulted in a reduction of HMGB1 expression at the mRNA (p < 0.05) (Fig. 3C) and protein (p < 0.05) (Fig. 3D) levels in MG-63 and Saos-2 cells. Collectively, HMGB1 is a direct target gene of miR-935 in OS.
Figure 3.
High-mobility group box 1 (HMGB1) is a direct target gene of miR-935 in OS. (A) Sequence alignment of wild-type (WT) and mutant (MUT) putative miR-935 binding sites in the 3′-untranslated region (3′-UTR) of HMGB1. (B) Luciferase activity was detected in MG-63 and Saos-2 cells cotransfected with miR-935 mimic or miR-NC and pMIR-HMGB1-3′-UTR WT and pMIR-HMGB1-3′-UTR MUT. Relative mRNA (C) and protein (D) levels of HMGB1 in MG-63 and Saos-2 cells after transfection with miR-935 mimics or miR-NC were determined using RT-qPCR and Western blot analysis, respectively. *p < 0.05.
HMGB1 Is Overexpressed in OS Tissues and Negatively Correlated With miR-935 Expression
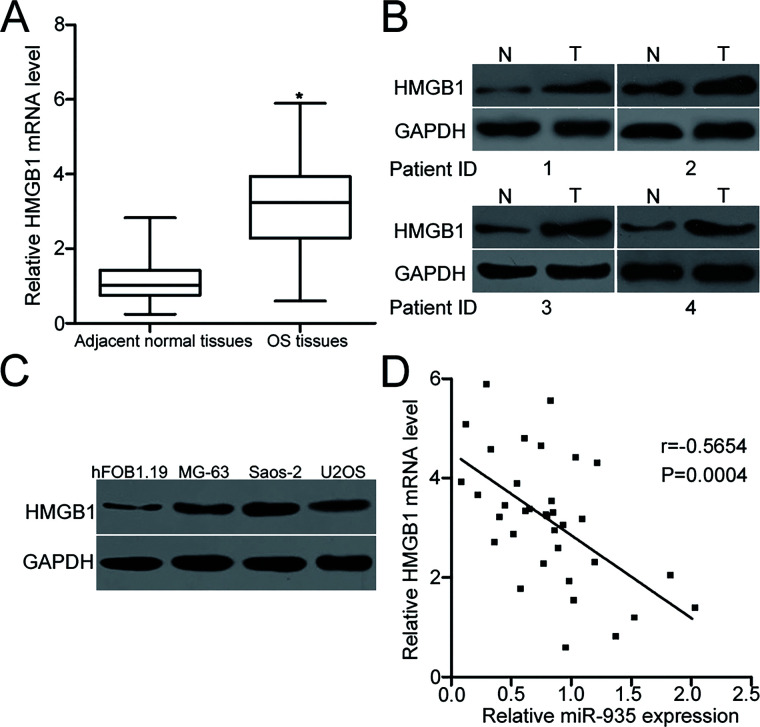
To further explore the association between miR-935 and HMGB1 in OS, we detected HMGB1 expression in human OS tissues and corresponding adjacent normal tissues. HMGB1 mRNA expression was upregulated in OS tissues compared with that in adjacent normal tissues (p < 0.05) (Fig. 4A). Western blot analysis indicated that protein levels of HMGB1 were increased in OS tissues than those of adjacent normal tissues (Fig. 4B). In addition, we detected HMGB1 protein expression in OS cell lines and found that all OS cell lines exhibited high HMGB1 expression (Fig. 4C). Furthermore, an inverse association was observed between miR-935 expression and HMGB1 mRNA levels in OS tissues (r = −0.5654, p = 0.0004) (Fig. 4D). These results suggest that the upregulation of HMGB1 in OS may be at least partly caused by the downregulation of miR-935.
Figure 4.
Upregulation of HMGB1 in OS was inversely correlated with miR-935 levels. (A) RT-qPCR analysis of HMGB1 mRNA expression in 34 primary human OS tissues and corresponding adjacent normal tissues. (B) HMGB1 protein levels in several pairs of OS tissues and corresponding adjacent normal tissues were detected using Western blot analysis. (C) Western blot analysis was conducted to measure HMGB1 protein level in three OS cell lines (MG-63, Saos-2, and U2OS) and a normal human osteoblast hFOB1.19. (D) Spearman’s correlation analysis indicated an inverse relationship between miR-935 and HMGB1 mRNA expression in OS tissues. r = −0.5654, p = 0.0004. *p < 0.05.
Recovery of HMGB1 Expression Reverses the Suppressive Effects of miR-935 in OS Cells
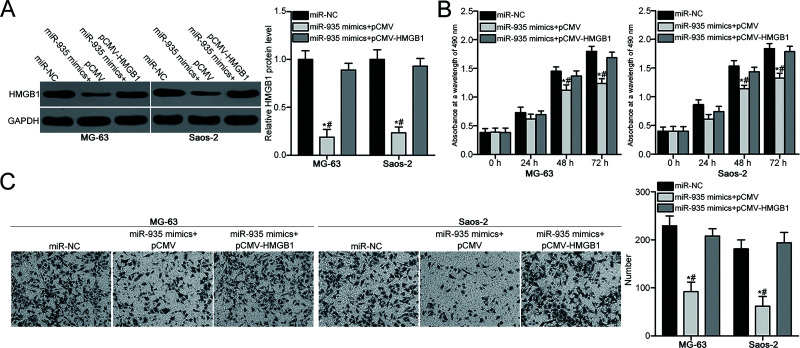
Based on the above results, the inhibitory roles of miR-935 in the proliferation and invasion of OS cells were hypothesized to be exerted through inhibiting HMGB1 expression. To confirm this hypothesis, a series of rescue experiments were carried out in MG-63 and Saos-2 cells, which were transfected with miR-935 mimic together with HMGB1 overexpression plasmid pCMV-HMGB1 or empty plasmid pCMV. Western blot analysis demonstrated that the decreased HMGB1 protein level caused by miR-935 overexpression was restored in MG-63 and Saos-2 cells following cotransfection with pCMV-HMGB1 (p < 0.05) (Fig. 5A). In addition, MTT and Transwell invasion assays showed that recovery of HMGB1 expression rescued the suppressive effects of miR-935 overexpression on proliferation (p < 0.05) (Fig. 5B) and invasion (p < 0.05) (Fig. 5C) of MG-63 and Saos-2 cells. These results further suggest that miR-935 inhibits OS cell proliferation and invasion at least partly by downregulating HMGB1 expression.
Figure 5.
Recovery of expression of HMGB1 rescues the suppressive effects of miR-935 overexpression in OS cell proliferation and invasion. MG-63 and Saos-2 cells were cotransfected with miR-935 mimic and pCMV-HMGB1 or pCMV and were used in the following experiments. (A) Relative protein expression of HMGB1 in indicated cells was determined using Western blot analysis. Cell proliferative (B) and invasion (C) abilities in the above-mentioned cells were evaluated using MTT and Transwell invasion assays, respectively. *p < 0.05. #p < 0.05.
DISCUSSION
Several studies have suggested that miRNAs are dysregulated in OS, which is implicated in OS initiation and progression19,20. Therefore, knowledge of aberrantly expressed miRNAs in OS may provide novel mechanistic insights into the tumorigenesis and tumor development of OS and facilitate therapeutic methods for patients with this aggressive bone neoplasm. In the present study, we explored the expression pattern, biological function, and regulatory mechanism of miR-935 in OS. The results showed that miR-935 expression was downregulated in OS tissues and cell lines. Enforced expression of miR-935 reduced cell proliferation and invasion in OS. Additionally, HMGB1 was identified as a direct target gene of miR-935 in OS. Furthermore, elevated expression of HMGB1 in OS tissues was negatively correlated with miR-935 level. Moreover, restoration of HMGB1 expression rescued the inhibitory effects on OS cell proliferation and invasion caused by miR-935 overexpression. These results suggest that miR-935 may play tumor-suppressive roles in OS by directly targeting HMGB1.
miR-935 has been found to play oncogenic roles in various cancer types. For example, miR-935 expression was upregulated in gastric cancer tissues and cell lines. Suppression of miR-935 attenuated cell proliferation in vitro and decreased tumor growth in vivo15. Liu et al. showed that miR-935 is highly expressed in hepatocellular carcinoma tissues and cell lines. Knockdown of miR-935 resulted in a significant reduction in cell proliferation, tumorigenesis, and cell cycle progression of hepatocellular carcinoma16. Wang et al. revealed that miR-935 is overexpressed in pancreatic carcinoma tissues and cell lines. Inhibition of miR-935 suppressed cell proliferation, migration, and epithelial–mesenchymal transition, and also induced apoptosis in pancreatic carcinoma17. However, miR-935 was underexpressed in gastric signet ring cell carcinoma tissues and cell lines. miR-935 played tumor-suppressive roles in gastric signet ring cell carcinoma by inhibiting cell growth and metastasis21. These findings demonstrate that the expression and biological roles of miR-935 in human malignancies have tissue specificity and also suggest that miR-935 may be a novel and effective target for clinical diagnosis and therapeutics in specific tumor types.
Several direct targets of miR-935 have been identified, including sex-determining region Y box 7 (SOX7) in gastric cancer15 and hepatocellular carcinoma16, inositol polyphosphate 4-phosphatase type I (INPP4A) in pancreatic carcinoma17, and Notch1 in gastric signet ring cell carcinoma21. In the current study, HMGB1, located in the human chromosome 13q12, was validated as a direct and functional target of miR-935 in OS. HMGB1 is a highly conserved DNA-binding protein that can translocate from the cytoplasm to the nucleus and interact with transcription factors, nucleosomes, and histones22. It has been previously reported to participate in multiple biological processes, including DNA and tissue repair, differentiation, inflammation, and cell death23–25. HMGB1 is overexpressed in many human cancer types, such as pancreatic cancer26, cervical cancer27, gastric cancer28, and colorectal cancer29. Aberrantly high expression of HMGB1 is involved in carcinogenesis and cancer progression by regulating numerous malignant biological behaviors, including proliferation, apoptosis, metastasis, epithelial–mesenchymal transition, autophagy, angiogenesis, radiotherapy, and chemotherapy30–33. These findings suggest that targeting HMGB1 may be a promising therapeutic method for antitumor therapy.
HMGB1 is found to be upregulated in OS, and this upregulation is significantly associated with tumor size, tumor stage, and nuclear grade. OS patients with higher HMGB1 levels exhibit poorer prognosis than patients with lower HMGB1 levels34. HMGB1 knockdown restricts cell proliferation, migration, invasion, and chemoresistance, and induces apoptosis35–38. HMGB1 can be negatively regulated by multiple miRNAs in OS, such as miR-50539, miR-2240, miR-142-3p35, and miR-129-5p35, therefore inhibiting OS progression. In this study, we also found that miR-935 targets HMGB1 to inhibit cell proliferation and invasion in OS. Hence, miRNA-based targeted therapy against the expression of HMGB1 might be an effective treatment for patients with OS.
In conclusion, miR-935 was underexpressed in OS tissues and cell lines. Recovery of the expression of miR-935 repressed OS development by directly targeting HMGB1. Therefore, miR-935 might be a therapeutic target in the treatment of patients with this malignancy.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Gelderblom H, Jinks RC, Sydes M, Bramwell VH, van Glabbeke M, Grimer RJ, Hogendoorn PC, McTiernan A, Lewis IJ, Nooij MA, Taminiau AH, Whelan J, European osteosarcoma I. survival after recurrent osteosarcoma: Data from 3 European Osteosarcoma Intergroup (EOI) randomized controlled trials. Eur J Cancer 2011;47:895–902. [DOI] [PubMed] [Google Scholar]
- 2. Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. [DOI] [PubMed] [Google Scholar]
- 3. Gianferante DM, Mirabello L, Savage SA. Germline and somatic genetics of osteosarcoma—Connecting aetiology, biology and therapy. Nat Rev Endocrinol. 2017;13:480–91. [DOI] [PubMed] [Google Scholar]
- 4. Ottaviani G, Jaffe N. The etiology of osteosarcoma. Cancer Treat Res. 2009;152:15–32. [DOI] [PubMed] [Google Scholar]
- 5. Hattinger CM, Biason P, Iacoboni E, Gagno S, Fanelli M, Tavanti E, Vella S, Ferrari S, Roli A, Roncato R, Giodini L, Scotlandi K, Picci P, Toffoli G, Serra M. Candidate germline polymorphisms of genes belonging to the pathways of four drugs used in osteosarcoma standard chemotherapy associated with risk, survival and toxicity in non-metastatic high-grade osteosarcoma. Oncotarget 2016;7:61970–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fagioli F, Aglietta M, Tienghi A, Ferrari S, Brach del Prever A, Vassallo E, Palmero A, Biasin E, Bacci G, Picci P, Madon E. High-dose chemotherapy in the treatment of relapsed osteosarcoma: An Italian sarcoma group study. J Clin Oncol. 2002;20:2150–6. [DOI] [PubMed] [Google Scholar]
- 7. Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, Sharon E, Spector Y, Bentwich Z. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet. 2005;37:766–70. [DOI] [PubMed] [Google Scholar]
- 8. Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004;116:281–97. [DOI] [PubMed] [Google Scholar]
- 9. Liu J, Chen B, Yue B, Yang J. MicroRNA-212 suppresses the proliferation and migration of osteosarcoma cells by targeting forkhead box protein A1. Exp Ther Med. 2016;12:4135–41. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10. Le F, Luo P, Yang QO, Zhong XM. MiR-181a promotes growth of thyroid cancer cells by targeting tumor suppressor RB1. Eur Rev Med Pharmacol Sci. 2017;21:5638–47. [DOI] [PubMed] [Google Scholar]
- 11. Lin Y, Xu T, Zhou S, Cui M. MicroRNA-363 inhibits ovarian cancer progression by inhibiting NOB1. Oncotarget 2017;8:101649–58. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12. Ren X, Shen Y, Zheng S, Liu J, Jiang X. miR-21 predicts poor prognosis in patients with osteosarcoma. Br J Biomed Sci. 2016;73:158–62. [DOI] [PubMed] [Google Scholar]
- 13. Li X, Sun X, Wu J, Li Z. MicroRNA-613 suppresses proliferation, migration and invasion of osteosarcoma by targeting c-MET. Am J Cancer Res. 2016;6:2869–79. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14. Fei D, Zhao K, Yuan H, Xing J, Zhao D. MicroRNA-187 exerts tumor-suppressing functions in osteosarcoma by targeting ZEB2. Am J Cancer Res. 2016;6:2859–68. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15. Yang M, Cui G, Ding M, Yang W, Liu Y, Dai D, Chen L. miR-935 promotes gastric cancer cell proliferation by targeting SOX7. Biomed Pharmacother. 2016;79:153–8. [DOI] [PubMed] [Google Scholar]
- 16. Liu X, Li J, Yu Z, Li J, Sun R, Kan Q. miR-935 promotes liver cancer cell proliferation and migration by targeting SOX7. Oncol Res. 2017;25:427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang C, Feng Z, Jiang K, Zuo X. Upregulation of microRNA-935 promotes the malignant behaviors of pancreatic carcinoma PANC-1 cells via targeting inositol polyphosphate 4-phosphatase type I gene (INPP4A). Oncol Res. 2017;25:559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 19. Jiang R, Zhang C, Liu G, Gu R, Wu H. MicroRNA-101 inhibits proliferation, migration and invasion in osteosarcoma cells by targeting ROCK1. Am J Cancer Res. 2017;7:88–97. [PMC free article] [PubMed] [Google Scholar]
- 20. Yang H, Peng Z, Da Z, Li X, Cheng Y, Tan B, Xiang X, Zheng H, Li Y, Chen L, Mo N, Yan X, Li X, Hu X. MicroRNA-148a acts as a tumor suppressor in osteosarcoma via targeting Rho-associated coiled-coil kinase. Oncol Res. 2017;25:1231–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yan C, Yu J, Kang W, Liu Y, Ma Z, Zhou L. miR-935 suppresses gastric signet ring cell carcinoma tumorigenesis by targeting Notch1 expression. Biochem Biophys Res Commun. 2016;470:68–74. [DOI] [PubMed] [Google Scholar]
- 22. Bianchi ME, Agresti A. HMG proteins: Dynamic players in gene regulation and differentiation. Curr Opin Genet Dev. 2005;15:496–506. [DOI] [PubMed] [Google Scholar]
- 23. Salgado J, Villalain J, Gomez-Fernandez JC. Effects of platelet-activating factor and related lipids on dielaidoylphosphatidylethanolamine by DSC, FTIR and NMR. Biochim Biophys Acta 1993;1145:284–92. [DOI] [PubMed] [Google Scholar]
- 24. Rauvala H, Huttunen HJ, Fages C, Kaksonen M, Kinnunen T, Imai S, Raulo E, Kilpelainen I. Heparin-binding proteins HB-GAM (pleiotrophin) and amphoterin in the regulation of cell motility. Matrix Biol. 2000;19:377–87. [DOI] [PubMed] [Google Scholar]
- 25. Ulloa L, Messmer D. High-mobility group box 1 (HMGB1) protein: Friend and foe. Cytokine Growth Factor Rev. 2006;17:189–201. [DOI] [PubMed] [Google Scholar]
- 26. Kang R, Xie Y, Zhang Q, Hou W, Jiang Q, Zhu S, Liu J, Zeng D, Wang H, Bartlett DL, Billiar TR, Zeh HJ 3rd, Lotze MT, Tang D. Intracellular HMGB1 as a novel tumor suppressor of pancreatic cancer. Cell Res. 2017;27:916–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu Y, Chen Z, Zhang G, Xi Y, Sun R, Chai F, Wang X, Guo J, Tian L. HMGB1 overexpression correlates with poor prognosis in early-stage squamous cervical cancer. Tumour Biol. 2015;36:9039–47. [DOI] [PubMed] [Google Scholar]
- 28. Zhang J, Zhang R, Lu WW, Zhu JS, Xia LQ, Lu YM, Chen NW. Clinical significance of HMGB1 expression in human gastric cancer. Int J Immunopathol Pharmacol. 2014;27:543–51. [DOI] [PubMed] [Google Scholar]
- 29. Ueda M, Takahashi Y, Shinden Y, Sakimura S, Hirata H, Uchi R, Takano Y, Kurashige J, Iguchi T, Eguchi H, Sugimachi K, Yamamoto H, Doki Y, Mori M, Mimori K. Prognostic significance of high mobility group box 1 (HMGB1) expression in patients with colorectal cancer. Anticancer Res. 2014;34:5357–62. [PubMed] [Google Scholar]
- 30. Chen X, Liu X, He B, Pan Y, Sun H, Xu T, Hu X, Wang S. MiR-216b functions as a tumor suppressor by targeting HMGB1-mediated JAK2/STAT3 signaling way in colorectal cancer. Am J Cancer Res. 2017;7:2051–69. [PMC free article] [PubMed] [Google Scholar]
- 31. Gao D, Lv AE, Li HP, Han DH, Zhang YP. LncRNA MALAT-1 elevates HMGB1 to promote autophagy resulting in inhibition of tumor cell apoptosis in multiple myeloma. J Cell Biochem. 2017;118:3341–8. [DOI] [PubMed] [Google Scholar]
- 32. Jiang D, Wang H, Li Z, Li Z, Chen X, Cai H. MiR-142 inhibits the development of cervical cancer by targeting HMGB1. Oncotarget 2017;8:4001–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shrivastava S, Mansure JJ, Almajed W, Cury F, Ferbeyre G, Popovic M, Seuntjens J, Kassouf W. The role of HMGB1 in radioresistance of bladder cancer. Mol Cancer Ther. 2016;15:471–9. [DOI] [PubMed] [Google Scholar]
- 34. He J, Zhang P, Li Q, Zhou D, Liu P. Expression of high mobility group box 1 protein predicts a poorer prognosis for patients with osteosarcoma. Oncol Lett. 2016;11:293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu K, Huang J, Ni J, Song D, Ding M, Wang J, Huang X, Li W. MALAT1 promotes osteosarcoma development by regulation of HMGB1 via miR-142-3p and miR-129-5p. Cell Cycle 2017;16:578–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang L, Zhang H, Sun M, Yin Z, Qian J. High mobility group box 1-mediated autophagy promotes neuroblastoma cell chemoresistance. Oncol Rep. 2015;34:2969–76. [DOI] [PubMed] [Google Scholar]
- 37. Meng Q, Zhao J, Liu H, Zhou G, Zhang W, Xu X, Zheng M. HMGB1 promotes cellular proliferation and invasion, suppresses cellular apoptosis in osteosarcoma. Tumour Biol. 2014;35:12265–74. [DOI] [PubMed] [Google Scholar]
- 38. Huang J, Liu K, Yu Y, Xie M, Kang R, Vernon P, Cao L, Tang D, Ni J. Targeting HMGB1-mediated autophagy as a novel therapeutic strategy for osteosarcoma. Autophagy 2012;8:275–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu YJ, Li W, Chang F, Liu JN, Lin JX, Chen DX. MicroRNA-505 is downregulated in human osteosarcoma and regulates cell proliferation, migration and invasion. Oncol Rep. 2018;39:491–500. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40. Guo S, Bai R, Liu W, Zhao A, Zhao Z, Wang Y, Wang Y, Zhao W, Wang W. miR-22 inhibits osteosarcoma cell proliferation and migration by targeting HMGB1 and inhibiting HMGB1-mediated autophagy. Tumour Biol. 2014;35:7025–34. [DOI] [PubMed] [Google Scholar]