Abstract
Prostate cancer (PCa) is the second most commonly diagnosed malignancy and the fifth leading cause of cancer-related deaths in males worldwide. MicroRNAs (miRNAs) may serve as important regulators in PCa occurrence and development. Therefore, understanding the expression and functions of PCa-related miRNAs may be beneficial for the identification of novel therapeutic methods for patients with PCa. In this study, miRNA-212 (miR-212) was evidently downregulated in PCa tissues and several PCa cell lines. Functional assays showed that the resumption of miR-212 expression attenuated cell proliferation and invasion and increased the apoptosis of PCa. In addition, mitogen-activated protein kinase 1 (MAPK1), a well-known oncogene, was identified as a novel target of miR-212 in PCa, as confirmed by bioinformatics, luciferase reporter assay, qRT-PCR, and Western blot analysis. Furthermore, MAPK1 expression was upregulated in PCa tissues and inversely correlated with miR-212 expression. Rescue experiments also demonstrated that restored MAPK1 expression reversed the tumor-suppressing effects of miR-212 on PCa cell proliferation, invasion, and apoptosis. In conclusion, miR-212 may exert tumor-suppressing roles in PCa by regulating MAPK1 and could be a novel therapeutic target for treatment of patients with this malignancy.
Key words: Prostate cancer, MicroRNA-212, Proliferation, Invasion, Apoptosis, Mitogen-activated protein kinase 1 (MAPK1)
INTRODUCTION
Prostate cancer (PCa) is the second most commonly diagnosed malignancy and the fifth leading cause of cancer-related deaths in males worldwide1. In China, the morbidity and mortality of PCa have been increasing steadily in the past decade2. Currently, the major treatment strategies for patients with PCa include surgery, chemotherapy, radiotherapy, and new therapeutic agents3. Despite the considerable improvement in the therapy and diagnosis, the long-term prognosis of patients with PCa remains unsatisfactory4,5. Recurrence, metastasis, and development of hormone refractory disease remain the major reasons for poor prognosis6. Moreover, the mechanism associated with prostate carcinogenesis and progression remains largely unknown, despite a large number of genetic or epigenetic alterations that have been identified to be involved in this process7. Therefore, the molecular mechanisms underlying PCa occurrence and development must be completely understood. Furthermore, novel effective therapeutic methods for patients with this malignancy must be developed.
Regulation of microRNAs (miRNAs) and their role in the formation and progression of PCa have become hot research topics8,9. miRNAs are a large subset of highly conserved, noncoding, and single-stranded small RNA molecules with a length of approximately 21 nucleotides10. These molecules negatively modulate gene expression primarily through specific interactions with the 3′-untranslated regions (3′-UTRs) of their target genes, leading to mRNA degradation and/or translational inhibition11. About two thirds of proteins coding the human transcripts are regulated by miRNAs12. An increasing number of studies have reported that miRNA deregulation is implicated in various types of human cancer, such as PCa13, bladder cancer14, gastric cancer15, lung cancer16, and ovarian cancer17. Deregulated miRNAs may serve as either oncogenes or tumor suppressors in the malignant progression of various cancers and may regulate various biological processes, including cell proliferation, cell cycle, apoptosis, differentiation, migration, metabolism, and metastasis18–20. Hence, miRNAs may be developed as novel therapeutic targets for anticancer therapy.
miR-212 is aberrantly expressed in several cancers, such as non-small cell lung cancer21, nasopharyngeal carcinoma22, and hepatocellular carcinoma23. miR-212 is also downregulated in PCa compared with that in benign prostatic hyperplasia tissues24. The resumption of miR-212 expression inhibits the transforming growth factor-β (TGF-β)-mediated epithelial–mesenchymal transition (EMT) and castration resistance of PCa cells24,25. In the present study, our data showed that miR-212 was significantly downregulated in PCa tissues and cell lines compared with that in adjacent normal prostate tissues and normal prostatic epithelial cell line (RWPE-1), respectively. miR-212 overexpression suppressed PCa cell proliferation and invasion and promoted apoptosis by regulating its novel target gene, namely, mitogen-activated protein kinase 1 (MAPK1).
MATERIAL AND METHODS
Clinical Specimens and Cell Lines
A total of 34 pairs of PCa tissues and their corresponding adjacent normal prostate tissues were acquired from patients who underwent surgery at the Shandong Provincial Hospital affiliated to Shandong University (Shandong, P.R. China). None of the patients had been treated with chemotherapy, radiotherapy, or androgen-deprivation treatment prior to surgery. All tissue samples were frozen in liquid nitrogen and then stored at −80°C. This study was approved by the Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong University. In addition, written informed consent was provided by all of the patients who participated in the study.
Human PCa cell lines, including DU145, PC3, 22RV1, and LNCaP, were acquired from the Institute of Biochemistry and Cell Biology at the Chinese Academy of Sciences (Shanghai, P.R. China) and cultured in RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Invitrogen), 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). Normal prostatic epithelial cell line (RWPE-1) was purchased from the American Type Culture Collection (Manassas, VA, USA) and maintained in keratinocyte serum-free medium (Invitrogen) supplemented with 50 mg/ml bovine pituitary extract and 5 ng/ml recombinant human epidermal growth factor (Invitrogen). All cells were grown at 37°C in a humidified atmosphere of 5% CO2.
Oligonucleotides, Plasmids, and Transfection
Negative control miRNA mimics (miR-NC) and miR-212 mimics were designed and synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, P.R. China). MAPK1 overexpression plasmid (pcDNA3.1-MAPK1) and an empty pcDNA3.1 plasmid were acquired from Guangzhou RiboBio Co., Ltd. (Guangzhou, P.R. China). For transfection, cells were seeded into six-well plates with a density of 8 × 105 cells/well. Oligonucleotide and plasmids were transfected into cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions.
RNA Extraction and Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
Total RNA from tissue specimens or cells were isolated using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). For detection of miR-212 expression, cDNA was synthesized using a TaqMan miRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) following the manufacturer’s protocol. A TaqMan MicroRNA PCR Kit (Applied Biosystems) was used to detect miR-212 expression, with U6 snRNA as an internal control. For the analysis of MAPK1 mRNA expression, reverse transcription was performed using Moloney murine leukemia virus (M-MLV) Reverse Transcription System (Promega Corporation, Madison, WI, USA), followed by qPCR with SYBR Premix Ex Taq™ Kit (Takara Biotechnology, Co., Ltd., Dalian, P.R. China). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as an endogenous control for MAPK1 mRNA expression. The relative gene expression was analyzed using the 2−ΔΔCt method21.
3-(4,5-Dimethyl-2-Thiazolyl)-2,5-Diphenyl-2-H-Tetrazoliumbromide (MTT) Assay
MTT assay was applied to examine the cell proliferation of PCa. After transfection for 24 h, cells were collected and plated into 96-well plates with a density of 1 × 103 cells/well. After 0, 24, 48, and 72 h of incubation, MTT assay was carried out according to the manufacturer’s protocol. In brief, 20 μl of the MTT reagent (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added into each well and incubated for an additional 4 h at 37°C with 5% CO2. Afterward, the medium was removed, and 150 μl of dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA) was added per well. Finally, the optical density value at a wavelength of 490 nm was determined using a microplate reader (BioTek Instruments, Inc., Winooski, VT, USA). All experiments were performed in triplicate.
Matrigel Invasion Assay
Transwell chambers (Corning Incorporated, Corning, NY, USA) precoated with Matrigel (BD Bioscience, San Jose, CA, USA) were used to evaluate the invasion ability of PCa cells. Transfected cells were collected at 48 h posttransfection, and single-cell suspension was prepared in FBS-free RPMI-1640 medium. A total of 5 × 104 cells were seeded in the upper chamber, and 500 μl of RPMI-1640 medium containing 10% FBS was added into the lower chamber. After 24 h of incubation at 37°C with 5% CO2, cells remaining on the upper surface of the chamber were gently scraped using cotton swabs. Subsequently, the invasive cells were fixed in methanol (Beyotime Institute of Biotechnology, Jiangsu, P.R. China), stained with 0.1% crystal violet (Beyotime Institute of Biotechnology), photographed, and counted under an inverted microscope (IX83; Olympus Corporation, Tokyo, Japan) using five randomly selected visual fields per well.
Flow Cytometry Analysis
After transfection for 48 h, cells were collected and washed with ice-cold phosphate-buffered saline (PBS). An Annexin-V-fluorescein isothiocyanate (FITC) apoptosis detection kit (Invitrogen Corporation) was utilized to examine the cell apoptosis rate. The cells were incubated in 300 μl of 1× binding buffer. Five microliters of FITC-annexin V and 5 μl of propidium iodide were added to the solution and incubated at room temperature in the dark for 20 min. The percentage of apoptotic cells was quantified with flow cytometry (Beckman Coulter Corp., Brea, CA, USA).
Bioinformatics Analysis
The potential target genes for miR-212 were predicted using TargetScan (targetscan.org) and miRanda (microrna.org).
Western Blot Analysis
Total proteins were isolated from tissues or cells using RIPA lysis buffer (Beyotime Institute of Biotechnology) supplemented with protease inhibitor PMSF (Beyotime Institute of Biotechnology). The total protein concentration was detected by a BCA assay kit (Beyotime Institute of Biotechnology). Equal amounts of proteins were fractionated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (EMD Millipore, Billerica, MA, USA). After blocking in 5% nonfat milk in Tris-buffered saline-Tween (TBST) for 1 h, the membranes were incubated at 4°C overnight with primary antibodies against the following proteins: MAPK1 (sc-81459; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and GAPDH (sc-47724; Santa Cruz Biotechnology, Inc.). The membranes were subsequently washed with TBST three times and probed with goat anti-mouse horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Inc.) for 2 h at room temperature. An electrochemiluminescence substrate Western blot detection system (Pierce; Thermo Fisher Scientific, Inc.) was added to visualize the protein blots. GAPDH served as a loading control.
Luciferase Reporter Assay
The luciferase reporter plasmids, pmirGLO-MAPK1-3′-UTR wild-type (Wt) and pmirGLO-MAPK1-3′-UTR mutant (Mut), were designed and chemically synthesized by GenePharma Co., Ltd. Cells were seeded into 24-well plates with a density of 2 × 105 cells each well. miR-212 mimics or miR-NC was transfected into cells using Lipofectamine 2000, together with pmirGLO-MAPK1-3′-UTR Wt or pmirGLO-MAPK1-3′-UTR Mut. After being incubated at 37°C with 5% CO2 for 48 h, luciferase activities were examined using a Dual-Luciferase Reporter assay system (Promega Corporation) as per the manufacturer’s protocols. Renilla luciferase activity was used as an internal control.
Statistical Analysis
Data are shown as the mean ± standard deviation. All data were analyzed with SPSS version 13.0 software (SPSS Inc., Chicago, IL, USA) using Student’s t-test or one-way ANOVA. Student–Newman–Keuls test was used as a post hoc test following ANOVA. A value of p < 0.05 was considered to indicate a statistically significant difference.
RESULTS
miR-212 Levels Are Downregulated in PCa Tissues and Cell Lines
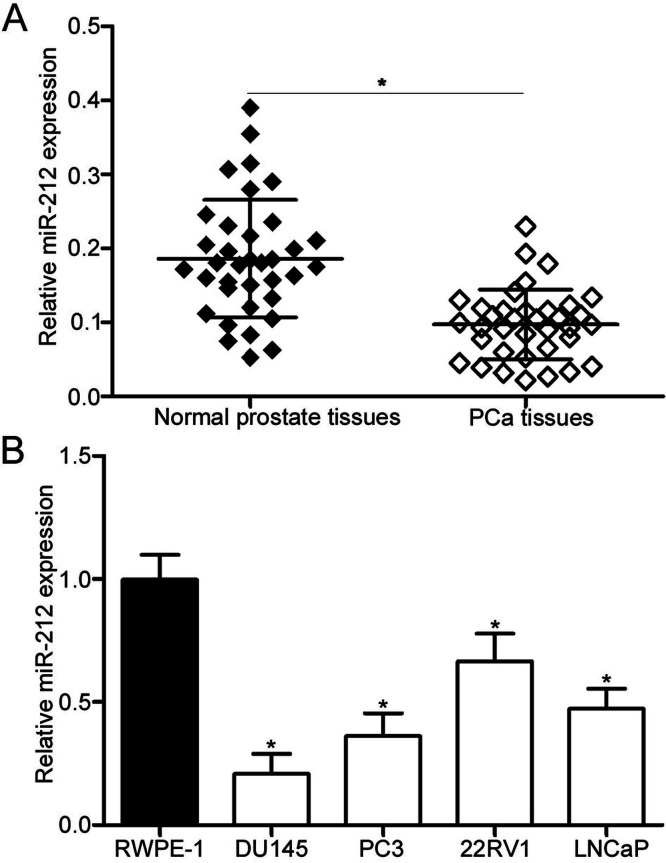
To investigate the biological functions of miR-212 in PCa, we first measured the miR-212 expression in 34 pairs of PCa tissues and their corresponding adjacent normal prostate tissues. qRT-PCR data showed that the miR-212 expression was significantly downregulated in PCa tissues compared with that in adjacent normal prostate tissues (p < 0.05) (Fig. 1A). Additionally, the expression level of miR-212 in PCa cell lines (DU145, PC3, 22RV1, and LNCaP) and RWPE-1 was detected using qRT-PCR. As presented in Figure 1B, the miR-212 expression level was also significantly decreased in all examined PCa cell lines compared with that in RWPE-1 (p < 0.05). Given that these two cell lines expressed much lower miR-212 expression than those of the 22RV1 and LNCaP cell lines, the DU145 and PC3 cell lines were selected for further experiments. These results suggest that miR-212 may be associated with PCa progression.
Figure 1.
MicroRNA-212 (miR-212) is downregulated in human prostate cancer (PCa) tissues and cell lines. (A) The relative miR-212 expression in 34 pairs of PCa tissues and their corresponding adjacent normal prostate tissues was analyzed using quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR). (B) qRT-PCR analysis was performed to detect the miR-212 expression level in PCa cell lines and normal prostatic epithelial cell line (RWPE-1). *p < 0.05 versus their respective control.
miR-212 Inhibits Cell Proliferation and Invasion and Induces Apoptosis in PCa
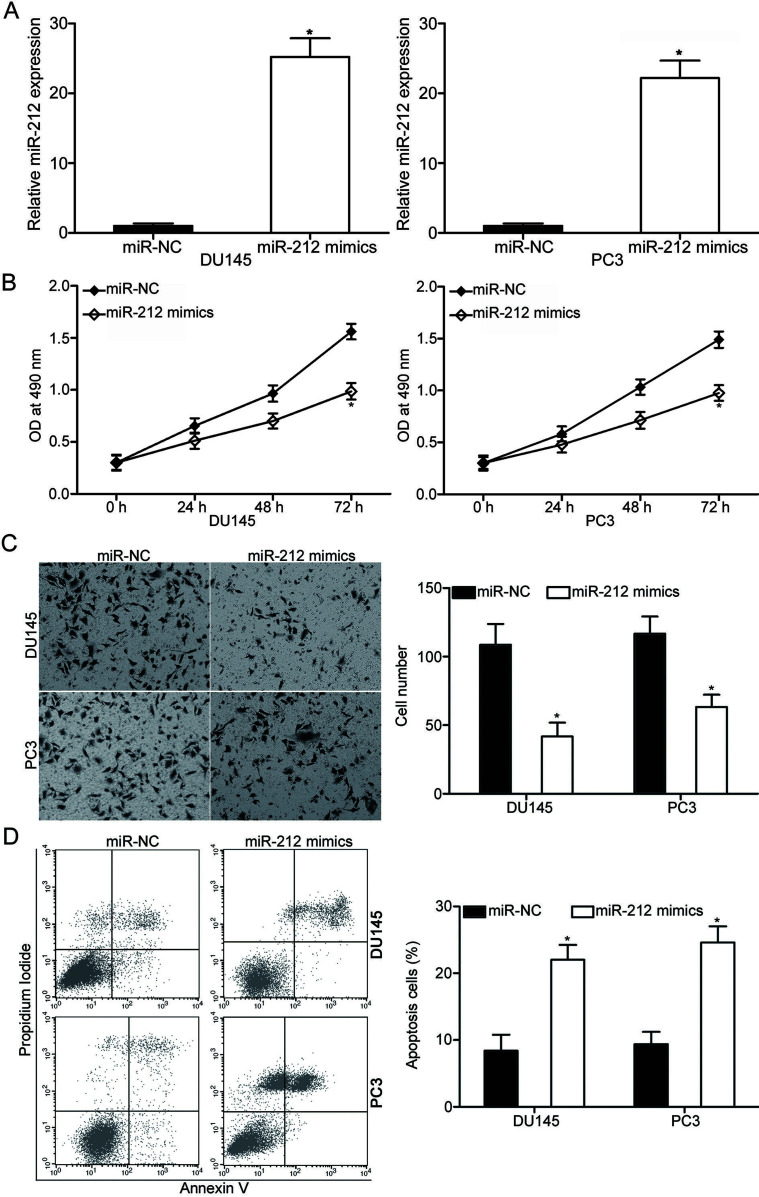
To determine the biological significance of miR-212 in PCa, miR-212 mimics were transfected into DU145 and PC3 cells to increase the miR-212 expression. After transfection, the qRT-PCR analysis data showed that miR-212 was evidently upregulated in DU145 and PC3 cell lines after transfection with miR-212 mimics (p < 0.05) (Fig. 2A). The effect of miR-212 overexpression on PCa cell proliferation was evaluated using the MTT assay. As shown in Figure 2B, enforced expression of miR-212 attenuated the proliferation of DU145 and PC3 cells (p < 0.05) (Fig. 2B). In addition, Matrigel invasion assay was adopted to detect the cell invasion ability in DU145 and PC3 cells following transfection with miR-212 mimics or miR-NC. The results showed that the DU145 and PC3 cells overexpressing miR-212 showed lower invasion ability than that of the miR-NC group (p < 0.05) (Fig. 2C). Furthermore, flow cytometry analysis was conducted to examine the cell apoptosis rate in DU145 and PC3 cells transfected with miR-212 mimics or miR-NC. Data revealed that the ectopic expression of miR-212 promoted the apoptosis of DU145 and PC3 cells compared with those of cells transfected with miR-NC (p < 0.05) (Fig. 2D). Taken together, miR-212 may play tumor suppressor phenotypic roles in PCa.
Figure 2.
miR-212 overexpression inhibits proliferation and invasion and promotes apoptosis of DU145 and PC3 cells. (A) miR-212 expression in DU145 and PC3 cells after transfection of miR-212 mimics or miR-negative control (miR-NC) was analyzed with qRT-PCR. (B) MTT assay was carried out to examine the effect of miR-212 overexpression on DU145 and PC3 cell proliferation in vitro. (C) The cell invasion capacity of DU145 and PC3 cells following transfection with miR-212 mimics or miR-NC was evaluated using Matrigel invasion assay. (D) Flow cytometry analysis was performed to detect the cell apoptosis rate in DU145 and PC3 cells after transfection with miR-212 mimics or miR-NC. *p < 0.05 versus their respective control.
miR-212 Inhibits MAPK1 Expression by Targeting its 3′-UTR
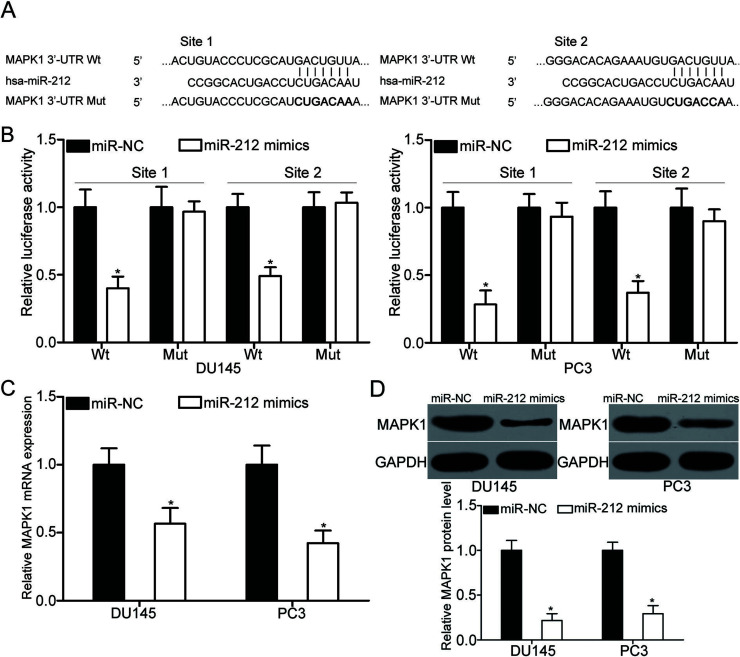
miRNAs exert their roles through negative regulation of their target genes11. To explore the mechanisms by which miR-212 inhibits PCa cell proliferation and invasion, bioinformatics analysis was carried out to predict the potential target genes for miR-212. MAPK1, also known as extracellular signal-regulated kinase 2 (ERK2), was predicted as a major target of miR-212 and selected for further confirmation. As illustrated in Figure 3A, the 3′-UTR of MAPK1 contains two predicted binding sites for miR-212. Luciferase reporter assays were conducted to investigate whether the 3′-UTR of MAPK1 could be directly targeted by miR-212. The results indicated that miR-212 upregulation reduced the luciferase activities of the pmirGLO-MAPK1-3′-UTR Wt (p < 0.05) (Fig. 3B). However, mutation with seed sites of miR-212 abrogated this effect, thereby suggesting that miR-212 can directly target the two binding sites in the 3′-UTR of MAPK1. To illustrate that miR-212 can modulate the endogenous expression of MAPK1, qRT-PCR and Western blot analysis were utilized to detect the MAPK1 expression in DU145 and PC3 cells transfected with miR-212 mimics or miR-NC. As shown in Figure 3C and D, miR-212 overexpression decreased the MAPK1 mRNA (p < 0.05) and protein (p < 0.05) expression in DU145 and PC3 cells. According to these findings, MAPK1 is a direct target of miR-212 in PCa.
Figure 3.
Mitogen-activated protein kinase 1 (MAPK1) is a direct target of miR-212 in PCa. (A) Predicted miR-212 target sequences in the 3′-untranslated regions (3′-UTRs) of MAPK1 and positions of mutated nucleotides (bold) in the 3′-UTR of MAPK1. (B, C) Relative luciferase activities in DU145 and PC3 cells cotransfected with miR-212 mimics or miR-NC and pmirGLO-MAPK1-3′-UTR wild type (Wt) (1 and 2) or pmirGLO-MAPK1-3′-UTR mutant (Mut) (1 and 2) were detected using a Dual-Luciferase Reporter assay system. Changes in MAPK1 mRNA (C) and protein (D) expression in DU145 and PC3 cells after transfection with miR-212 mimics or miR-NC were determined with qRT-PCR and Western blot analysis respectively. *p < 0.05 versus their respective control.
MAPK1 Is Highly Expressed in PCa Tissues and Is Negatively Correlated With the Expression Levels of miR-212
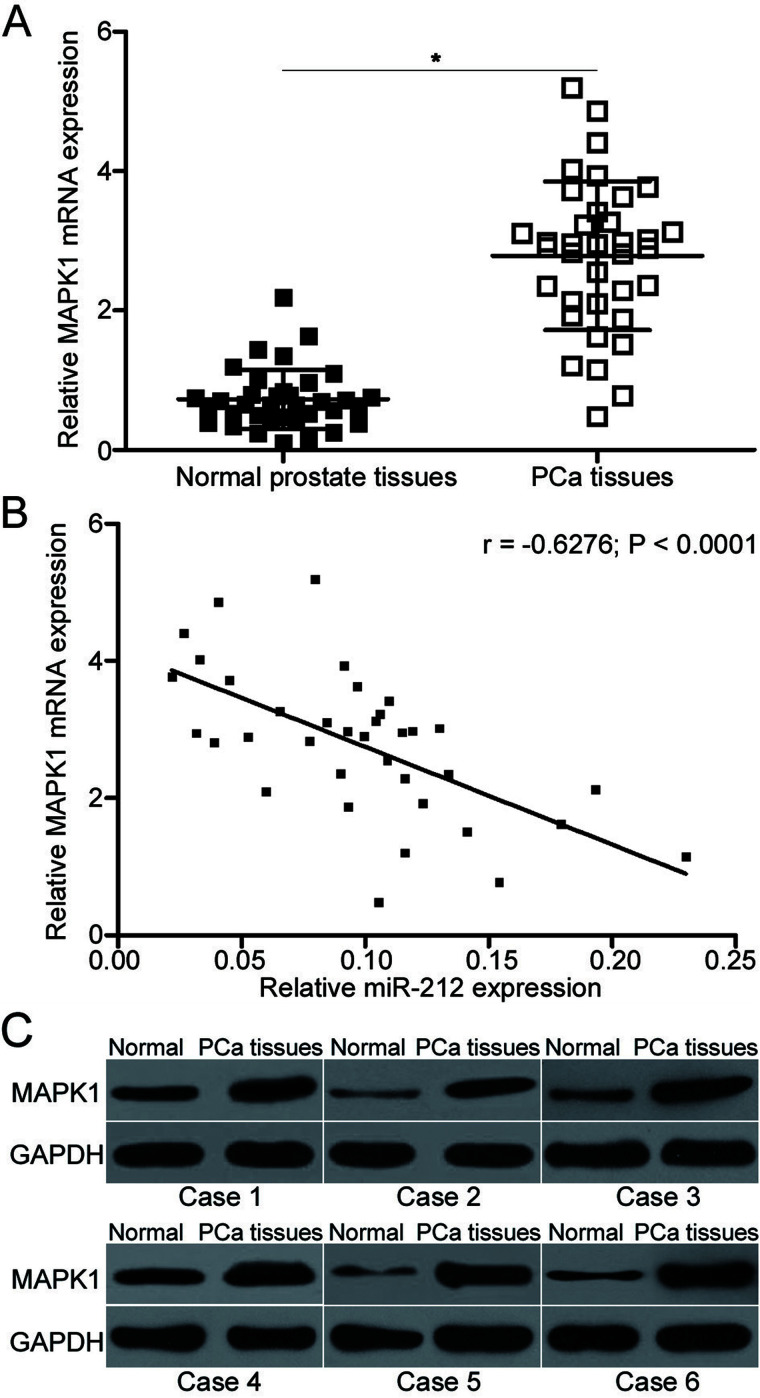
To further evaluate the association between miR-212 and MAPK1, MAPK1 mRNA expression was detected in 34 pairs of PCa tissues and their corresponding adjacent normal prostate tissues using qRT-PCR. The results indicated that MAPK1 mRNA was evidently upregulated in PCa tissues compared with that in adjacent normal prostate tissues (p < 0.05) (Fig. 4A). Additionally, a negative correlation between miR-212 and MAPK1 mRNA was observed in PCa tissues using Spearman’s correlation analysis (r = −0.6276, p < 0.0001) (Fig. 4B). Furthermore, Western blot analysis revealed that the expression level of the MAPK1 protein was higher in PCa tissues than that in adjacent normal prostate tissues (Fig. 4C).
Figure 4.
MAPK1 is upregulated in PCa tissues and inversely correlated with miR-212 expression. (A) qRT-PCR analysis of MAPK1 mRNA expression in 34 pairs of PCa tissues and their corresponding adjacent normal prostate tissues. (B) Spearman’s correlation analysis of the association between miR-212 and MAPK1 mRNA expression in PCa tissues (r = −0.6276, p < 0.0001). (C) Western blot analysis of MAPK1 protein expression in PCa tissues and their corresponding adjacent normal prostate tissues in six cases. *p < 0.05 versus their respective control.
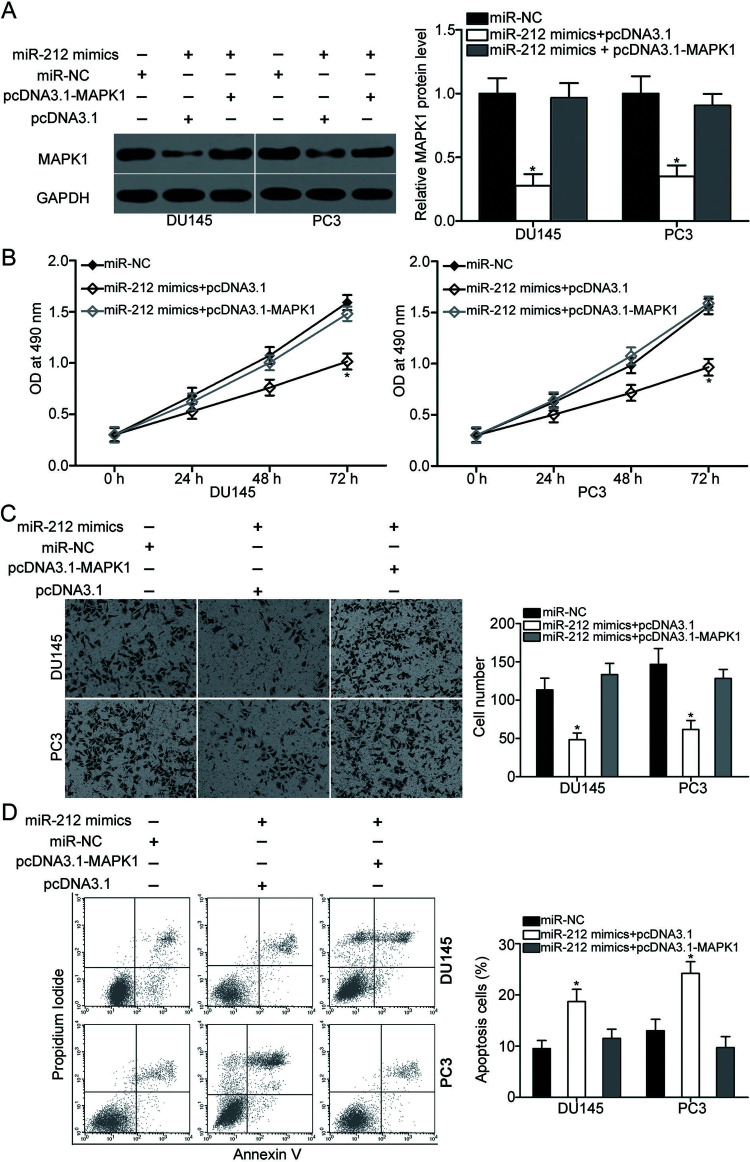
MAPK1 Overexpression Reverses the Effects of miR-212 on PCa Cell Proliferation, Invasion, and Apoptosis
To investigate whether miR-212 exerts its tumor-suppressive roles in PCa by regulation of MAPK1, a series of rescue experiments was carried out. DU145 and PC3 cells were transfected with miR-212 mimics, together with empty pcDNA3.1 or MAPK1 overexpression plasmid (pcDNA3.1-MAPK1). After transfection, Western blot analysis confirmed that the decreased MAPK1 expression induced by miR-212 overexpression could be recovered by cotransfection with pcDNA3.1-MAPK1 in DU145 and PC3 cells (p < 0.05) (Fig. 5A). Data from the MTT assay, Matrigel invasion assay, and flow cytometry analysis revealed that restored MAPK1 expression significantly rescued the effects of miR-212 mimics on DU145 and PC3 cell proliferation (p < 0.05) (Fig. 5B), invasion (p < 0.05) (Fig. 5C), and apoptosis (p < 0.05) (Fig. 5D). These results demonstrated that miR-212 may inhibit the malignant biological behavior of PCa cells by downregulating MAPK1 expression.
Figure 5.
Upregulation of MAPK1 partially rescues the tumor-suppressing effects on PCa cell proliferation, invasion, and apoptosis induced by miR-212 mimics. DU145 and PC3 cells overexpressing miR-212 were transfected with pcDNA3.1 or pcDNA3.1-MAPK1. (A) After 72 h of transfection, Western blot analysis was used to detect the protein expression of MAPK1. Cells were treated as above. Afterward, MTT assay, Matrigel invasion assay, and flow cytometry analysis were conducted to evaluate cell proliferation (B), invasion (C), and apoptosis rate (D), respectively. *p < 0.05 versus their respective control.
DISCUSSION
miRNAs may serve as important regulators in PCa occurrence and development26–28. Therefore, understanding the expression and functions of PCa-related miRNAs may be beneficial for the identification of novel therapeutic methods for patients with PCa. In the present study, we found that miR-212 was downregulated in PCa tissues and cell lines. The resumption of miR-212 expression attenuated cell proliferation and invasion and promoted apoptosis in PCa. Additionally, MAPK1 is a direct target of miR-212 in PCa. MAPK1 mRNA and protein expression levels were upregulated in PCa tissues. Furthermore, the expression level of MAPK1 in PCa tissues was much higher than that in normal prostate tissues, and its mRNA expression level was inversely correlated with miR-212 expression. Moreover, the restored MAPK1 expression rescued the tumor-suppressing effects of miR-212 on PCa cell proliferation, invasion, and apoptosis. Thus, these results provided evidence of a mechanism by which miR-212 plays tumor-suppressive roles in PCa.
miR-212 is lowly expressed in a number of cancer types. For example, miR-212 is downregulated in non-small cell lung cancer tissues and cell lines. Decreased miR-212 expression is significantly correlated with lymph node metastasis and tumor-node-metastasis (TNM) stage. In addition, low miR-212 expression is a novel independent prognostic marker for patients with non-small cell lung cancer21. In nasopharyngeal carcinoma, the miR-212 expression level is reduced in both tumor tissues and cell lines. Low miR-212 expression is significantly correlated with TNM stage and metastasis of patients with nasopharyngeal carcinoma22. Downregulation of miR-212 is also observed in hepatocellular carcinoma23, cervical cancer29,30, ovarian cancer31, glioblastoma32, osteosarcoma33, and gastric cancer34. By contrast, miR-212 is upregulated in pancreatic ductal adenocarcinoma tissues and cell lines35,36. Increased miR-212 expression is correlated with tumor size and tumor stage. Furthermore, pancreatic ductal adenocarcinoma patients with high miR-212 expression show poorer prognosis than those patients with low miR-212 expression level35. These conflicting findings suggest that the expression pattern of miR-212 exhibits tissue specificity, and it may be a biomarker of the progression of human cancer.
miR-212 plays tumor-suppressive roles in the formation and progression of multiple types of human cancers. For instance, restored miR-212 expression inhibits cell proliferation, metastasis, and EMT in non-small cell lung cancer21. Jiang et al. reported that miR-212 overexpression decreases the cell motility of nasopharyngeal carcinoma22. Enforced expression of miR-212 attenuates hepatocellular carcinoma cell proliferation, promotes apoptosis in vitro, and reduces tumor growth in vivo23,37. According to Zhou et al., miR-212 upregulation suppresses cervical cancer cell proliferation, invasion, and EMT, and arrests G1/S phase transition29. The research of Luo et al. indicated that the ectopic expression of miR-212 suppresses cell proliferation and invasion in vitro and decreases tumor growth in nude mice33. Multiple studies showed that the restoration of miR-212 expression reduces gastric cancer cell proliferation, invasion, and metastasis34,38,39. Nevertheless, miR-212 is an oncogene in pancreatic ductal adenocarcinoma affecting cell proliferation, cell cycle, and invasion36. These findings indicated conflicting results in that miR-212 is identified as a tumor suppressor in certain cancer types and an oncogene in others.
To date, a number of miR-212 targets have been validated; these targets include sex-determining region Y box 4 (SOX4)21 in non-small cell lung cancer, forkhead box A1 (FOXA1)23,37 in hepatocellular carcinoma, transcription factor 7-like 2 (TCF7L2)29, mothers against decapentaplegic homolog 2 (SMAD2)30 in cervical cancer, heparin-binding epidermal growth factor-like growth factor (HBEGF)31 in ovarian cancer, retinol-binding protein 2 (RBP2)34, paxillin (PXN)38, methyl-CpG-binding protein 2 (MECP2)39 in gastric cancer, and retinoblastoma 1 (Rb1)36 in pancreatic ductal adenocarcinoma. In our current study, MAPK1 was confirmed as a novel target of miR-212 in PCa. MAPK1 is a member of the MAPK family, and it is upregulated in various types of human malignancy, such as ovarian cancer40, bladder cancer41, non-small cell lung cancer42, and gastric cancer43. The MAPK1 expression level is increased in PCa; MAPK1 also serves important roles in the occurrence and development of PCa through regulating cell proliferation, cycle, apoptosis, metastasis, and EMT44,45. These findings suggested that MAPK1 may be developed as a potential target for the inhibition of PCa growth and metastasis.
In conclusion, this study demonstrated that miR-212 targets MAPK1 to serve tumor-suppressive roles in PCa. The miR-212/MAPK1 pathway may be used as a theoretical basis for its application in the treatment of patients with PCa.
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 3. Chang SS. Treatment options for hormone-refractory prostate cancer. Rev Urol. 2007;9(Suppl 2):S13–8. [PMC free article] [PubMed] [Google Scholar]
- 4. Hanna C, Jones RJ. Emerging treatments for recurrent prostate cancer. Future Oncol. 2015;11:2873–80. [DOI] [PubMed] [Google Scholar]
- 5. Deng X, He G, Liu J, Luo F, Peng X, Tang S, Gao Z, Lin Q, Keller JM, Yang T, Keller ET. Recent advances in bone-targeted therapies of metastatic prostate cancer. Cancer Treat Rev. 2014;40:730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mukherji D, Eichholz A, De Bono JS. Management of metastatic castration-resistant prostate cancer: Recent advances. Drugs 2012;72:1011–28. [DOI] [PubMed] [Google Scholar]
- 7. Xu L, Wang Z, Li XF, He X, Guan LL, Tuo JL, Wang Y, Luo Y, Zhong HL, Qiu SP, Cao KY. Screening and identification of significant genes related to tumor metastasis and PSMA in prostate cancer using microarray analysis. Oncol Rep. 2013;30:1920–8. [DOI] [PubMed] [Google Scholar]
- 8. Ni J, Bucci J, Chang L, Malouf D, Graham P, Li Y. Targeting microRNAs in prostate cancer radiotherapy. Theranostics 2017;7:3243–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Massillo C, Dalton GN, Farre PL, De Luca P, De Siervi A. Implications of microRNA dysregulation in the development of prostate cancer. Reproduction 2017;154:R81–R97. [DOI] [PubMed] [Google Scholar]
- 10. Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–39. [DOI] [PubMed] [Google Scholar]
- 11. Lai EC. Micro RNAs are complementary to 3′ UTR sequence motifs that mediate negative post-transcriptional regulation. Nat Genet. 2002;30:363–4. [DOI] [PubMed] [Google Scholar]
- 12. Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang J, Wang X, Wang Y, Peng R, Lin Z, Wang Y, Hu B, Wang J, Shi G. Low expression of microRNA-30c promotes prostate cancer cells invasion involved in downregulation of KRAS protein. Oncol Lett. 2017;14:363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu X, Liu X, Wu Y, Wu Q, Wang Q, Yang Z, Li L. MicroRNAs in biofluids are novel tools for bladder cancer screening. Oncotarget 2017;8:32370–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Y, Guan DH, Bi RX, Xie J, Yang CH, Jiang YH. Prognostic value of microRNAs in gastric cancer: A meta-analysis. Oncotarget 2017;8:55489–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang P, Liu X, Shao Y, Wang H, Liang C, Han B, Ma Z. MicroRNA-107-5p suppresses non-small cell lung cancer by directly targeting oncogene epidermal growth factor receptor. Oncotarget 2017;8:57012–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li C, Duan P, Wang J, Lu X, Cheng J. miR-320 inhibited ovarian cancer oncogenicity via targeting TWIST1 expression. Am J Transl Res. 2017;9:3705–13. [PMC free article] [PubMed] [Google Scholar]
- 18. Bouyssou JM, Manier S, Huynh D, Issa S, Roccaro AM, Ghobrial IM. Regulation of microRNAs in cancer metastasis. Biochim Biophys Acta 2014;1845:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vimalraj S, Miranda PJ, Ramyakrishna B, Selvamurugan N. Regulation of breast cancer and bone metastasis by microRNAs. Dis Markers 2013;35:369–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Esquela-Kerscher A, Slack FJ. Oncomirs—MicroRNAs with a role in cancer. Nat Rev Cancer 2006;6:259–69. [DOI] [PubMed] [Google Scholar]
- 21. Tang T, Huan L, Zhang S, Zhou H, Gu L, Chen X, Zhang L. MicroRNA-212 functions as a tumor-suppressor in human non-small cell lung cancer by targeting SOX4. Oncol Rep. 2017;38:2243–50. [DOI] [PubMed] [Google Scholar]
- 22. Jiang C, Wang H, Zhou L, Jiang T, Xu Y, Xia L. MicroRNA-212 inhibits the metastasis of nasopharyngeal carcinoma by targeting SOX4. Oncol Rep. 2017;38:82–8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23. Tu H, Wei G, Cai Q, Chen X, Sun Z, Cheng C, Zhang L, Feng Y, Zhou H, Zhou B, Zeng T. MicroRNA-212 inhibits hepatocellular carcinoma cell proliferation and induces apoptosis by targeting FOXA1. Onco Targets Ther. 2015;8:2227–35. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24. Fu W, Tao T, Qi M, Wang L, Hu J, Li X, Xing N, Du R, Han B. MicroRNA-132/212 upregulation inhibits TGF-beta-mediated epithelial-mesenchymal transition of prostate cancer cells by targeting SOX4. Prostate 2016;76:1560–70. [DOI] [PubMed] [Google Scholar]
- 25. Yang Y, Jia D, Kim H, Abd Elmageed ZY, Datta A, Davis R, Srivastav S, Moroz K, Crawford BE, Moparty K, Thomas R, Hudson RS, Ambs S, Abdel-Mageed AB. Dysregulation of miR-212 promotes castration resistance through hnRNPH1-mediated regulation of AR and AR-V7: Implications for racial disparity of prostate cancer. Clin Cancer Res. 2016;22:1744–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lv Z, Rao P, Li W. MiR-592 represses FOXO3 expression and promotes the proliferation of prostate cancer cells. Int J Clin Exp Med. 2015;8:15246–53. [PMC free article] [PubMed] [Google Scholar]
- 27. Huang K, Tang Y, He L, Dai Y. MicroRNA-340 inhibits prostate cancer cell proliferation and metastasis by targeting the MDM2-p53 pathway. Oncol Rep. 2016;35:887–95. [DOI] [PubMed] [Google Scholar]
- 28. Khanmi K, Ignacimuthu S, Paulraj MG. MicroRNA in prostate cancer. Clin Chim Acta 2015;451:154–60. [DOI] [PubMed] [Google Scholar]
- 29. Zhou C, Tan DM, Chen L, Xu XY, Sun CC, Zong LJ, Han S, Zhang YZ. Effect of miR-212 targeting TCF7L2 on the proliferation and metastasis of cervical cancer. Eur Rev Med Pharmacol Sci. 2017;21:219–26. [PubMed] [Google Scholar]
- 30. Zhao JL, Zhang L, Guo X, Wang JH, Zhou W, Liu M, Li X, Tang H. miR-212/132 downregulates SMAD2 expression to suppress the G1/S phase transition of the cell cycle and the epithelial to mesenchymal transition in cervical cancer cells. IUBMB Life 2015;67:380–94. [DOI] [PubMed] [Google Scholar]
- 31. Wei LQ, Liang HT, Qin DC, Jin HF, Zhao Y, She MC. MiR-212 exerts suppressive effect on SKOV3 ovarian cancer cells through targeting HBEGF. Tumour Biol. 2014;35:12427–34. [DOI] [PubMed] [Google Scholar]
- 32. Liu H, Li C, Shen C, Yin F, Wang K, Liu Y, Zheng B, Zhang W, Hou X, Chen X, Wu J, Wang X, Zhong C, Zhang J, Shi H, Ai J, Zhao S. MiR-212-3p inhibits glioblastoma cell proliferation by targeting SGK3. J Neurooncol. 2015;122:431–9. [DOI] [PubMed] [Google Scholar]
- 33. Luo XJ, Tang DG, Gao TL, Zhang YL, Wang M, Quan ZX, Chen J. MicroRNA-212 inhibits osteosarcoma cells proliferation and invasion by down-regulation of Sox4. Cell Physiol Biochem. 2014;34:2180–8. [DOI] [PubMed] [Google Scholar]
- 34. Jiping Z, Ming F, Lixiang W, Xiuming L, Yuqun S, Han Y, Zhifang L, Yundong S, Shili L, Chunyan C, Jihui J. MicroRNA-212 inhibits proliferation of gastric cancer by directly repressing retinoblastoma binding protein 2. J Cell Biochem. 2013;114:2666–72. [DOI] [PubMed] [Google Scholar]
- 35. Wu Z, Zhou L, Ding G, Cao L. Overexpressions of miR-212 are associated with poor prognosis of patients with pancreatic ductal adenocarcinoma. Cancer Biomark. 2017;18:35–9. [DOI] [PubMed] [Google Scholar]
- 36. Park JK, Henry JC, Jiang J, Esau C, Gusev Y, Lerner MR, Postier RG, Brackett DJ, Schmittgen TD. miR-132 and miR-212 are increased in pancreatic cancer and target the retinoblastoma tumor suppressor. Biochem Biophys Res Commun. 2011;406:518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dou C, Wang Y, Li C, Liu Z, Jia Y, Li Q, Yang W, Yao Y, Liu Q, Tu K. MicroRNA-212 suppresses tumor growth of human hepatocellular carcinoma by targeting FOXA1. Oncotarget 2015;6:13216–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li D, Li Z, Xiong J, Gong B, Zhang G, Cao C, Jie Z, Liu Y, Cao Y, Yan Y, Xiong H, Qiu L, Yang M, Chen H, Jiang S, Yang X, Chen H. MicroRNA-212 functions as an epigenetic-silenced tumor suppressor involving in tumor metastasis and invasion of gastric cancer through down-regulating PXN expression. Am J Cancer Res. 2015;5:2980–97. [PMC free article] [PubMed] [Google Scholar]
- 39. Wada R, Akiyama Y, Hashimoto Y, Fukamachi H, Yuasa Y. miR-212 is downregulated and suppresses methyl-CpG-binding protein MeCP2 in human gastric cancer. Int J Cancer 2010;127:1106–14. [DOI] [PubMed] [Google Scholar]
- 40. Yiwei T, Hua H, Hui G, Mao M, Xiang L. HOTAIR interacting with MAPK1 regulates ovarian cancer skov3 cell proliferation, migration, and invasion. Med Sci Monit. 2015;21:1856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang X, Wu G, Cao G, Chen X, Huang J, Jiang X, Hou J. MicroRNA335 inhibits bladder cancer cell growth and migration by targeting mitogen-activated protein kinase 1. Mol Med Rep. 2016;14:1765–70. [DOI] [PubMed] [Google Scholar]
- 42. You B, Yang YL, Xu Z, Dai Y, Liu S, Mao JH, Tetsu O, Li H, Jablons DM, You L. Inhibition of ERK1/2 down-regulates the Hippo/YAP signaling pathway in human NSCLC cells. Oncotarget 2015;6:4357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fei B, Wu H. MiR-378 inhibits progression of human gastric cancer MGC-803 cells by targeting MAPK1 in vitro. Oncol Res. 2012;20:557–64. [DOI] [PubMed] [Google Scholar]
- 44. Chen QG, Zhou W, Han T, Du SQ, Li ZH, Zhang Z, Shan GY, Kong CZ. MiR-378 suppresses prostate cancer cell growth through downregulation of MAPK1 in vitro and in vivo. Tumour Biol. 2016;37:2095–103. [DOI] [PubMed] [Google Scholar]
- 45. Amatangelo MD, Goodyear S, Varma D, Stearns ME. c-Myc expression and MEK1-induced Erk2 nuclear localization are required for TGF-beta induced epithelial-mesenchymal transition and invasion in prostate cancer. Carcinogenesis 2012;33:1965–75. [DOI] [PMC free article] [PubMed] [Google Scholar]