Abstract
This study was aimed to investigate the function and mechanism of microRNA-200c (miR-200c) in the progression of non-small cell lung cancer (NSCLC). A total of 76 patients diagnosed as having NSCLC were enrolled in this study. The expression level of miR-200c in NSCLC tissues and cell lines was investigated using the quantitative real-time polymerase chain reaction (RT-qPCR) method. We found that the expression of miR-200c was significantly reduced in NSCLC tissues and cell lines compared with normal lung tissues and the human bronchial epithelial cell line. Overexpression of miR-200c using the miR-200c mimic significantly suppressed cell proliferation and migration of NSCLC cell lines. The results of the luciferase reporter assay identified lactate dehydrogenase A (LDHA) as a direct target of miR-200c. The expression of LDHA was shown to be suppressed in NSCLC cell lines with miR-200c mimic transfection. Furthermore, the transfection of small interfering RNA (siRNA) targeting LDHA suppressed the proliferation and migration of NSCLC cell lines. In summary, our results presented in this study suggested that miR-200c was able to inhibit the proliferation and migration of NSCLC cells by downregulating LDHA. Therefore, miR-200c may be considered as a potential candidate for the treatment of NSCLC.
Key words: miR-200c, Non-small cell lung cancer (NSCLC), Lactate dehydrogenase A (LDHA), Cell proliferation, Cell migration
INTRODUCTION
Lung cancer (LC) is the leading cause of cancer-related deaths worldwide, accounting for about 1.4 million cancer deaths annually1. The annual LC mortality rate in China was estimated to reach 1 million by 2025, presenting a major public health issue and imposing an enormous burden on patients2,3. Statistically, 85% of LC cases were non-small cell lung cancer (NSCLC) according to pathology type4. Smoking and environmental pollution are two major risk factors for LC in China5,6. Extensive efforts have been made regarding the screening and treatment methods of NSCLC, but the 5-year overall survival rate of patients with NSCLC remains poor because the patients are often diagnosed at a late stage5,6. Therefore, a more effective biomarker that can contribute to early detection is urgently needed.
The Warburg effect (aerobic glycolysis) is a common feature of cancer cells, which facilitates tumor cell proliferation and progression with elevated glucose uptake and lactate production7. Lactate dehydrogenase A (LDHA), one of the subunits of lactate dehydrogenase (LDH), participates in the final step of aerobic glycolysis process by catalyzing pyruvate into lactate8,9. Elevated expression of LDHA has been found in a number of human cancers including breast cancer, hepatocellular carcinoma, gastric cancer, pancreatic cancer, and colorectal cancer10–14. The abnormal expression of LDHA was also associated with poor clinical outcome10–14. Moreover, several studies have shown that several known oncogenes or transcription factors could stimulate the expression of LDHA, such as forkhead box protein (FOXM1), miR-30a-5p, and MYC10,15,16.
MicroRNAs (miRNAs) are a class of small noncoding RNAs with a length of 18 to 25 bases, which can inhibit relevant gene expression through binding to the seed sequences within the 3′-untranslational region (3′-UTR) of target messenger RNAs (mRNAs)17. It has previously been demonstrated that miRNAs are crucial for tumor initiation and progression18,19. miR-200c, one member of the miR-200 family, has been demonstrated to be downregulated in several human cancers20–23. Furthermore, miR-200c also regulates cell proliferation, invasion, metastasis, and drug resistance through interacting with the corresponding target genes20–22. However, although these findings indicate that miR-200c plays an important role in cancer cell migration and survival, the molecular mechanisms underlying these processes require further elucidation.
We investigated miR-200c expression and biological function in NSCLC. Furthermore, we analyzed whether miR-200c can regulate the expression of LDHA in NSCLC. Our data indicated that miR-200c attenuated tumor cell proliferation and migration through decreasing the expression of LDHA. Additionally, these results support miR-200c as a tumor suppressor and a potential treatment target in NSCLC.
MATERIALS AND METHODS
Patients and Specimens
Seventy-six patients diagnosed with NSCLC who underwent surgical resection at Nanjing Jinling Hospital between September 2009 and December 2011 were enrolled in this study. The approval of this study was obtained from the Ethics Committee of Nanjing Jinling Hospital. Written informed consent was obtained from the patients according to the Declaration of Helsinki. The clinicopathological parameters of all the enrolled patients were collected and analyzed.
Cell Culture and Transfection
Two NSCLC cell lines (A549 and NCI-H460) and the human bronchial epithelial cell line (16HBE) were obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA). The cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% of fetal bovine serum (FBS; Thermo Fisher Scientific). The cells were maintained at 37°C in a humidified atmosphere containing 5% CO2.
For cell transfection, Lipofectamine 2000 (Invitrogen, Thermo Fisher Scientific) was used in this study according to the manufacturer’s instructions. miR-200c mimic, miR-200c inhibitor, negative control (NC) miRNA, small interfering RNA (siRNA) targeting LDHA, and NC siRNA were purchased from Genechem Co., Ltd. (Shanghai, P.R. China). The mixture containing the synthesized nucleotide and Lipofectamine 2000 was incubated at room temperature for 10 min and then added to the cultured cell lines. Cells were collected after 48 h of transfection for the following usage.
RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR) Analysis
Total RNA including miRNA was extracted from the NSCLC tissues and cell lines using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The first-strand cDNA was synthesized using the cDNA synthesis kit (Beyotime, Shanghai, P.R. China). RT-qPCR analysis was performed using SYBR Green qPCR Mix (Beyotime) on Applied Biosystems 7500 equipment (Foster City, CA, USA). The PCR conditions were as follows: 95°C for 5 min, and 40 cycles of denaturation at 95°C for 15 s and annealing/elongation at 60°C for 30 s. U6 snRNA and β-actin were used as internal control to normalize the expression of miR-200c and LDHA. The primers used in this study were as follows: miR-200c, 5′-CTTAAAGCCCCTTCGTCTCC-3′ (forward) and 5′-AGGGGTGAAGGTCAGAGGTT-3′ (reverse); U6 snRNA, 5′-TGCGGGTGCTCGCTTCGGCAGC-3′ (forward) and 5′-CCAGTGCAGGGTCCGAGGT-3′ (reverse); LDHA, 5′-GGTTGGTGCTGTTGGCATGG-3′ (forward) and 5′-TGCCCCAGCCGTGATAATGA-3′ (reverse); β-actin, 5′-TGGCACCCAGCACAATGAA-3′ (forward) and 5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ (reverse). The relative expression level was measured with the 2−ΔΔCT method.
Cell Proliferation Analysis
The cell proliferation was measured by MTT [3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide] assay. Briefly, 100 μl of transfected cells was seeded into 96-well plates at a density of about 5 × 103 cells/well. Cell proliferation was detected after the cells were incubated for 0, 24, 48, and 72 h. MTT (10 μl, 5 mg/ml; Beyotime) was added into every well. The cells were cultured for 4 h in a 37°C incubator. Subsequently, dimethyl sulfoxide (DMSO; 100 μl) was added into each well and shaken gently for 10 min at room temperature to dissolve the crystal. The absorbance was measured at 570 nm using a microplate reader (SpectraMAX Plus; Molecular Devices, Sunnyvale, CA, USA).
Cell Migration Analysis
Cell migration was measured by in vitro scratch assay. Cells (1 × 105) were plated in a six-well plate and cultured to confluence. The cell monolayer was scraped in a straight line to generate wound with a p100 pipet tip. The cells were then washed twice to remove debris. The migration distance was assessed at 0 and 24 h after wounding using ImageJ 1.48 software (NIH, Bethesda, MA, USA).
Western Blot
Total protein was extracted from the cells and tissues using RIPA lysis buffer according to the manufacturer’s instructions. Proteins were separated using 10% SDS-polyacrylamide gel and then transferred to a polyvinylidene difluoride (PVDF) membrane (GE Healthcare Life Sciences, Chalfont, UK). The PVDF membrane was blocked with phosphate-buffered saline (PBS) buffer containing 5% fat-free milk overnight at 4°C. The membranes were incubated with rabbit anti-LDHA monoclonal antibody (1:1,000; 3582; Cell Signaling Technology, Danvers, MA, USA) and rabbit anti-β-actin monoclonal antibody (1:1,000; 4970; Cell Signaling Technology) at room temperature for 3 h. Subsequently, the membrane was incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:1,000; 7074; Cell Signaling Technology) at room temperature for 2 h. The signals on the PVDF membrane were detected using the Enhanced Chemiluminescent Kit (Beyotime) according to the manufacturer’s protocol. The relative protein expression levels were analyzed using Quantity One v4.63 (Bio-Rad, Hercules, CA, USA) and are presented as the density ratio versus β-actin.
Dual-Luciferase Reporter Assay
Dual-luciferase reporter assay was used to confirm whether LDHA is a direct target of miR-200c. The wild-type (WT) and mutant (Mut) 3′-UTR of LDHA was amplified and cloned into the pmirGLO luciferase reporter vector (Promega, Madison, WI, USA). The WT and Mut LDHA constructs were cotransfected with either miR-200c mimic or NC miRNA into the NSCLC cell lines using Lipofectamine 2000. Forty-eight hours following transfection, the luciferase activity was measured using a Dual-Luciferase Reporter Assay Kit (Promega) following the manufacturer’s protocol. Data are presented as the ratio of Renilla luciferase to firefly luciferase.
Statistical Analysis
Data are presented as mean ± standard deviation from at least three independent experiments. SPSS 16.0 software (Chicago, IL, USA) was used for statistical analysis. The differences between groups were analyzed with Student’s t-test (two groups) and one-way analysis of variance and Tukey’s test (three groups or above). Chi-square test was used to examine the correlation between miR-200c expression and clinicopathological parameters. Kaplan–Meier curve and log-rank test were used to analyze the effect of miR-200c on the 5-year overall survival of NSCLC patients. Univariate and multivariate analyses with Cox regression model were used to identify the independent factors for the prognosis of NSCLC patients. A value of p < 0.05 was considered statistically significant.
RESULTS
miR-200c Was Significantly Downregulated in NSCLC Tissues and Cell Lines and Was Associated With Tumor Progression
The expression status of miR-200c in NSCLC tissues and cell lines was assessed using RT-qPCR. First, we analyzed the expression of miR-200c in 76 pairs of NSCLC tumor tissues and adjacent noncancerous tissues. We observed that the expression level of miR-200c was significantly reduced in NSCLC tumor tissues compared with adjacent noncancerous tissues (Fig. 1A). Next, we investigated the expression of miR-200c in NSCLC cell lines (A549 and NCI-H460) and in normal human lung epithelial cell line (16HBE). We found that the expression level of miR-200c in the A549 and NCI-H460 cell lines was also significantly decreased when compared with the 16HBE cell line (Fig. 1B). The A549 cell line exhibited the most significant decrease in the expression level of miR-200c (Fig. 1B).
Figure 1.
MicroRNA-200c (miR-200c) was downregulated in non-small cell lung cancer (NSCLC) and correlated with poor prognosis of NSCLC patients. (A) The expression of miR-200c in tumor tissues and adjacent noncancerous tissues was detected by quantitative real-time polymerase chain reaction (RT-qPCR). (B) The expression of miR-200c in NSCLC cell lines (A549 and NCI-H460) and the human bronchial epithelial cell line (16HBE) was detected by RT-qPCR. ***p < 0.001. (C) Low expression of miR-200c predicts poorer 5-year overall survival of NSCLC patients.
To determine the clinical significance of miR-200c expression in NSCLC, we tested whether the expression of miR-200c was associated with clinicopathological characteristics of NSCLC patients. We revealed that miR-200c downregulation was frequently found in NSCLC with large tumor size (p = 0.033) and later tumor stage (p = 0.013). Also, we investigated the effect of miR-200c expression on the 5-year overall survival of NSCLC patients. The results presented in Figure 1C revealed that the patients with low miR-200c had poorer overall survival than those with high miR-200c (p = 0.014).
Overexpression of miR-200c Suppresses the Proliferation and Migration of NSCLC Cells
Because the expression of miR-200c was found downregulated in NSCLC, we hypothesized that miR-200c plays important roles in the progression and development of human liver cancer. Therefore, the effect of miR-200c expression on the NSCLC cell proliferation and migration was investigated. The NSCLC cell lines were transfected with miR-200c mimic, miR-200c inhibitor, and NC miRNA. Posttransfection, RT-qPCR was performed to detect the expression level of miR-200c. As depicted in Figure 2A, the expression of miR-200c was significantly increased in the miR-200c mimic-transfected group compared with the NC miRNA-transfected group. Conversely, the expression of miR-200c in the miR-200c inhibitor-transfected group was significantly decreased compared with the NC miRNA-transfected group (Fig. 2A). Subsequently, the MTT assay was performed to evaluate the effect of miR-200c on the proliferation of miRNA-transfected A549 and NCI-H460 cell lines. The results indicate that the ectopic expression of miR-200c significantly reduced the cell proliferation rate of the A549 and NCI-H460 cell lines, whereas transfection with the miR-200c inhibitor significantly increased the cell proliferation rate in the A549 and NCI-H460 cell lines (Fig. 2B and C). A wound-healing assay was performed to determine the effect of miR-200c on the cell migration of miRNA-transfected A549 and NCI-H460 cell lines. As shown in Figure 2D and E, overexpression of miR-200c by the miR-200c mimic markedly suppressed A549 and NCI-H460 cell migration compared with the miR-200c inhibitor and NC miRNA-transfected cell lines. Taken together, our results suggested that miR-200c may function as a negative regulator on the proliferation and migration of NSCLC cell lines.
Figure 2.
Overexpression of miR-200c inhibits A549 and NCI-H460 cell proliferation and migration in vitro. (A) The expression of miR-200c in A549 and NCI-H460 cell lines after miR-200c mimic, inhibitor, and negative control (NC) miRNA transfection was detected by RT-qPCR. The cell proliferation of (B) A549 and (C) NCI-H460 cell lines after miR-200c mimic, inhibitor, and NC miRNA transfection was detected by MTT assay. The cell migration of (D) A549 and (E) NCI-H460 cell lines after miR-200c mimic, inhibitor, and NC miRNA transfection was detected by wound-healing assay. *p < 0.05, **p < 0.01, ***p < 0.001.
LDHA Was a Direct Target of miR-200c in NSCLC Cells
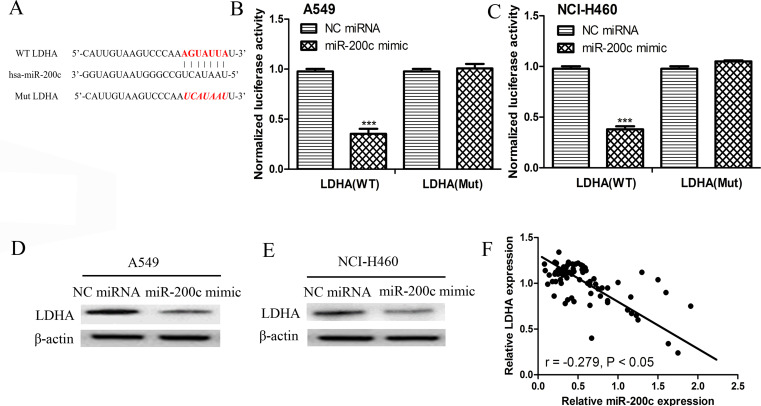
The computational algorithms TargetScan and miRanda were used to predict the potential targets of miR-200c. Among all the putative targets of miR-200c, LDHA was selected because the seed sequence in the 3′-UTR of LDHA perfectly paired with miR-200c (Fig. 3A). Subsequently, the luciferase reporter assay was used to determine whether miR-200c was able to directly bind to seed sequences in the LDHA 3′-UTR in the NSCLC A549 and NCI-H460 cell lines. As shown in Figure 3B and C, the luciferase activity was markedly reduced in both A549 and NCI-H460 cell lines cotransfected with the WT LDHA 3′-UTR construct and miR-200c mimic; however, no difference was detected in the A549 and NCI-H460 cells cotransfected with the Mut LDHA 3′-UTR construct and miR-200c mimic, compared with the control group. These findings indicate that LDHA was a direct target of miR-200c in NSCLC. Next, we analyzed the expression of LDHA in the miR-200c mimic- or NC miRNA-transfected A549 and NCI-H460 cell lines. The results presented in Figure 3D and E illustrate that the transfection of the miR-200c mimic could significantly reduce the expression of LDHA in both A549 and NCI-H460 cell lines. Moreover, we found that the expression of LDHA was inversely correlated with miR-200c in NSCLC (Fig. 3F), which implies that miR-200c could negatively regulate the expression of LDHA by directly binging to the 3′-UTR of LDHA.
Figure 3.
Lactate dehydrogenase A (LDHA) was a direct target of miR-200c. (A) The putative binding sequence for miR-200c in the 3′-untranslated region (3′-UTR) of LDHA. The luciferase activity assay revealed the miR-200c mimic suppressed LDHA 3′-UTR wild-type (WT) luciferase activity, whereas it had no effect on LDHA 3′-UTR mutant (Mut) luciferase activity compared with control in (B) A549 and (C) NCI-H460 cells. ***p < 0.001. Protein expression of LDHA in (D) A549 and (E) NCI-H460 cell line after miR-200c mimic and NC miRNA transfection was detected by Western blot assay. (F) The expression of LDHA was inversely correlated with the expression of miR-200c in NSCLC.
Knockdown of LDHA Inhibits NSCLC Proliferation and Migration
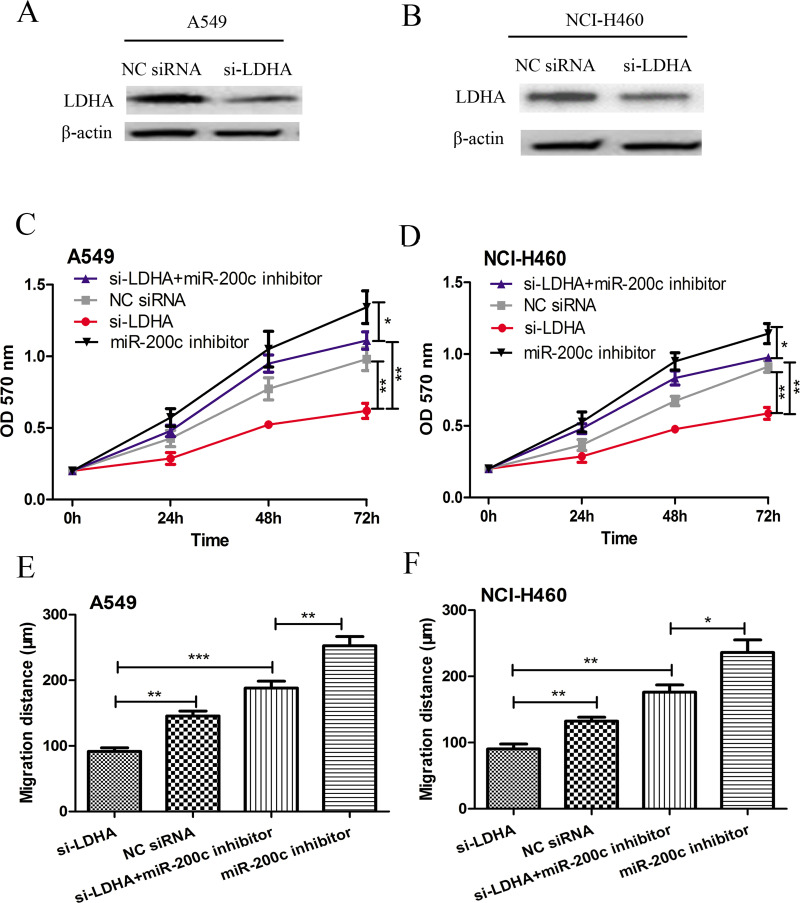
Because miR-200c could negatively regulate LDHA expression, we therefore investigated whether knockdown of LDHA exhibited similar effects on cell proliferation and migration in A549 and NCI-H460 cells, as overexpression of miR-200c. The A549 and NCI-H460 cell lines were transfected with LDHA siRNA. The results in Figure 4A and B indicated that the protein expression level of LDHA was significantly reduced posttransfection. The MTT assay and wound-healing assay were then conducted to measure the cell proliferation rate and cell migration distance in order to assess the function of LDHA on NSCLC progression. As shown in Figure 4C and D, knocking down the expression of LDHA suppressed the cell proliferation of both the A549 and NCI-H460 cell lines. Similarly, cell migration was also impaired by the downregulation of LDHA (Fig. 4E and F). The results presented in Figure 4C–F revealed that the knockdown of LDHA could partly abolish the effect of the miR-200c inhibitor on cell proliferation and migration. Collectively, these results suggest that miR-200c may negatively mediate cell proliferation and migration in NSCLC, probably via inhibition of LDHA.
Figure 4.
Knockdown expression of LDHA inhibits A549 and NCI-H460 cell proliferation and migration in vitro. The protein expression of LDHA in (A) A549 and (B) NCI-H460 cell lines after small interfering (si)-LDHA and NC siRNA transfection was detected by Western blot assay. The cell proliferation of (C) A549 and (D) NCI-H460 cell lines after si-LDHA and NC siRNA transfection was detected by MTT assay. The cell migration of (E) A549 and (F) NCI-H460 cell lines after si-LDHA and NC siRNA transfection was detected by wound-healing assay. *p < 0.05, **p < 0.01, ***p < 0.001.
DISCUSSION
Currently, accumulating studies have suggested that miRNAs were dysregulated in multiple types of human cancers and play important roles in the tumor initiation and progression processes18–23. Several miRNAs, including miR-200c, have been found abnormally expressed in NSCLC and correlate with poor outcome of NSCLC20–22,24–26. The present study demonstrated that miR-200c was significantly downregulated in NSCLC tissues and cell lines compared with adjacent noncancerous tissues and normal lung epithelial cells. The biological function of miR-200c was investigated in NSCLC cell lines in vitro. Gain-of-function studies indicated that overexpression of miR-200c significantly inhibited the proliferation and migration of NSCLC cells, which suggested that the decreased expression of miR-200c may contribute to the progression of NSCLC. Furthermore, the clinical significance of miR-200c in NSCLC was investigated. We found that low expression of miR-200c was correlated with the poor overall survival of NSCLC patients.
A number of studies have explored the role of miR-200c in NSCLC20,22,27,28. Shan et al. reported that overexpression of miR-200c could suppress cell invasion and restore methotrexate sensitivity in the LC A549 cell line27. Another study conducted by Jiao et al. found that miR-200c could also regulate the migration of the A549 cell line and the epithelial–mesenchymal transition process20. Recently, ZEB1 was identified as a target of miR-200c in NSCLC in two independent studies22,28. However, the underlying mechanism of miR-200c in regulating these biological processes remains to be explored.
Our present study identified LDHA as a direct target gene of miR-200c and demonstrated that miR-200c negatively regulates the protein expression of LDHA in A549 and NCI-H460 NSCLC cell lines. LDHA is a cancer-specific isoform of LDH that regulates glycolytic flux by converting cytoplasmic pyruvate to lactate, an important energy-producing step for cancer cells9. Liu et al. found that LDHA was overexpressed in gastric cancer, and the decreased expression of LDHA by oxamate could inhibit cell proliferation and invasion, which suggested that LDHA may be a potential therapeutic target for gastric cancer12. In this present study, knockdown of LDHA expression using LDHA targeting siRNA significantly suppressed the proliferation and migration of A549 and NCI-H460 NSCLC cells, similar to the effects observed following miR-200c overexpression. In addition, LDHA has been identified as a direct target of miR-200c in bladder cancer29. Therefore, the present study provides further evidence regarding the relationship between miR-200c and LDHA in human cancer.
In conclusion, the present study presents a functional role of miR-200c in regulating NSCLC. Our results demonstrated that miR-200c exerts suppressive effects on the proliferation and migration of NSCLC cells, at least partly by targeting LDHA, thus suggesting that miR-200c acts as a tumor suppressor in NSCLC. Therefore, miR-200c may be a candidate target for cancer therapy of NSCLC.
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136(5):E359–86. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Cancer: Fact sheet No. 297. http://www.who.int/mediacentre/factsheets/fs297/en/
- 3. She J, Yang P, Hong QY, Bai CX. Lung cancer in China: Challenges and interventions. Chest 2013;143(4):1117–26. [DOI] [PubMed] [Google Scholar]
- 4. Ramalingam SS, Owonikoko TK, Khuri FR. Lung cancer: New biological insights and recent therapeutic advances. CA Cancer J Clin. 2011;61(2):91–112. [DOI] [PubMed] [Google Scholar]
- 5. Chen WQ, Zheng RS, Baade PD, Zhang SW, Zeng HM, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32. [DOI] [PubMed] [Google Scholar]
- 6. Gao J, Woodward A, Vardoulakis S, Kovats S, Wilkinson P, Li L, Xu L, Li J, Yang J, Li J, Cao L, Liu X, Wu H, Liu Q. Haze, public health and mitigation measures in China: A review of the current evidence for further policy response. Sci Total Environ. 2017;578:148–57. [DOI] [PubMed] [Google Scholar]
- 7. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009;324(5930):1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mirebeau-Prunier D, Le Pennec S, Jacques C, Fontaine JF, Gueguen N, Boutet-Bouzamondo N, Donnart A, Malthiery Y, Savagner F. Estrogen-related receptor alpha modulates lactate dehydrogenase activity in thyroid tumors. PLoS One 2013;8(3):e58683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang Y, Su D, Zhao L, Zhang D, Xu J, Wan J, Fan S, Chen M. Different effects of LDH-A inhibition by oxamate in non-small cell lung cancer cells. Oncotarget 2014;5(23):11886–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li L, Kang L, Zhao W, Feng YY, Liu WP, Wang T, Mai HX, Huang J, Chen SY, Liang YC, Han JQ, Xu XJ, Ye QN. MiR-30a-5p suppresses breast tumor growth and metastasis through inhibiting of LDHA-mediated Warburg effect. Cancer Lett. 2017;400:89–98. [DOI] [PubMed] [Google Scholar]
- 11. Li X, Lu P, Li B, Yang R, Chu Y, Zhang Z, Wan H, Niu C, Wang C, Luo K. Sensitization of hepatocellular carcinoma cells to irradiation by miR-34a through targeting lactate dehydrogenase-A. Mol Med Rep. 2016;13(4):3661–7. [DOI] [PubMed] [Google Scholar]
- 12. Liu X, Yang Z, Chen Z, Chen R, Zhao D, Zhou Y, Qiao L. Effects of the suppression of lactate dehydrogenase A on the growth and invasion of human gastric cancer cells. Oncol Rep. 2015;33(1):157–62. [DOI] [PubMed] [Google Scholar]
- 13. Mohammad GH, Olde Damink SW, Malago M, Dhar DK, Pereira SP. Pyruvate kinase M2 and lactate dehydrogenase A are overexpressed in pancreatic cancer and correlate with poor outcome. PLoS One 2016;11(3):e0151635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang J, Wang H, Liu A, Fang C, Hao J, Wang Z. Lactate dehydrogenase A negatively regulated by miRNAs promotes aerobic glycolysis and is increased in colorectal cancer. Oncotarget 2015;6(23):19456–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang WH, Zhou F, Li N, Li Q, Wang LW. FOXM1-LDHA signaling promoted gastric cancer glycolytic phenotype and progression. Int J Clin Exp Pathol. 2015;8(6):6756–63. [PMC free article] [PubMed] [Google Scholar]
- 16. Dang CV, Le A, Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res. 2009;15(21):6479–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Engelsvold DH, Utheim TP, Olstad OK, Gonzalez P, Eidet JR, Lyberg T, Troseid AMS, Dartt DA, Raeder S. miRNA and mRNA expression profiling identifies members of the miR-200 family as potential regulators of epithelial-mesenchymal transition in pterygium. Exp Eye Res. 2013;115:189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597–610. [DOI] [PubMed] [Google Scholar]
- 19. Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer 2006;6(11):857–66. [DOI] [PubMed] [Google Scholar]
- 20. Jiao AH, Sui MH, Zhang LM, Sun P, Geng DM, Zhang WW, Wang XW, Li JX. MicroRNA-200c inhibits the metastasis of non-small cell lung cancer cells by targeting ZEB2, an epithelial-mesenchymal transition regulator. Mol Med Rep. 2016;13(4):3349–55. [DOI] [PubMed] [Google Scholar]
- 21. Liu PL, Liu WL, Chang JM, Chen YH, Liu YP, Kuo HF, Hsieh CC, Ding YS, Chen WW, Chang IW. MicroRNA-200c inhibits epithelial mesenchymal transition, invasion, and migration of lung cancer by targeting HMGB1. PLoS One 2017;12(7):e0180844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gao HX, Yan L, Li CZ, Zhao LM, Liu W. miR-200c regulates crizotinib-resistant ALK-positive lung cancer cells by reversing epithelial-mesenchymal transition via targeting ZEB1. Mol Med Rep. 2016;14(5):4135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang H, Sun Z, Li Y, Fan D, Jiang H. MicroRNA-200c binding to FN1 suppresses the proliferation, migration and invasion of gastric cancer cells. Biomed Pharmacother. 2017;88:285–92. [DOI] [PubMed] [Google Scholar]
- 24. Li YW, Zhang HB, Dong YL, Fan YG, Li Y, Zhao CL, Wang C, Liu JH, Li X, Dong M, Liu HY, Chen J. MiR-146b-5p functions as a suppressor miRNA and prognosis predictor in non-small cell lung cancer. J Cancer 2017;8(9):1704–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou F, Nie L, Feng D, Guo S, Luo R. MicroRNA-379 acts as a tumor suppressor in non-small cell lung cancer by targeting the IGF-1R-mediated AKT and ERK pathways. Oncol Rep. 2017;38(3):1857–66. [DOI] [PubMed] [Google Scholar]
- 26. Wang Y, Zhao M, Liu J, Sun Z, Ni J, Liu H. miRNA-125b regulates apoptosis of human non-small cell lung cancer via the PI3K/Akt/GSK3β signaling pathway. Oncol Rep. 2017;38(3):1715–23. [DOI] [PubMed] [Google Scholar]
- 27. Shan WL, Zhang XL, Li M, Deng F, Zhang J. Over expression of miR-200c suppresses invasion and restores methotrexate sensitivity in lung cancer A549 cells. Gene 2016;593(2):265–71. [DOI] [PubMed] [Google Scholar]
- 28. Zhou G, Zhang F, Guo Y, Huang J, Xie Y, Yue S, Chen M, Jiang H, Li M. miR-200c enhances sensitivity of drug-resistant non-small cell lung cancer to gefitinib by suppression of PI3K/Akt signaling pathway and inhibits cell migration via targeting ZEB1. Biomed Pharmacother. 2017;85:113–9. [DOI] [PubMed] [Google Scholar]
- 29. Yuan DZ, Zheng SS, Wang LY, Li J, Yang JN, Wang B, Chen X, Zhang XB. MiR-200c inhibits bladder cancer progression by targeting lactate dehydrogenase A. Oncotarget 2017;8(40):67663–9. [DOI] [PMC free article] [PubMed] [Google Scholar]