Abstract
Background:
Evidence links gestational exposure to particulate matter with an aerodynamic diameter of less than 2.5 microns (PM2.5) with changes in leukocyte telomere length in cord blood with some studies showing sex-specific effects. PM2.5 exposure in utero increases oxidative stress, which can impact telomere biology. Thus, maternal antioxidant intakes may also modify the particulate air pollution effects.
Methods:
We examined associations among prenatal PM2.5 exposure and newborn relative leukocyte telomere length (rLTL), and the modifying effects of maternal antioxidant intake and infant sex. We estimated daily PM2.5 exposures over gestation using a validated spatiotemporally resolved satellite-based model. Maternal dietary and supplemental antioxidant intakes over the prior three months were ascertained during the second trimester using the modified Block98 food frequency questionnaire; high and low antioxidant intakes were categorized based on a median split. We employed Bayesian distributed lag interaction models (BDLIMs) to identify both sensitive windows of exposure and cumulative effect estimates for prenatal PM2.5 exposure on newborn rLTL, and to examine effect modification by maternal antioxidant intakes. A 3-way interaction between PM2.5, maternal antioxidant intake and infant sex was also explored.
Results:
For the main effect of PM2.5, BDLIMs identified a sensitive window at 12-20 weeks gestation for the association between increased prenatal PM2.5 exposure and shorter newborn rLTL and a cumulative effect of PM2.5 over gestation on newborn telomere length [cumulative effect estimate (CEE) = −0.29 (95% CI −0.49 to −0.10) per 1μg/m3 increase in PM2.5]. In models examining maternal antioxidant intake effects, BDLIMs found that children born to mothers reporting low antioxidant intakes were most vulnerable [CEE of low maternal antioxidant intake = −0.31 (95% CI −0.55 to −0.06) vs high maternal antioxidant intake = −0.07 (95% CI −0.34 to 0.17) per 1μg/m3 increase in PM2.5]. In exploratory models examining effect modification by both maternal antioxidant intakes and infant sex, the cumulative effect remained significant only in boys whose mothers reported low antioxidant intakes [CEE = −0.38 (95% CI −0.80 to −0.004)]; no sensitive windows were identified in any group.
Conclusions:
Prenatal PM2.5 exposure in mid-gestation was associated with reduced infant telomere length. Higher maternal antioxidant intakes mitigated these effects.
Keywords: prenatal, particulate air pollution, telomere length, antioxidant intakes, sex-specific effects
Introduction
Prenatal ambient air pollution exposure, specifically particulate matter with an aerodynamic diameter of less than 2.5 microns (PM2.5), has been linked to adverse birth outcomes (e.g., low birth weight, preterm delivery) and a range of adverse child health outcomes, including neurodevelopment, respiratory, and cardiometabolic disorders (Block et al. 2012; Fleisch et al. 2017; Korten et al. 2017). Epidemiological and biological data support the central role of oxidative stress (OS) in the toxic effects of particulate air pollution, including during pregnancy (Li et al. 2019; Rao et al. 2018; Romieu et al. 2008). Fetal toxicity may result from the induction of OS either related to systemic effects in the mothers or direct toxic effects when particles are translocated to the placenta and/or fetus (Slama et al. 2008). The fetus is particularly vulnerable to oxidants due to immature antioxidant defenses. Particulate matter-induced OS may result from the direct generation of reactive oxygen species (ROS), altered oxygen-hungry mitochondrial functioning, and inflammatory activation (Li et al. 2019). Notably, our group has previously demonstrated associations between prenatal particulate air pollution exposure and reduced mitochondrial DNA copy number in placenta and cord blood, a recognized biomarker of enhanced OS (Brunst et al. 2018; Kaali et al. 2018).
Overlapping evidence suggests that environmentally-induced OS alters other cellular processes, including telomere biology and telomere length. Telomeres are repeating nucleotide sequences located at the ends of chromosomes that both protect from degradation and regulate cellular function (Turner et al. 2019). The reverse transcriptase telomerase prevents telomere shortening and, while active in early pregnancy, is largely silent in later gestation suggesting that the prenatal period may be a period where telomeres are particularly sensitive to environmental exposures (Ozturk et al. 2014). Telomeres are sensitive to OS given their guanine-rich structure, with increased OS exposure resulting in shorter telomere length (Oikawa and Kawanishi 1999). Leukocyte telomere length shortens with successive cellular divisions and may trigger senescence when very short. Shorter leukocyte telomere length in adulthood has been associated with a number of chronic diseases (e.g., cardiovascular, neurologic) (Rizvi et al. 2014). Telomere length over the lifespan is determined by telomere length at birth and subsequent rate of attrition (Factor-Litvak et al. 2016). In addition to genetic factors and maternal and paternal characteristics such as body mass index or age, emerging evidence suggests that prenatal exposures, such as air pollution, contribute to the telomere length “set-point” at birth, making cord blood telomere length a promising biomarker of environmental risk starting in utero (Bosquet Enlow et al. 2019; Entringer et al. 2018; Hjelmborg et al. 2015; Martens et al. 2017; Rosa et al. 2019).
Diet is a major source of antioxidants (AOs) which may mitigate the effects of chemical toxins that influence health by promoting oxidative stress. Specifically, experimental animal studies demonstrate that dietary antioxidant supplementation can modify the effects of pollutants (Romieu et al. 2008). Human studies have shown that some harmful effects of particulate air pollution, including respiratory and cardiovascular effects in non-pregnant samples, can be modified by the intake of antioxidant nutrients (Liu et al. 2019; Peter et al. 2015; Zeng et al. 2018; Zhong et al. 2017). While studies considering the protective effects of prenatal antioxidant intakes for air pollution effects on children’s health are sparse, supporting evidence from the tobacco literature, specifically a randomized clinical trial demonstrating that maternal antioxidant supplementation during pregnancy can reverse the effect of maternal smoking on infant lung function and wheeze provides proof of concept (McEvoy et al. 2014; McEvoy et al. 2019). One epidemiologic study found that antioxidant intakes in pregnancy reduced the increased risk of neurobehavioral outcomes in young children (Guxens et al. 2012). A recent study in children linked antioxidant intakes with longer telomeres (Garcia-Calzon et al. 2015). A study in Argentina examined associations between various toxic metals and telomere length in cord blood as well as the potential modifying effects of antioxidants (zinc, selenium, folate, and vitamin D3) (Herlin et al. 2019). To our knowledge, there have been no studies that have examined dietary intakes among pregnant women in relation to newborn telomere length nor whether prenatal antioxidant intakes modify effects of PM exposures in utero on telomere biomarkers at birth.
Emerging epidemiological studies link prenatal particulate matter exposure with changes in telomere length at birth (Martens et al. 2017; Perera et al. 2018; Rosa et al. 2019; Song et al. 2019). Maternal particulate matter exposure during pregnancy has been shown to affect fetal development in a sex-differential manner. Sex-specific associations between prenatal maternal air pollution exposure and child asthma and lung function, for example, suggest that while all children are affected, boys may be particularly vulnerable (A Lee et al. 2018; AG Lee et al. 2018). Notably, telomere length may similarly demonstrate sex-differential responses (Bosquet Enlow et al. 2019). Among the handful of studies that have examined associations between PM2.5 exposure during gestation and telomere length at birth, only three have considered sex-specific effects with conflicting results (Martens et al. 2017; Rosa et al. 2019; Song et al. 2019). In a Mexico City prenatal cohort, Rosa et al (Rosa et al. 2019) found that leukocyte telomere length in girls was differentially affected by prenatal PM2.5 exposure. Martens et al (Martens et al. 2017) performed similar analyses in a predominantly white, European cohort and found no sex-specific effects. Conversely, Song et al reported sex-differential effects in a Chinese population, with males more susceptible to the effects of prenatal air pollution exposures. These studies also suggest that both dose and timing of prenatal PM exposure are important. Underlying differences in population and PM2.5 exposure levels may account for the discrepancy in findings.
Leveraging an inner city, ethnically diverse pregnancy cohort, we examined associations among PM2.5, maternal antioxidant intake, and infant sex. We hypothesized that increased exposure to PM2.5 in pregnancy would be associated with reduced leukocyte telomere length in cord blood. We further hypothesized that higher maternal antioxidant intake status would be associated with longer newborn leukocyte telomere length and that higher antioxidant intakes during pregnancy would mitigate the effect of higher PM2.5 exposures on leukocyte telomere length at birth. Finally, given our prior findings focused on another biomarker of OS (mitochondrial DNA copy number) within this cohort demonstrating that boys were more vulnerable to prenatal maternal PM2.5 exposure, we also hypothesized that the effect of PM2.5 would impact boys more than girls (Brunst et al. 2018). In exploratory analyses, we examined the 3-way interaction between prenatal PM2.5 exposure, maternal antioxidant intake, and infant sex.
Materials/Methods
Participants
Analyses included pregnant women enrolled in the PRogramming of Intergenerational Stress Mechanisms (PRISM) study, a prospective pregnancy cohort designed to examine the role of early life environmental exposures, including ambient air pollution, on child development. Beginning in 2011, pregnant women were recruited from Boston and New York City hospitals and affiliated pregnancy clinics during the first or second trimester of pregnancy. Women were eligible if they were: English or Spanish-speaking; 18 years or older at enrollment; and pregnant with a singleton fetus. Exclusion criteria included HIV+ status or self-report of ≥7 alcoholic drinks per week before or any alcohol consumption after pregnancy recognition. Approximately nine months after study recruitment began, additional funding was obtained to collect cord blood. Procedures were approved by human studies committees at the Icahn School of Medicine at Mount Sinai and the Brigham and Women’s Hospital (BWH) in Boston; Beth Israel Deaconess Medical Center relied on BWH oversight. Written informed consent was obtained by all mothers in their primary language.
Daily prenatal PM2.5 exposure
Maternal geocoded addresses, obtained at enrollment and updated for moves over gestation, were used to estimate residence-specific daily PM2.5 exposures over pregnancy. As previously detailed, we regressed daily surface PM2.5 measurements obtained by U.S. Environmental Protection Agency Air Quality System and Interagency Monitoring of Protected Visual Environments Network with daily spectroradiometer satellite-derive aerosol optical depth measurements (1 x 1km spatial resolution), meteorological variables and land-use terms to determine residence-specific daily PM2.5 and temperature exposures (Kloog et al. 2014). The models were calibrated daily and validated with a robust out of sample 10-fold cross-validation (R2 = 0.88) (Kloog et al. 2014). For these analyses, we averaged daily PM2.5 exposures into weekly averages over gestation.
Leukocyte Cord Blood Analyses
We assessed newborn relative leukocyte telomere length (rLTL) using banked cord blood DNA. As has been described elsewhere, cord blood was collected at delivery in EDTA tubes and the buffy coat fraction was isolated and stored at −80°C (Bosquet Enlow et al. 2018). Once all samples were collected, DNA extraction was performed using the Promega Wizard DNA extraction system (Madison, WI, USA). We used Implen NanoPhotometer Pearl (Westlake Village, CA) to assess DNA quantity and quality; values were found to be within acceptable ranges. Quantitative real-time polymerase chain reaction (PCR) was used to measure the ratio of telomeric repeat copy number (T) to a nuclear single copy gene (S, human beta-globulin gene) copy number (T/S ratio) in each sample on a 384-well plate. All samples were measured in triplicate. To control for inter-assay variability, a pooled DNA sample was included in each run (See Bosquet Enlow et al 2018, Supplementary material Telomere Assaying Procedures for more information) and showed little variation (median 0.96, IQR 0.96-1.05) across plates. The coefficient of variation for the average T: S ratio of samples analyzed over 3 consecutive days was 8.7%, similar to the reproducibility originally reported for this method. For each plate, we divided the T/S ratio of each pooled DNA sample by the average T/S ratio for all pooled samples across all plates to obtain a normalizing factor. We then divided samples on a given plate by the plate-specific normalizing factor to adjust for potential batch effects (Lin et al. 2010). Upon initial examination of the distribution of rLTL, plate 7 results appeared skewed compared to the others (n=2 samples) and one outlier was noted (value >3 SD above mean), thus these three samples were excluded from further analyses.
Maternal Antioxidant Dietary Intake
We assessed maternal dietary and supplement antioxidant intakes over the prior three months during the second trimester, using the modified Block98 Food Frequency Questionnaire (FFQ) (Boucher et al. 2006). The Block98 FFQ consists of approximately 120 food and beverage items and was based on the National Health and Nutrition Examination Survey III (NHANES III) dietary recall data and modified to include additional fish and seafood items (Siega-Riz et al. 2002). The measure has been validated in multi-ethnic populations, including in the PRISM study (Brunst et al. 2016). The Block98 FFQ was administered by trained, bilingual research staff in English or Spanish and ascertained the quantity (small, medium, or large serving with portion size pictorially presented) and frequency (daily, weekly, monthly, rarely/never) of each food or beverage item reported. The FFQ also assessed dietary supplement intake, including prenatal and regular vitamins, minerals and omega-3 supplements.
Each item reported on the FFQ, including frequency and portion size, was processed using the online Block Dietary Data Systems (Berkeley, CA) to calculate daily energy and macro- and micronutrient intakes using NHANES and United States Department of Agriculture (USDA) Nutrient Database for Standard Reference (NDSR) (Block et al. 1986; Block 2001; Laboratory 1998). Micronutrient data including antioxidant intakes were validated using 24-hour dietary recall within a subset of 42 participants (Brunst et al. 2016). For these analyses, we first calculated the energy-adjusted intakes of antioxidant micronutrients including β-carotene, vitamins A, C, and E. and trace minerals including magnesium, selenium, and zinc. Total antioxidant micronutrient intakes were then calculated by combining dietary and supplement intakes. Finally, a composite antioxidant intake measure was derived by averaging each participant’s relative rank across the 7 micronutrients evaluated. Higher scores reflect higher total antioxidant intakes.
Covariates
We used Directed Acyclic Graph (DAG) theory to select covariates for adjustment (Greenland et al. 1999). Based on the dependencies encoded by our DAG, we adjusted models for maternal age at delivery (continuous), self-reported ethnicity (Black vs Hispanic vs White vs other), marital status (single vs married or living with partner) and education level (less than high school vs high school or higher) which were determined by questionnaires at time of enrollment. Prior research by our group found that cumulative lifetime maternal traumatic stress was associated with decreased rLTL (Bosquet Enlow et al. 2018) which we conceptualized as a precision variable; adjusting for these variables did not open any back door paths based on our conceptual models. Lifetime exposure to traumatic and non-traumatic stressful events was ascertained using the Life Stressor Checklist-Revised (LSC-R) (Wolfe and Kimerling 1997). Child sex was recorded at delivery. We are interested in the total effect of PM2.5 on telomere length, therefore we did not adjust models for newborn gestational length, which we conceptualized as potentially on the pathway between PM2.5 and cord blood telomere length based on prior research. Additionally, to maintain equal exposure time between newborns, we subset analyses on term births (>37 weeks), thereby reducing inter-individual variability in gestational length.
Statistical Analyses
These analyses included N=155 mother-infant dyads with complete data on maternal prenatal PM2.5 exposures, dietary intake and cord blood rLTL. We took the following stepwise approach to examining associations among PM2.5 exposure over gestation, maternal antioxidant intake, and infant sex. Correlation analysis was used to examine the relationship between maternal antioxidant intakes and cord blood rLTL. We used Bayesian distributed lag interaction models (BDLIMs) to estimate time-varying associations and identify sensitive windows between weekly prenatal PM2.5 exposures across gestation and rLTL. As previously described (Wilson et al. 2017b), the BDLIM extends distributed lag models to account for within window effects and to test for effect modification, in addition to identifying windows of susceptibility and estimating a cumulative effect over gestation (Gasparrini 2014). When examining interactions, the BDLIM partitions the distributed lag function into weights to identify the sensitive windows and coefficients to identify the magnitude of within-window effects. The model is then able to identify whether the groups, herein antioxidant status, sex and/or sex-by-antioxidant status, have the same or different sensitive windows and magnitude of the within-window effect. The distribution of maternal antioxidant status was skewed (Median 56.36, IQR 37.68-70.96), therefore, we a priori dichotomized maternal antioxidant status around the median into high versus low categories to test effect modification.
First, we assessed the main effect of PM2.5 exposure on newborn rLTL. We employed BDLIM to identify sensitive windows of exposure and estimate the cumulative effect of prenatal PM2.5 on newborn rLTL over gestation in the sample as a whole. The cumulative effect estimate is the expected change in rLTL associated with a 1-unit increase in exposure simultaneously at each week of pregnancy. The main effect model was adjusted for maternal age, self-reported ethnicity, marital status, education level, trauma as indexed by LSC-R, maternal antioxidant intake and infant sex. We next examined effect modification by maternal antioxidant intake and infant sex considered in separate 2-way interaction models. Models examining effect modification by maternal antioxidant intake status were adjusted for the same covariates. Finally, we explored the 3-way interaction between PM2.5 exposure, maternal antioxidant status, and infant sex. For all models, we examined model fit and optimal number of knots and pattern (i.e., best fitting weights and effects) using the deviance information criteria (DIC) and model stability by fitting models with varying iterations, burn in and thinning criteria (Spiegelhalter 2002). Sensitivity models performed additional adjustment for average temperature over pregnancy and storage time. Analyses were implemented in R Studio statistical software (v1.2.1335-1). BLDIMs were performed using the regimes package.
Results
Participant characteristics are reported in Table 1. Mothers were largely ethnic minorities (39% Black, 12% Hispanic) with 13% reporting less than a high school degree. Average prenatal PM2.5 exposure over pregnancy was 8.8 ± 0.8 μg/m3 (IQR 8.2-9.2) and average newborn rLTL was 2.4 ± 0.9. The median time from cord blood collection to telomere assay was 1.9 years (IQR 1.4-2.3). Mothers reporting high total antioxidant intakes (N=76) as compared to low total antioxidant intakes (N=76) were more likely to be married [N=58 (76%) vs N=32 (42%)], older [32 years (4.4SD) vs 29 years (5.6SD)], have a high school or higher education [72 (95%) vs 61 (80%)], report lower lifetime stress [LSC-R 9.2 (9.2SD) vs 14 (12.3SD)], and gave birth to newborns with longer rLTL [2.6 (0.9SD) vs 2.1 (0.9)], respectively. Maternal antioxidant intakes were linearly related to newborn rLTL (Pearson r=0.25, p=0.002, Figure 1). No differences in average prenatal PM2.5 exposure or infant sex were seen by maternal total antioxidant intake status. There were no significant differences in covariates or rLTL by infant sex.
Table 1.
Sample characteristics
| All infants N=152 | Maternal Antioxidant Intakes * |
||
|---|---|---|---|
| Low N=76 (50%) | High N=76 (50%) | ||
| Continuous Variables [mean (sd)] | |||
| Maternal Age (years) | 30.7 (5.2) | 29.3 (5.6) | 32.1 (4.4) |
| Maternal trauma (LSC-R) † | 11.6 (11.1) | 14 (12.3) | 9.2 (9.2) |
| Maternal prenatal PM2.5 exposure (μg/m3) | 8.8 (0.8) | 8.8 (0.9) | 8.7 (0.7) |
| Newborn leukocyte telomere length ± | 2.4 (0.9) | 2.1 (0.9) | 2.6 (0.9) |
| Categorical Variables [N(%)] | |||
| Infant Sex, Male | 72 (47.4%) | 36 (47.4%) | 36 (47.4%) |
| Ethnicity | |||
| White | 58 (38.2) | 16 (21.1) | 42 (55.3) |
| Black | 59 (38.8) | 39 (51.3) | 20 (26.3) |
| Hispanic | 18 (11.8) | 14 (18.4) | 4 (5.3) |
| Other | 17 (11.2) | 7 (9.2) | 10 (13.2) |
| Maternal education | |||
| < High School | 19 (12.5) | 15 (19.7) | 4 (5.3) |
| ≥ High School | 133 (87.5) | 61 (80.3) | 72 (94.7) |
| Maternal marital status | |||
| Married | 90 (59.2) | 32 (42.1) | 58 (76.3) |
| Living with partner | 24 (15.8) | 18 (23.7) | 6 (7.9) |
| Single | 19 (12.5) | 14 (18.4) | 5 (6.6) |
| Other (Divorced, separated, widowed) | 19 (12.5) | 12 (15.8) | 7 (9.2) |
Ranked composite of energy-adjusted intake of dietary and supplemental antioxidant micronutrients, determined by the Block98 FFQ
Life Stressor Checklist-Revised (LSC-R) measured self-reported lifetime exposure to traumatic events
T/S ratio normalized against plate pool average
Figure 1.

Scatterplot of the relationship between maternal total antioxidant intakes and newborn rLTL.
Main effect of prenatal PM2.5 exposure on newborn rLTL
In the sample as a whole, BDLIMs identified a sensitive window of exposure (12-20 weeks gestation) during which children born to mothers exposed to higher PM2.5 had lower rLTL at birth, after adjusting for maternal age, self-reported race/ethnicity, marital status, education level, lifetime stressors as indexed by the LSC-R, antioxidant intakes during pregnancy and child sex (Figure 2). The estimated cumulative effect of PM2.5 over pregnancy on newborn rLTL was also significant (cumulative effect estimate = −0.29 per 1μg/m3 increase in PM2.5, 95% CI −0.49 to −0.10). A sensitivity model additionally adjusting for average temperature over pregnancy and storage time identified a similar sensitive window of exposure (7-18 weeks gestation) and cumulative effect (cumulative effect estimate = −0.27 per 1μg/m3 increase in PM2.5, 95% CI −0.49 to −0.04).
Figure 2. Associations between weekly PM2.5 levels over gestation and newborn relative leukocyte telomere length (rLTL).
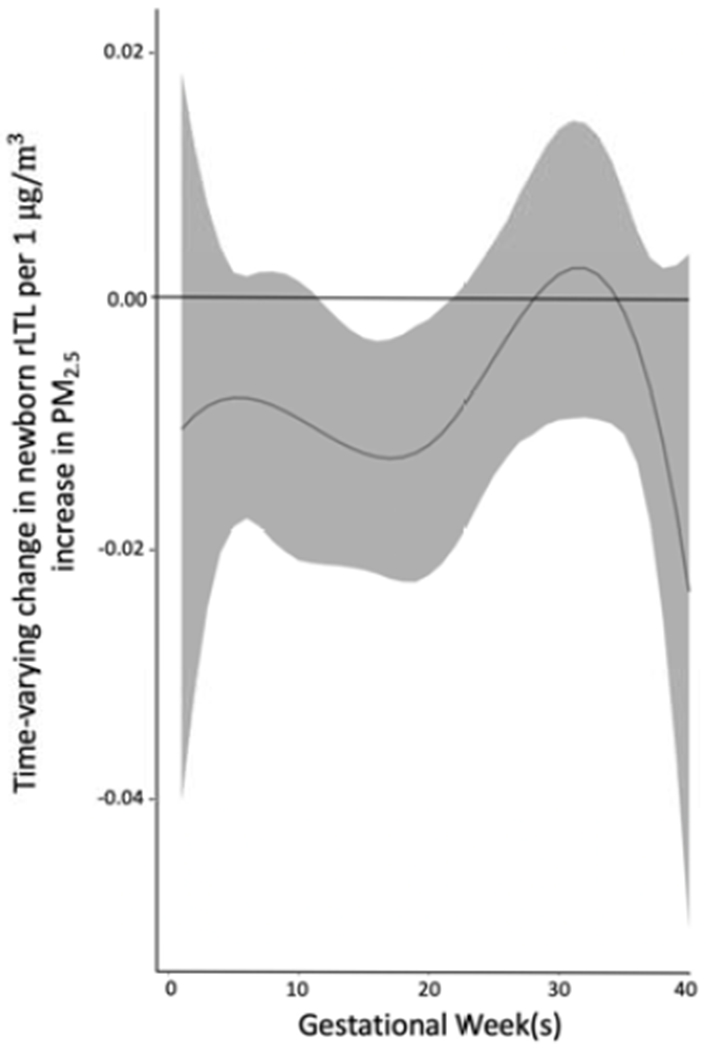
This figure demonstrates the association between PM2.5 exposures over pregnancy and rLTL using a BDLIM assuming week-specific effects for the overall sample. The model was adjusted for maternal age, self-reported ethnicity, marital status, education level, maternal lifetime stress, antioxidant intake and infant sex. The y-axis represents the change in rLTL per 1 μg/m3 increase in PM2.5. The x-axis represents gestational age in weeks. The solid line shows the estimated change in rLTL while gray areas indicate 95% credible intervals (CIs). A sensitive window is identified for weeks 12-20 of gestation, where the estimated pointwise 95% CI does not include zero. BDLIMs also identified a cumulative estimated effect of PM2.5 over pregnancy on rLTL of −0.29 (95% CI −0.49 to −0.10) per 1μg/m3 increase in PM2.5.
Maternal antioxidant intake-specific effects of prenatal PM2.5 on newborn telomere rLTL
When examining effect modification by maternal antioxidant intake, BDLIMs did not identify a sensitive window of exposure for high or low maternal antioxidant intake although the shape of the curve in children born to mothers reporting high antioxidant intake was relatively flat while that of low antioxidant intake was suggestive of a mid-gestation effect. BDLIMs did identify an estimated cumulative effect of PM2.5 over pregnancy on newborn rLTL in children born to mothers with low antioxidant intake [cumulative effect estimate: low maternal antioxidant intake = −0.31 (95% CI −0.55 to −0.06) vs high maternal antioxidant intake = −0.07 (95% CI −0.34 to 0.17) per 1μg/m3 increase in PM2.5] (Figure 3). Sensitivity models additionally adjusting for average temperature over pregnancy and storage time did not substantively change these findings [cumulative effect estimate: low maternal antioxidant intake = −0.27 (95% CI −0.52 to −0.03) vs high maternal antioxidant intake = −0.05 (95% CI −0.31 to 0.19) per 1μg/m3 increase in PM2.5].
Figure 3. Maternal antioxidant intake-specific associations between PM2.5 exposure over gestation and newborn relative telomere length (rLTL).

This figure demonstrates the maternal antioxidant intake-specific associations between PM2.5 exposures over pregnancy and rLTL using a BDLIM assuming week-specific effects for children born to mothers reporting low versus high antioxidant intake during pregnancy. Models were adjusted for maternal age, self-reported ethnicity, marital status, education level, LSC-R, and infant sex. Panel A demonstrates the cumulative effect on newborn rLTL per 1 μg/m3 increase in PM2.5 by maternal antioxidant intake [cumulative effect estimate: low maternal antioxidant intake = −0.31 (95% CI −0.55 to −0.06) vs high maternal antioxidant intake = −0.07 (95% CI −0.34 to 0.17) per 1μg/m3 increase in PM2.5.5]. In panel B, the y-axis represents the change in rLTL per 1 μg/m3 increase in PM2.5. The x-axis represents gestational age in weeks. The solid line shows the estimated change in rLTL while gray areas indicate 95% credible intervals (CIs). No sensitive window is identified.
Sex-specific effects of prenatal PM2.5 on newborn rLTL
When examining effect modification by child sex, BDLIMs found a similarly shaped curve in both boys and girls however only identified a sensitive window of exposure in boys. Specifically, the model suggests that boys born to mothers exposed to higher PM2.5 during 16 to 19 weeks gestation had lower rLTL at birth, after adjusting for maternal age, self-reported race/ethnicity, marital status, education level, lifetime stressors, and prenatal antioxidant intakes (Figure 4). The estimated cumulative effect of PM2.5 over pregnancy on newborn rLTL also suggested a larger effect in boys [cumulative effect estimates: boys = −0.30 (95% CI −0.58 to −0.01) vs girls = −0.15 (95% CI −0.40 to 0.05) per 1μg/m3 increase in PM2.5].
Figure 4. Sex-specific associations between weekly PM2.5 levels over gestation and newborn relative telomere length (rLTL).
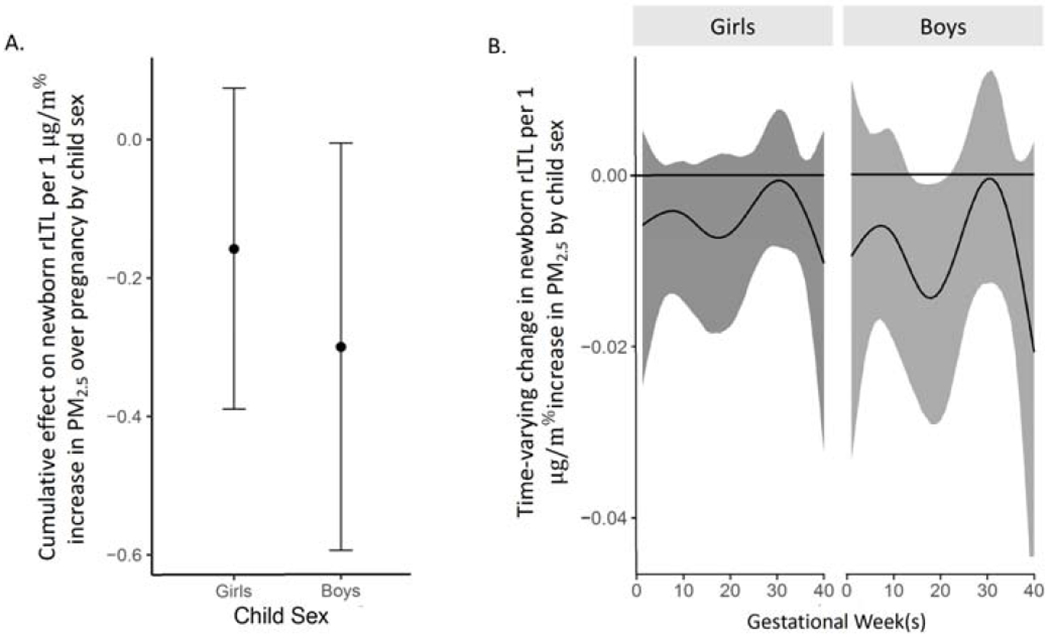
This figure demonstrates the sex-specific associations between PM2.5 exposures over pregnancy and rLTL using a BDLIM assuming week-specific effects for the girls and boys. Models were adjusted for maternal age, self-reported ethnicity, marital status, education level, LSC-R, and antioxidant intake. Panel A demonstrates the cumulative effect on newborn rLTL per 1 μg/m3 increase in PM2.5 by infant sex [cumulative effect estimate: girls = −0.15 (95% CI −0.40 to −0.05) vs boys = −0.30 (95% CI −0.58 to −0.01) per 1μg/m3 increase in PM2.5.5]. In panel B, the y-axis represents the change in rLTL per 1 μg/m3 increase in PM2.5. The x-axis represents gestational age in weeks, for girls and boys separately. The solid line shows the estimated change in rLTL while gray areas indicate 95% credible intervals (CIs). No sensitive window is identified in girls whereas a sensitive window is identified in boys from 16-19 weeks gestation where the estimated pointwise 95% CI does not include zero.
Sex- and maternal antioxidant intake-specific effects of prenatal PM2.5 on newborn rLTL
We next explored a 3-way interaction between prenatal PM2.5 exposure, maternal prenatal total antioxidant intake, and infant sex in relation to newborn rLTL. Following adjustment for the same covariates, this model did not identify a sensitive window of exposure between prenatal PM2.5 and newborn rLTL regardless of child sex or maternal antioxidant intake status. However, the estimated cumulative effect of PM2.5 over pregnancy on newborn rLTL was significantly reduced in boys whose mothers reported low antioxidant intakes compared with boys born to mothers reporting high antioxidant intakes [among boys, cumulative effect estimates: low maternal AO intakes = −0.38 (95% CI −0.80 to −0.004) vs high AO intakes = −0.04 (95% CI −0.45 to 0.33) per 1μg/m3 increase in PM2.5, Figure 4]. Similarly, in girls whose mothers reported low antioxidant intakes, rLTL was lower than in girls whose mothers reported high antioxidant intakes, however this effect did not reach statistical significance [among girls, cumulative effect estimates: low maternal AO intake = −0.26 (95% CI −0.59 to 0.02) vs high = 0.02 (−0.26 to 0.30) per 1μg/m3 increase in PM2.5, Figure 4]. Sensitivity analyses additionally adjusting for maternal BMI, storage time and average temperature over pregnancy did not substantively change these findings.
Conclusions
This study provides evidence that maternal antioxidant intakes during pregnancy may ameliorate the effects of prenatal air pollution exposure on newborn telomere length. Infants born to mothers with increased PM2.5 exposures over gestation, and specifically during mid-gestation, had shorter telomeres with those born to mothers reporting low antioxidant intake during pregnancy being most vulnerable. In models examining sex-specific effects of particulate air pollution exposure on cord blood rLTL suggested that boys were more impacted. Exploratory analyses examining sex- and antioxidant-specific effects of the association between PM2.5 and newborn rLTL showed a similar pattern in both boys and girls, with those born to mothers reporting low antioxidant intake having shorter rLTL compared to their counterparts. The suggested cumulative effect was more evident among boys.
Use of advanced biostatistical methods enabled us to identify sensitive windows of prenatal air pollution exposure on newborn telomere length, as compared to averaging exposure across gestation or trimester which could miss temporal associations or bias effect estimates (Wilson et al. 2017a). For all children, the identified window of susceptibility of 12-20 weeks gestation overlaps with significant windows of susceptibility to maternal air pollution exposures on various child health outcomes reported in previous research. For example, work by our group found that increased maternal PM2.5 exposure during midgestation was associated with body composition by age 4 years, neurodevelopment by age 6 years, and asthma by age 7 years, also demonstrating sex-specific effects (Chiu et al. 2016; Chiu et al. 2017; Hsu et al. 2015). Additional evidence suggests associations between mid-gestation air pollution exposures and measures of fetal growth and asthma by 3.5 years (Jung et al. 2019; Lamichhane et al. 2018). Within an individual, telomere length is proportional across cell types and cells with shortened telomere length may induce release of proteases, inflammatory cytokines, and growth factors which act on neighboring cells and activate the immune system, thus predisposing to future disease (Daniali et al. 2013; Turner et al. 2019).
Main effects analyses corroborated findings linking increased exposure to PM cumulatively over pregnancy on shortened leukocyte telomere length at birth (Martens et al. 2017; Perera et al. 2018; Rosa et al. 2019; Song et al. 2019). Other work using biomarkers of prenatal exposure at a single time point, for example polycyclic aromatic hydrocarbons-DNA adducts, has also demonstrated inverse associations between prenatal air pollution and newborn telomere length (Perera et al. 2018). Notably, the implementation of distributed lag models identified different windows of vulnerability compared to other published research. For example, recent work by Rosa et al leveraged the PROGRESS birth cohort and BDLIMs to demonstrate that increased PM2.5 exposure in early pregnancy, specifically 4-9 weeks gestation, was associated with shortened newborn telomere length whereas increased PM2.5 exposure in later pregnancy, specifically 14-19 and 34-36 weeks, was associated with elongated telomere length at birth (Rosa et al. 2019). Notably this Mexico City-based cohort had higher prenatal PM2.5 exposures (median average prenatal PM2.5 22.8 μg/m3) than estimated in our study sample from the northeastern United States. Distributed lag models were also employed in the ENVIRONAGE birth cohort, a primarily white European sample with prenatal PM2.5 exposures again higher than in the PRISM cohort (average prenatal PM2.5 13.4 μg/m3), and demonstrated that higher PM2.5 exposures during mid-gestation (12-25 weeks) and late gestation (32-34 weeks) were associated with shortened and elongated cord blood telomere length, respectively (Martens et al. 2017). Heterogeneity in identifying the window of vulnerability across these studies may in part be explained by differences in the underlying populations (e.g., genetic differences), range of air pollution exposure, or distribution of other covariates.
While the underlying mechanisms contributing to the effects of particulate air pollution on telomere biology beginning in pregnancy are not clear, overlapping research highlights the importance of oxidative stress. Air pollution, and specifically particulate matter, exposure may lead to a pro-oxidant and anti-oxidant imbalance inducing oxidative stress and triggering a cascade of redox sensitive pathways. Oxidative stress induces site-specific damage in telomeres at the triple guanine nucleotide sequence for which repair is slow and incomplete (Oikawa and Kawanishi 1999; Petersen et al. 1998).
These findings also extend research considering the potential beneficial effects of prenatal dietary antioxidant intakes to reduce effects of particulate air pollution on fetal development. Our analyses suggest that the effect of prenatal PM2.5 exposure on newborn telomere length was ameliorated in children born to mothers reporting higher antioxidant intakes during pregnancy. These findings are in line with prior animal research suggesting a protective effect of dietary antioxidant intakes on the adverse health effects of air pollution exposure; human studies are largely focused on adults or in children are cross-sectional and therefore do not extend to the prenatal period (Romieu et al. 2008). In adults, dietary intake of antioxidants has been found to be protective against telomere shortening (Freitas-Simoes et al. 2016). Cross-sectional studies in children similarly show a positive relationship between dietary antioxidant intakes and telomere length (Garcia-Calzon et al. 2015). Maternal antioxidant intake during pregnancy may improve neurodevelopment outcomes (Guxens et al. 2012). This is the first study implicating maternal antioxidant intakes in pregnancy contribute to the telomere length set point at birth. Moreover, if our findings are replicated in future studies, these results suggest that nutritional interventions enhancing antioxidant intakes/supplementation may reduce the effects of air pollution exposures on fetal development.
Our data suggest that, while all children are affected, boys may be more vulnerable to the effects of prenatal PM2.5 exposure. Prior research investigating the relationship between prenatal air pollution exposures and child health outcomes has demonstrated sex differential effects, although findings have been mixed. For example, girls in the PROGRESS cohort were more vulnerable to the effects of prenatal PM2.5 on newborn telomere length whereas no sex-specific effect was found in the ENVIRONAGE cohort. Due to sample size restraints, sex-specific effects were not examined by Perrera et al(Martens et al. 2017; Perera et al. 2018; Rosa et al. 2019). Adult males also appear more vulnerable to the effects of increased air pollution, specifically black carbon, on telomere length (Ward-Caviness et al. 2016). Oxidative stress may operate in sex-differential ways. For example, sex hormones including estrogen and testosterone can interact with reactive oxygen species. Estrogens themselves have antioxidant properties and can induce upregulation of antioxidant genes via mitogen-activated protein (MAP) kinase and nuclear factor kappa B (Ruiz-Larrea et al. 1997; Vina et al. 2005). Testosterone, conversely, has no antioxidant properties (Alonso-Alvarez et al. 2007). Testosterone has also been shown to generate oxidative stress in a number of tissues including the placenta, thereby suggesting that sex steroids may modify in utero oxidative stress effects on placental function and fetal development (Zhu et al. 1997).
Notably, a number of maternal environmental exposures, including chemical and non-chemical exposures thought to likely operate through similar mechanistic pathways (i.e., enhanced oxidative stress), have been found to have a stronger effect on newborn telomere length in males (Herlin et al. 2019). Within PRISM, Enlow et al previously reported that maternal smoking, higher body mass index, and depressive symptoms during pregnancy or sexual abuse experienced by mothers in her own childhood were associated with shortened newborn telomere length among boys while higher maternal education and household income were protective; none of these factors were predictive of telomere length in girls (Bosquet Enlow et al. 2018). While exploratory analyses in this sample suggested that higher antioxidant intake in pregnancy mitigated the effect of particulate air pollution on reduced telomere length at in both male and female newborns, the impact was more evident among boys. However, these findings need to be interpreted with caution given the relatively small sample size in the examined subgroups. Further work is needed to elucidate the sex-specific effects of prenatal PM2.5 exposure on newborn rLTL relative to maternal antioxidant intake during pregnancy.
Our study has several strengths. First, we leverage a prospective cohort of ethnically and socioeconomically diverse mother-infant dyads at increased risk for exposure to ambient air pollution. We assessed daily air pollution exposures over pregnancy using a validated spatiotemporal model and maternal nutrient intakes using a validated FFQ. Cord blood was collected at delivery and rLTL was analyzed using quantitative real-time PCR. The applied data-driven statistical methods allowed us to identify sensitive windows of exposure effects and cumulative effects in pregnancy as well as enhanced the power to determine effect modification by maternal antioxidant intake status and infant sex. Our well-characterized cohort allowed for the adjustment of a number of important covariates. As we continue to follow our cohort as they age, it will be important to examine whether newborn telomere length may serve as an early biomarker of future child health outcomes.
We also acknowledge limitations. Our air pollution model leverages remote-sensing satellite and land use data and therefore does not account for indoor sources of pollution, such as cooking or tobacco smoke. However, we note that prior studies demonstrate a lack of correlation between indoor and ambient particles (Wilson et al. 2000), suggesting that this would not confound associations between ambient PM2.5 and newborn telomere length. We also note that currently BDLIMs only allow investigation of interaction by a categorical variable and therefore we are not able to explore interactions with continuous measure of antioxidant intake. Notably, sensitivity analyses using other cutoffs (i.e., at or above the 75th percentile) did not substantively change our findings. We cannot rule out potential confounding by unmeasured or unknown factors. We do not have cell count and thus our relative measure reflects the average telomere length across all chromosomes in all cells sampled for a given individual. A larger sample size may have allowed us to detect sensitive windows of exposure in addition to differences in cumulative effect over gestation when assessing higher level interactions, in particular sex differences in antioxidant intake status-specific effects on the link between prenatal PM2.5 and newborn rLTL. Finally, findings from our ethnically mixed sample from the northeastern US may not be broadly generalizable.
In summary, these findings add to a growing literature linking increased ambient PM2.5 exposure during gestation to shortened telomere length at birth. In our sample, infants born to mothers reporting low antioxidant intakes during pregnancy were most vulnerable to PM2.5 effects. Our findings suggesting that higher antioxidant intakes in pregnant women may mitigate the effects of PM2.5 may inform future prevention strategies to optimize fetal development and thus warrant replication.
Supplementary Material
Figure 5. Sex- and maternal antioxidant (AO) intake-specific associations between cumulative prenatal PM2.5 levels and newborn relative telomere length (rLTL).
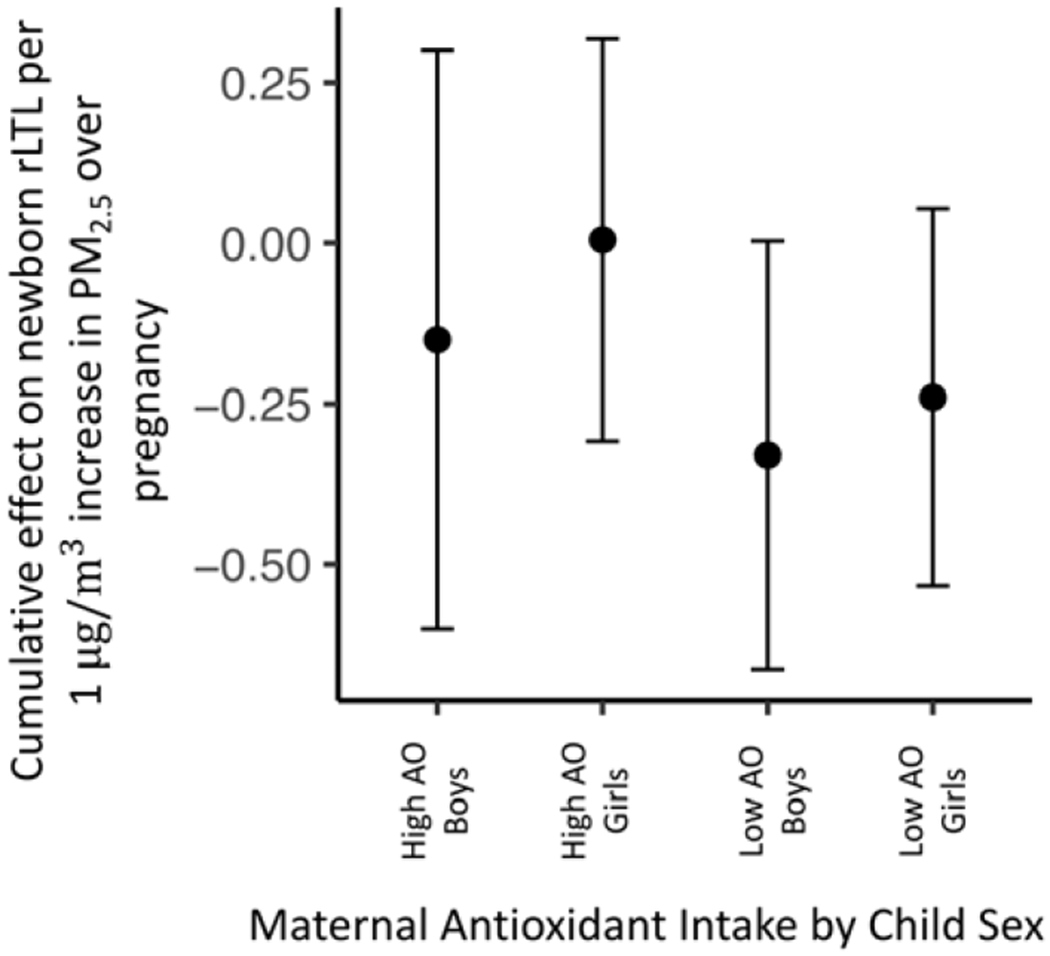
This figure demonstrates the group-specific cumulative estimated effect and 95% credible intervals (CIs) between PM2.5 exposures over pregnancy and rLTL using a BDLIM, specifically for girls and boys by maternal total AO intakes dichotomized around the median. Models were adjusted for maternal age, selfreported race/ethnicity, marital status, education level and lifetime stress. By group, the cumulative estimated effects were: boys with high maternal AO intake (N=36) = −0.04 (95% CI −0.45 to 0.33); girls with high maternal AO intake (N=40) = 0.02 (−0.26 to 0.30); boys with low maternal AO intake (N=36) = −0.38 (95% CI −0.80 to −0.004); and girls with low maternal AO intake (N=40) = −0.26 (95% CI −0.59 to 0.02), per 1μg/m3 increase in PM2.5.
Highlights.
Examination of associations between prenatal fine particulate matter exposure and newborn relative leukocyte telomere length suggested that infants born to mothers exposed to higher levels of fine particulate matter in mid-gestation had shorter telomeres.
When considering effect modification by maternal antioxidant intake during pregnancy, infants born to mothers reporting low antioxidant intake were most vulnerable to these effects.
Exploratory analyses examining joint effects of maternal antioxidant intake during pregnancy and infant sex found that that boys born to mothers reporting low antioxidant intakes were most vulnerable.
Acknowledgments
Funding: R01 HL095606, R01 HL114396, P30 ES023515 from the National Institutes of Health; during preparation of this manuscript AGL was supported by K23 HL135349 and WC was supported by T32 HD049311.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Alonso-Alvarez C, Bertrand S, Faivre B, Chastel O, Sorci G. 2007. Testosterone and oxidative stress: The oxidation handicap hypothesis. Proc Biol Sci 274:819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. 1986. A data-based approach to diet questionnaire design and testing. Am J Epidemiol 124:453–469. [DOI] [PubMed] [Google Scholar]
- Block G 2001. Invited commentary: Another perspective on food frequency questionnaires. Am J Epidemiol 154:1103–1104; discussion 1105-1106. [DOI] [PubMed] [Google Scholar]
- Block ML, Elder A, Auten RL, Bilbo SD, Chen H, Chen JC, et al. 2012. The outdoor air pollution and brain health workshop. Neurotoxicology 33:972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosquet Enlow M, Bollati V, Sideridis G, Flom JD, Hoxha M, Hacker MR, et al. 2018. Sex differences in effects of maternal risk and protective factors in childhood and pregnancy on newborn telomere length. Psychoneuroendocrinology 95:74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosquet Enlow M, Sideridis G, Bollati V, Hoxha M, Hacker MR, Wright RJ. 2019. Maternal cortisol output in pregnancy and newborn telomere length: Evidence for sex-specific effects. Psychoneuroendocrinology 102:225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher B, Cotterchio M, Kreiger N, Nadalin V, Block T, Block G. 2006. Validity and reliability of the block98 food-frequency questionnaire in a sample of canadian women. Public health nutrition 9:84–93. [DOI] [PubMed] [Google Scholar]
- Brunst KJ, Kannan S, Ni YM, Gennings C, Ganguri HB, Wright RJ. 2016. Validation of a food frequency questionnaire for estimating micronutrient intakes in an urban us sample of multiethnic pregnant women. Maternal and child health journal 20:250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunst KJ, Sanchez-Guerra M, Chiu YM, Wilson A, Coull BA, Kloog I, et al. 2018. Prenatal particulate matter exposure and mitochondrial dysfunction at the maternal-fetal interface: Effect modification by maternal lifetime trauma and child sex. Environ Int 112:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Hsu HH, Coull BA, Bellinger DC, Kloog I, Schwartz J, et al. 2016. Prenatal particulate air pollution and neurodevelopment in urban children: Examining sensitive windows and sex-specific associations. Environ Int 87:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YM, Hsu HL, Wilson A, Coull BA, Pendo MP, Baccarelli A, et al. 2017. Prenatal particulate air pollution exposure and body composition in urban preschool children: Examining sensitive windows and sex-specific associations. Environ Res 158:798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniali L, Benetos A, Susser E, Kark JD, Labat C, Kimura M, et al. 2013. Telomeres shorten at equivalent rates in somatic tissues of adults. Nature communications 4:1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, de Punder K, Buss C, Wadhwa PD. 2018. The fetal programming of telomere biology hypothesis: An update. Philos Trans R Soc Lond B Biol Sci 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Factor-Litvak P, Susser E, Kezios K, McKeague I, Kark JD, Hoffman M, et al. 2016. Leukocyte telomere length in newborns: Implications for the role of telomeres in human disease. Pediatrics 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch AF, Luttmann-Gibson H, Perng W, Rifas-Shiman SL, Coull BA, Kloog I, et al. 2017. Prenatal and early life exposure to traffic pollution and cardiometabolic health in childhood. Pediatric obesity 12:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas-Simoes TM, Ros E, Sala-Vila A. 2016. Nutrients, foods, dietary patterns and telomere length: Update of epidemiological studies and randomized trials. Metabolism 65:406–415. [DOI] [PubMed] [Google Scholar]
- Garcia-Calzon S, Moleres A, Martinez-Gonzalez MA, Martinez JA, Zalba G, Marti A, et al. 2015. Dietary total antioxidant capacity is associated with leukocyte telomere length in a children and adolescent population. Clin Nutr 34:694–699. [DOI] [PubMed] [Google Scholar]
- Gasparrini A 2014. Modeling exposure-lag-response associations with distributed lag nonlinear models. Statistics in medicine 33:881–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S, Pearl J, Robins JM. 1999. Causal diagrams for epidemiologic research. Epidemiology (Cambridge, Mass) 10:37–48. [PubMed] [Google Scholar]
- Guxens M, Aguilera I, Ballester F, Estarlich M, Fernandez-Somoano A, Lertxundi A, et al. 2012. Prenatal exposure to residential air pollution and infant mental development: Modulation by antioxidants and detoxification factors. Environ Health Perspect 120:144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlin M, Broberg K, Igra AM, Li H, Harari F, Vahter M. 2019. Exploring telomere length in mother-newborn pairs in relation to exposure to multiple toxic metals and potential modifying effects by nutritional factors. BMC medicine 17:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmborg JB, Dalgard C, Moller S, Steenstrup T, Kimura M, Christensen K, et al. 2015. The heritability of leucocyte telomere length dynamics. J Med Genet 52:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HH, Chiu YH, Coull BA, Kloog I, Schwartz J, Lee A, et al. 2015. Prenatal particulate air pollution and asthma onset in urban children. Identifying sensitive windows and sex differences. American journal of respiratory and critical care medicine 192:1052–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CR, Chen WT, Tang YH, Hwang BF. 2019. Fine particulate matter exposure during pregnancy and infancy and incident asthma. The Journal of allergy and clinical immunology. [DOI] [PubMed] [Google Scholar]
- Kaali S, Jack D, Delimini R, Hu L, Burkart K, Opoku-Mensah J, et al. 2018. Prenatal household air pollution alters cord blood mononuclear cell mitochondrial DNA copy number: Sex-specific associations. International journal of environmental research and public health 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog I, Chudnovsky AA, Just AC, Nordio F, Koutrakis P, Coull BA, et al. 2014. A new hybrid spatio-temporal model for estimating daily multi-year pm2.5 concentrations across northeastern USA using high resolution aerosol optical depth data. Atmos Environ (1994) 95:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korten I, Ramsey K, Latzin P. 2017. Air pollution during pregnancy and lung development in the child. Paediatric respiratory reviews 21:38–46. [DOI] [PubMed] [Google Scholar]
- Laboratory ND. 1998. Usda national nutrient database for standard reference. Beltsville, MD:Department of Agriculture. [Google Scholar]
- Lamichhane DK, Ryu J, Leem JH, Ha M, Hong YC, Park H, et al. 2018. Air pollution exposure during pregnancy and ultrasound and birth measures of fetal growth: A prospective cohort study in korea. Sci Total Environ 619-620:834–841. [DOI] [PubMed] [Google Scholar]
- Lee A, Leon Hsu HH, Mathilda Chiu YH, Bose S, Rosa MJ, Kloog I, et al. 2018. Prenatal fine particulate exposure and early childhood asthma: Effect of maternal stress and fetal sex. The Journal of allergy and clinical immunology 141:1880–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AG, Le Grand B, Hsu HL, Chiu YM, Brennan KJ, Bose S, et al. 2018. Prenatal fine particulate exposure associated with reduced childhood lung function and nasal epithelia gstp1 hypermethylation: Sex-specific effects. Respiratory research 19:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Tang Y, Song X, Lazar L, Li Z, Zhao J. 2019. Impact of ambient pm2.5 on adverse birth outcome and potential molecular mechanism. Ecotoxicol Environ Saf 169:248–254. [DOI] [PubMed] [Google Scholar]
- Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M, et al. 2010. Analyses and comparisons of telomerase activity and telomere length in human t and b cells: Insights for epidemiology of telomere maintenance. J Immunol Methods 352:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang Y, Yang X. 2019. Vitamin e reduces the extent of mouse brain damage induced by combined exposure to formaldehyde and pm2.5. Ecotoxicol Environ Saf 172:33–39. [DOI] [PubMed] [Google Scholar]
- Martens DS, Cox B, Janssen BG, Clemente DBP, Gasparrini A, Vanpoucke C, et al. 2017. Prenatal air pollution and newborns’ predisposition to accelerated biological aging. JAMA Pediatr 171:1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy CT, Schilling D, Clay N, Jackson K, Go MD, Spitale P, et al. 2014. Vitamin c supplementation for pregnant smoking women and pulmonary function in their newborn infants: A randomized clinical trial. JAMA 311:2074–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy CT, Shorey-Kendrick LE, Milner K, Schilling D, Tiller C, Vuylsteke B, et al. 2019. Oral vitamin c (500 mg/d) to pregnant smokers improves infant airway function at 3 months (vcsip). A randomized trial. American journal of respiratory and critical care medicine 199:1139–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa S, Kawanishi S. 1999. Site-specific DNA damage at ggg sequence by oxidative stress may accelerate telomere shortening. FEBS Lett 453:365–368. [DOI] [PubMed] [Google Scholar]
- Ozturk S, Sozen B, Demir N. 2014. Telomere length and telomerase activity during oocyte maturation and early embryo development in mammalian species. Molecular human reproduction 20:15–30. [DOI] [PubMed] [Google Scholar]
- Perera F, Lin CJ, Qu L, Tang D. 2018. Shorter telomere length in cord blood associated with prenatal air pollution exposure: Benefits of intervention. Environ Int 113:335–340. [DOI] [PubMed] [Google Scholar]
- Peter S, Holguin F, Wood LG, Clougherty JE, Raederstorff D, Antal M, et al. 2015. Nutritional solutions to reduce risks of negative health impacts of air pollution. Nutrients 7:10398–10416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S, Saretzki G, von Zglinicki T. 1998. Preferential accumulation of single-stranded regions in telomeres of human fibroblasts. Exp Cell Res 239:152–160. [DOI] [PubMed] [Google Scholar]
- Rao X, Zhong J, Brook RD, Rajagopalan S. 2018. Effect of particulate matter air pollution on cardiovascular oxidative stress pathways. Antioxid Redox Signal 28:797–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi S, Raza ST, Mahdi F. 2014. Telomere length variations in aging and age-related diseases. Curr Aging Sci 7:161–167. [DOI] [PubMed] [Google Scholar]
- Romieu I, Castro-Giner F, Kunzli N, Sunyer J. 2008. Air pollution, oxidative stress and dietary supplementation: A review. Eur Respir J 31:179–197. [DOI] [PubMed] [Google Scholar]
- Rosa MJ, Hsu HL, Just AC, Brennan KJ, Bloomquist T, Kloog I, et al. 2019. Association between prenatal particulate air pollution exposure and telomere length in cord blood: Effect modification by fetal sex. Environ Res 172:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Larrea MB, Leal AM, Martin C, Martinez R, Lacort M. 1997. Antioxidant action of estrogens in rat hepatocytes. Rev Esp Fisiol 53:225–229. [PubMed] [Google Scholar]
- Siega-Riz AM, Bodnar LM, Savitz DA. 2002. What are pregnant women eating? Nutrient and food group differences by race. Am J Obstet Gynecol 186:480–486. [DOI] [PubMed] [Google Scholar]
- Slama R, Darrow L, Parker J, Woodruff TJ, Strickland M, Nieuwenhuijsen M, et al. 2008. Meeting report: Atmospheric pollution and human reproduction. Environ Health Perspect 116:791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Zhang B, Liu B, Wu M, Zhang L, Wang L, et al. 2019. Effects of maternal exposure to ambient air pollution on newborn telomere length. Environment international 128:254–260. [DOI] [PubMed] [Google Scholar]
- Spiegelhalter DJ, Best NG, Carlin BP, & Van Der Linde A, 2002. Bayesian measures of model complexity and fit. Journal of the Royal Statistical Society: Series B 64:583–616. [Google Scholar]
- Turner KJ, Vasu V, Griffin DK. 2019. Telomere biology and human phenotype. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vina J, Borras C, Gambini J, Sastre J, Pallardo FV. 2005. Why females live longer than males? Importance of the upregulation of longevity-associated genes by oestrogenic compounds. FEBS Lett 579:2541–2545. [DOI] [PubMed] [Google Scholar]
- Ward-Caviness CK, Nwanaji-Enwerem JC, Wolf K, Wahl S, Colicino E, Trevisi L, et al. 2016. Longterm exposure to air pollution is associated with biological aging. Oncotarget 7:74510–74525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Chiu YM, Hsu HL, Wright RO, Wright RJ, Coull BA. 2017a. Potential for bias when estimating critical windows for air pollution in children’s health. Am J Epidemiol 186:1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Chiu YM, Hsu HL, Wright RO, Wright RJ, Coull BA. 2017b. Bayesian distributed lag interaction models to identify perinatal windows of vulnerability in children’s health. Biostatistics 18:537–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WE, Mage DT, Grant LD. 2000. Estimating separately personal exposure to ambient and nonambient particulate matter for epidemiology and risk assessment: Why and how. J Air Waste Manag Assoc 50:1167–1183. [DOI] [PubMed] [Google Scholar]
- Wolfe J, Kimerling R. 1997. Gender issues in the assessment of posttraumatic stress disorder In: Assessing psychological trauma and ptsd. New York, NY, US:Guilford Press, 192–238. [Google Scholar]
- Zeng X, Liu J, Du X, Zhang J, Pan K, Shan W, et al. 2018. The protective effects of selenium supplementation on ambient pm2.5-induced cardiovascular injury in rats. Environmental science and pollution research international 25:22153–22162. [DOI] [PubMed] [Google Scholar]
- Zhong J, Trevisi L, Urch B, Lin X, Speck M, Coull BA, et al. 2017. B-vitamin supplementation mitigates effects of fine particles on cardiac autonomic dysfunction and inflammation: A pilot human intervention trial. Scientific reports 7:45322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XD, Bonet B, Knopp RH. 1997. 17beta-estradiol, progesterone, and testosterone inversely modulate low-density lipoprotein oxidation and cytotoxicity in cultured placental trophoblast and macrophages. Am J Obstet Gynecol 177:196–209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


