Abstract
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-related deaths worldwide. Currently, only chemoembolization and sorafenib have shown survival benefits for advanced HCC. There are major unmet needs in HCC management and the discovery of new therapeutic targets. Here we identified NCAPG (non-SMC condensin I complex, subunit G) as a novel mitotic gene required for HCC cell proliferation and migration through siRNA knockdown of a panel of novel overexpressed genes in HCC based on The Cancer Genome Atlas (TCGA) dataset. We found that knockdown of NCAPG induces HCC cell mitosis and inhibits cell growth, proliferation, and migration in vitro. Tetracycline-inducible shRNA knockdown of NCAPG inhibits tumor growth of HCC cells in vivo. Moreover, overexpression of NCAPG in clinical HCC samples was associated with recurrence and survival of patients. The overexpression of NCAPG was significantly correlated with the overexpression of CCNB1 (G2/mitotic-specific cyclin B1), a regulatory protein involved in mitosis. Therefore, NCAPG may provide a promising novel therapeutic target for the treatment of advanced HCC in the future.
Key words: Hepatocellular carcinoma (HCC), NCAPG, Proliferation, Migration
INTRODUCTION
Hepatocellular carcinoma (HCC) represents approximately 90% of all cases of primary liver cancer1. It is the second leading cause of cancer-related deaths worldwide and has an incidence of approximately 850,000 new cases per year, with China alone accounting for about 50% of the total number of cases and deaths2,3. The main risk factors for HCC include hepatitis B and C virus infection, alcohol intake, aflatoxin B1, and nonalcoholic steatohepatitis. However, the molecular mechanisms underlying hepatocarcinogenesis are not well characterized4. The current management of HCC patients includes surgical resection, liver transplantation, radiofrequency ablation, transcatheter arterial chemoembolization (TACE), or sorafenib5. The median overall survival (OS) of patients who are diagnosed with HCC is less than 1 year due to the absence of effective treatments1–3. There are major unmet needs in HCC management that might be addressed through the discovery of new therapeutic targets6.
To identify molecular biomarkers and disease subgroups, as well as to analyze cancer-related genes and pathways, more than 300 human liver HCC (LIHC) samples have been sequenced by The Cancer Genome Atlas (TCGA) project. We systematically evaluated the TCGA whole-transcriptome sequencing data of HCC by comparing the global gene expression profiles between tumors and their corresponding nontumor liver tissues (https://gdc-portal.nci.nih.gov/). Based on the differential gene expression analysis, we selected a number of novel overexpressed genes that have never been reported in HCC. We used RNA interference (RNAi)7 to knock down the gene expression and examined the cell growth and mitosis. Here we reported that non-SMC condensin I complex subunit G (NCAPG)8 is a novel mitotic gene required for liver cancer cell proliferation and migration. NCAPG in cancers has been less well studied, but it is overexpressed in melanomas9 and gliomas10 and downregulated in out-of-niche primary tumor cells from multiple myelomas and acute myeloid leukemias10,11. In this study, we demonstrated that NCAPG was significantly associated with recurrence and survival of HCC patients, indicating that NCAPG may be a promising novel target for treating HCC in the future.
MATERIALS AND METHODS
Cell Culture and Tissues
The HuH7 cells were bought from the Japanese Collection of Research Bioresources (JCRB) Cell Bank (Osaka, Japan). The HCCLM3 cell line was obtained from the Liver Cancer Institute (Zhongshan Hospital, Fudan University, Shanghai, P.R. China) and has been described previously12. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum with streptomycin (100 μg/ml) and penicillin (100 U/ml). Tumor tissues and adjacent normal tissues were collected in Affiliated Zhongda Hospital of Southeast University. None of the patients had received chemotherapy before surgical resection. The protocol was approved by the Affiliated Zhongda Hospital of Southeast University. Written informed consent was obtained from each participant.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA from siRNA-transfected HCC cells and tissue samples was isolated using TRIzol (Takara, Japan). The EXPRESS One-Step SuperScript qRT-PCR Kit was used for PCR amplification for the quantification of NCAPG and HPRT1. The following primers were used: NCAPG, ggctgctgtcgattaaggag (forward) and ttatcatccatcgtgcggta (reverse); HPRT1, tgacactggcaaaacaatgca (forward) and ggtccttttcaccagcaagct (reverse). HPRT1 was used as internal control of NCAPG. The relative expression was measured by the 2−ΔCT method.
Cell Viability Assay
The HuH7 and HCCLM3 cells were seeded in a 96-well plate and transfected with siScramble or siNCAPG-1 and siNCAPG-2 (Sigma-Aldrich, St. Louis, MO, USA). Seventy-two hours after transfection, the cell viability was assessed by the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetra zolium] assay using the CellTiter 96 AQueous One Solution Cell Proliferation Assay kit from Promega (Madison, WI, USA) following the manufacturer’s instructions. Each experiment was repeated three times.
Soft Agar Colony Assay
The HuH7 and HCCLM3 cells were seeded in 24-well plates and transfected with siScramble or siNCAPG-1 and siNCAPG-2 (Sigma-Aldrich). Twenty-four hours after transfection, the transfected cells were collected and mixed with tissue culture medium containing 0.6% low-melting-point agarose (Sigma-Aldrich), resulting in a final agar concentration of 0.3%. Then 500 μl of the cell suspension (800 cells) was immediately plated in 24-well plates coated with 500 μl of 0.6% agar in tissue culture medium and cultured at 37°C with 5% CO2. The plates were kept in the incubator, and the number of colonies formed was counted under an inverted light microscope (40× objective) after 2–3 weeks. The assay was analyzed in duplicate in three independent experiments.
Immunofluorescence Analysis of the Mitosis Marker
The HCC cells were seeded in the BD Falcon™ eight-well CultureSlide and transfected with 50 nM siScramble or siNCAPG-1 and siNCAPG-2. Cells were fixed and incubated with primary antibodies, phospho-Histone H3 (Ser10; Cell Signaling Technology, Danvers, MA, USA), or isotype control and then incubated with Alexa Fluor® 488 goat anti-rabbit IgG (Invitrogen, Carlsbad, CA, USA). The culture slides were imaged with a confocal laser-scanning microscope (Carl Zeiss, Oberkochen, Germany). Data were processed with Adobe Photoshop 7.0 software for analysis.
Transwell Migration Assay
Cell migration was detected using 24-well Transwell chambers without Matrigel matrix (8-μm pore size; BD Biosciences, San Jose, CA, USA). In brief, 600 μl of culture medium was added to the bottom chamber, and siRNA-transfected HCC cells (1 × 105 cells) were suspended in serum-free medium in the upper chamber. After 36 h, the cells on the top surface of the membrane were mechanically removed using a cotton swab, and the cells on the bottom surface of the membrane were fixed in ethanol and stained with crystal violet solution. The migrated cells were counted in five randomly selected areas under a 40× microscope field. Each experiment was repeated three times.
Western Blot
Following siRNA transfection, 20 mg of protein lysates per sample was separated by SDS-PAGE gel (10%–12%). Proteins were then transferred to PVDF membranes (Bio-Rad, Hercules, CA, USA). After blocking in 10% milk for 30 min, the blots were incubated with NCAPG primary antibody and GAPDH (Sigma-Aldrich), and the secondary antibody (Sigma-Aldrich). GAPDH was used as internal control. Detection of interested bands was through West Pico ECL Reagents (Pierce, Rockford, IL, USA). ImageJ software was applied to quantify the bands.
Immunohistochemistry (IHC)
The paraffin-embedded HCC tissue samples were cut in 5-μm sections and placed on polylysine-coated slides, deparaffinized in xylene, and rehydrated using a series of graded alcohols. Antigen retrieval was performed by heat mediation in citrate buffer (pH 6; Dako, Carpinteria, CA, USA). Samples were blocked with 10% goat serum before incubating with primary antibody NCAPG (Sigma-Aldrich) in a humidified container at 4°C. Immunohistochemical staining was performed with the Dako Envision Plus System (Dako) according to the manufacturer’s instructions.
Tumor Xenograft Assay
The BALB/c nude mice (male, 5 weeks old, 18–20 g weight) were provided by the Animal Center of Affiliated Zhongda Hospital of Southeast University (Nanjing, P.R. China). The stable HuH7 and HCCLM3 cells, expressing the tetracycline-inducible NCAPG shRNA (sequence: CCGGGCTATGCAGAAGCATCTTCTTCTCGAGAAGAAGATGCTTCTGCATAGCTTTTTTG), were inoculated subcutaneously into the flanks of mice. Palpable tumors started to appear in 2 weeks. The size of the tumors was measured weekly by a caliper, and tumor volume was calculated according to the formula V = ab 2/2. All animal procedures were performed according to the IACUC of all the authors’ institutions.
Statistical Analysis
The results were presented as mean ± standard deviation from triplicate experiments repeated three separate times and analyzed with SPSS software (version 22.0; SPSS Inc., Chicago, IL, USA) unless otherwise indicated. The significance of the differences was determined by one-way analysis of variance among multiple groups, and a value of p < 0.05 was considered statistically significant.
RESULTS
Knockdown of NCAPG Inhibits Liver Cancer Cell Growth and Proliferation
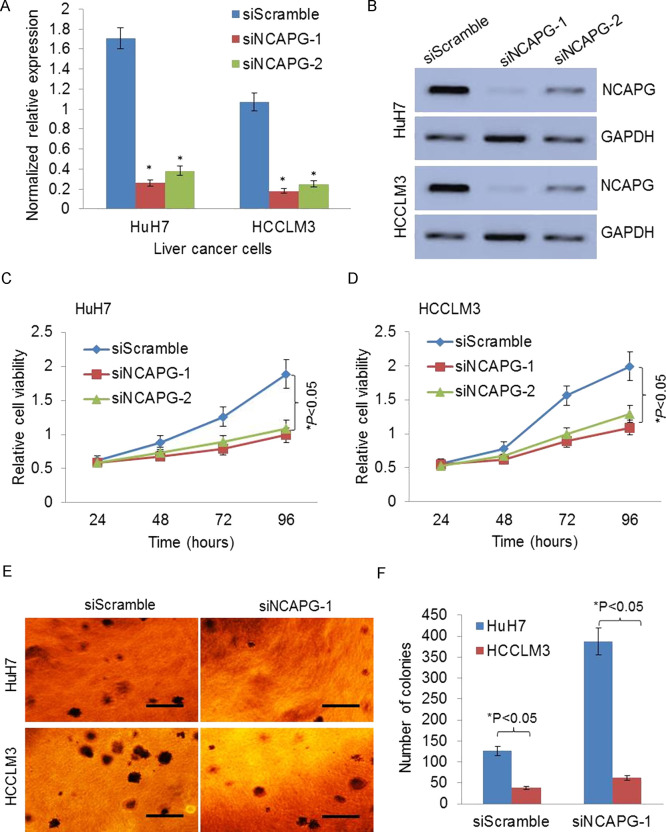
To investigate the role of NCAPG in HCC, we transfected two HCC cell lines with siScramble, siNCAPG-1, and siNCAPG-2 to knock down the NCAPG expression. The qRT-PCR analysis showed that the expression of NCAPG was significantly decreased in both HCC cell lines by siNCAPG-1 or siNCAPG-2 (Fig. 1A). The knockdown effects of siNCAPG were further validated by Western blotting analysis (Fig. 1B). The cell growth was dramatically reduced by knockdown of NCAPG in HuH7 and HCCLM3 cells (p < 0.05) (Fig. 1C and D). Knockdown of NCAPG also significantly inhibited the colony formation ability of HuH7 and HCCLM3 in soft agar, suggesting that NCAPG inhibits cellular anchorage-independent growth of HCC cells in vitro (Fig. 1E and F). Thus, these data revealed that knockdown of NCAPG inhibits HCC cell growth and proliferation.
Figure 1.
Knockdown of non-SMC condensin I complex, subunit G (NCAPG) inhibits liver cancer cell growth and proliferation. (A) The expression of NCAPG was significantly silenced in both hepatocellular carcinoma (HCC) cell lines by siNCAPG-1 or siNCAPG-2 by quantitative real-time polymerase chain reaction (qRT-PCR) analysis. (B) The representative images of Western blotting show the knockdown effects of siNCAPG. (C, D) The anchorage-independent growth was dramatically reduced by knockdown of NCAPG in HuH7 and HCCLM3 cells by MTS assay analysis. (E, F) The representative images and quantification of the colony formation ability of HuH7 and HCCLM3 in soft agar show that knockdown of NCAPG significantly inhibits colony formation. *p < 0.05.
Knockdown of NCAPG Induces Mitosis and Inhibits Migration In Vitro
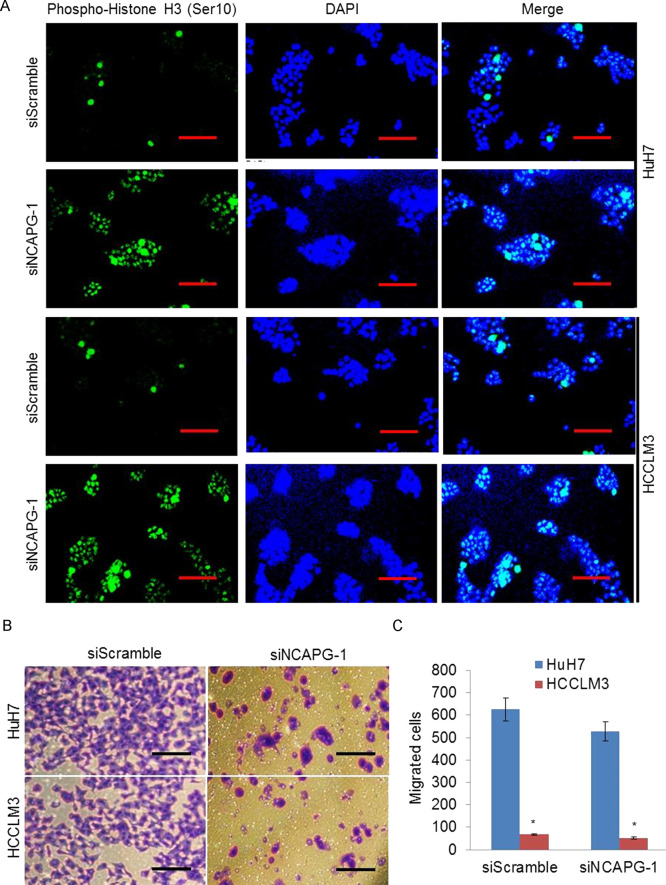
Phosphorylation at a highly conserved serine residue (Ser-10) in the histone H3 tail is considered to be a crucial event for the onset of mitosis, and it has been suggested that NCAPG was involved in the mitotic cell cycle. We then examined the effect of NCAPG knockdown on HCC cell mitosis arrest. Confocal immunofluorescence studies showed that knockdown of NCAPG significantly increased the numbers of positive cells with anti-phospho histone H3 (Ser-10) staining (Fig. 2A), suggesting that inhibition of NCAPG causes mitotic arrest, which led to an increase in mitosis marker, phospho-Histone H3 (Ser10). To further investigate the role of NCAPG on HCC cell migration, we examined whether NCAPG was a critical molecule on HCC cell migration by the Transwell migration assay. As shown, knockdown of NCAPG significantly suppressed the migration rates of HuH7 and HCCLM3 cells (Fig. 2B and C). These data indicated that knockdown of NCAPG induces mitosis and inhibits migration in vitro.
Figure 2.
Knockdown of NCAPG induces mitosis and inhibits migration in vitro. (A) The representative confocal immunofluorescence analysis images show that knockdown of NCAPG increases the phospho-histone H3 (mitotic marker)-positive cell number in both HuH7 and HCCLM3 cells with siNCAPG transfection. (B) The representative images of Transwell cell migration show that knockdown of NCAPG inhibits migration ability of HCC cells. (C) The number of migrated cells was quantified by plotting them as the average number of cells per field of view from three different experiments as described. *p < 0.05.
Tetracycline-Inducible shRNA Knockdown of NCAPG Inhibits Tumor Growth In Vivo
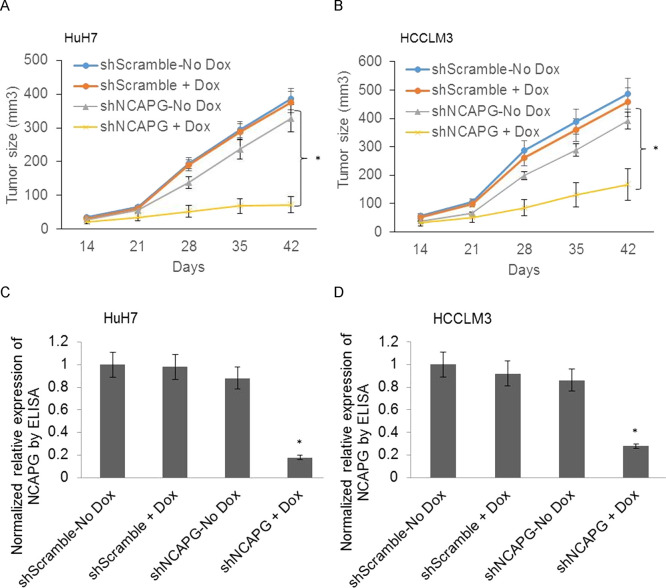
In order to validate that NCAPG is indeed a significant regulator of liver tumor growth in vivo, we studied the ability of NCAPG knockdown to inhibit growth of HuH7 and HCCLM3 tumor xenografts. We first established subcutaneous tumor xenografts in male nude mice using shScramble or shNCAPG HCC cells from our tetracycline-inducible knockdown model system. Five days after inoculation of the tumor cells, doxycycline was administered to half the mice. Tumor volume was measured once a week for 42 days at which time point some of the tumors were nearing 10 mm in diameter, necessitating euthanasia according to IACUC guidelines. While doxycycline itself had no effect on tumor growth in the shScramble mice, a significant reduction in tumor growth was observed in doxycycline-treated mice bearing shNCAPG tumors (Fig. 3A and B). ELISA analysis of lysates prepared from each group of the tumors revealed a significant reduction in NCAPG in shNCAPG +Dox tumors. Taken together, these results indicate that NCAPG is necessary for the growth of HCC tumors in vivo.
Figure 3.
Tetracycline-inducible shRNA knockdown of NCAPG inhibits tumor growth in vivo. (A, B) Tumor volume was measured every week, and the growth curve is shown. Mice were injected subcutaneously with either shScramble- or shNCAPG-transfected HuH7 (A) and HCCLM3 (B) cells. The experiment was performed using five to six mice per group and was independently repeated twice (n = 10–11 mice per group). (C, D) Equal amounts of protein from tumor lysates were subjected to ELISA for the detection of NCAPG. For each tumor sample, duplicate measurements were determined, and tumors from four to five mice in each group were analyzed. *p < 0.05.
Overexpression of NCAPG in Clinical HCC Samples Was Associated With Recurrence and Survival
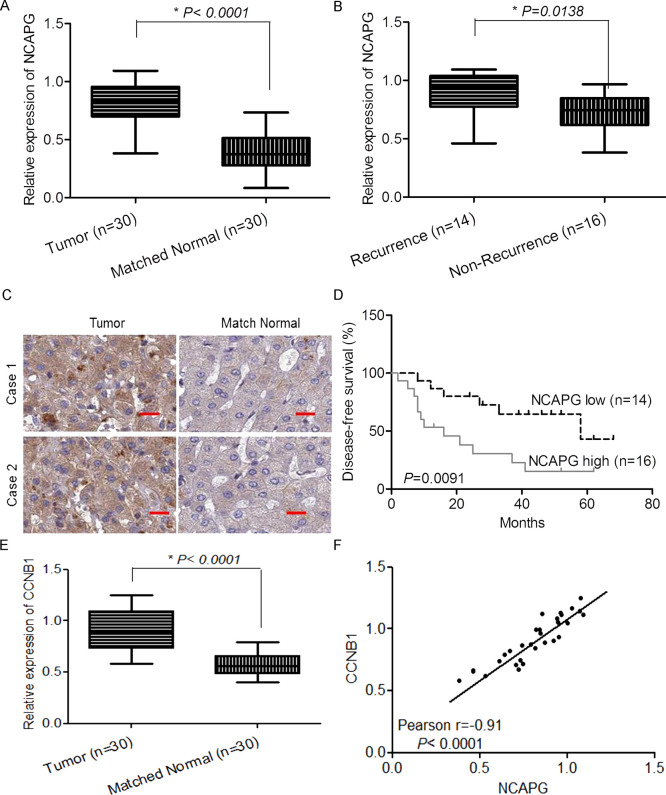
To validate the expression of NCAPG in clinical HCC samples, we analyzed 30 pairs of HCC and adjacent normal tissue samples by qRT-PCR. The results showed that the expression of NCAPG was higher in the HCC samples than in the matched normal tissue samples (Fig. 4A). Moreover, we divided the 30 HCC tumor samples into two groups according to their recurrence (within 5 years) and analyzed the expression of NCAPG in each group. The results indicated that the samples from the group of recurrence had significantly higher expression of NCAPG than those from the nonrecurrence group (Fig. 4B). The overexpression of NCAPG in tumor tissue compared to that in matched normal tissues was also detected by IHC (Fig. 4C). For further analysis about whether expression of NCAPG was associated with patients’ survival, we separated the 30 HCC samples into high expression of NCAPG and low expression of NCAPG groups according to the average expression level. The results showed that the high expression group had significantly shorter OS (Fig. 4D). Moreover, we also examined CCNB1, a critical regulatory gene involved in mitosis, in our HCC samples. The result showed that CCNB1 is significantly overexpressed in tumors compared to matched normal tissues. Interestingly, the expression of NCAPG was significantly positively correlated with the expression of CCNB1. Therefore, overexpression of NCAPG in clinical HCC samples was associated with recurrence and survival of patients.
Figure 4.
Overexpression of NCAPG in clinical HCC samples was associated with recurrence and survival. (A) The normalized relative expression of NCAPG in 30 pairs of HCC and adjacent normal samples by RT-qPCR analysis. (B) The normalized relative expression of NCAPG in 30 pairs of HCC samples with recurrence and nonrecurrence. (C) The representative immunohistochemistry (IHC) images show the expression and localization of NCAPG in HCC tissues. (D) The expression of miR-1258 was significantly associated with patients’ survival. (E) The normalized relative expression of CCNB1 in 30 pairs of HCC and adjacent normal samples by RT-qPCR analysis. (F) The significant correlation between the expression of NCAPG and CCNB1 was observed in our tissue samples.
DISCUSSION
NCAPG is a subunit of the condensin complex, which is responsible for the condensation and stabilization of chromosomes during mitosis and meiosis13. NCAPG-related pathways are cell cycle, mitotic, and cell cycle chromosome condensation in prometaphase according to gene ontology (GO) analysis11. Previously, RNA-seq was performed on tissue samples from 95 human individuals representing 27 different tissues in order to determine tissue specificity of all protein-coding genes. NCAPG has shown to be mainly expressed in the bone marrow, lymph node, and testis of normal human tissues. There is low expression of NCAPG in normal human liver14. Interestingly, NCAPG was significantly overexpressed in HCC according to the TCGA dataset, suggesting that it may play an important role in the development of HCC. However, there are few functional studies of NCAPG in cancers. A recent bioinformatics study showed that proteins encoded by differentially expressed genes CDK1, BUB1, CDC20, NCAPG, NDC80, CDCA8, MAD2L1, CCNB1, CCNA2, and BIRC5 were hub genes with high degrees in the protein–protein interaction network by analyzing the HCC gene expression profile dataset GSE29721 (10 HCC and 10 control samples)15. In gliomas, CENPE, KIF14, and NCAPG levels were significantly higher in pediatric high- and low-grade gliomas and were shown as the direct targets of miR-137 or miR-6500-3p. Furthermore, knockdown of CENPE, KIF14, or NCAPG combined with temozolomide treatment resulted in a combined suppressive effect on pediatric high-grade glioma cell proliferation10. To our knowledge, this is the first report studying NCAPG expression and its biological functions in HCC cells.
The results of this study suggest that NCAPG might be an important oncogene of HCC. First, NCAPG is only expressed in human liver cancer tissues and HCC cells, but not in normal human liver tissues. Second, siRNA-mediated NCAPG knockdown inhibited cell growth and proliferation and induced mitosis of HCC cells. Importantly, tetracycline-inducible shRNA knockdown of NCAPG inhibits tumor growth in vivo. Together, the results of this preclinical study suggest that NCAPG might be an important oncogene or oncotarget protein of HCC. Moreover, overexpression of NCAPG in clinical HCC samples was significantly associated with recurrence and survival of patients. The expression of NCAPG was significantly positively correlated with the expression of CCNB1, a critical regulatory gene involved in mitosis16, suggesting the potential interaction between NCAPG and CCNB1. A previous study showed that two threonine residues in the NCAPG subunit of condensin, threonines 308 and 332, are targets of cdc2/cyclin B phosphorylation17. Therefore, our study indicated that NCAPG may be a promising therapeutic target for HCC treatment in the future. Future studies will be needed to further understand the underlying mechanisms of NCAPG-mediated mitosis and NCAPG/CCNB1 interaction.
ACKNOWLEDGMENT
This work was supported by funding from the Health and Family Planning Commission of Nanjing (YKK16272).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394–9. [DOI] [PubMed] [Google Scholar]
- 2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 3. Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers 2016;2:16019. [DOI] [PubMed] [Google Scholar]
- 4. Sia D, Villanueva A, Friedman SL, Llovet JM. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology 2017;152:745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tovoli F, Negrini G, Bolondi L. Comparative analysis of current guidelines for the treatment of hepatocellular carcinoma. Hepatic Oncol. 2016;3:119–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344–54. [DOI] [PubMed] [Google Scholar]
- 7. Ngo VN, Davis RE, Lamy L, Yu X, Zhao H, Lenz G, Lam LT, Dave S, Yang L, Powell J. A loss-of-function RNA interference screen for molecular targets in cancer. Nature 2006;441:106–10. [DOI] [PubMed] [Google Scholar]
- 8. Eberlein A, Takasuga A, Setoguchi K, Pfuhl R, Flisikowski K, Fries R, Klopp N, Fürbass R, Weikard R, Kühn C. Dissection of genetic factors modulating fetal growth in cattle indicates a substantial role of the non-SMC condensin I complex, subunit G (NCAPG) gene. Genetics 209;183:951–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ryu B, Kim DS, DeLuca AM, Alani RM. Comprehensive expression profiling of tumor cell lines identifies molecular signatures of melanoma progression. PLoS One 2007;2:e594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liang ML, Hsieh TH, Ng KH, Tsai YN, Tsai CF, Chao ME, Liu DJ, Chu SS, Chen W, Liu YR. Downregulation of miR-137 and miR-6500-3p promotes cell proliferation in pediatric high-grade gliomas. Oncotarget 2016;7:19723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohen Y, Gutwein O, Garach-Jehoshua O, Bar-Haim A, Kornberg A. The proliferation arrest of primary tumor cells out-of-niche is associated with widespread downregulation of mitotic and transcriptional genes. Hematology 2014;19:286–92. [DOI] [PubMed] [Google Scholar]
- 12. Li Y, Tang Z, Ye S, Liu B, Liu Y, Chen J, Xue Q. Establishment of a hepatocellular carcinoma cell line with unique metastatic characteristics through in vivo selection and screening for metastasis-related genes through cDNA microarray. J Cancer Res Clin Oncol. 2003;129:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sutani T, Sakata T, Nakato R, Masuda K, Ishibashi M, Yamashita D, Suzuki Y, Hirano T, Bando M, Shirahige K. Condensin targets and reduces unwound DNA structures associated with transcription in mitotic chromosome condensation. Nat Commun. 2015;6:7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fagerberg L, Hallström BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S, Danielsson A, Edlund K. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics 2014;13:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yan H, Li Z, Shen Q, Wang Q, Tian J, Jiang Q, Gao L. Aberrant expression of cell cycle and material metabolism related genes contributes to hepatocellular carcinoma occurrence. Pathol Res Pract. 2017;213:316–21. [DOI] [PubMed] [Google Scholar]
- 16. Nath S, Ghatak D, Das P, Roychoudhury S. Transcriptional control of mitosis: Deregulation and cancer. Front Endocrinol. 2015;6:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murphy LA, Sarge KD. Phosphorylation of CAP-G is required for its chromosomal DNA localization during mitosis. Biochem Biophys Res Commun. 2008;377:1007–11. [DOI] [PMC free article] [PubMed] [Google Scholar]