Abstract
The human immunodeficiency virus (HIV) protease inhibitor nelfinavir acts against malignancies by inducing endoplasmic reticulum (ER) stress. The HIV protease inhibitor ritonavir, on the other hand, not only induces ER stress but also inhibits P-glycoprotein’s pump activity and thereby enhances the effects of its substrate drugs. We therefore postulated that ritonavir in combination with nelfinavir would kill bladder cancer cells effectively by inducing ER stress cooperatively and also enhancing nelfinavir’s effect. Nelfinavir was shown to be a P-glycoprotein substrate, and the combination of nelfinavir and ritonavir inhibited bladder cancer cell growth synergistically. It also suppressed colony formation significantly. The combination significantly increased the number of cells in the sub-G1 fraction and also the number of annexin V+ cells, confirming robust apoptosis induction. The combination induced ER stress synergistically, as evidenced by the increased expression of glucose-regulated protein 78, ER-resident protein 44, and endoplasmic oxidoreductin-1-like protein. It also increased the expression of the mammalian target of rapamycin (mTOR) inhibitor AMP-activated protein kinase and caused dephosphorylation of S6 ribosomal protein, demonstrating that the combination also inhibited the mTOR pathway. We also found that the combination enhanced histone acetylation synergistically by decreasing the expression of HDACs 1, 3, and 6.
Key words: Nelfinavir, Ritonavir, Endoplasmic reticulum stress, Bladder cancer
INTRODUCTION
There is currently no curative treatment for advanced bladder cancer. Cisplatin-based chemotherapies have been the mainstay of the treatment of metastatic bladder cancer for decades, but their efficacy is quite limited1. The anti-programmed cell death ligand 1 (PD-L1) antibody atezolizumab has recently been shown to have clinical activity in patients with metastatic cancer and approved for the treatment of bladder cancer by the US Food and Drug Administration2, but the objective response rate was not satisfactory. It is evident that novel treatment approaches are urgently needed.
Drug repositioning is a powerful strategy for finding new anticancer drugs3, and our laboratory has been trying to kill urological cancer cells by combining drugs already used for other purposes. Furthermore, to develop a therapy more effective than those currently used, it is necessary to use a novel strategy for killing cancer cells. We have focused on two innovative anticancer mechanisms, inducing endoplasmic reticulum (ER) stress and acetylating histones, and reported the effective drug combinations that act by these mechanisms against renal cancer4–6 and prostate cancer7.
In the present study, we used two clinically feasible non-anticancer drugs in combination to kill bladder cancer cells by inducing ER stress. The human immunodeficiency virus (HIV) protease inhibitors have recently attracted attention as a new class of anticancer drugs with multiple effects8. Nelfinavir is an HIV protease inhibitor widely used to treat HIV infection and has recently been shown to induce ER stress and act against malignancies9. Ritonavir is another clinically available HIV protease inhibitor. It not only induces ER stress10 but also inhibits P-glycoprotein11, enhancing the effects of its substrates by impeding their efflux from cells. We thought that ritonavir in combination with nelfinavir would kill bladder cancer cells effectively by inducing ER stress cooperatively and also enhancing nelfinavir’s effect.
MATERIALS AND METHODS
Cell Cultures
Bladder cancer cells (5637, J82, and UMUC3) were purchased from the American Type Culture Collection (Rockville, MD, USA) and grown in RPMI or MEM media containing 10% fetal bovine serum and 0.3% penicillin–streptomycin (Invitrogen, Carlsbad, CA, USA).
Reagents
Ritonavir (purchased from Toronto Research Chemicals, North York, ON, Canada), nelfinavir (purchased from Tocris Bioscience, Minneapolis, MN, USA), and valspodar (purchased from Santa Cruz Biotechnology, Santa Cruz, CA, USA) were dissolved in dimethyl sulfoxide and stored at −20°C until use.
Cell Viability Assay
For the cell viability assay, 5 × 103 cells were plated in 96-well culture plates 1 day before being cultured for 48 h under the indicated conditions. Cell viability was assayed using the MTS assay (Promega, Madison, WI, USA) according to the manufacturer’s protocol.
Colony Formation Assay
For the colony formation assay, 300 cells were plated in 6-cm plates 1 day before being cultured for 48 h in media containing 20 μM nelfinavir and/or 25 or 50 μM ritonavir. They were then given fresh media and allowed to grow for 1–2 weeks before being fixed with 100% methanol, stained with Giemsa’s solution, and counted.
Flow Cytometry
Induction of apoptosis was detected by measuring the number of cells in the sub-G1 fraction on cell cycle analysis and by annexin V assay. Cells (1.5 × 105) were plated in six-well culture plates 1 day before being cultured for 48 h in media containing 20 μM nelfinavir and/or 50 μM ritonavir. They were then washed with phosphate-buffered saline and harvested by trypsinization. For the cell cycle analysis, the harvested cells were resuspended in citrate buffer, stained with propidium iodide, and analyzed by flow cytometry. For the annexin V assay, the harvested cells were stained with annexin V and 7-amino-actinomycin D according to the manufacturer’s instruction (Beckman Coulter, Marseille, France) and then analyzed by flow cytometry. Data were analyzed using CellQuest Pro Software (BD Biosciences, San Jose, CA, USA).
Western Blotting
Cells were treated under the indicated conditions, and whole-cell lysates were obtained using radioimmunoprecipitation buffer. The lysates were then subjected to Western blot analysis as reported previously6. The primary antibodies used were anti-cyclin D1, anti-cyclin-dependent kinase 4 (CDK4), anti-glucose-regulated protein 78 (GRP78), anti-ubiquitinated protein, and anti-histone deacetylases (HDACs) 1, 3, and 6 (Santa Cruz Biotechnology); anti-ER-resident protein 44 (ERP44), anti-endoplasmic oxidoreductin-1-like protein (Ero1L), anti-S6 ribosomal protein, and anti-phosphorylated S6 ribosomal protein (Cell Signaling Technology, Danvers, MA, USA); anti-AMP-activated protein kinase (AMPK; Proteintech, Rosemont, IL, USA); anti-acetylated histone (Abcam, Cambridge, UK); and anti-actin (Millipore, Billerica, MA, USA).
Statistical Analysis
Combination indexes were calculated using CalcuSyn software (Biosoft, Cambridge, UK). The differences observed in the colony formation assay, cell cycle analysis, and annexin V assay were tested for statistical significance using the Mann–Whitney U-test (StatView software; SAS Institute, Cary NC, USA). Differences were considered significant with a value of p < 0.05.
RESULTS
Nelfinavir Was a P-glycoprotein Substrate
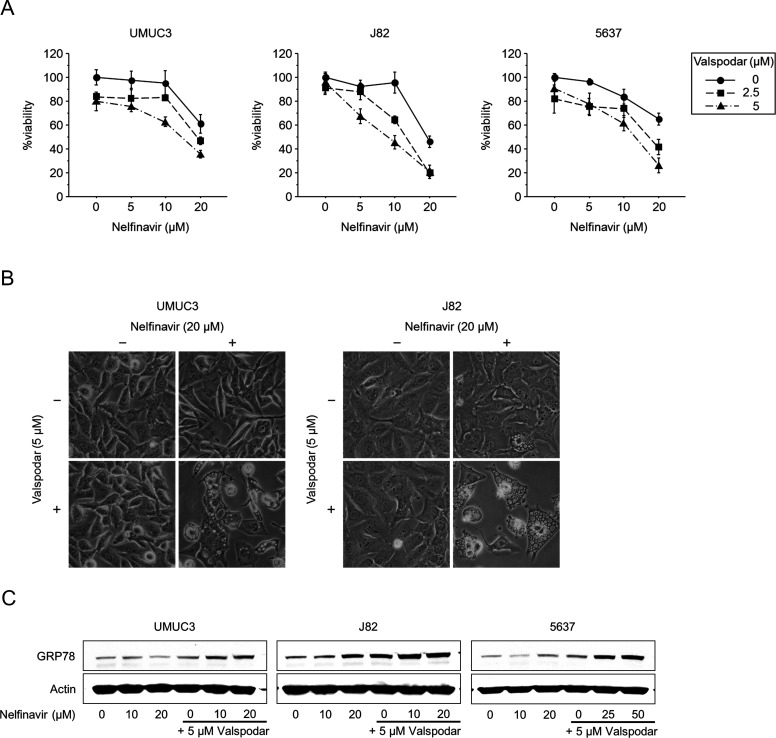
We examined whether nelfinavir was a substrate of P-glycoprotein using the specific P-glycoprotein inhibitor valspodar. The cancer cells were treated for 48 h with 5–20 μM nelfinavir in combination with 2.5–5 μM valspodar. MTS assay results showed that valspodar enhanced the cytotoxic effect of nelfinavir, whereas valspodar itself had only slight cytotoxicity (Fig. 1A). According to the combination indexes, the combined effect was synergistic (combination index <1) under many of the treatment conditions (Table 1). Interestingly, the cells treated with nelfinavir and valspodar displayed massive cytoplasmic vacuolization (Fig. 1B), a morphological change associated with ER stress12. Valspodar also enhanced nelfinavir-induced ER stress as evidenced by the increased expression of GRP78, a central regulator of ER function13 (Fig. 1C). From these results, we inferred that nelfinavir was a P-glycoprotein substrate.
Figure 1.
Nelfinavir was a P-glycoprotein substrate. (A) MTS assay. Cells were treated for 48 h with 5–20 μM nelfinavir with or without 2.5–5 μM valspodar. Bars represent mean ± SD, n = 6. (B) Photomicrographs after 48 h of treatment with 20 μM nelfinavir and/or 5 μM valspodar. Note marked cytoplasmic vacuolization after treatment with nelfinavir and valspodar. Original magnification: 200×. (C) Western blotting for glucose-regulated protein 78 (GRP78) after 48 h of treatment with 10–20 μM nelfinavir and/or 5 μM valspodar. Actin was used for the loading control. Representative blots are shown.
Table 1.
Combination Indexes (CIs) for the Combination of Nelfinavir and Valspodar in Bladder Cancer Cells
| Bladder Cancer Cells | Nelfinavir | ||
|---|---|---|---|
| 5 μM | 10 μM | 20 μM | |
| UMUC3 | |||
| 2.5 μM valspodar | 1.176 | 1.632 | 0.692 |
| 5 μM valspodar | 0.84 | 0.559 | 0.562 |
| J82 | |||
| 2.5 μM valspodar | 1.047 | 0.607 | 0.423 |
| 5 μM valspodar | 0.354 | 0.403 | 0.434 |
| 5637 | |||
| 2.5 μM valspodar | 0.353 | 0.664 | 0.637 |
| 5 μM valspodar | 0.395 | 0.487 | 0.444 |
CI < 1 indicates synergy.
The Combination of Nelfinavir and Ritonavir Inhibited Bladder Cancer Growth Synergistically
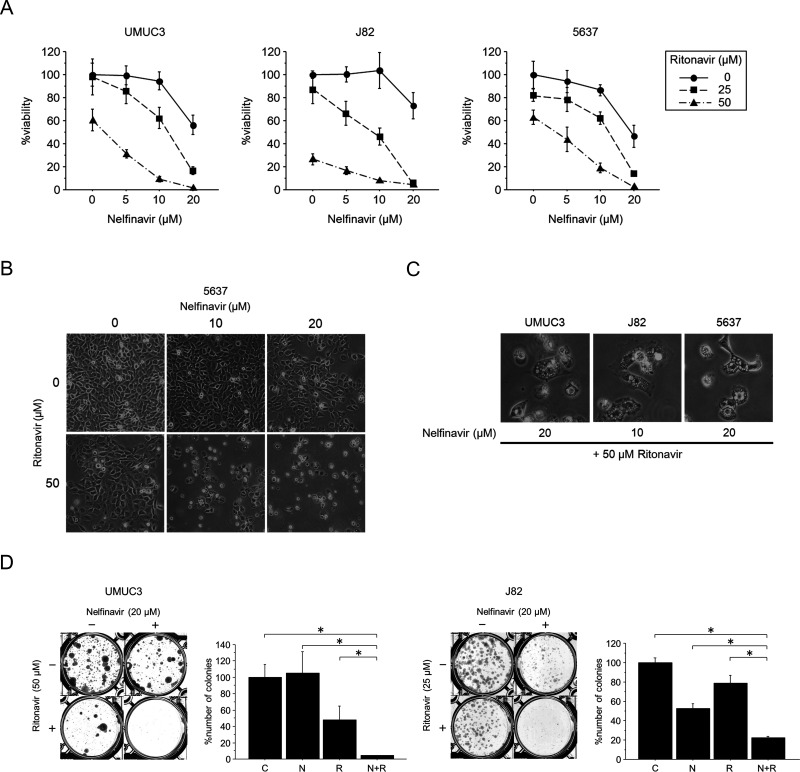
We treated the cells with 5–20 μM nelfinavir for 48 h with or without 25–50 μM ritonavir. MTS assay results showed that the combination effectively inhibited the viability of bladder cancer cells (Fig. 2A). This combined effect was also evident on microscopic examination (Fig. 2B): each agent alone had only a moderate effect on the morphology of the cancer cells, whereas most of the combination-treated cells were floating. We also calculated combination indexes and found the combined effect to be synergistic under many of the treatment conditions (Table 2). Furthermore, the combination-treated cells displayed massive cytoplasmic vacuolization (Fig. 2C), indicating the induction of ER stress. The combination also significantly inhibited colony formation by the bladder cancer cells (Fig. 2D). Thus, the combination of nelfinavir and ritonavir was shown to effectively inhibit bladder cancer growth.
Figure 2.
The combination of nelfinavir and ritonavir inhibited bladder cancer growth synergistically. (A) MTS assay. Cells were treated for 48 h with 5–20 μM nelfinavir with or without 25–50 μM ritonavir. Bars represent mean ± SD, n = 6. (B) Photomicrographs after 48 h of treatment with 10–20 μM nelfinavir and/or 50 μM ritonavir. Note that most of the cells treated with the combinations are floating. Original magnification: 100×. (C) High-power field image of the cells treated by the combination, showing marked cytoplasmic vacuolization. Original magnification: 200×. (D) Colony formation assay. A total of 300 cells were cultured for 48 h in media containing 20 μM nelfinavir and/or 25 or 50 μM ritonavir. They were then given fresh media and allowed to grow for 1–2 weeks. Bars represent mean ± SE, n = 3. C, control; N, nelfinavir; R, ritonavir. *p = 0.0495.
Table 2.
Combination Indexes (CIs) for the Combination of Nelfinavir and Ritonavir in Bladder Cancer Cells
| Bladder Cancer Cells | Nelfinavir | ||
|---|---|---|---|
| 5 μM | 10 μM | 20 μM | |
| UMUC3 | |||
| 25 μM ritonavir | 1.033 | 1.04 | 0.936 |
| 50 μM ritonavir | 0.977 | 0.838 | 0.737 |
| J82 | |||
| 25 μM ritonavir | 0.936 | 0.955 | 0.813 |
| 50 μM ritonavir | 1.009 | 0.967 | 1.081 |
| 5637 | |||
| 25 μM ritonavir | 1.284 | 1.107 | 0.595 |
| 50 μM ritonavir | 0.796 | 0.532 | 0.306 |
CI < 1 indicates synergy.
The Combination of Nelfinavir and Ritonavir Perturbed the Cell Cycle
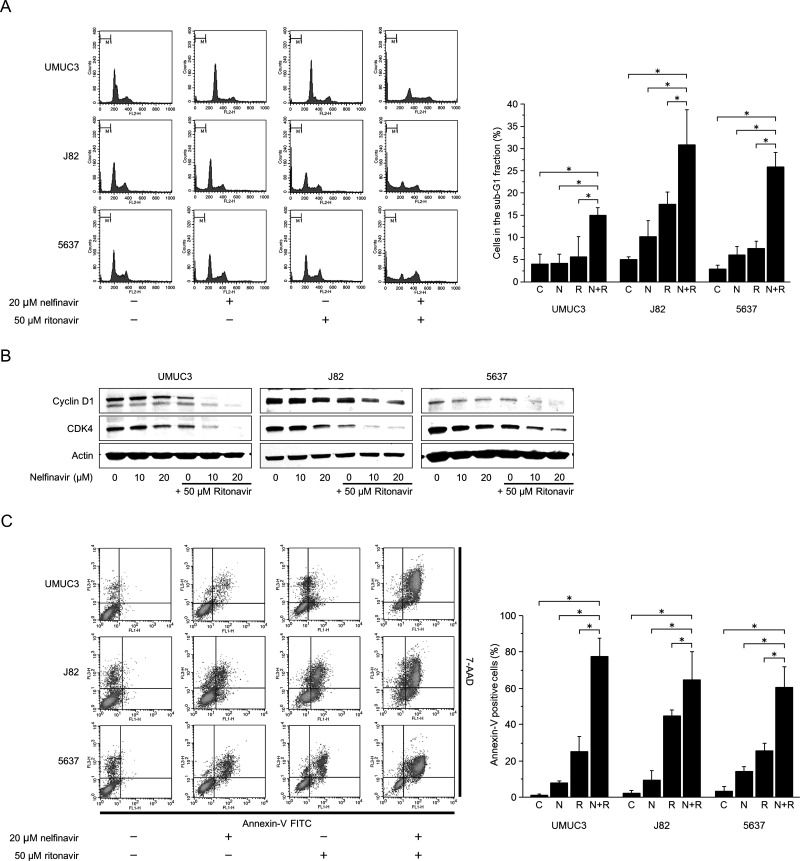
Cells were cultured for 48 h in medium containing 20 μM nelfinavir and/or 50 μM ritonavir. Each agent alone caused a slight to moderate increase in the number of the cells in the sub-G1 fraction, but in combination increased it significantly (Fig. 3A). These changes were in accordance with the decreased expression of the cell cycle regulators cyclin D1 and CDK4 (Fig. 3B).
Figure 3.
The combination of nelfinavir and ritonavir perturbed the cell cycle and caused apoptosis. (A) Cell cycle analysis. Cells were treated for 48 h with 20 μM nelfinavir and/or 50 μM ritonavir. A total of 10,000 cells were counted, and changes in cell cycle were analyzed using flow cytometry. Bar graphs show cells in the sub-G1 fraction. Data are expressed as mean ± SD from three independent experiments. *p = 0.0495. (B) Western blotting for cyclin D1 and cyclin-dependent kinase 4 (CDK4) after 48 h of treatment with 10–20 μM nelfinavir and/or 50 μM ritonavir. Actin was used for the loading control. Representative blots are shown. (C) Annexin V assay. Cells were treated for 48 h with 20 μM nelfinavir and/or 50 μM ritonavir. A total of 10,000 cells were counted, and induction of apoptosis was evaluated by annexin V assay using flow cytometry. Bar graphs show percentage of annexin V+ cells. Data are expressed as mean ± SD from three independent experiments. FITC, fluorescein isothiocyanate; 7-AAD, 7- amino-actinomycin D. *p = 0.0495.
The Combination of Nelfinavir and Ritonavir Induced Apoptosis
Cells were treated for 48 h with 20 μM nelfinavir and/or 50 μM ritonavir. Nelfinavir or ritonavir alone increased the number of annexin V+ cells only moderately, but together increased it significantly (Fig. 3C). This, along with the increased number of cells in the sub-G1 fraction, showed that the combination of nelfinavir and ritonavir induced apoptosis in bladder cancer cells.
The Combination of Nelfinavir and Ritonavir Induced ER stress
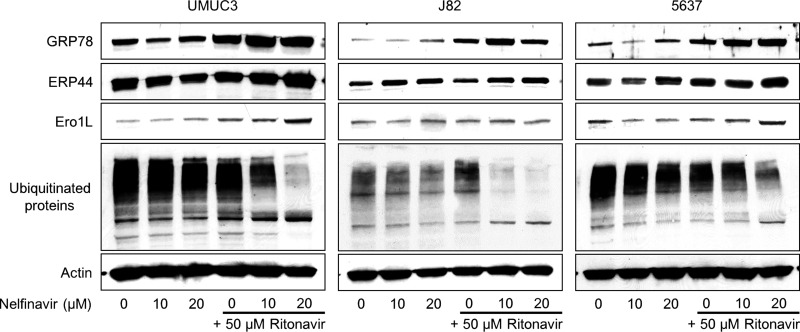
Ritonavir or nelfinavir alone induced ER stress, and the combination enhanced this ER stress, as indicated by the increased expression of the ER stress markers GRP78, ERP44, and Ero1L (Fig. 4). Our hypothesis is that the combination increases the amount of unfolded proteins in the cell and induces ER stress, so we also examined the change in the amount of ubiquitinated unfolded proteins. Surprisingly, the expression of ubiquitinated proteins was seemingly decreased by the combination.
Figure 4.
The combination of nelfinavir and ritonavir induced endoplasmic reticulum (ER) stress. Western blotting for ER stress markers and ubiquitinated proteins after 48 h of treatment with 10–20 μM nelfinavir and/or 50 μM ritonavir. Actin was used for the loading control. Representative blots are shown. ERP44, endoplasmic reticulum-resident protein 44; Ero1L, endoplasmic oxidoreductin-1-like protein.
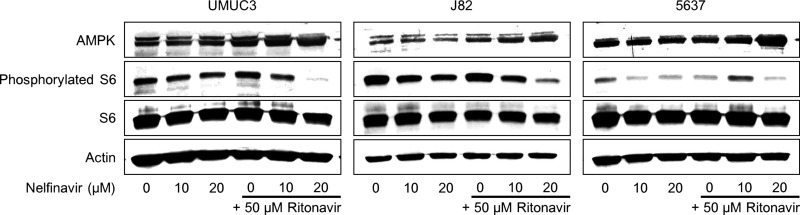
The Combination of Nelfinavir and Ritonavir Inhibited the Mammalian Target of Rapamycin (mTOR) Pathway
Because induction of ER stress has been reported to inhibit the mTOR pathway14, a relevant pathway for cancer proliferation, we thought that the combination could also inhibit it. The combination increased the expression of the mTOR inhibitor AMPK and caused dephosphorylation of the S6 ribosomal protein (Fig. 5), demonstrating that the combination indeed inhibited the mTOR pathway.
Figure 5.
The combination of nelfinavir and ritonavir inhibited the mammalian target of rapamycin pathway. Western blotting for AMP-activated protein kinase (AMPK), phosphorylated S6 ribosomal protein, and total S6 ribosomal protein after 48 h of treatment with 10–20 μM nelfinavir and/or 50 μM ritonavir. Actin was used for the loading control. Representative blots are shown.
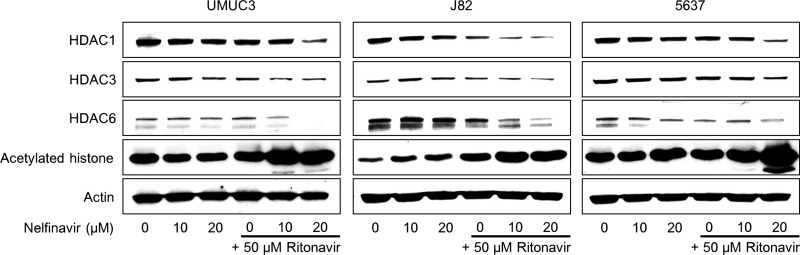
The Combination of Nelfinavir and Ritonavir Induced Histone Acetylation
We have previously shown that a combination causing ER stress in renal cancer cells also induced histone acetylation in them5. Therefore, we thought that the combination of nelfinavir and ritonavir might also induce histone acetylation. As expected, the combination caused histone acetylation synergistically (Fig. 6). In J82 cells, either 10 or 20 μM nelfinavir or 50 μM ritonavir caused slight histone acetylation, and in 5637 cells 20 μM nelfinavir did, whereas in UMUC3 cells neither agent alone caused histone acetylation. However, given in combination, they caused drastic histone acetylation in all the cell lines.
Figure 6.
The combination of nelfinavir and ritonavir induced histone acetylation. Western blotting for histone deacetylases (HDACs) 1, 3, and 6, and acetylated histone after 48 h of treatment with 10–20 μM nelfinavir and/or 50 μM ritonavir. Actin was used for the loading control. Representative blots are shown.
To explore the mechanism of this histone acetylation, we then evaluated the expression of HDACs and found that the combination decreased the expression of HDACs 1, 3, and 6 in all the cell lines.
DISCUSSION
Cisplatin-based chemotherapies have been the mainstay of the treatment of advanced bladder cancer for decades, but their efficacy is quite limited1. Recently, the PD-L1 antibody atezolizumab was approved for the treatment of bladder cancer by the US Food and Drug Administration, but the objective response rate was only 26%2. Development of a novel treatment is clearly needed.
Inducing ER stress is an emerging strategy to treat malignancies15. Starting with the combination of ritonavir and suberoylanilide hydroxamic acid in renal cancer16, we have investigated ways to kill urological cancer cells by inducing ER stress4–7. In the present study, we tried to introduce the concept of killing cancer cells by inducing ER stress to bladder cancer treatment. The most important thing was effectively inducing this ER stress.
Developing a new agent costs much, and therefore recent innovative anticancer agents such as kinase inhibitors, mTOR inhibitors, anti-PD-1 antibodies, and anti-PD-L1 antibodies are all very expensive. Furthermore, because development processes are long and approval rates are low, it takes a long time for a new agent to be applied clinically. Therefore, drug repositioning has emerged as an alternative approach to finding novel anticancer agents, an approach better in terms of both economic cost and time efficiency17. In the present study, using the concept of drug repositioning, we combined clinically feasible common non-anticancer agents to induce ER stress effectively.
Because ritonavir not only induces ER stress10 but also inhibits P-glycoprotein11, we thought that ritonavir in combination with nelfinavir kills bladder cancer cells effectively by inducing ER stress cooperatively and also enhancing nelfinavir’s effect. Ritonavir or nelfinavir alone induced ER stress and in combination enhanced this stress, which was in accordance with our hypothesis. Nelfinavir alone inhibited cell viability in a dose-dependent fashion, and the specific P-glycoprotein inhibitor valspodar enhanced this inhibition. Furthermore, enhanced expression of GRP78 confirmed that valspodar enhanced the nelfinavir-induced ER stress. Nelfinavir was thus shown to be one of the substrates of P-glycoprotein, and its inhibition is thought to be one mechanism of the combination’s action. On the other hand, ritonavir itself caused ER stress and inhibited bladder cancer viability. Ritonavir reportedly inhibits the proteasome18 and molecular chaperones19, inducing ER stress10. Therefore, cooperative ER stress induction would be another important mechanism of the combination’s action.
The combination of nelfinavir and ritonavir decreased the expression of ubiquitinated proteins. This result seems to be inconsistent with our hypothesis. Because the combination actually induced ER stress or unfolded protein response, unfolded ubiquitinated proteins were thought to accumulate. Mimnaugh et al.20 reported that the ER stress-inducing combination of the HSP90 inhibitor geldanamycin and the proteasome inhibitor bortezomib decreased the expression of ubiquitinated proteins by causing their aggregation and shift into the detergent-insoluble fraction, which may explain the seeming decrease in the expression of ubiquitinated proteins in the present study. Another possible mechanism of the decreased ubiquitinated protein expression would be protein synthesis inhibition due to the extensive ER stress induction21, but further study will be needed to clarify the exact mechanism of this phenomenon.
Inhibition of the mTOR pathway is another important mechanism by the combination. Genetic variations in the phosphoinositide-3 kinase–AKT–mTOR pathway were reported to increase bladder cancer risk22. The expression of phosphorylated-S6 ribosomal protein, a downstream target of mTOR, was shown to be an independent predictor of disease-specific survival in bladder cancer patients23. Furthermore, mTOR inhibition by rapamycin reportedly reduced cancer cell proliferation in RT4, T24, J82, and UMUC3 cells, corresponding to reduced phosphorylated-S6 ribosomal protein levels24. Thus, the mTOR pathway is thought to play an important role in bladder cancer proliferation. In the present study, the combination of nelfinavir and ritonavir caused dephosphorylation of S6 ribosomal protein, showing that the combination inhibited the mTOR pathway. As reported by Brüning et al.14, this mTOR inhibition would be secondary to the ER stress. The increased expression of the mTOR inhibitor AMPK is one important mechanism of the mTOR inhibition in the present study. AMPK is activated under conditions that deplete cellular ATP and elevate AMP levels such as glucose deprivation, hypoxia, ischemia, and heat shock25 and inhibits the mTOR pathway26,27. The increased expression of AMPK is thought to also be due to the ER stress because the ER stressor bortezomib was reported to increase the expression of AMPK28. This mTOR inhibition is an attractive important mechanism of the combination’s action because targeting AMPK–mTOR has recently emerged as novel cancer therapy29.
Surprisingly, the combination of nelfinavir and ritonavir caused histone acetylation, although we did not use any HDAC inhibitor in the present study. To our knowledge, there has been no report that an HIV protease inhibitor itself caused histone acetylation. The acetylation and deacetylation of histones play important roles in the regulation of gene transcription and in the modulation of chromatin structure30, and the acetylation status is determined by the activities of histone acetyltransferases and HDACs31. Because aberrant HDAC activity is associated with tumorigenicity and survival of cancer cells, compounds targeting HDACs (i.e., HDAC inhibitors) have generated great interest as anticancer drugs32,33. Because nelfinavir and ritonavir are not so-called HDAC inhibitors, we examined the expression of HDACs themselves in pursuit of a mechanism of the histone acetylation and found that the combination actually decreased the expression of HDACs. Another possible mechanism of the histone acetylation would be the ER stress induction, which has been shown to induce histone acetylation in other cancer cells5,7. Clarifying the exact mechanism will require further study, but inhibition of HDACs might play an important role in the combination’s antineoplastic activity because it not only causes histone acetylation but also causes acetylation of non-histone proteins such as transcription factors, signal transduction mediators, and DNA repair enzymes, modulating their function34.
There are also limitations in the present study. In the treatment of HIV infection, ritonavir is normally administered with other HIV protease inhibitors such as saquinavir, indinavir, lopinavir, and amprenavir35, but the combination of nelfinavir and ritonavir has not been used routinely. However, the results of some clinical studies36–38 indicate that the combination of nelfinavir and ritonavir could be administered safely. Careful drug monitoring would be needed, however, because ritonavir inhibits CYP3A4 in the liver35 and may increase the serum concentration of nelfinavir.
In summary, the combination of nelfinavir and ritonavir killed bladder cancer cells by inducing ER stress and histone acetylation. Inhibition of the mTOR pathway was also an important mechanism of action. Because both agents are common HIV protease inhibitors and have already been used clinically, a clinical trial using the combination would be more cost effective and feasible than one using novel agents even though careful drug monitoring would be needed.
ACKNOWLEDGMENT
This work was supported by JSPS KAKENHI Grant No. JP26462434.
REFERENCES
- 1. von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A, Arning M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23(21):4602–8. [DOI] [PubMed] [Google Scholar]
- 2. Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O’Donnell PH, Balmanoukian A, Loriot Y, Srinivas S, Retz MM, Grivas P, Joseph RW, Galsky MD, Fleming MT, Petrylak DP, Perez-Gracia JL, Burris HA, Castellano D, Canil C, Bellmunt J, Bajorin D, Nickles D, Bourgon R, Frampton GM, Cui N, Mariathasan S, Abidoye O, Fine GD, Dreicer R. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016;387(10031):1909–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Würth R, Thellung S, Bajetto A, Mazzanti M, Florio T, Barbieri F. Drug-repositioning opportunities for cancer therapy: Novel molecular targets for known compounds. Drug Discov Today 2016;21(1):190–9. [DOI] [PubMed] [Google Scholar]
- 4. Sato A, Asano T, Ito K, Sumitomo M, Asano T. Suberoylanilide hydroxamic acid (SAHA) combined with bortezomib inhibits renal cancer growth by enhancing histone acetylation and protein ubiquitination synergistically. BJU Int. 2012;109(8):1258–68. [DOI] [PubMed] [Google Scholar]
- 5. Sato A, Asano T, Isono M, Ito K, Asano T. Panobinostat synergizes with bortezomib to induce endoplasmic reticulum stress and ubiquitinated protein accumulation in renal cancer cells. BMC Urol. 2014;71:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Isono M, Sato A, Okubo K, Asano T, Asano T. Ritonavir interacts with belinostat to cause endoplasmic reticulum stress and histone acetylation in renal cancer cells. Oncol Res. 2016;24(5):327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sato A, Asano T, Ito K, Asano T. Vorinostat and bortezomib synergistically cause ubiquitinated protein accumulation in prostate cancer cells. J Urol. 2012;188(6):2410–8. [DOI] [PubMed] [Google Scholar]
- 8. Chow WA, Jiang C, Guan M. Anti-HIV drugs for cancer therapeutics: Back to the future? Lancet Oncol. 2009;10(1):61–71. [DOI] [PubMed] [Google Scholar]
- 9. Pyrko P, Kardosh A, Wang W, Xiong W, Schönthal AH, Chen TC. HIV-1 protease inhibitors nelfinavir and atazanavir induce malignant glioma death by triggering endoplasmic reticulum stress. Cancer Res. 2007;67(22):10920–8. [DOI] [PubMed] [Google Scholar]
- 10. Sato A. The human immunodeficiency virus protease inhibitor ritonavir is potentially active against urological malignancies. Onco Targets Ther. 2015;8:761–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Drewe J, Gutmann H, Fricker G, Török M, Beglinger C, Huwyler J. HIV protease inhibitor ritonavir: A more potent inhibitor of P-glycoprotein than the cyclosporine analog SDZ PSC 833. Biochem Pharmacol. 1999;57(10):1147–52. [DOI] [PubMed] [Google Scholar]
- 12. Ram BM, Ramakrishna G. Endoplasmic reticulum vacuolation and unfolded protein response leading to paraptosis like cell death in cyclosporine A treated cancer cervix cells is mediated by cyclophilin B inhibition. Biochim Biophys Acta 2014;1843(11):2497–512. [DOI] [PubMed] [Google Scholar]
- 13. Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6(1):45–54. [DOI] [PubMed] [Google Scholar]
- 14. Brüning A, Rahmeh M, Friese K. Nelfinavir and bortezomib inhibit mTOR activity via ATF4-mediated sestrin-2 regulation. Mol Oncol. 2013;7(6):1012–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Y, Ye Y. Proteostasis regulation at the endoplasmic reticulum: A new perturbation site for targeted cancer therapy. Cell Res. 2011;21(6):867–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sato A, Asano T, Horiguchi A, Ito K, Sumitomo M, Asano T. Combination of suberoylanilide hydroxamic acid and ritonavir is effective against renal cancer cells. Urology 2010;76(3):764.e7–13. [DOI] [PubMed] [Google Scholar]
- 17. Bhattarai D, Singh S, Jang Y, Hyeon Han S, Lee K, Choi Y. An insight into drug repositioning for the development of novel anti-cancer drugs. Curr Top Med Chem. 2016;16(19):2156–68. [DOI] [PubMed] [Google Scholar]
- 18. Gaedicke S, Firat-Geier E, Constantiniu O, Lucchiari-Hartz M, Freudenberg M, Galanos C, Niedermann G. Antitumor effect of the human immunodeficiency virus protease inhibitor ritonavir: Induction of tumor-cell apoptosis associated with perturbation of proteasomal proteolysis. Cancer Res. 2002;62(23):6901–8. [PubMed] [Google Scholar]
- 19. Srirangam A, Mitra R, Wang M, Gorski JC, Badve S, Baldridge L, Hamilton J, Kishimoto H, Hawes J, Li L, Orschell CM, Srour EF, Blum JS, Donner D, Sledge GW, Nakshatri H, Potter DA. Effects of HIV protease inhibitor ritonavir on Akt-regulated cell proliferation in breast cancer. Clin Cancer Res. 2006;12(6):1883–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mimnaugh EG, Xu W, Vos M, Yuan X, Isaacs JS, Bisht KS, Gius D, Neckers L. Simultaneous inhibition of hsp 90 and the proteasome promotes protein ubiquitination, causes endoplasmic reticulum-derived cytosolic vacuolization, and enhances antitumor activity. Mol Cancer Ther. 2004;3(5):551–66. [PubMed] [Google Scholar]
- 21. Paschen W. Shutdown of translation: Lethal or protective? Unfolded protein response versus apoptosis. J Cereb Blood Flow Metab. 2003;23(7):773–9. [DOI] [PubMed] [Google Scholar]
- 22. Chen M, Cassidy A, Gu J, Delclos GL, Zhen F, Yang H, Hildebrandt MA, Lin J, Ye Y, Chamberlain RM, Dinney CP, Wu X. Genetic variations in PI3K-AKT-mTOR pathway and bladder cancer risk. Carcinogenesis 2009;30(12):2047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schultz L, Albadine R, Hicks J, Jadallah S, DeMarzo AM, Chen YB, Nielsen ME, Gonzalgo ML, Sidransky D, Schoenberg M, Netto GJ. Expression status and prognostic significance of mammalian target of rapamycin pathway members in urothelial carcinoma of urinary bladder after cystectomy. Cancer 2010;116(23):5517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hansel DE, Platt E, Orloff M, Harwalker J, Sethu S, Hicks JL, De Marzo A, Steinle RE, Hsi ED, Theodorescu D, Ching CB, Eng C. Mammalian target of rapamycin (mTOR) regulates cellular proliferation and tumor growth in urothelial carcinoma. Am J Pathol. 2010;176(6):3062–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kemp BE, Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, Gupta A, Adams JJ, Katsis F, van Denderen B, Jennings IG, Iseli T, Michell BJ, Witters LA. AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans. 2003;31(Pt 1):162–8. [DOI] [PubMed] [Google Scholar]
- 26. Kimura N, Tokunaga C, Dalal S, Richardson C, Yoshino K, Hara K, Kemp BE, Witters LA, Mimura O, Yonezawa K. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signaling pathway. Genes Cells 2003;8(1):65–79. [DOI] [PubMed] [Google Scholar]
- 27. Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: An overview. Clin Sci. 2012;122(6):253–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deshmukh RR, Dou QP. Proteasome inhibitors induce AMPK activation via CaMKKβ in human breast cancer cells. Breast Cancer Res Treat. 2015;153(1):79–88. [DOI] [PubMed] [Google Scholar]
- 29. Yue W, Yang CS, DiPaola RS, Tan XL. Repurposing of metformin and aspirin by targeting AMPK-mTOR and inflammation for pancreatic cancer prevention and treatment. Cancer Prev Res. 2014;7(4):388–97. [DOI] [PubMed] [Google Scholar]
- 30. Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: Causes and therapies. Nat Rev Cancer 2001;1(3):194–202. [DOI] [PubMed] [Google Scholar]
- 31. Wade PA. Transcriptional control at regulatory checkpoints by histone deacetylases: Molecular connections between cancer and chromatin. Hum Mol Genet. 2001;10(7):693–8. [DOI] [PubMed] [Google Scholar]
- 32. Spange S, Wagner T, Heinzel T, Krämer OH. Acetylation of non-histone proteins modulates cellular signalling at multiple levels. Int J Biochem Cell Biol. 2009;41(1):185–98. [DOI] [PubMed] [Google Scholar]
- 33. West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest. 2014;124(1):30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: Molecular mechanisms of action. Oncogene 2007;26(37):5541–52. [DOI] [PubMed] [Google Scholar]
- 35. Zeldin RK, Petruschke RA. Pharmacological and therapeutic properties of ritonavir-boosted protease inhibitor therapy in HIV-infected patients. J Antimicrob Chemother. 2004;53(1):4–9. [DOI] [PubMed] [Google Scholar]
- 36. la Porte CJ, de Graaff-Teulen MJ, Colbers EP, Voncken DS, Ibanez SM, Koopmans PP, Hekster YA, Burger DM. Effect of efavirenz treatment on the pharmacokinetics of nelfinavir boosted by ritonavir in healthy volunteers. Br J Clin Pharmacol. 2004;58(6):632–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aarnoutse RE, Droste JA, van Oosterhout JJ, Koopmans PP, Popescu M, Reiss P, Hekster YA, Burger DM. Pharmacokinetics, food intake requirements and tolerability of once-daily combinations of nelfinavir and low-dose ritonavir in healthy volunteers. Br J Clin Pharmacol. 2003;55(2):115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kurowski M, Kaeser B, Sawyer A, Popescu M, Mrozikiewicz A. Low-dose ritonavir moderately enhances nelfinavir exposure. Clin Pharmacol Ther. 2002;72(2):123–32. [DOI] [PubMed] [Google Scholar]