Abstract
Lung cancer is the leading cause of cancer deaths worldwide. Given that the major threat of cancer is metastasis, delineation of the molecular mechanism underlying it would help devise therapeutic strategies. Transglutaminase 2 (TG2), belonging to the transglutaminase superfamily, is a versatile protein with enzymatic and nonenzymatic functions. It mainly localizes inside the cell, but also appears extracellularly. Recent findings have demonstrated the involvement of TG2 in cancer development. Here we examine the role of TG2 in metastasis of lung cancer using a lung cancer cell line CL1-0, which exhibits low invasiveness, and its invasive subline CL1-5. Our results show that CL1-5 cells express a higher amount of TG2 than CL1-0 cells. Overexpression of TG2 in CL1-0 enhances cell migration and invasion, and lowering TG2 expression in CL1-5 cells reduces their ability to do so. The transamidase activity of TG2 is not required since cells expressing the inactive TG2 mutant or treated with a TG2 inhibitor are still able to migrate and invade. TG2-stimulated migration and invasion are, at least in part, mediated by Rac, as inhibition of Rac activity suppresses cell migration and invasion. Lastly, exogenous application of recombinant TG2 protein to CL1-0 cells substantially augments cell migration and invasion, suggesting the significance of extracellular TG2 in promoting these events. Collectively, our results show that TG2 plays a positive role in cell migration and invasion, and this might help metastasis of lung cancer cells.
Key words: Transglutaminase 2 (TG2), Lung cancer, Migration, Invasion, Rac
INTRODUCTION
Lung cancer is the leading cause of cancer-related deaths worldwide, and one major cause of death is metastasis1. Tumor metastasis is a complex process that involves tumor invasion at the primary site, survival in the circulation, and eventually colonization at new sites2. Identification of metastasis-related genes and pathways would help devise strategies for cancer therapy.
Transglutaminases (TGs; EC 2.3.2.13) are a family of enzymes that catalyze Ca2+-dependent protein transamidation. Transglutaminase 2 (TG2), belonging to the TG superfamily, has functions beyond catalysis of protein transamidation. It also possesses GTPase, protein kinase, and protein disulfide isomerase activities3,4. Moreover, TG2 exhibits nonenzymatic scaffolding function for cell adhesion and signaling3,5,6. Most TG2 proteins are localized intracellularly, but some are present outside the cell, both on the cell surface and in the extracellular matrix3. Given its wide distribution and multiple functions, TG2 regulates a number of responses, such as cell survival/apoptosis, adhesion, migration, differentiation, and inflammation3,4.
Mounting evidence has shown the involvement of TG2 in cancer development. TG2 expression is higher in tumor than in normal tissues in melanoma, pancreatic, and ovarian cancers7–10. It is also positively correlated with the metastatic status in these cancer types and in breast cancer7,8,11,12. Enhancing TG2 expression in various types of cancer cells promotes invasion and drug resistance7,8,10,13–19. This might be due to the induction of epithelial–mesenchymal transition (EMT)20–24. In breast and ovarian cancers, cells with increased TG2 expression acquire properties of stem cells that help self-renewal and survival25,26. Recent studies have demonstrated a novel act of TG2 in pancreatic cancer. Secreted TG2 promotes tumor growth and drug resistance by affecting extracellular matrix and stromal fibroblasts in the tumor microenvironment9,27.
The role of TG2 in cancer development has been extensively studied in breast, ovarian, and pancreatic cancers8–11,13–15,20,21,25–30. There is some evidence for the involvement of TG2 in lung cancer progression17–19,22. Here we used a lung cancer cell line, CL1-0, which displays low invasiveness, and its invasive subline, CL1-5, to explore the possibility that TG2 controls metastasis of lung cancer cells.
MATERIALS AND METHODS
Reagents
Recombinant human TG2 protein and antibody to TG2 were purchased from Thermo Fisher Scientific (Fremont, CA, USA). Monodansylcadaverine (MDC) and antibody to actin were obtained from Sigma-Aldrich (St. Louis, MO, USA). The Rac activation assay kit was from Millipore (Temecula, CA, USA).
Cell Culture and Transfection
Human lung adenocarcinoma cell lines CL1-0 and CL1-5 were provided by Dr. Pan-Chyr Yang (National Taiwan University, Taiwan, Republic of China). CL1-5, a subline of CL1-0, was isolated from CL1-0 through progressive in vitro invasion screening. CL1-0 and CL1-5 exhibit low and high invasion ability, respectively31. These cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco), 100 IU/ml penicillin, and 100 μg/ml streptomycin (Gibco). CL1-0 cells were transfected with pcDNA3.1+ vector (CL1-0/vector), wild-type TG2 construct (CL1-0/TG2), or TG2-C277S construct (CL1-0/TG2-C277S) using the Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA). These TG2 constructs were provided by Dr. Gail V. W. Johnson (University of Rochester, USA). Stable transfectants of CL1-0 were selected and maintained in medium containing 700 μg/ml of G418 (Gibco). Transient transfection of siRNA and Rac1N17 construct into cells was carried out using the Lipofectamine 2000 reagent. The target sequence of TG2 siRNA is 5′-AAGGGCGAACCACCTGAACAA-3′. The Rac1N17 construct was donated by Dr. Tzuu-Shuh Jou (National Taiwan University, Taiwan, Republic of China).
TG Activity Assay
TG activity was measured as previously described32. Briefly, microplates were coated with 20 mg/ml of N,N′-dimethylcasein (Sigma-Aldrich) and blocked with nonfat dry milk (0.5% in 0.1 M Tris-HCl, pH 8.5). Cell lysates were extracted by a buffer containing 5 mM Tris-HCl, pH 7.4, 0.25 M sucrose, 0.2 mM MgSO4, 2 mM dithiothreitol (DTT), 0.4 mM phenylmethylsulfonyl fluoride, and 0.4% Triton X-100, sonicated for 10 s, and loaded onto the coated microplates. The lysates were then incubated for 1 h in a reaction mix containing 100 mM Tris, pH 8.5, 20 mM CaCl2, 40 mM DTT, and 2 mM 5-(biotinamido)pentylamine (Pierce, Rockford, IL, USA), followed by incubating with streptavidin alkaline phosphatase (Sigma-Aldrich) and p-NPP (Sigma-Aldrich). The absorbance at 405 nm was measured by an ELISA plate reader.
Cell Migration and Invasion Assay
A commercially available modified Boyden chamber (Neuro Probe, Inc., Gaithersburg, MD, USA) without or layered with Matrigel (BD Biosciences, Bedford, MA, USA) was used to detect cell migration and invasion, respectively. Polycarbonate membranes (8-μm pore size; Neuro Probe, Inc.) were coated without or with 1 μg/ml Matrigel. Cells were trypsinized, centrifuged, and resuspended in DMEM containing 10% FBS and counted. An equal number of cells (4 × 105 cells/well) was loaded onto the upper chamber. The same medium was added in the lower chamber. After 24 h of incubation, cells were fixed with methanol and stained with Giemsa (Sigma-Aldrich). Cells on the upper side of membranes were removed, and cells that had migrated/invaded to the lower surface of membranes were counted.
Immunoblotting
Cells were lysed in lysis buffer containing 50 mM Tris, pH 7.4, 150 mM NaCl, 2 mM EDTA, 1 mM Na3VO4, 10 mM NaF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride, and 1% Triton X-100. Cell lysates containing equal amounts of protein were subjected to SDS-PAGE, transferred to a PVDF membrane (PerkinElmer, Boston, MA, USA), and probed with antibodies to TG2 (1:500) and actin (1:1,000). Proteins were visualized using an ECL kit (Cell Signaling Technology, Beverly, MA, USA).
Rac Activity Assay
Measurement of Rac activity was performed according to the manufacturer’s instructions (Millipore). Briefly, ∼1.5 mg of cell lysates was incubated with GST-PAK-1 p21-binding domain bound to glutathione–agarose beads to precipitate GTP-bound Rac. Total lysates and precipitates were analyzed by immunoblotting with antibody to Rac.
Statistical Analysis
Data are expressed as mean ± SEM of at least three independent experiments and were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s or Dunnett’s multiple comparisons test. A value of p < 0.05 was considered statistically significant. GraphPad Prism 5 software (GraphPad Software, La Jolla, CA, USA) was used for statistical analyses.
RESULTS
TG2 Promotes Migration and Invasion of Lung Cancer Cells
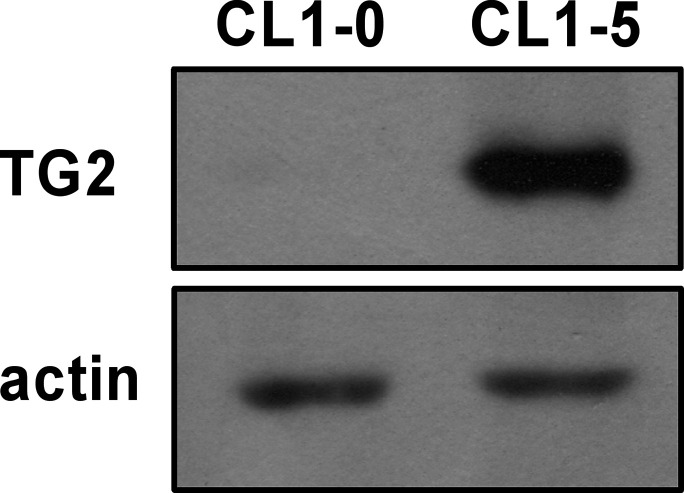
TG2 has been implicated in metastasis of various cancer types8,10,11,14,16,20. To elucidate its role in metastasis of lung cancer, we used a lung adenocarcinoma cell line, CL1-0, and its subline, CL1-5. CL1-5 was isolated from CL1-0 through progressive in vitro invasion screening. CL1-0 cells exhibit epithelial phenotype with low invasiveness, whereas CL1-5 cells display fibroblast-like morphology and are highly invasive31. Examination of TG2 expression in these cells revealed that TG2 levels were substantially higher in CL1-5 than in CL1-0 cells (Fig. 1), implying a positive correlation between TG2 expression and cell invasiveness.
Figure 1.
Transglutaminase 2 (TG2) expression positively correlates with cell invasiveness in lung cancer cell lines. Total cell lysates collected from CL1-0 and its highly invasive subline CL1-5 were analyzed by immunoblotting with antibodies to TG2 and actin.
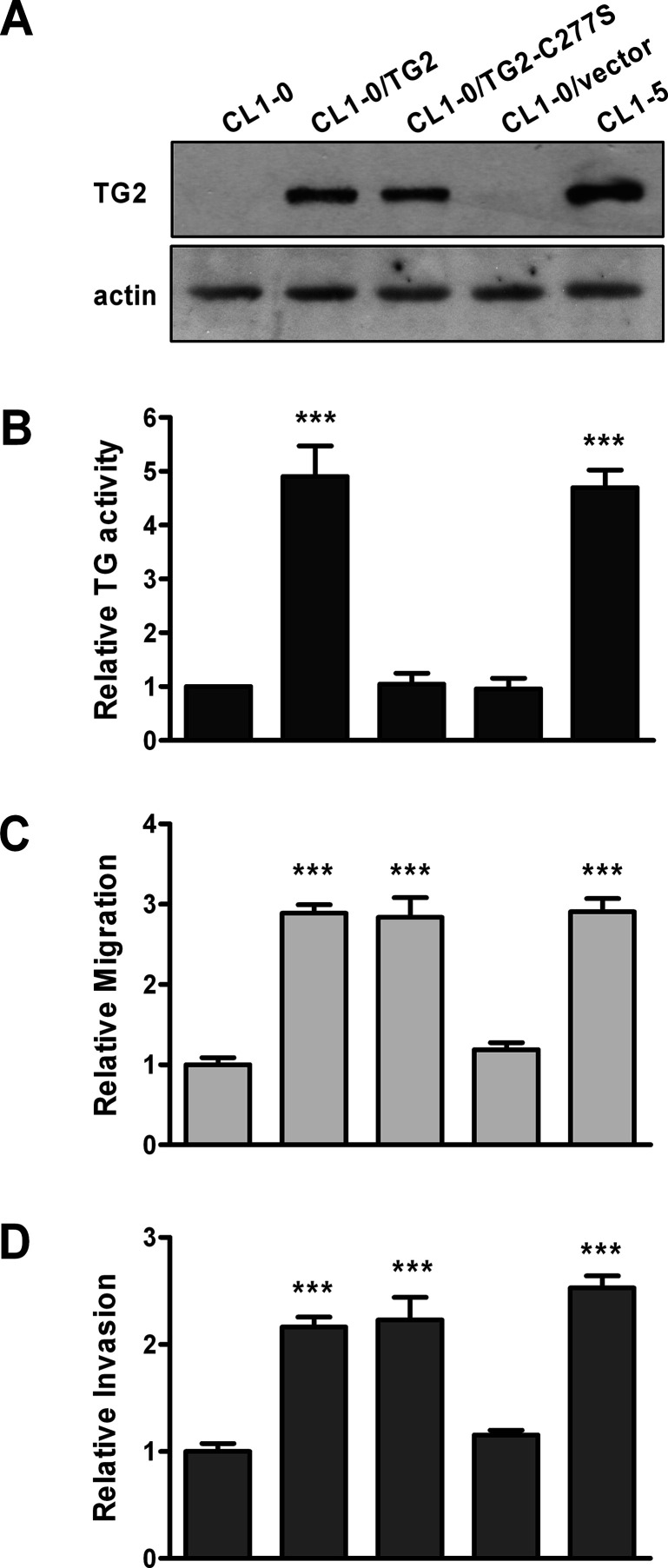
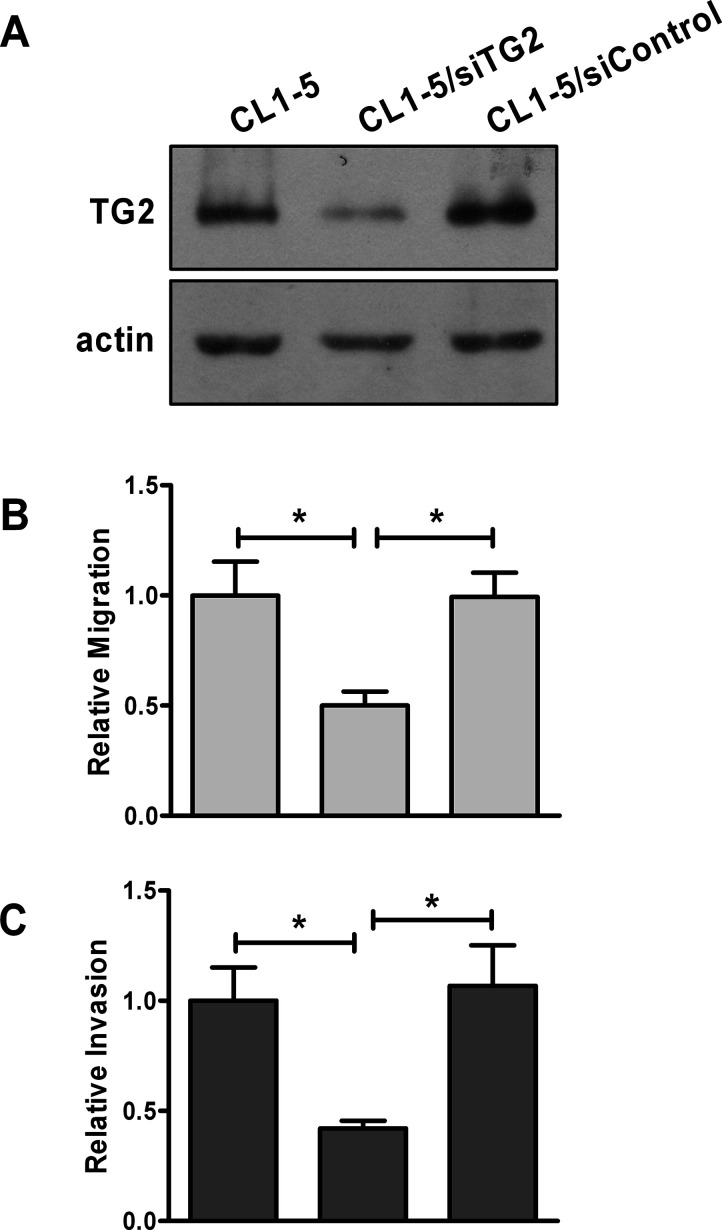
To determine if TG2 promotes cell invasion, we established stable lines of CL1-0 expressing wild-type TG2 and empty vector, designated as CL1-0/TG2 and CL1-0/vector, respectively. Expression of TG2 in these cells was confirmed by immunoblotting (Fig. 2A). In agreement with previous findings, CL1-5 cells were more motile and invasive than CL1-0 cells31. Overexpression of TG2 in CL1-0 enhanced cell migration and invasion (Fig. 2C and D). Alternatively, TG2 expression in CL1-5 was knocked down, and its effect on cell migration and invasion was examined. As shown in Figure 3, TG2 siRNA (siTG2) effectively reduced TG2 expression, and this caused a decrease in the migratory and invasive abilities of CL1-5 cells. Collectively, these results suggest that TG2 promotes migration and invasion of lung cancer cells.
Figure 2.
Overexpression of TG2 promotes migration and invasion of lung cancer cells. Stable lines of CL1-0 were established by transfecting cells with DNA construct containing sequences of wild-type TG2 (CL1-0/TG2) or the inactive TG2 mutant TG2-C277S (CL1-0/TG2-C277S), or vector control (CL1-0/vector). CL1-0, CL1-0 stable transfectants, and CL1-5 were subjected to immunoblotting (A), TG activity assay (B), migration assay (C), and invasion assay (D). (B–D) Data are expressed as fold change relative to CL1-0. ***p < 0.005, compared with CL1-0.
Figure 3.
Lowering TG2 expression reduces the migratory and invasive abilities of lung cancer cells. CL1-5 cells were mock transfected or transiently transfected with control siRNA (siControl) or TG2 siRNA (siTG2). CL1-5 and its transfectants were subjected to immunoblotting (A), migration assay (B), and invasion assay (C). (B, C) Data are expressed as fold change relative to mock-transfected CL1-5. *p < 0.05.
The Transamidase Activity of TG2 Is Not Required for Migration and Invasion of Lung Cancer Cells
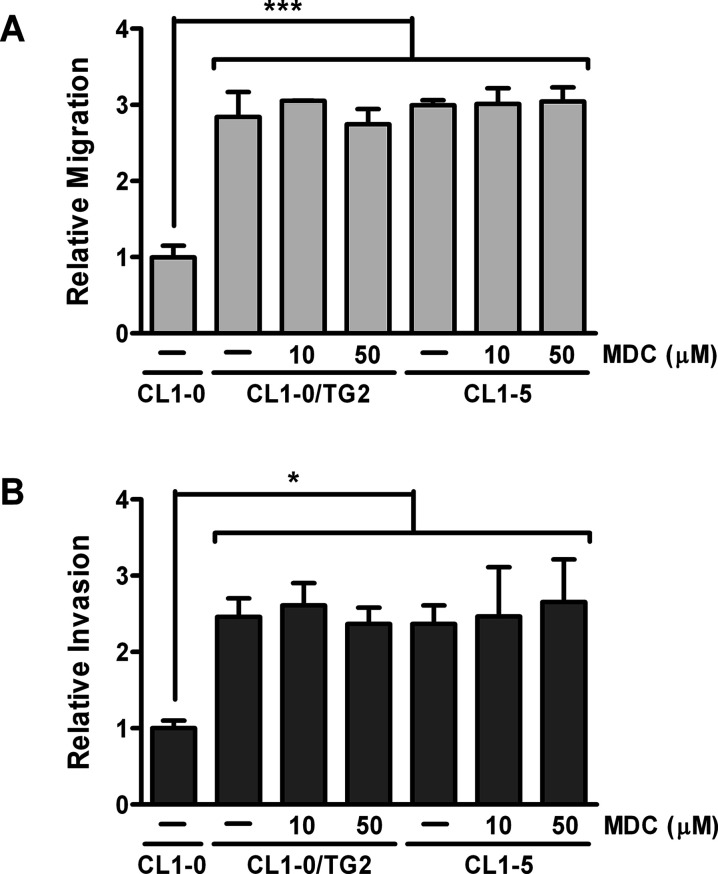
One major function of TG2 is to catalyze protein transamidation3,4. To examine if the transamidase activity of TG2 is required for cell migration and invasion, the inactive TG2 mutant TG2-C277S, in which the cysteine residue in the active site is mutated to serine, was stably expressed in CL1-0 cells. Expression of TG2-C277S was confirmed by immunoblotting (Fig. 2A). TG activity assay was conducted to assure that TG2-C277S lacked transamidase activity (Fig. 2B). Overexpression of TG2-C277S also promoted migration and invasion of CL1-0 cells (Fig. 2C and D), suggesting that the transamidase activity of TG2 is dispensable in these events. To further confirm it, a TG2 inhibitor, MDC, was used to treat cells. MDC did not inhibit migration and invasion in CL1-0/TG2 and CL1-5 cells (Fig. 4). Similar results were obtained by exposing cells to cystamine, another TG2 inhibitor (data not shown). These data indicate that TG2 facilitates cell migration and invasion through a mechanism that does not need its transamidase activity.
Figure 4.
Inhibition of the transamidase activity of TG2 by monodansylcadaverine (MDC) does not suppress cell migration and invasion. CL1-0 cells were untreated, and CL1-0/TG2 and CL1-5 cells were treated with 10 or 50 μM MDC. Cells were then subjected to migration assay (A) and invasion assay (B). Data are expressed as fold change relative to CL1-0. *p < 0.05, ***p < 0.005, compared with CL1-0.
Exogenous Application of Recombinant TG2 Protein Augments Migration and Invasion of Lung Cancer Cells
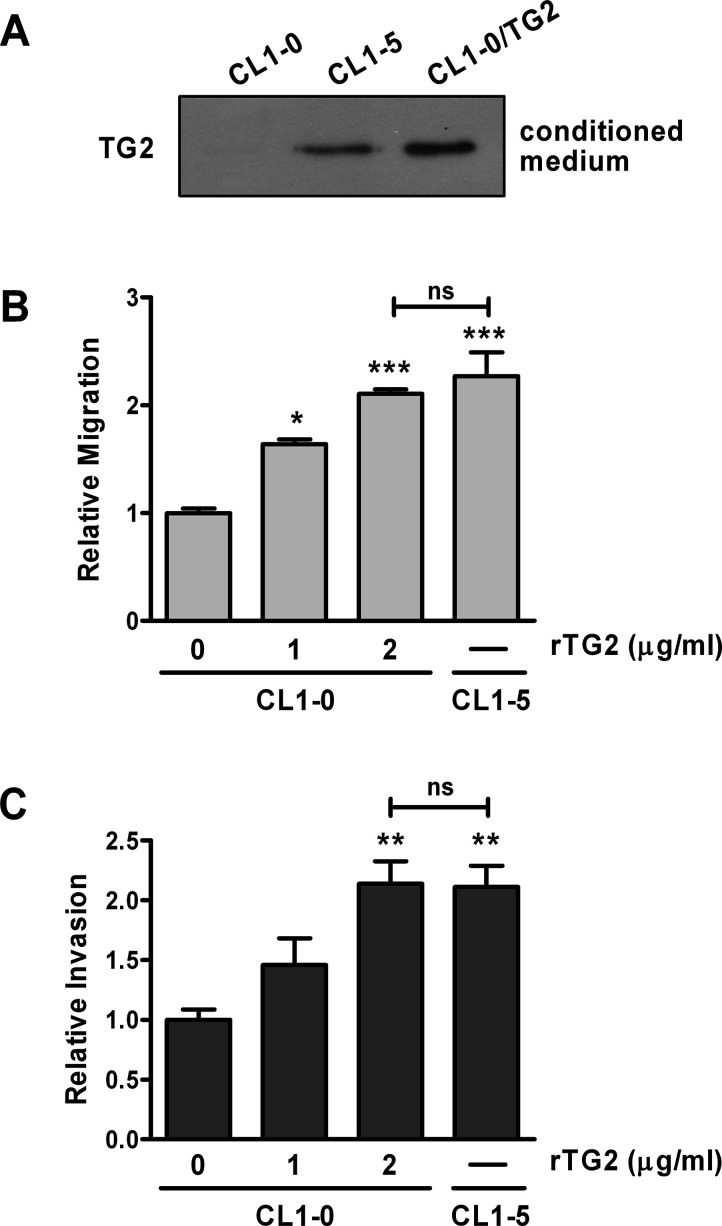
An intriguing feature of TG2 is its diverse localizations, both inside and outside a cell3,33. In ovarian cancer, TG2 is secreted into the extracellular milieu, and extracellular TG2 facilitates tumor metastasis10,11. Here we examined the possibility that extracellular TG2 exerted similar effects in lung cancer cells. Our results revealed that cells with higher TG2 expression including CL1-5 and CL1-0/TG2 had greater amounts of TG2 in culture medium (Fig. 5A). Application of conditioned medium collected from CL1-5 into CL1-0 cells augmented migration and invasion, although the extents were lower than those in CL1-5 (data not shown). To demonstrate that the stimulatory effect of conditioned medium is ascribed to secreted TG2 instead of other components in it, recombinant human TG2 protein was used to treat CL1-0 cells. This treatment stimulated cell migration and invasion in a dose-dependent manner (Fig. 5B and C), suggesting that extracellular TG2 is capable of promoting migration and invasion in lung cancer cells.
Figure 5.
Exogenous application of recombinant human TG2 protein augments cell migration and invasion. (A) Culture medium (conditioned medium) collected from CL1-0, CL1-0/TG2, and CL1-5 was analyzed by immunoblotting for TG2. (B, C) CL1-0 cells were untreated or treated with 1 or 2 μg/ml recombinant TG2 protein and subjected to migration assay (B) and invasion assay (C). (B, C) Data are expressed as fold change relative to untreated CL1-0. *p < 0.05, **p < 0.01, ***p < 0.005, compared with untreated CL1-0; ns, not significant.
TG2 Promotes Migration and Invasion by Increasing Rac Activity in Lung Cancer Cells
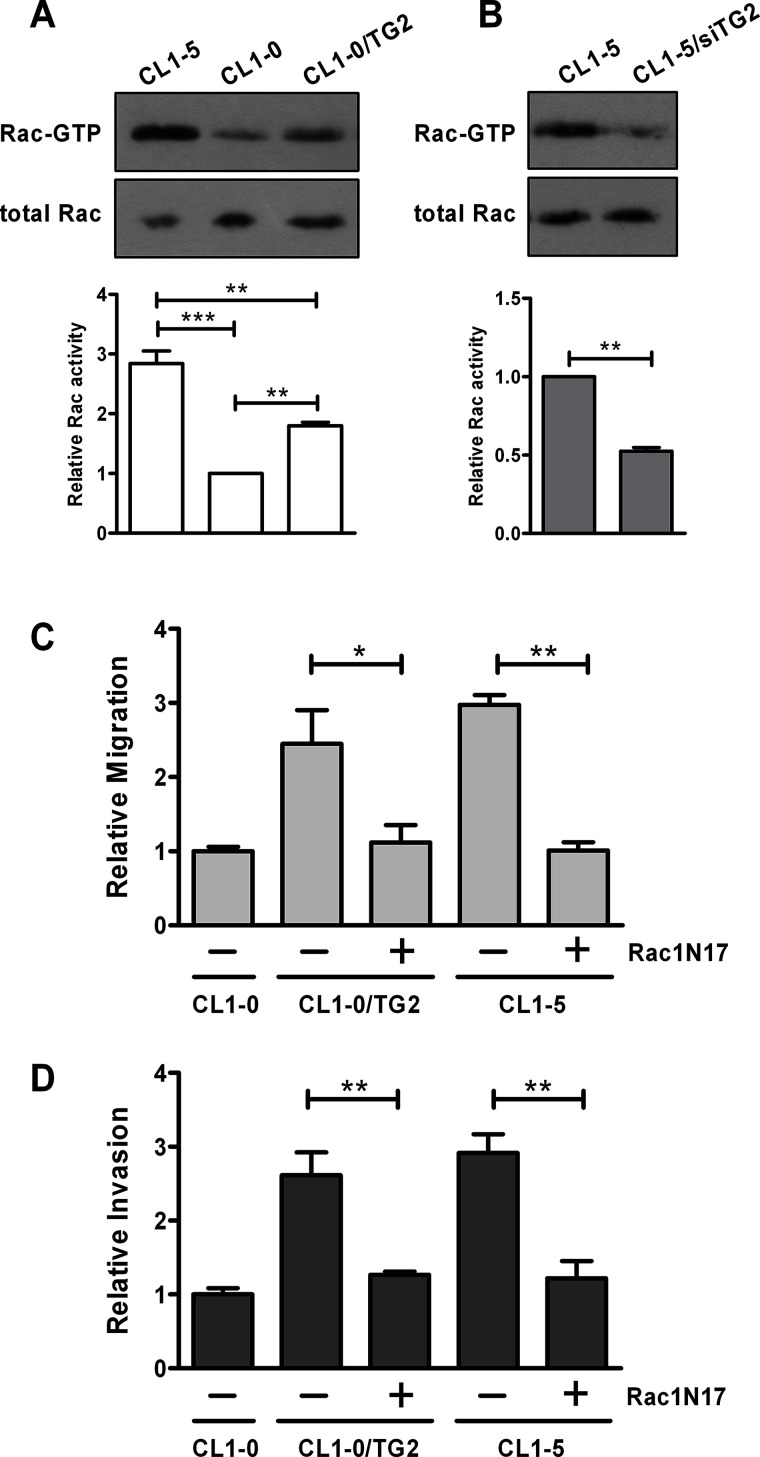
We then explored the mechanism for TG2-stimulated migration and invasion. Several signaling pathways have been documented to be activated by TG2, such as focal adhesion kinase (FAK), Akt, NF-κB, and Rac GTPase11,15,29,34–36. We found that overexpression of TG2 in CL1-0 cells did not alter levels of I-κB, FAK phosphorylation, and Akt phosphorylation (data not shown). Rac activity was higher in CL1-5 than in CL1-0, and overexpression of TG2 caused an increase in Rac activity in CL1-0 (Fig. 6A). Lowering TG2 expression decreased Rac activity in CL1-5 (Fig. 6B). These data imply that TG2 positively regulates the activation of Rac. Moreover, expression of a dominant negative form of Rac1 (Rac1N17) in CL1-0/TG2 and CL1-5 cells reduced their migratory and invasive abilities, suggesting that TG2 promotes cell migration and invasion through, at least in part, activating Rac (Fig. 6C and D).
Figure 6.
TG2 promotes cell migration and invasion through activating Rac. (A) Total cell lysates collected from CL1-0, CL1-0/TG2, and CL1-5 were incubated with GST-PAK1 p21-binding domain bound to glutathione–agarose beads to precipitate GTP-bound Rac. Total lysates and precipitates were then analyzed by immunoblotting for Rac. Data were quantified, normalized to levels of total Rac, and expressed as fold change relative to CL1-0. **p < 0.01, ***p < 0.005. (B) CL1-5 cells were mock transfected or transiently transfected with TG2 siRNA (siTG2). Total cell lysates were subjected to Rac activity assay. Data are expressed as fold change relative to mock-transfected CL1-5. **p < 0.01. (C, D) CL1-0 cells were mock transfected, and CL1-0/TG2 and CL1-5 cells were mock transfected or transiently transfected with Rac1N17 plasmid. After 24 h, cells were subjected to migration assay (C) and invasion assay (D). (C, D) Data are expressed as fold change relative to mock-transfected CL1-0. *p < 0.05, **p < 0.01.
DISCUSSION
TG2 is a multifunctional protein with various cellular localizations, and its overexpression has been implicated in cancer development4. Here we show that the expression of TG2 is positively correlated with invasiveness of lung cancer cells. Overexpression of TG2 promotes cell migration and invasion through activating Rac. The effect of TG2 on these events might, at least in part, rely on its function outside the cell as application of recombinant TG2 protein to cells substantially augments migration and invasion. Thus, our results suggest that TG2 plays a role in lung cancer metastasis by facilitating cell migration and invasion.
Both intracellular and cell surface TG2 have been documented to promote metastasis. Intracellular TG2 activates NF-κB, leading to expression of hypoxia-inducible factor-1 (HIF-1), which in turn induces EMT by stimulating expression of EMT regulators, such as Snail, Twist, and Zeb1/234. Cancer cells undergoing EMT acquire the ability to metastasize20,37. This process requires the GTP-binding activity but not the transamidase activity of TG228. Extracellular TG2 proteins are localized on the cell surface and in the extracellular matrix. Cell surface TG2 interacts with fibronectin as well as β1 and β3 integrins, thereby facilitating cell adhesion and integrin signaling. Disruption of the adhesive effect of surface TG2 hinders cell migration5,6. In cancer cells, TG2 was shown to associate with β1, β3, and β5 integrins, and TG2 expression positively correlates with cell adhesion, migration, and invasion10,14,16. Likewise, these effects are independent of the transamidase activity of TG25,14. TG2 in the extracellular matrix cross-links matrix proteins to enhance their stability and rigidity, thus affecting cell proliferation, survival, and invasion4. There is evidence that TG2-modified matrix prevents tumor invasion, perhaps due to increased stability of the matrix38. However, it was reported recently that TG2 influenced tumor stroma by enhancing deposition and cross-linking of collagen and activating stromal fibroblasts, leading to tumor growth and chemoresistance9,27.
Consistent with other reports, we found that TG2 promoted migration and invasion in lung cancer cells, and this was independent of protein transamidation8,14,24,28. Application of recombinant TG2 protein to cells substantially augments migration and invasion, suggesting that extracellular TG2 is fairly sufficient to confer invasiveness. Interestingly, higher levels of TG2 were detected in bronchoalveolar lavage fluid from patients with primary lung adenocarcinoma compared to that from individuals without lung cancer39. This indicates the likelihood for extracellular TG2 to facilitate metastasis of lung cancer in vivo. Likewise, abundant TG2 was detected in ascites from patients with malignant ovarian cancer, and application of recombinant TG2 protein enhanced peritoneal metastasis in vivo and cell invasion in vitro10,11.
The mechanism for TG2-stimulated tumor metastasis has been ascribed to its induction of EMT4. Both NF-κB and Akt were shown to mediate this effect11,23,28,34. However, we found that overexpression of TG2 in CL1-0 affected neither NF-κB activity as assessed by I-κB degradation nor Akt phosphorylation. These cells also maintained epithelial morphology, indicating no induction of EMT (data not shown). Based on our results that cells with higher TG2 expression secreted more TG2 and application of recombinant TG2 protein greatly augmented migration and invasion (Fig. 5), we speculated that secreted TG2 had a role in these events by stimulating cell adhesion-related signaling. Indeed, Rac GTPase, a signaling molecule involved in cell adhesion and migration as well as tumor metastasis, was upregulated by TG2 (Fig. 6A)40. Inhibition of Rac activity alleviated the effect of TG2, suggesting that Rac plays a part in TG2-mediated migration and invasion in lung cancer cells. Similarly, elevating TG2 expression in macrophages led to activation of Rac, and application of recombinant TG2 protein facilitates efferocytosis35. Noncanonical NF-κB pathway was shown to be activated by extracellular TG2 in ovarian cancer cells. It conferred the effect of TG2 on EMT and possibly tumor metastasis11. We suspect that this pathway is involved as CL1-0/TG2 cells show no signs of EMT (data not shown). However, this possibility cannot be completely excluded.
Owing to its multiple localizations and diverse functions, TG2 influences numerous responses during cancer development. Our results here reveal a significant role of extracellular TG2 in promoting migration and invasion of lung cancer cells. Hence, targeting extracellular TG2 might be a means to block tumor metastasis as it is more accessible to therapeutic agents.
ACKNOWLEDGMENTS
The authors thank Dr. Gail V. W. Johnson (University of Rochester, USA) for providing the pcDNA3.1+-TG2 and pcDNA3.1+-TG2-C277S plasmids, and Dr. Tzuu-Shuh Jou (National Taiwan University, Taiwan, ROC) for providing the pEGFP-C1-Rac1N17 plasmid. This work was funded by the Ministry of Science and Technology, Republic of China (MOST 103-2320-B-040-020), Chung Shan Medical University, and Antai Tian-Sheng Memorial Hospital (CSMU-TSMH-099-004).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136(5):E359–86. [DOI] [PubMed] [Google Scholar]
- 2. Steeg PS. Tumor metastasis: Mechanistic insights and clinical challenges. Nat Med. 2006;12(8):895–904. [DOI] [PubMed] [Google Scholar]
- 3. Nurminskaya MV, Belkin AM. Cellular functions of tissue transglutaminase. Int Rev Cell Mol Biol. 2012;294:1–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eckert RL, Kaartinen MT, Nurminskaya M, Belkin AM, Colak G, Johnson GV, Mehta K. Transglutaminase regulation of cell function. Physiol Rev. 2014;94(2):383–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akimov SS, Krylov D, Fleischman LF, Belkin AM. Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J Cell Biol. 2000;148(4):825–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Akimov SS, Belkin AM. Cell surface tissue transglutaminase is involved in adhesion and migration of monocytic cells on fibronectin. Blood 2001;98(5):1567–76. [DOI] [PubMed] [Google Scholar]
- 7. Fok JY, Ekmekcioglu S, Mehta K. Implications of tissue transglutaminase expression in malignant melanoma. Mol Cancer Ther. 2006;5(6):1493–503. [DOI] [PubMed] [Google Scholar]
- 8. Verma A, Wang H, Manavathi B, Fok JY, Mann AP, Kumar R, Mehta K. Increased expression of tissue transglutaminase in pancreatic ductal adenocarcinoma and its implications in drug resistance and metastasis. Cancer Res. 2006;66(21):10525–33. [DOI] [PubMed] [Google Scholar]
- 9. Lee J, Condello S, Yakubov B, Emerson R, Caperell-Grant A, Hitomi K, Xie J, Matei D. Tissue transglutaminase mediated tumor-stroma interaction promotes pancreatic cancer progression. Clin Cancer Res. 2015;21(19):4482–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Satpathy M, Cao L, Pincheira R, Emerson R, Bigsby R, Nakshatri H, Matei D. Enhanced peritoneal ovarian tumor dissemination by tissue transglutaminase. Cancer Res. 2007;67(15):7194–202. [DOI] [PubMed] [Google Scholar]
- 11. Yakubov B, Chelladurai B, Schmitt J, Emerson R, Turchi JJ, Matei D. Extracellular tissue transglutaminase activates noncanonical NF-κB signaling and promotes metastasis in ovarian cancer. Neoplasia 2013;15(6):609–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mehta K, Fok J, Miller FR, Koul D, Sahin AA. Prognostic significance of tissue transglutaminase in drug resistant and metastatic breast cancer. Clin Cancer Res. 2004;10(23):8068–76. [DOI] [PubMed] [Google Scholar]
- 13. Herman JF, Mangala LS, Mehta K. Implications of increased tissue transglutaminase (TG2) expression in drug-resistant breast cancer (MCF-7) cells. Oncogene 2006;25(21):3049–58. [DOI] [PubMed] [Google Scholar]
- 14. Mangala LS, Fok JY, Zorrilla-Calancha IR, Verma A, Mehta K. Tissue transglutaminase expression promotes cell attachment, invasion and survival in breast cancer cells. Oncogene 2007;26(17):2459–70. [DOI] [PubMed] [Google Scholar]
- 15. Cao L, Petrusca DN, Satpathy M, Nakshatri H, Petrache I, Matei D. Tissue transglutaminase protects epithelial ovarian cancer cells from cisplatin-induced apoptosis by promoting cell survival signaling. Carcinogenesis 2008;29(10):1893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen SH, Lin CY, Lee LT, Chang GD, Lee PP, Hung CC, Kao WT, Tsai PH, Schally AV, Hwang JJ, Lee MT. Up-regulation of fibronectin and tissue transglutaminase promotes cell invasion involving increased association with integrin and MMP expression in A431 cells. Anticancer Res. 2010;30(10):4177–86. [PubMed] [Google Scholar]
- 17. Frese-Schaper M, Schardt JA, Sakai T, Carboni GL, Schmid RA, Frese S. Inhibition of tissue transglutaminase sensitizes TRAIL-resistant lung cancer cells through upregulation of death receptor 5. FEBS Lett. 2010;584(13):2867–71. [DOI] [PubMed] [Google Scholar]
- 18. Park KS, Kim HK, Lee JH, Choi YB, Park SY, Yang SH, Kim SY, Hong KM. Transglutaminase 2 as a cisplatin resistance marker in non-small cell lung cancer. J Cancer Res Clin Oncol. 2010;136(4):493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Z, Xu X, Bai L, Chen W, Lin Y. Epidermal growth factor receptor-mediated tissue transglutaminase overexpression couples acquired tumor necrosis factor-related apoptosis-inducing ligand resistance and migration through c-FLIP and MMP-9 proteins in lung cancer cells. J Biol Chem. 2011;286(24):21164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumar A, Xu J, Brady S, Gao H, Yu D, Reuben J, Mehta K. Tissue transglutaminase promotes drug resistance and invasion by inducing mesenchymal transition in mammary epithelial cells. PLoS One 2010;5(10):e13390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shao M, Cao L, Shen C, Satpathy M, Chelladurai B, Bigsby RM, Nakshatri H, Matei D. Epithelial-to-mesenchymal transition and ovarian tumor progression induced by tissue transglutaminase. Cancer Res. 2009;69(24):9192–201. [DOI] [PubMed] [Google Scholar]
- 22. Park MK, You HJ, Lee HJ, Kang JH, Oh SH, Kim SY, Lee CH. Transglutaminase-2 induces N-cadherin expression in TGF-β1-induced epithelial mesenchymal transition via c-Jun-N-terminal kinase activation by protein phosphatase 2A down-regulation. Eur J Cancer 2013;49(7):1692–705. [DOI] [PubMed] [Google Scholar]
- 23. Lin CY, Tsai PH, Kandaswami CC, Chang GD, Cheng CH, Huang CJ, Lee PP, Hwang JJ, Lee MT. Role of tissue transglutaminase 2 in the acquisition of a mesenchymal-like phenotype in highly invasive A431 tumor cells. Mol Cancer 2011;10:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fisher ML, Adhikary G, Xu W, Kerr C, Keillor JW, Eckert RL. Type II transglutaminase stimulates epidermal cancer stem cell epithelial-mesenchymal transition. Oncotarget 2015;6(24):20525–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kumar A, Gao H, Xu J, Reuben J, Yu D, Mehta K. Evidence that aberrant expression of tissue transglutaminase promotes stem cell characteristics in mammary epithelial cells. PLoS One 2011;6(6):e20701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cao L, Shao M, Schilder J, Guise T, Mohammad KS, Matei D. Tissue transglutaminase links TGF-β, epithelial to mesenchymal transition and a stem cell phenotype in ovarian cancer. Oncogene 2012;31(20):2521–34. [DOI] [PubMed] [Google Scholar]
- 27. Lee J, Yakubov B, Ivan C, Jones DR, Caperell-Grant A, Fishel M, Cardenas H, Matei D. Tissue transglutaminase activates cancer-associated fibroblasts and contributes to gemcitabine resistance in pancreatic cancer. Neoplasia 2016;18(11):689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kumar A, Xu J, Sung B, Kumar S, Yu D, Aggarwal BB, Mehta K. Evidence that GTP-binding domain but not catalytic domain of transglutaminase 2 is essential for epithelial-to-mesenchymal transition in mammary epithelial cells. Breast Cancer Res. 2012;14(1):R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Verma A, Guha S, Wang H, Fok JY, Koul D, Abbruzzese J, Mehta K. Tissue transglutaminase regulates focal adhesion kinase/AKT activation by modulating PTEN expression in pancreatic cancer cells. Clin Cancer Res. 2008;14(7):1997–2005. [DOI] [PubMed] [Google Scholar]
- 30. Verma A, Guha S, Diagaradjane P, Kunnumakkara AB, Sanguino AM, Lopez-Berestein G, Sood AK, Aggarwal BB, Krishnan S, Gelovani JG, Mehta K. Therapeutic significance of elevated tissue transglutaminase expression in pancreatic cancer. Clin Cancer Res. 2008;14(8):2476–83. [DOI] [PubMed] [Google Scholar]
- 31. Chu YW, Yang PC, Yang SC, Shyu YC, Hendrix MJ, Wu R, Wu CW. Selection of invasive and metastatic subpopulations from a human lung adenocarcinoma cell line. Am J Respir Cell Mol Biol. 1997;17(3):353–60. [DOI] [PubMed] [Google Scholar]
- 32. Slaughter TF, Achyuthan KE, Lai TS, Greenberg CS. A microtiter plate transglutaminase assay utilizing 5-(biotinamido)pentylamine as substrate. Anal Biochem. 1992;205(1):166–71. [DOI] [PubMed] [Google Scholar]
- 33. Park D, Choi SS, Ha KS. Transglutaminase 2: A multi-functional protein in multiple subcellular compartments. Amino Acids 2010;39(3):619–31. [DOI] [PubMed] [Google Scholar]
- 34. Kumar S, Mehta K. Tissue transglutaminase constitutively activates HIF-1α promoter and nuclear factor-κB via a non-canonical pathway. PLoS One 2012;7(11):e49321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Toth B, Garabuczi E, Sarang Z, Vereb G, Vamosi G, Aeschlimann D, Blasko B, Becsi B, Erdodi F, Lacy-Hulbert A, Zhang A, Falasca L, Birge RB, Balajthy Z, Melino G, Fésüs L, Szondy Z. Transglutaminase 2 is needed for the formation of an efficient phagocyte portal in macrophages engulfing apoptotic cells. J Immunol. 2009;182(4):2084–92. [DOI] [PubMed] [Google Scholar]
- 36. Tong L, Png E, Aihua H, Yong SS, Yeo HL, Riau A, Mendoz E, Chaurasia SS, Lim CT, Yiu TW, Iismaa SE. Molecular mechanism of transglutaminase-2 in corneal epithelial migration and adhesion. Biochim Biophys Acta 2013;1833(6):1304–15. [DOI] [PubMed] [Google Scholar]
- 37. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mangala LS, Arun B, Sahin AA, Mehta K. Tissue transglutaminase-induced alterations in extracellular matrix inhibit tumor invasion. Mol Cancer 2005;4:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Almatroodi SA, McDonald CF, Collins AL, Darby IA, Pouniotis DS. Quantitative proteomics of bronchoalveolar lavage fluid in lung adenocarcinoma. Cancer Genomics Proteomics 2015;12(1):39–48. [PubMed] [Google Scholar]
- 40. Mack NA, Whalley HJ, Castillo-Lluva S, Malliri A. The diverse roles of Rac signaling in tumorigenesis. Cell Cycle 2011;10(10):1571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]