Abstract
Dysregulation of SUMO-specific protease 1 (SENP1) expression has been reported in several kinds of cancer, including human colorectal and prostate cancers, proposing SENP1 as an oncogene with a critical role in cancer progression. miR-133a-3p has been reported as a tumor suppressor in several malignant neoplasias. However, the precise molecular mechanisms underlying its role in colorectal cancer remain largely unknown. The aim of this work was to investigate the relationship between miR-133a-3p and SENP1 in colorectal cancer cells. We found that miR-133a-3p expression was downregulated in colorectal cancer tissues. In silico analyses indicated that SENP1 is one of the target genes of miR-133a-3p. Overexpression of miR-133a-3p mimics was able to inhibit cell growth with G1 arrest of colorectal cancer cells. Overexpression of miR-133a-3p antisense promoted cell growth of colorectal cancer cells. The luciferase reporter experiments showed that miR-133a-3p regulated the expression of SENP1 by combining with its 3′-UTR and resulted in downregulation of SENP1 and upregulation of CDK inhibitors such as p16, p19, p21, and p27. These results suggest that the miR-133a-3p–SENP1 axis might play a role in cell proliferation and cell cycle regulation of colorectal cancer cells.
Key words: Colorectal cancer, miR-133a-3p, SUMO-specific protease 1 (SENP1), CDK inhibitors
INTRODUCTION
Colorectal cancer is the fourth leading cause of cancer-related deaths worldwide and the second leading cause in the US1,2. Because of early detection, the death rates of colorectal cancer have declined over the past 20 years; however, its incidence still remains high, and new treatments have been slowing down. Surgical removal of tumors followed with chemotherapy remains the first choice of treatment3. Novel drugs that have been recently approved are primarily biologics that target tumor angiogenesis or the epidermal growth factor receptor. However, these drugs have had limited success because the oncogenic mutations in KRAS confer resistance. Thus, it is critical to provide new or complementary targets for therapeutic and preventative applications for colorectal cancer.
SUMO (small-ubiquitin-like modifier) posttranslational modification (PTM) affects the function, subcellular localization, and/or stability of target proteins4. SUMOylation is a dynamic and reversible process that is catalyzed by SUMO-specific activating (E1), conjugating (E2), and ligating (E3) enzymes and is reversed by a family of sentrin/SUMO-specific proteases (SENPs)5. SENP1 deconjugates a large number of SUMOylated proteins, the mutation of which causes embryonic lethality due to a defective hypoxia-inducible factor-1a (HIF-1a) pathway6. SENP1 functions as an oncogene and was overexpressed in several malignant neoplasias, including colon cancers7–9. SENP1 played an important role in colon cancer cell proliferation, tumor formation, and cell cycle progression by controlling the expression of CDK inhibitors7. However, how to regulate SENP1 in colon cancer cells remains largely unknown.
MicroRNAs (miRNAs) are a significant class of short endogenous noncoding RNAs that are evolutionarily conserved among different species, controlling gene expression at the posttranscriptional level through direct base pairing with their target mRNAs to degradation or by inhibiting their target mRNA translation10–12. miRNAs play critical roles in many biological pathways, and more than half of the sequences encoding miRNAs are located in cancer-associated genomic regions or fragile sites, suggesting they may regulate cancer at a systematically fundamental level. Recent studies indicate that aberrant expression of miRNAs plays a crucial role in the pathogenesis of cancer. The human genome codes more than 1,900 miRNAs; about 250 miRNAs are reported to have changes in abundance or altered functions in colorectal cancer13. For example, miR-155 and miR19a were upregulated to promote colorectal cancer cell growth. miR-141 and miR-455-3p were downregulated in colorectal cancer and exerted an antitumor effect14–17. miR-133a-3p has been reported as a tumor suppressor in several malignant neoplasias18–20. However, the role of miR-133a-3p in the pathogenesis of colorectal cancer remains largely unknown.
In the current study, we found that miR-133a-3p expression was downregulated in colorectal cancer tissues. miR-133a-3p functioned as a tumor suppressor in colorectal cancer cells by targeting SENP1 and resulted in downregulation of SENP1 and upregulation of CDK inhibitors such as p16, p19, p21, and p27.
MATERIALS AND METHODS
Human Tissues
Twenty pairs of frozen material from primary colorectal cancers and the adjacent normal colorectal tissues were obtained from our department. The diagnoses of these tissue samples were verified by pathologists from our hospital. This study was approved by the ethics committee of our hospital.
Cell Culture
Human colon cancer cell lines HCT116 and SW480 were purchased from the ATCC (Manassas, VA, USA). Cells were cultured in DMEM supplemented with 10% FBS and 1,000 U/ml penicillin/streptomycin and incubated in 5% CO2 at 37°C.
Cell Transfection
miR-133a-3p mimics, antisense, and negative controls (NCs) were purchased from Genepharm Company (Shanghai, P.R. China). Transfections were carried out using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol.
Bromodeoxyuridine (BrdU) Incorporation Assay
A cell proliferation enzyme-linked immunosorbent assay kit (Beyotime, Shanghai, P.R. China) was used for BrdU incorporation assay to analyze the incorporation of BrdU during DNA synthesis following the manufacturer’s protocols.
Real-Time PCR
Total RNAs were isolated from cells and patient tissues by TRIzol reagent (Invitrogen), and reverse transcriptions were performed by the Takara RNA PCR kit (Takara, Dalian, P.R. China) following the manufacturer’s instructions. To determine the transcripts of the genes of interest, real-time PCR was performed using a SYBR Green Premix Ex Taq (Takara) on an ABI 7500 machine.
Western Blot
Tissues and cells were lysed with ice-cold lysis buffer (50 mM Tris-HCl, pH 6.8, 100 mM DTT, 2% SDS, and 10% glycerol). After centrifugation at 20,000 rpm for 10 min at 4°C, proteins in the supernatants were quantified and separated by 10–12% SDS-PAGE, and then transferred to a NC membrane (Beyotime). After blocking with 10% nonfat milk in PBS, membranes were immunoblotted with antibodies as indicated, followed by HRP-linked secondary antibodies (Beyotime). The signals were detected by Chemiluminescent Substrate Kit (Beyotime) according to the manufacturer’s instructions. Anti-SENP1, p16, p19, p21, p27, and GAPDH antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Protein levels were normalized to GAPDH.
Luciferase Assay
Synthetic oligonucleotides with three copies of the SENP1 3′-UTR (ACCUUGACCAUGUGGGGGACCAG), which were predicted to bind miR-133a-3p, or three copies of a mutated version of the sequence (ACCUUGACCAUGUGGGGATCCAG) that contained BamH1 restriction site were cloned into the pMIR-REPORT firefly luciferase miRNA expression reporter vector (Applied Biosystems, Framingham, MA, USA).
pMIR-REPORT vector and pRL-TK Renilla luciferase control vector (Promega, Madison, WI, USA) were cotransfected into HCT116 cells using Lipofectamine 2000 (Invitrogen). Twenty-four hours after transfection, the cells were further transfected with miR-133a-3p or the NC miRNA for an additional 24 h. The luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s instructions. Firefly luciferase activities were normalized to Renilla luciferase activities.
Flow Cytometric Assays for Cell Cycle
Cells were collected, rinsed, and fixed overnight in 75% cold ethanol at −20°C. The cells were treated with Tris-HCl buffer supplemented with RNase A and stained with propidium iodide (BD Biosciences, San Jose, CA, USA). Cell cycle distribution was determined by flow cytometry (Becton Dickinson, San Jose, CA, USA); 10,000 cells were acquired and analyzed for DNA content. All data were collected, stored, and analyzed by ModFit software.
Statistical Analysis
Values are displayed as mean ± standard error of mean (SEM). Statistical analysis was performed with GraphPad version 6.0 software (La Jolla, CA, USA). Statistical significance was considered with a value of p < 0.05.
RESULTS
miR-133a-3p Was Downregulated in Primary Colorectal Cancer Tissues
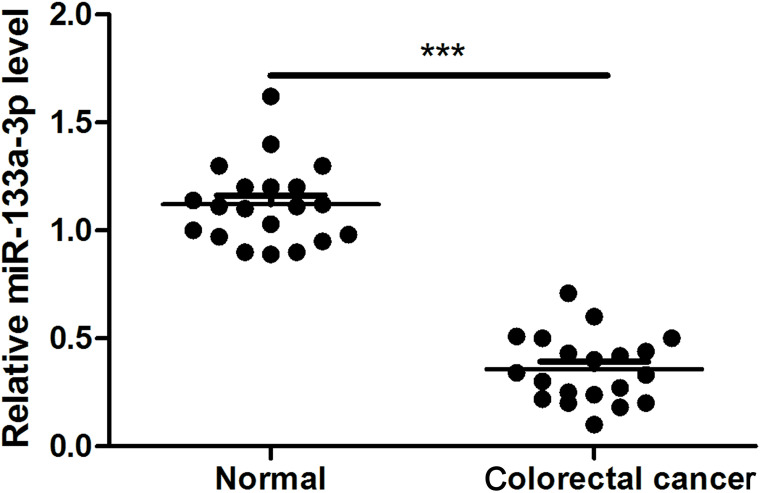
The expression of miR-133a-3p in primary colorectal cancer tissues is still unknown. Thus, we first checked the expression of miR-133a-3p in 20 pairs of frozen material from primary colorectal cancers and the adjacent normal colorectal tissues by means of quantitative real-time PCR. Our data indicated that miR-133a-3p was significantly decreased in colorectal cancer tissues compared with adjacent normal tissues (Fig. 1).
Figure 1.
miR-133a-3p was downregulated in primary colorectal cancer tissues. miR-133a-3p expression was determined by real-time PCR in human colorectal cancer tissues and adjacent normal tissues. ***p < 0.001.
miR-133a-3p Inhibits Colorectal Cell Proliferation and Promotes G1 Arrest
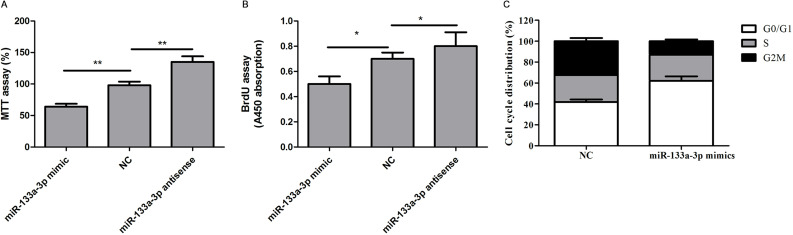
We then investigate the function of miR-133a-3p in colorectal cells. Gain- and loss-of-function experiments by introducing the mimics or antisense of miR-133a-3p into HCT116 cells were performed. We also used the scramble sequences as NCs. As examined by the BrdU incorporation assay and the MTT assay, we found that the cell viability and proliferation ability were significantly decreased in HCT116 cells when miR-133a-3p was overexpressed (Fig. 2A and B). Moreover, overexpression of miR-133a-3p mimics significantly induced G1 arrest (Fig. 2C). In contrast, inhibition of endogenous miR-133a-3p by antisense oligos promoted the abilities of cell growth and proliferation (Fig. 2A and B). Similar results were also observed in SW480 cells (data not shown).
Figure 2.
miR-133a-3p inhibits colorectal cell proliferation and promotes G1 arrest. (A) The cell viability assay of HCT116 cells transfected with indicated microRNAs (miRNAs) was determined by MTT assay. **p < 0.01. (B) The cell proliferation assay of HCT116 cells transfected with indicated miRNAs was determined by bromodeoxyuridine (BrdU) incorporation assay. *p < 0.05. (C) The cell cycle distribution of HCT116 cells transfected with indicated miRNAs was determined by FACS assay.
SENP1 Was a Target of miR-133a-3p in Colorectal Cancer Cells
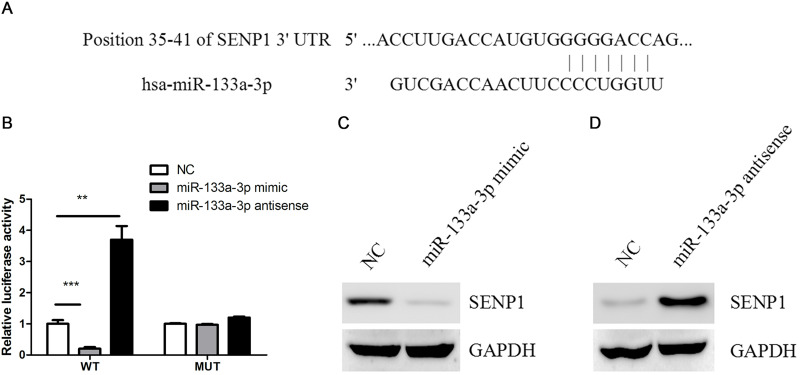
To screen the function target of miR-133a-3p in colorectal cancer cells, Bioinformatics software TargetScan and miRWalk were used to screen the target genes of miR-133a-3p. Among them, SENP1 was predicted to be a direct target of miR-133a-3p (Fig. 3A). Luciferase activity assay found that miR-133a-3p mimics significantly suppressed the activity of the wild-type 3′-UTR of SENP1, whereas its antisense had the opposite effect on the activity of the wild-type 3′-UTR of SENP1. However, either miR-133a-3p mimic or antisense had a detectable effect on the mutant 3′-UTR of SENP1 (Fig. 3B). Consistently, miR-133a-3p mimics dramatically reduced, whereas miR-133a-3p antisense increased, SENP1 protein content (Fig. 3C and D).
Figure 3.
SUMO-specific protease 1 (SENP1) was a target of miR-133a-3p in colorectal cancer cells. (A) Prediction of miR-133a-3p binding sites in the 3′-UTRs of the human SENP1 gene by bioinformatics software TargetScan and miRWalk. (B) HCT116 cells were transfected with wild-type or mutant 3′-UTR reporter constructs together with miR-133a-3p mimics, antisense, or negative control (NC). Luciferase reporter assays were then performed. **p < 0.01, ***p < 0.001. (C) Protein levels of SENP1 were determined by Western blot in HCT116 cells transfected with miR-133a-3p mimics or NC. (D) Protein levels of SENP1 were determined by Western blot in HCT116 cells transfected with miR-133a-3p antisense or NC.
miR-133a-3p Inhibits the Proliferation of Colorectal Cancer Cells via Downregulation of SENP1
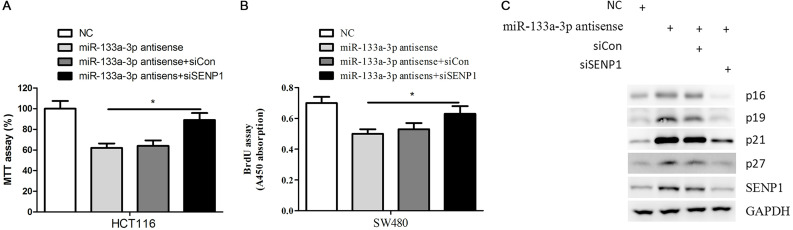
Given the critical role of SENP1 in colorectal cancer cell proliferation and cell cycle progress, we proposed that downregulation of SENP1 might contribute to the biology function of miR-133a-3p. To this end, we silenced the expression of SENP1 in miR-133a-3p antisense-transfected HCT116 and SW480 cells and found that SENP1 inhibition significantly attenuated miR-133a-3p antisense-induced colorectal cancer cell growth and proliferation (Fig. 4A and B). At the molecular level, we found that miR-133a-3p antisense could significantly increase the expression of CDK inhibitors, such as p16, p19, p21, and p27, in HCT116 cells. Consistent with this observation, silencing the expression of SENP1 in miR-133a-3p antisense-transfected HCT116 cells dramatically decreased the expression of these proteins (Fig. 4C). Taken together, these data suggested that SENP1 and its downstream genes contributed to the impaired proliferation of colorectal cancer cells by overexpression of the miR-133a-3p mimic.
Figure 4.
miR-133a-3p inhibits the proliferation of colorectal cancer cells via downregulation of SENP1. (A) The cell viability assay of HCT116 cells transfected with indicated miRNAs and siRNAs was determined by MTT assay. *p < 0.05. (B) The cell proliferation assay of SW480 cells transfected with indicated miRNAs and siRNAs was determined by BrdU incorporation assay. *p < 0.05. (C) HCT116 cells transfected with indicated miRNAs and siRNAs were subjected to Western blot with indicated antibodies.
DISCUSSION
In this study, we found that miR-133a-3p showed significantly lower expression in colorectal cancer tissues compared to adjacent normal tissues. However, the molecular mechanisms underlying the downregulation of miR-133a-3p remain unclear. DNA methyltransferases, such as DNMT3B, was usually abnormally upregulated in colorectal cancer tissue. Therefore, we proposed that epigenetic factors might contribute to the downregulation of miR-133a-3p in colorectal cancer tissue, which requires further investigation in the future.
It has been well established that a single miRNA could regulate multiple targeted genes. A previous study identified miR-133a-3p to exert inhibitory effects on gallbladder carcinoma via targeting RBPJ18. Aberrant expression of miR-133a-3p was also associated with tumor differentiation degree and lymph node metastasis18. Therefore, the roles of miR-133a-3p in tumorigenesis might be cell or tissue specific. In our study, we found that miR-133a-3p overexpression inhibited, while its suppression increased, colorectal cancer cell growth and proliferation. We also identified SENP1 as a direct target of miR-133b in colorectal cancer cells. Therefore, our results suggest that miR-133b could inhibit colorectal cancer progression, at least in part, by repressing SENP1 expression.
It has been reported that expression of SENP1, a SUMO-specific protease, was elevated in colorectal cancer7. SENP1 played an important role in colon cancer cell proliferation, tumor formation, and cell cycle progression by controlling the expression of CDK inhibitors20. Moreover, SENP1 is also required for the proper accumulation of the HIF-1a protein during hypoxia response21. Given that SENP1 plays a crucial role in promoting progression of human cancers, molecules or chemical compounds targeting SENP1 might be an effective new therapeutic for malignant progression. Indeed, several kinds of small molecule inhibitors targeting SENP1 has been reported and exhibited promising antitumor activity22–24. We observed that miR-133a-3p could regulate the expression of CDK inhibitors in colorectal cancer cells, further indicating the tumor-suppressive role of miR-133a-3p.
In summary, for the first time, this study explored the function of miR-133a-3p in colorectal cancer progression and identified SENP1 as its critical targeted gene. Future studies, for example, generating miR-133a-3p knockout mice, will be needed to establish the physiological role of miR-133a-3p in colorectal tumorigenesis.
ACKNOWLEDGMENTS
This study was supported by the Project of Nature Science Foundation of China (81672348), the Project of Medical Science and Technology Development Foundation of Jiangsu Province of China (YG201406), the Science and Technology Research Project of Changshu City of China (CS201504), the Six Major Talent Peak Project of Jiangsu Province of China (2015-WSW-014), the Six One Project for Advanced Medical Talent of Jiangsu Province of China (LGY2016031), and Jiangsu Provincial Medical Youth Talent of China (QNRC2016735).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Seton-Rogers S. Colorectal cancer: A circuitous way to target p53. Nat Rev Cancer 2015;15:318–9. [DOI] [PubMed] [Google Scholar]
- 2. Seton-Rogers S. Colorectal cancer: Elongation is essential. Nat Rev Cancer 2014;14:764–5. [DOI] [PubMed] [Google Scholar]
- 3. Stagnitti A, Barchetti F, Barchetti G, Pasqualitto E, Sartori A, Glorioso M, Gigli S, Buonocore V, Monti ML, Marini A, Mele C, Stagnitti F, Laghi A. Preoperative staging of colorectal cancer using virtual colonoscopy: Correlation with surgical results. Eur Rev Med Pharmacol Sci. 2015;19:1645–51. [PubMed] [Google Scholar]
- 4. Muller S, Hoege C, Pyrowolakis G, Jentsch S. SUMO, ubiquitin’s mysterious cousin. Nat Rev Mol Cell Biol. 2001;2:202–10. [DOI] [PubMed] [Google Scholar]
- 5. Bawa-Khalfe T, Yeh ET. SUMO losing balance: SUMO proteases disrupt SUMO homeostasis to facilitate cancer development and progression. Genes Cancer 2010;1:748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng J, Kang X, Zhang S, Yeh ET. SUMO-specific protease 1 is essential for stabilization of HIF1alpha during hypoxia. Cell 2007;131:584–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu Y, Li J, Zuo Y, Deng J, Wang LS, Chen GQ. SUMO-specific protease 1 regulates the in vitro and in vivo growth of colon cancer cells with the upregulated expression of CDK inhibitors. Cancer Lett. 2011;309:78–84. [DOI] [PubMed] [Google Scholar]
- 8. Bawa-Khalfe T, Cheng J, Lin SH, Ittmann MM, Yeh ET. SENP1 induces prostatic intraepithelial neoplasia through multiple mechanisms. J Biol Chem. 2010;285:25859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaikkonen S, Jaaskelainen T, Karvonen U, Rytinki MM, Makkonen H, Gioeli D, Paschal BM, Palvimo JJ. SUMO-specific protease 1 (SENP1) reverses the hormone-augmented SUMOylation of androgen receptor and modulates gene responses in prostate cancer cells. Mol Endocrinol. 2009;23:292–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer 2015;15:321–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McCarthy N. MicroRNA: Lacking in maturity. Nat Rev Cancer 2013;13:377. [DOI] [PubMed] [Google Scholar]
- 12. Seton-Rogers S. MicroRNAs: Editing changes the meaning. Nat Rev Cancer 2012;12:797. [DOI] [PubMed] [Google Scholar]
- 13. Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA 2004;101:2999–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Forzati F, De Martino M, Esposito F, Sepe R, Pellecchia S, Malapelle U, Pellino G, Arra C, Fusco A. miR-155 is positively regulated by CBX7 in mouse embryonic fibroblasts and colon carcinomas, and targets the KRAS oncogene. BMC Cancer 2017;17:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hu J, Xu Y, Cai S. Specific microRNAs as novel biomarkers for combination chemotherapy resistance detection of colon adenocarcinoma. Eur J Med Res. 2015;20:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Giraldez MD, Lozano JJ, Ramirez G, Hijona E, Bujanda L, Castells A, Gironella M. Circulating microRNAs as biomarkers of colorectal cancer: Results from a genome-wide profiling and validation study. Clin Gastroenterol Hepatol. 2013;11:681–8. [DOI] [PubMed] [Google Scholar]
- 17. Cheng H, Zhang L, Cogdell DE, Zheng H, Schetter AJ, Nykter M, Harris CC, Chen K, Hamilton SR, Zhang W. Circulating plasma miR-141 is a novel biomarker for metastatic colon cancer and predicts poor prognosis. PLoS One 2011;6:e17745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang Y, Wu Y, Dong J, Han D, Yang S, Jiang L. MicroRNA-133a-3p exerts inhibitory effects on gallbladder carcinoma via targeting RBPJ. Am J Cancer Res. 2016;6:2448–62. [PMC free article] [PubMed] [Google Scholar]
- 19. Li CY, Liang GY, Yao WZ, Sui J, Shen X, Zhang YQ, Peng H, Hong WW, Ye YC, Zhang ZY, Zhang WH, Yin LH, Pu YP. Identification and functional characterization of microRNAs reveal a potential role in gastric cancer progression. Clin Transl Oncol. 2017;19:162–72. [DOI] [PubMed] [Google Scholar]
- 20. Benson EA, Skaar TC, Liu Y, Nephew KP, Matei D. Carboplatin with decitabine therapy, in recurrent platinum resistant ovarian cancer, alters circulating miRNAs concentrations: A pilot study. PLoS One 2015;10:e141279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ulrich HD. SUMO teams up with ubiquitin to manage hypoxia. Cell 2007;131:446–7. [DOI] [PubMed] [Google Scholar]
- 22. Wu J, Lei H, Zhang J, Chen X, Tang C, Wang W, Xu H, Xiao W, Gu W, Wu Y. Momordin Ic, a new natural SENP1 inhibitor, inhibits prostate cancer cell proliferation. Oncotarget 2016;7:58995–9005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao Y, Wang Z, Zhang J, Zhou H. Identification of SENP1 inhibitors through in silico screening and rational drug design. Eur J Med Chem. 2016;122:178–84. [DOI] [PubMed] [Google Scholar]
- 24. Madu IG, Namanja AT, Su Y, Wong S, Li YJ, Chen Y. Identification and characterization of a new chemotype of noncovalent SENP inhibitors. ACS Chem Biol. 2013;8:1435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]