Abstract
Abnormal expression of long noncoding RNAs (lncRNAs) often contributes to the unrestricted growth and invasion of cancer cells. lncRNA X-inactive specific transcript (XIST) expression is upregulated in several cancers; however, its underlying mechanism in osteosarcoma (OS) has not been elucidated. In the present study, we found that XIST expression was significantly increased in OS tissues and cell lines by LncRNA Profiler and qRT-PCR. The effects of XIST and miR-320b on OS cell proliferation and invasion were studied by MTT and Transwell invasion assays. The competing relationship between XIST and miR-320b was confirmed by luciferase reporter assay. Our results showed that XIST knockdown strikingly inhibited cell proliferation and invasion. Furthermore, XIST could directly bind to miR-320b and repress miR-320b expression. Moreover, XIST overexpression significantly relieved the inhibition on OS cell proliferation and invasion mediated by miR-320b overexpression, which involved the derepression of Ras-related protein RAP2B. We propose that XIST is responsible for OS cell proliferation and invasion and that XIST exerts its function through the miR-320b/RAP2B axis. Our findings suggest that lncRNA XIST may be a candidate prognostic biomarker and a target for new therapies in OS patients.
Key words: Long noncoding RNAs (lncRNAs), XIST, Osteosarcoma (OS), miR-320b, RAP2B, Invasion
INTRODUCTION
Osteosarcoma (OS) is a common, aggressive, malignant mesenchymal neoplasm and the second leading cause of cancer-related mortality in the pediatric age group1. Despite significantly developed therapeutic strategies for OS in recent years, the 5-year survival rate for patients has not changed much due to local relapse or lung metastasis after the resection of primary OS2. To treat malignant OS, studies to identify molecular targets associated with OS cell growth, apoptosis, differentiation, invasion, and migration have been performed. However, it is still a challenge to develop effective, targeted therapies to overcome this complex disease. Therefore, to understand the molecular pathogenesis of OS, find novel diagnostic biomarkers, and develop therapeutic treatments, it is essential to design new, effective therapeutic strategies to improve the survival rate of patients with OS.
More than 90% of the human DNA sequence is actively transcribed, but protein-coding genes only account for about 2%, whereas the majority of transcripts are referred to as noncoding RNAs (ncRNAs)3,4. ncRNAs are generally assigned into two major classes according to transcript size: long noncoding RNAs (lncRNAs) and small ncRNAs5. lncRNAs are a group of ncRNAs that are composed of more than 200 nucleotides in length with little or no protein-coding capacity6. Abnormal expression of lncRNAs often contributes to tumor initiation, metastasis, and cell growth7, and lncRNAs are dysregulated in many cancers. lncRNA X-inactive specific transcript (XIST) is required for transcriptional silence in one X chromosome during development in mammals8. It plays a significant role in the differentiation, proliferation, and genome maintenance of human cells9, and abnormal expression often contributes to the development of human cancer10. For instance, lncRNA XIST expression is upregulated in glioma tissues and promotes cell proliferation and invasion11. Furthermore, its overexpression is highly associated with occurrence, growth, invasion, and metastasis in ovarian cancer12. However, the role of lncRNA XIST in OS is still poorly understood.
MicroRNAs (miRNAs) are a class of small ncRNA molecules, usually 18–25 nucleotides in length, which posttranscriptionally regulate gene expression by binding to the 3′-untranslated region (3′-UTR) of mRNA and subsequently leads to mRNA degradation or translation repression13. It is well documented that dysregulated expression of miRNAs plays a critical role in the regulation of various critical biological processes, including cell development, differentiation, proliferation, and apoptosis14,15. Previous studies have shown that miR-320b is significantly downregulated in several cancers including colorectal cancer16, glioblastoma17, gastric cancer18, and bladder cancer19. Given that downregulation of miR-320b is common in a number of cancers, it has been hypothesized that miR-320b may play an important role in tumor development and function as a tumor suppressor. However, a more detailed role for miR-320b in the suppression of cell growth and invasion in OS has not yet been elucidated.
It has been reported that lncRNA may serve as a competing endogenous RNA (ceRNA), also known as a sponge or antagomir, which interacts with miRNAs and modulates the expression of miRNA target genes20. In the present study, we found that XIST is highly expressed in OS cells, and it may play an oncogenic role in OS. Further investigation showed that XIST may function as an endogenous sponge to compete with Rab-related protein RAP2B through directly binding to miR-320b. Our study, for the first time, presents the XIST/miR-320b/RAP2B pathway in human OS.
MATERIALS AND METHODS
Patients and Cancer Specimens
Fresh frozen human gastric OS samples and their corresponding normal tissue samples were obtained from the Tumor Bank Facility of Tianjin Medical University Cancer Institute and Hospital and National Foundation of Cancer Research (TBF of TMUCIH and NFCR, Tianjin, P.R. China). All the tumor types were confirmed by pathologic analysis. The use of all human material was in accordance with the ethical guidelines of the Declaration of Helsinki (1975) and was approved by the Institutional Review Board of Tianjin Hospital.
Cell Culture
Human OS cell lines U2OS, Saos-2, MG63, and MNNG/HOS and the human conditionally immortalized fetal osteoblast cell line HFOB 1.19 were obtained from the Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, P.R. China). HFOB 1.19 cells were cultured in F12 containing 10% fetal bovine serum (FBS). The MNNG/HOS cell line was cultured in Eagle’s minimum essential medium (MEM), and all other cell lines were grown in Roswell Park Memorial Institute (RPMI)-1640 medium containing 10% FBS. All media and the FBS were purchased from Gibco Invitrogen Corporation (Carlsbad, CA, USA). The media above were also supplemented with 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA). The cell lines were maintained at 37°C in a humidified 5% CO2 incubator.
Bioinformatics Analysis of XIST and miR-130b
The online software program Starbase v2.0 (http://starbase.sysu.edu.cn/mirLncRNA.php) was used to identify potential binding partners for XIST, while the online software TargetScan (http://www.targetscan.org) was used to determine the bioinformatics of miR-130b. Interactions were confirmed using a luciferase assay (see below).
RNA Isolation and Quantitative Real-Time qRT-PCR
Total RNA was isolated from cultured cells using TRIzol reagent (Invitrogen) following the manufacturer’s instructions. A High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) was used to reversely transcribe RNA samples. Quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR) was performed using the Fast Start Universal SYBR Green Master Mix (Roche Diagnostics, Indianapolis, IN, USA). The primers to amplify XIST were 5′-CAGACGTG TGCTCTTC-3′ (forward) and 5′-CGATCTGTAAGTCCACCA-3′ (reverse), and for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were 5′-AACGTGTCAGTGGTGGACCTG-3′ (forward) and 5′-AGTGGGTG TCGCTGTTGAAGT-3′ (reverse). The primers for miR-320b and U6 snRNA were purchased from RiboBio (Guangzhou, P.R. China). Relative expression levels were calculated as ratios normalized against the endogenous control (GAPDH or U6 snRNA). The relative fold changes of candidate genes were analyzed using the 2−ΔΔCT method.
Cell Transfection
Cells were seeded into six-well plates at a concentration of 2 × 105 cells/well, and 50 nM miR-320b mimics or negative control (NC) was transfected using Lipofectamine™ 2000 transfection reagent (Invitrogen) following the protocol recommended by the manufacturer. The miR-320b and NC mimics were synthesized by Shanghai GenePharma Co. (Shanghai, P.R. China). Small interfering RNA (siRNA) targeting XIST (si-XIST), siRNA targeting RAP2B, and scrambled negative control (si-NC) were all purchased from Invitrogen. The full-length sequence of human XIST gene was synthesized and subcloned into pcDNA 3.1 vector (Invitrogen). After 48 h of transfection, the cells were collected and used for further analysis.
Cell Proliferation Assay
Cell proliferation was measured using an MTT kit (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s instructions. Briefly, the transfected cells (5,000 cells/well, six repeated wells) were seeded into 96-well plates and cultured for 24, 48, and 72 h. Subsequently, 20 μl of MTT solution (5 mg/ml) was added into each well. Following an additional 4 h of incubation, the supernatant was discarded, and 200 μl of dimethyl sulfoxide (DMSO) was supplemented to dissolve formazan crystals for 10 min. Absorbance value at a wavelength of 490 nm was detected on an enzyme-linked immunosorbent assay (ELISA) reader (Molecular Devices, Sunnyvale, CA, USA). All experiments were repeated three times.
Luciferase Reporter Assays
The 3′-UTR of XIST fragments containing the predicted miR-320b binding sites were amplified by PCR from human genomic DNA, subcloned and inserted downstream of the firefly luciferase gene of the pGL3 vector (Promega Corporation, Madison, WI, USA) to form pGL3-XIST-WT. The sequence of putative miR-320b binding sites in the XIST gene was replaced so as to construct the mutant pGL3-XIST-MUT. For the luciferase reporter assay, human embryonic kidney (HEK) 293T cells were seeded into 12-well plates and then cotransfected with 300 ng of pGL3-XIST-WT or pGL3-XIST-MUT and 50 nM miR-320b or miR-NC using Lipofectamine™ 2000 (Invitrogen). Cells were collected and lysed 48 h after transfection, and luciferase activity was analyzed with a Dual-Luciferase Reporter Assay System (Promega). The firefly luciferase activity of each sample was normalized to that of Renilla luciferase.
Disease-Related Human LncRNA Profiler
The Disease-Related Human LncRNA Profiler™ was purchased from System Biosciences (SBI; Mountain View, CA, USA). The detection of the lncRNAs was performed according to the standard protocol.
Western Blot
Radioimmunoprecipitation assay (RIPA) buffer (Sigma-Aldrich) supplemented with Complete Protease Inhibitor Cocktail (Roche) was used to lyse cells. Cell lysates were transferred to a 1.5-ml tube and stored at −20°C prior to use. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was conducted to separate the cellular proteins. Proteins were separated by 5% stacking gel and 10% running gel. The molecular weight of candidate proteins was confirmed by reference to the Prestained SeeBlue rainbow marker (Invitrogen) loaded in parallel. The following antibodies were used: RAP2B (Abcam, Cambridge, MA, USA) and GAPDH (Sigma-Aldrich).
Transwell Invasion Assay
We used Transwell chambers (8-μm pore size; BD Biosciences, San Jose, CA, USA) to measure cell invasiveness. Briefly, approximately 5 × 104 of transfected Saos-2 or MG63 cells were resuspended in 500 μl of serum-free media and then inoculated in the upper chambers of an insert coated with 50 μl of Matrigel. Complete medium (500 μl) containing FBS, as a chemoattractant, was added to the bottom chambers of the Transwell plates. Following incubation for 36 h at 37°C and 5% CO2, cells remaining in the upper chambers were scraped by a cotton swab. Subsequently, cells that attached to the bottom surface of the membrane were fixed with methanol for 10 min, stained with 0.1% crystal violet for 20 min, imaged, and counted using an IX71 inverted microscope (Olympus, Tokyo, Japan). Experiments were performed independently in triplicate.
Statistical Analysis
Statistical analysis was performed by the SPSS 15.0 software (SPSS, Chicago, IL, USA). All data were expressed as the mean ± standard deviation (SD) and derived from at least three independent experiments. For comparison, Student’s t-test and one-way analysis of variance (ANOVA) were performed. A value of p < 0.05 was considered to be statistically significant.
RESULTS
lncRNA XIST Plays a Vital Role in the Progression of OS
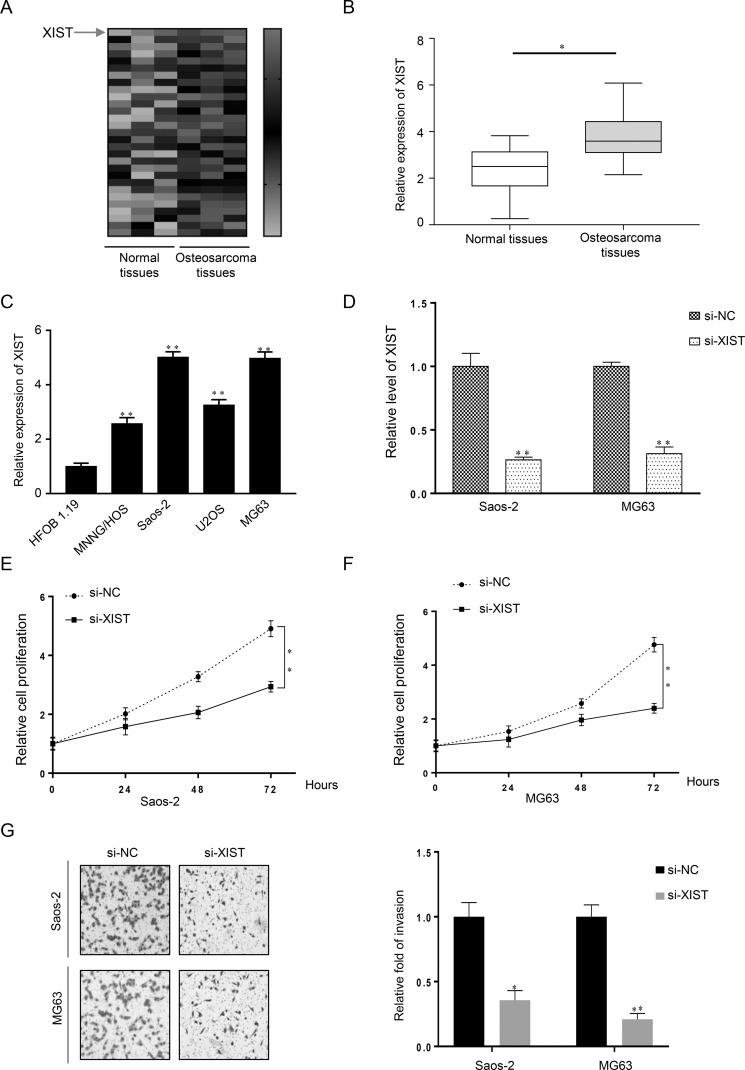
To investigate the biological function of lncRNA in OS carcinogenesis, we used the Disease-Related Human LncRNA Profiler to identify that lncRNA XIST was highly expressed in OS tissues when compared with normal tissues (Fig. 1A). This result was confirmed in OS tissues and matched normal tissue samples by qRT-PCR (p < 0.05) (Fig. 1B). Further analysis of the XIST expression profile among four different OS cell lines (U2OS, Saos-2, MG63, and MNNG/HOS) and the human normal osteoblast cell line HFOB 1.19 was completed. When normalized to HFOB 1.19 cells, XIST expression was obviously overexpressed in four OS cell lines, especially in the MG63 and Saos-2 cell lines (Fig. 1C). Therefore, it was suggested that lncRNA XIST may play a significant role in the initiation and progression of OS.
Figure 1.
Long noncoding RNA X-inactive specific transcript (lncRNA XIST) is upregulated in osteosarcoma (OS) tissues and cell lines and promotes the proliferation and invasion of OS cells. (A, B) XIST expression was significantly increased in OS tissues when compared with the normal counterparts using the Disease-Related Human LncRNA Profiler and quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR), respectively. (C) The four OS cell lines U2OS, Saos-2, MG63, and MNNG/HOS all had a higher level of XIST expression than the normal osteoblast cell line HFOB 1.19. (D) XIST knockdown was achieved by XIST small interfering RNA (si-XIST), and the inhibitory efficiency was verified by real-time PCR. (E, F) MTT assays revealed that knockdown of XIST attenuated the growth of both Saos-2 and MG63 cell lines for up to 3 days, compared with the si-NC (negative control) group. (G) Cell invasiveness in Saos-2 and MG63 cells transfected with si-XIST or si-NC was detected by Transwell invasion assay and is shown both pictorially and graphically. *p < 0.05, **p < 0.01 compared to normal tissue in (B), HFOB 1.19 cells in (C), and the si-NC groups in (D–G).
To determine the association of XIST expression with OS cell proliferation and invasion, XIST siRNA (si-XIST), as well as negative control (si-NC), was transfected into two human OS cell lines: Saos-2 and MG63. Compared with the si-NC group, XIST expression was decreased in cells transfected with si-XIST as measured by qRT-PCR (Fig. 1D). Cell proliferation and invasion were determined by MTT and Transwell assays, respectively. When compared with the si-NC group, knockdown of XIST attenuated the growth of both Saos-2 and MG63 cells for up to 3 days (Fig. 1E and F). Knockdown of XIST also reduced the invasion abilities in both Saos-2 and MG63 cells (Fig. 1G). Together, these data indicated that si-XIST successfully knocked down XIST expression and that XIST promotes OS cell growth and metastasis.
Inverse Correlation of miR-320b and XIST Expression in OS Tissues and Direct Interaction Between miR-320b and the 3′-UTR of XIST
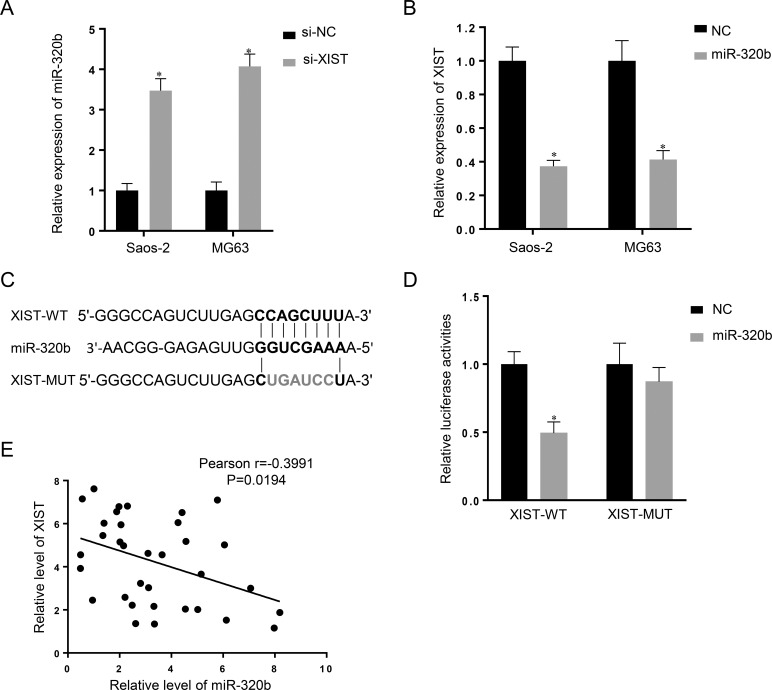
Recently, mounting evidence has demonstrated that lncRNA may serve as a ceRNA to sponge miRNAs and have an inhibitory effect on miRNA expression and activity21. To figure out the mechanism by which XIST is involved in the progression of OS (Fig. 2), we searched for miRNAs with complementary base pairing with XIST utilizing the online software program Starbase v2.0. Based on the results, we focused on miR-320b, a known suppressor of cancer cell proliferation and invasion (Fig. 2C). The qRT-PCR assay showed that miR-320b expression was increased in the si-XIST group when compared with the si-NC group (Fig. 2A), whereas miR-320b overexpression resulted in decreased XIST expression compared with the miR-NC group (Fig. 2B). Together, these data suggested that miR-320b expression is negatively correlated with XIST expression in OS.
Figure 2.
XIST is associated with microRNA-320b (miR-320b) expression in OS cells. (A) Real-time PCR assay showed that knockdown of XIST (si-XIST) caused upregulation of miR-320b in the Saos-2 and MG63 cell lines. (B) XIST expression was decreased in response to miR-320b overexpression, compared with the miR-NC (NC) group. Data are presented as mean ± SD of three independent experiments. (C) Generation of XIST-WT and XIST-MUT containing luciferase reporter vectors by sequentially mutating the predicted miR-320b binding site in the XIST 3′-untranslated region (3′-UTR). (D) The XIST-WT/XIST-MUT vectors and miR-NC/miR-320b mimics were cotransfected into human embryonic kidney (HEK) 293T cells, respectively. Luciferase activity of the XIST-WT vector was reduced in cells cotransfected with miR-320b mimics. Repression of luciferase activity by miR-320b was not shown in cells transfected with XIST-MUT. Data are presented as mean ± SD of three independent experiments. (E) An inverse correlation between XIST and miR-320b expression was observed. *p < 0.05 compared to the NC group.
To further investigate whether XIST is a functional target of miR-320b, we cloned the predicted miR-320b binding site of XIST (XIST-WT) and a mutated binding site (XIST-MUT) into a luciferase reporter plasmid. The results showed that cotransfection of miR-320b and XIST-WT strongly decreased luciferase activity, while cotransfection of miR-NC and XIST-WT did not change the luciferase activity. Interestingly, cotransfection of miR-320b and XIST-MUT did not change the luciferase activity either (Fig. 2D). To investigate whether miR-320b correlates with XIST in the regulation of OS cell growth, we performed expression analysis and found an inverse correlation between XIST and miR-320b expression in OS tissues (Fig. 2E). Taken together, these data suggested that miR-320b could directly bind to XIST and decrease XIST expression.
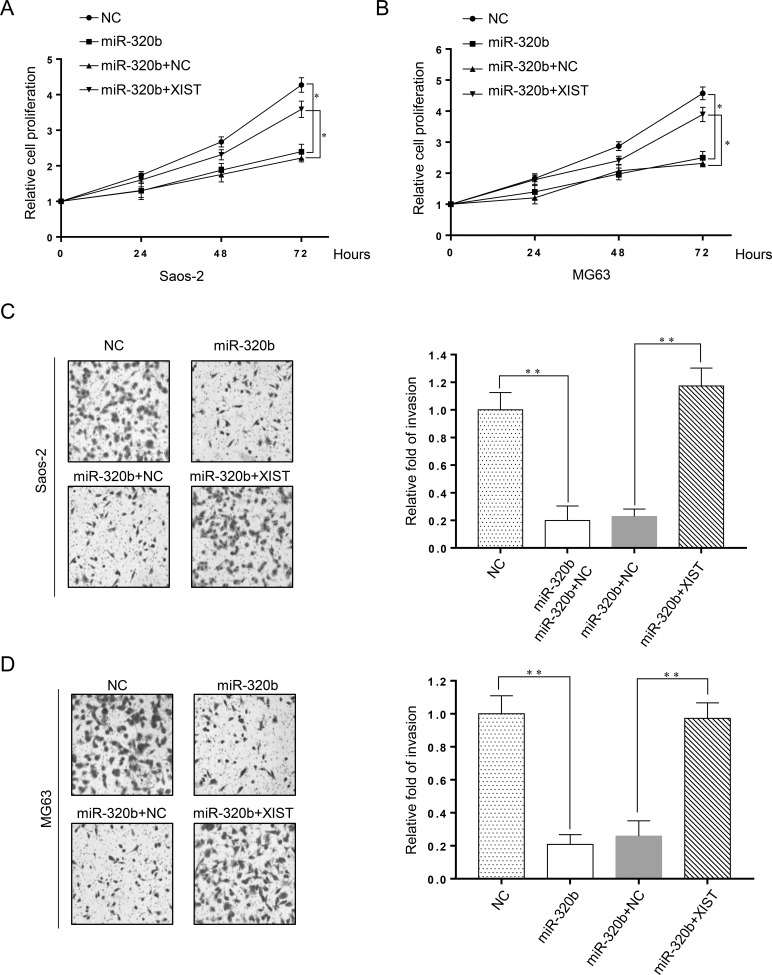
XIST Overturns the miR-320b-Induced Inhibitory Effect on Proliferation and Invasion of OS Cells
In consideration of the inhibitory effect of XIST on miR-320b expression in OS, we further investigated whether XIST can inhibit the biological function of miR-320b in Saos-2 and MG63 cells transfected with miR-320b, miR-NC, miR-320b + NC, or miR-320b + XIST. An MTT assay suggested that miR-320b overexpression obviously inhibited cell viability in Saos-2 and MG63 cells compared with the miR-NC transfected group, whereas XIST markedly relieved the inhibitory effect on cell proliferation mediated by miR-320b overexpression in vitro (Fig. 3A and B). Likewise, cell invasiveness was dramatically reduced by miR-320b overexpression, and this reduction was strikingly reversed by XIST overexpression in Saos-2 (Fig. 3C) and MG63 (Fig. 3D) cells compared with the miR-NC-transfected groups in vitro.
Figure 3.
XIST overturns the miR-320b-induced inhibitory effect on proliferation and invasion of OS cells. Saos-2 and MG63 cells were transfected with miR-320b, miR-NC, miR-320b + NC, or miR-320b + XIST. Cell viability was determined by MTT assay in transfected Saos-2 (A) and MG63 (B) cells at 24, 48, and 72 h. Cell invasiveness was evaluated by Transwell invasion assay and was represented both pictorially and graphically in transfected Saos-2 (C) and MG63 (D) cells. *p < 0.05, **p < 0.01 as shown.
Regulation of RAP2B by XIST and miR-320b in Human OS Cells and RAP2B as a Direct Target of miR-320b
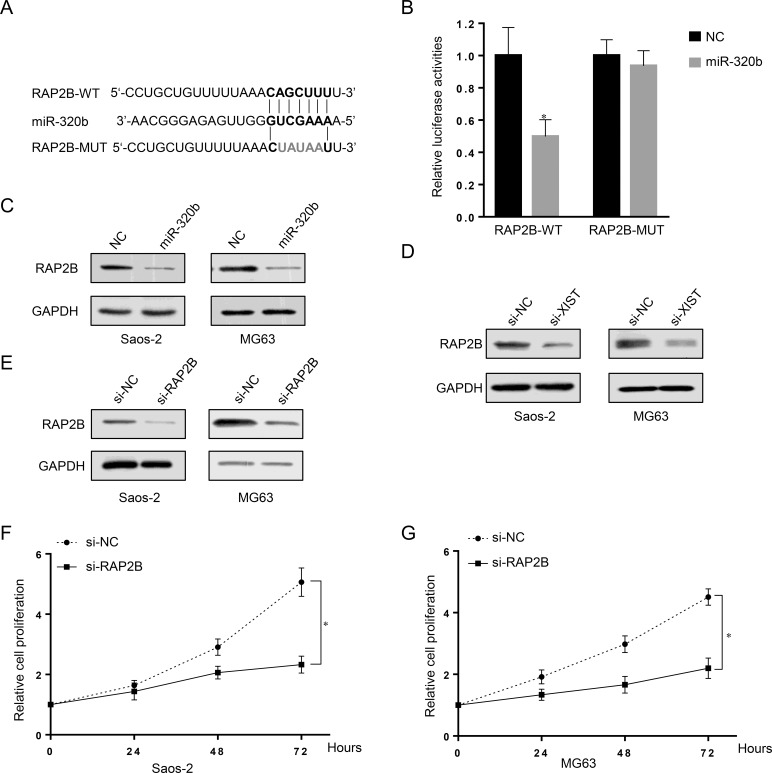
In view of the inhibitory effect of XIST on miR-320b expression and function in OS cells, we further investigated the effect of XIST on the miR-320b target RAP2B. Bioinformatics using the online software TargetScan revealed that miR-320b may bind to the 3′-UTR of RAP2B (Fig. 4A). In addition, we used a luciferase assay to confirm the bioinformatical prediction. The wild-type 3′-UTR of RAP2B or the corresponding mutant type was subcloned into the pGL3 luciferase reporter and cotransfected with miR-NC, miR-320b, into HEK 293T cells. The results showed that, compared with miR-NC, miR-320b reduced the luciferase activity of the pGL3 luciferase reporter containing wild-type 3′-UTR of RAP2B, but not the mutant reporter, confirming that RAP2B was a target of miR-320b (Fig. 4B).
Figure 4.
Regulation of RAP2B by XIST and miR-320b in human OS cells and RAP2B as a direct target of miR-320b. (A) The putative binding sequences of miR-320b and 3′-UTR of RAP2B. (B) The RAP2B-WT/RAP2B-MUT vectors and miR-NC/miR-320b mimics were cotransfected into HEK 293T cells, respectively. Luciferase activity of the RAP2B-WT vector was reduced in cells cotransfected with miR-320b mimics. The repression of luciferase activity by miR-320b was not shown in cells transfected with RAP2B-MUT. (C) Western blot assay showed that the expression of RAP2B was downregulated by miR-320b overexpression in both Saos-2 and MG63 cell lines. (D) Western blot results showed that knockdown of XIST also downregulated RAP2B in both Saos-2 and MG63 cells. (E) RAP2B knockdown was achieved by si-RAP2B as demonstrated by Western blot assay, which showed a much lower protein expression of RAP2B. (F, G) MTT assay results showed that OS cell growth was attenuated in response to RAP2B inhibition by si-RAP2B. Data are presented as mean ± SD of three independent experiments. *p < 0.05 compared to the NC group.
To determine the relationship of RAP2B with miR-320b and XIST, we measured the expression of RAP2B in response to miR-320b overexpression and XIST knockdown in human OS cells. RAP2B was downregulated by the overexpression of miR-320b, as demonstrated by Western blot (Fig. 4C). Knockdown of XIST also downregulated RAP2B in Saos-2 and MG63 cells (Fig. 4D).
We next investigated the effect of RAP2B on OS cell growth by knockdown of RAP2B in OS cells. RAP2B was successfully knocked down by si-RAP2B, as demonstrated by Western blots, showing a lower RAP2B protein expression (Fig. 4E). MTT assays revealed that OS cell growth was attenuated in response to RAP2B inhibition by si-RAP2B (Fig. 4F and G). Together, these results suggested that RAP2B promotes OS cell growth and proliferation.
DISCUSSION
lncRNAs may serve as effective therapeutic targets for cancer treatment including breast, prostate, colon, and gastric cancers, as well as OS. However, only a few lncRNAs have been functionally characterized. For example, abnormal expression of lncRNA nuclear paraspeckle assembly transcript 1 (NEAT1) has a close relationship to the development of ovarian cancer occurrence, growth, invasion, and metastasis12. Dysfunctional expression of XIST may have a pathological role in cancer. lncRNA XIST can promote cancer cell proliferation and invasion in glioma11. In addition, lncRNA XIST can be a predictive biomarker for screening non-small lung cancer22. Further investigation revealed that XIST acts as an oncogene in non-small cell lung cancer by epigenetically repressing Krüppel-like factor 2 (KLF2) expression23. lncRNA XIST can act as a molecular sponge of miR-101 to modulate enhancer of zeste homolog 2 (EZH2) and thereby promote the progression of gastric cancer24. In the present study, we revealed that lncRNA XIST was upregulated in OS patient tissues and cell lines compared with adjacent normal tissues and normal cell lines. We also found that knockdown of XIST by siRNA resulted in decreased cell proliferation and invasion in OS cells. These results encouraged us to investigate the underlying mechanism of XIST in regulating OS cell growth and invasion.
miR-320b, a member of the miR-320 family that inhibits cell proliferation, is downregulated in colorectal adenoma and is involved in the submucosal invasion of carcinoma tissues25. It was reported that miR-320a was downregulated from colorectal mucosa to low-grade dysplasia to high-grade dysplasia to adenocarcinomas in the same sample26, and the downregulation of miR-320b in colorectal adenoma, miR-320c and miR-320d in colorectal adenoma and colorectal cancer, and miR-320e in colorectal cancer25. Emerging evidence suggests that lncRNAs act as endogenous miRNA sponges that bind to miRNAs and regulate their function. To find out whether XIST serves as an miRNA sponge, we performed bioinformatics analysis and found that XIST contained binding sites for several miRNAs and focused on miR-320b. A luciferase assay indicated that miR-320b could bind to XIST directly by the putative miRNA response element. Furthermore, overexpression of miR-320b suppressed XIST expression. Interestingly, XIST knockdown displayed an elevated miR-320b expression. The above data suggest that there might be a reciprocal repression between XIST and miR-320b. Further studies suggested that XIST could dramatically overturn the inhibitory effect of miR-320b on cell proliferation and invasion, suggesting that XIST exerts its biological function by negatively regulating miR-320b in OS.
RAP2B was first isolated from the cDNA library of human platelets, and it belongs to the Ras superfamily of low-molecular mass guanosine triphosphate (GTP)-binding proteins, whose expression is elevated in a variety of human tumors27. Numerous studies have shown that Ras gene mutation or overexpression is responsible for the oncogenesis of various human tumors and results in poor prognostic significance for survival28. A recent study also demonstrated that RAP2B promotes proliferation, migration, and invasion of human breast cancer via modulating the calcium-related extracellular signal-regulated kinase 1/2 (ERK1/2) signaling pathway29. However, the role of RAP2B in OS is unknown. Using a bioinformatics tool, we identified that RAP2B was a potential target of miR-320b. Luciferase reporter assay and regulatory analysis in our study showed that miR-320b downregulated the expression level of RAP2B. We also observed that overexpression of miR-320b or knockdown of XIST both resulted in a markedly reduced RAP2B expression. The MTT assay also showed that a downregulated expression of RAP2B reduced the proliferation of OS cells. Thus, our data indicate that XIST may promote OS cell growth through the inhibition of miR-320b and by targeting RAP2B.
In summary, our current study suggests that XIST facilitates the invasion and proliferation of OS. We further revealed a novel XIST/miR-320b/RAP2B pathway regulatory axis in OS cells. Our findings provide further evidence of lncRNA as a ceRNA, which regulates the target gene of miRNA through directly sponging miRNA, thereby potentially promoting the development of OS.
ACKNOWLEDGMENTS
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009;115(7):1531–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meyers PA, Schwartz CL, Krailo MD, Healey JH, Bernstein ML, Betcher D, Ferguson WS, Gebhardt MC, Goorin AM, Harris M, Kleinerman E, Link MP, Nadel H, Nieder M, Siegal GP, Weiner MA, Wells RJ, Womer RB, Grier HE; Children’s Oncology Group. Osteosarcoma: The addition of muramyl tripeptide to chemotherapy improves overall survival—A report from the Children’s Oncology Group. J Clin Oncol. 2008;26(4):633–8. [DOI] [PubMed] [Google Scholar]
- 3. Martens-Uzunova ES, Bottcher R, Croce CM, Jenster G, Visakorpi T, Calin GA. Long noncoding RNA in prostate, bladder, and kidney cancer. Eur Urol. 2014;65(6):1140–51. [DOI] [PubMed] [Google Scholar]
- 4. Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F, Xue C, Marinov GK, Khatun J, Williams BA, Zaleski C, Rozowsky J, Röder M, Kokocinski F, Abdelhamid RF, Alioto T, Antoshechkin I, Baer MT, Bar NS, Batut P, Bell K, Bell I, Chakrabortty S, Chen X, Chrast J, Curado J, Derrien T, Drenkow J, Dumais E, Dumais J, Duttagupta R, Falconnet E, Fastuca M, Fejes-Toth K, Ferreira P, Foissac S, Fullwood MJ, Gao H, Gonzalez D, Gordon A, Gunawardena H, Howald C, Jha S, Johnson R, Kapranov P, King B, Kingswood C, Luo OJ, Park E, Persaud K, Preall JB, Ribeca P, Risk B, Robyr D, Sammeth M, Schaffer L, See LH, Shahab A, Skancke J, Suzuki AM, Takahashi H, Tilgner H, Trout D, Walters N, Wang H, Wrobel J, Yu Y, Ruan X, Hayashizaki Y, Harrow J, Gerstein M, Hubbard T, Reymond A, Antonarakis SE, Hannon G, Giddings MC, Ruan Y, Wold B, Carninci P, Guigó R, Gingeras TR. Landscape of transcription in human cells. Nature 2012;489(7414):101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ma MZ, Li CX, Zhang Y, Weng MZ, Zhang MD, Qin YY, Gong W, Quan ZW. Long non-coding RNA HOTAIR, a c-Myc activated driver of malignancy, negatively regulates miRNA-130a in gallbladder cancer. Mol Cancer 2014;13:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell 2011;43(6):904–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheetham SW, Gruhl F, Mattick JS, Dinger ME. Long noncoding RNAs and the genetics of cancer. Br J Cancer 2013;108(12):2419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown CJ, Ballabio A, Rupert JL, Lafreniere RG, Grompe M, Tonlorenzi R, Willard HF. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature 1991;349(6304):38–44. [DOI] [PubMed] [Google Scholar]
- 9. Engreitz JM, Pandya-Jones A, McDonel P, Shishkin A, Sirokman K, Surka C, Kadri S, Xing J, Goren A, Lander ES, Plath K, Guttman M. The Xist lncRNA exploits three-dimensional genome architecture to spread across the X chromosome. Science 2013;341(6147):1237973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McHugh CA, Chen CK, Chow A, Surka CF, Tran C, McDonel P, Pandya-Jones A, Blanco M, Burghard C, Moradian A, Sweredoski MJ, Shishkin AA, Su J, Lander ES, Hess S, Plath K, Guttman M. The Xist lncRNA interacts directly with SHARP to silence transcription through HDAC3. Nature 2015;521(7551):232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yao Y, Ma J, Xue Y, Wang P, Li Z, Liu J, Chen L, Xi Z, Teng H, Wang Z, Li Z, Liu Y. Knockdown of long non-coding RNA XIST exerts tumor-suppressive functions in human glioblastoma stem cells by up-regulating miR-152. Cancer Lett. 2015;359(1):75–86. [DOI] [PubMed] [Google Scholar]
- 12. Ren C, Li X, Wang T, Wang G, Zhao C, Liang T, Zhu Y, Li M, Yang C, Zhao Y, Zhang GM. Functions and mechanisms of long noncoding RNAs in ovarian cancer. Int J Gynecol Cancer 2015;25(4):566–9. [DOI] [PubMed] [Google Scholar]
- 13. Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol Med. 2014;20(8):460–9. [DOI] [PubMed] [Google Scholar]
- 14. Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004;116(2):281–97. [DOI] [PubMed] [Google Scholar]
- 15. Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer 2015;15(6):321–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dong Y, Wu WK, Wu CW, Sung JJ, Yu J, Ng SS. MicroRNA dysregulation in colorectal cancer: A clinical perspective. Br J Cancer 2011;104(6):893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roth P, Wischhusen J, Happold C, Chandran PA, Hofer S, Eisele G, Weller M, Keller A. A specific miRNA signature in the peripheral blood of glioblastoma patients. J Neurochem. 2011;118(3):449–57. [DOI] [PubMed] [Google Scholar]
- 18. Li X, Luo F, Li Q, Xu M, Feng D, Zhang G, Wu W. Identification of new aberrantly expressed miRNAs in intestinal-type gastric cancer and its clinical significance. Oncol Rep. 2011;26(6):1431–9. [DOI] [PubMed] [Google Scholar]
- 19. Song T, Xia W, Shao N, Zhang X, Wang C, Wu Y, Dong J, Cai W, Li H. Differential miRNA expression profiles in bladder urothelial carcinomas. Asian Pac J Cancer Prev. 2010;11(4):905–11. [PubMed] [Google Scholar]
- 20. Kallen AN, Zhou XB, Xu J, Qiao C, Ma J, Yan L, Lu L, Liu C, Yi JS, Zhang H, Min W, Bennett AM, Gregory RI, Ding Y, Huang Y. The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol Cell 2013;52(1):101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, Tramontano A, Bozzoni I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 2011;147(2):358–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tantai J, Hu D, Yang Y, Geng J. Combined identification of long non-coding RNA XIST and HIF1A-AS1 in serum as an effective screening for non-small cell lung cancer. Int J Clin Exp Pathol. 2015;8(7):7887–95. [PMC free article] [PubMed] [Google Scholar]
- 23. Fang J, Sun CC, Gong C. Long noncoding RNA XIST acts as an oncogene in non-small cell lung cancer by epigenetically repressing KLF2 expression. Biochem Biophys Res Commun. 2016;478(2):811–7. [DOI] [PubMed] [Google Scholar]
- 24. Chen DL, Ju HQ, Lu YX, Chen LZ, Zeng ZL, Zhang DS, Luo HY, Wang F, Qiu MZ, Wang DS, Xu DZ, Zhou ZW, Pelicano H, Huang P, Xie D, Wang FH, Li YH, Xu RH. Long non-coding RNA XIST regulates gastric cancer progression by acting as a molecular sponge of miR-101 to modulate EZH2 expression. J Exp Clin Cancer Res. 2016;35(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tadano T, Kakuta Y, Hamada S, Shimodaira Y, Kuroha M, Kawakami Y, Kimura T, Shiga H, Endo K, Masamune A, Takahashi S, Kinouchi Y, Shimosegawa T. MicroRNA-320 family is downregulated in colorectal adenoma and affects tumor proliferation by targeting CDK6. World J Gastrointest Oncol. 2016;8(7):532–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gattolliat CH, Uguen A, Pesson M, Trillet K, Simon B, Doucet L, Robaszkiewicz M, Corcos L. MicroRNA and targeted mRNA expression profiling analysis in human colorectal adenomas and adenocarcinomas. Eur J Cancer 2015;51(3):409–20. [DOI] [PubMed] [Google Scholar]
- 27. Zhang X, He Y, Lee KH, Dubois W, Li Z, Wu X, Kovalchuk A, Zhang W, Huang J. Rap2b, a novel p53 target, regulates p53-mediated pro-survival function. Cell Cycle 2013;12(8):1279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mascaux C, Iannino N, Martin B, Paesmans M, Berghmans T, Dusart M, Haller A, Lothaire P, Meert AP, Noel S, Lafitte JJ, Sculier JP. The role of RAS oncogene in survival of patients with lung cancer: A systematic review of the literature with meta-analysis. Br J Cancer 2005;92(1):131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Di J, Huang H, Qu D, Tang J, Cao W, Lu Z, Cheng Q, Yang J, Bai J, Zhang Y, Zheng J. Rap2B promotes proliferation, migration, and invasion of human breast cancer through calcium-related ERK1/2 signaling pathway. Sci Rep. 2015;5:12363. [DOI] [PMC free article] [PubMed] [Google Scholar]