Abstract
Growing evidence has demonstrated that numerous microRNAs (miRNAs) may participate in the regulation of gastric carcinogenesis and progression. This phenomenon suggests that gastric cancer-related miRNAs can be identified as effective therapeutic targets for this disease. miRNA-708 (miR-708) has recently been reported to be aberrantly expressed in several types of cancer and contribute to carcinogenesis and progression. However, the expression level, biological roles, and underlying mechanisms of miR-708 in gastric cancer are poorly understood. Here we found that miR-708 was downregulated in gastric cancer tissues and cell lines. Downregulated miR-708 expression was significantly associated with lymphatic metastasis, invasive depth, and TNM stage. Further investigation indicated that ectopic expression of miR-708 prohibited cell proliferation and invasion in gastric cancer. Bioinformatics analysis showed that Notch1 was a potential target of miR-708. Notch1 was further confirmed as a direct target gene of miR-708 in gastric cancer by dual-luciferase reporter assay, reverse transcription quantitative polymerase chain reaction, and Western blot analysis. Furthermore, an inverse association was found between miR-708 and Notch1 mRNA levels in gastric cancer tissues. In addition, restored Notch1 expression rescued the inhibitory effects on gastric cancer cell proliferation and invasion induced by miR-708 overexpression. Our findings highlight the tumor-suppressive roles of miR-708 in gastric cancer and suggest that miR-708 may be investigated as a novel target for gastric cancer treatment.
Key words: MicroRNA-708, Gastric cancer, Notch1, Proliferation, Invasion
INTRODUCTION
Gastric cancer ranks the fifth most common cancer and the third leading cause of cancer-related mortality worldwide1. Approximately 950,000 new cases and over 720,000 deaths caused by gastric cancer per year have been estimated worldwide2. Several risk factors, such as Helicobacter pylori infection, dietary habits, smoking, obesity, pernicious anemia, and chronic atrophic gastritis, contribute to gastric cancer initiation and progression3,4. The primary treatments for gastric cancer are surgery resection, chemotherapy, and radiotherapy5. Despite considerable improvement in diagnostic approaches and treatment strategies, the prognosis of gastric cancer patients at an advanced stage remains poor6. The 5-year survival rate and median overall survival period for advanced gastric cancer patients are only 5–20% and less than 1 year, respectively7. Therefore, further clarification of the underlying molecular mechanisms of gastric cancer onset and progression may provide a foundation to develop efficient therapeutic strategies for gastric cancer.
MicroRNAs (miRNAs) have been widely implicated in tumorigenesis and tumor development8,9. miRNAs are ∼22-nt, single-stranded, and noncoding short RNA molecules that serve as key gene regulators by complementarily binding to the corresponding 3′-untranslated regions (3′-UTRs) of their target genes. This process results in translation suppression and/or mRNA degradation10. Emerging literature has highlighted that the expression level of miRNAs is altered in almost all types of human malignancy and that miRNAs may be involved in the regulation of various pathological processes, such as cell proliferation, cell cycle, apoptosis, differentiation, angiogenesis, invasion, epithelial–mesenchymal transition, and metastasis11–13. Dysregulated miRNAs may function as either oncogenes or tumor suppressors in different types of cancer, and this depends largely on the biological roles of their target genes14. Therefore, further investigation on the expression and functions of miRNAs in gastric cancer is beneficial to identify novel therapeutic targets for patients with this disease.
miR-708 has recently been reported to be aberrantly expressed in several types of cancer and contribute to carcinogenesis and progression15–19. However, the expression level, biological roles, and underlying mechanisms of miR-708 in gastric cancer are poorly understood. This study was performed to detect the expression levels of miR-708 in gastric cancer and evaluate the association between the miR-708 expression level and clinicopathologic factors of gastric cancer patients. The biological effects of miR-708 on gastric cancer cell proliferation and invasion were also investigated. Moreover, the molecular mechanisms underlying the action of miR-708 in gastric cancer cells were explored.
MATERIALS AND METHODS
Tissue Specimens
This study was approved by the Ethics Committee of Huizhou Central People’s Hospital. Written informed consent for research purposes was provided by all patients. A total of 53 paired gastric cancer tissues and adjacent nontumor gastric tissues were obtained from gastric cancer patients who underwent surgical resection at the Huizhou Central People’s Hospital between October 2014 and July 2016. None of these gastric cancer patients had received chemotherapy or radiotherapy prior to surgery. Collected tissue specimens were immediately frozen in liquid nitrogen and subsequently stored at −80°C.
Cell Lines, Culture Condition, and Transfection
A human gastric epithelial cell line, GES-1, was acquired from the American Type Culture Collection (Manassas, VA, USA). Four human gastric cancer cell lines, BGC-823, MKN-45, SGC-7901, and MGC-803, were purchased from Shanghai Institute of Biochemistry and Cell Biology (Shanghai, P.R. China). All cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 mg/ml streptomycin (all from Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), and grown at 37°C in a humidified atmosphere containing 5% CO2 and 95% air.
miR-708 mimic and miRNA mimic negative control (miR-NC) were obtained from Shanghai GenePharma Co., Ltd (Shanghai, P.R. China). Notch1 overexpression plasmid (pcDNA3.1-Notch1) and the corresponding empty plasmid were designed and synthesized by Guangzhou GeneCopoeia Co., Ltd (Guangzhou, P.R. China). miRNA mimic or plasmid was transfected into cells using Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) in accordance with the manufacturer’s protocol.
RNA Isolation and Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA of tissue specimens or cells was isolated by use of TRIzol reagent (Tiangen, Bejing, P.R. China) according to the manufacturer’s protocol. For analysis of miR-708, a TaqMan microRNA reverse transcription kit was utilized to synthesize complementary DNA (cDNA), followed by qPCR with a TaqMan microRNA assay kit (all from Applied Biosystems, Carlsbad, CA, USA). For detection of the mRNA level of Notch1, cDNA was synthesized from total RNA using a PrimeScript RT Reagent Kit (Takara Bio, Dalian, P.R. China) following the manufacturer’s protocols. Afterward, the cDNA was subjected to PCR amplification using a SYBR Premix Ex Taq™ Kit (Takara Bio). The expression of miR-708 and Notch1 mRNA was normalized to U6 snRNA and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), respectively. Data were calculated by the 2−ΔΔCt method20.
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
Transfected cells were collected at 24 h posttransfection and inoculated in 96-well plates with a density of 3,000 cells per well. Cell proliferation was detected using MTT assay after incubation at 37°C for 0, 24, 48, and 72 h. Briefly, 20 μl of MTT solution (5 mg/ml; Beyotime Institute of Biotechnology, Haimen, P.R. China) was added into each well and incubated at 37°C for another 4 h. Then the culture medium containing MTT solution was discarded, and 150 μl of DMSO (Beyotime Institute of Biotechnology) was added into each well to dissolve the formazan crystals. Finally, the absorbance of each well at a wavelength of 490 nm was determined using a microplate reader (Bio-Rad Laboratories Inc., Hercules, CA, USA).
Matrigel Invasion Assay
Matrigel-coated Transwell chambers with 8-μm pores (all from BD Biosciences, San Jose, CA, USA) were used to investigate the cell invasion ability. At 48 h posttransfection, the cells were harvested and suspended in FBS-free DMEM. A total of 5 × 104 transfected cells in 200 μl of FBS-free DMEM were plated in the top chamber of a Transwell chamber. Then 500 μl of DMEM containing 10% FBS was added into the lower chamber. After 24 h of incubation at 37°C with 5% CO2, noninvasive cells that did not pass through the chambers were gently removed with cotton swabs. The invasive cells were fixed with 4% formaldehyde and stained with 0.5% crystal violet. After three washes, the stained cells were photographed and counted under an inverted light microscope (Olympus Corporation, Tokyo, Japan) in five randomly selected visual fields.
Bioinformatics Analysis
TargetScan (http://www.targetscan.org) and miRBase (http://www.mirbase.org/) were employed to predict the potential targets of miR-708.
Dual-Luciferase Reporter Assay
A wild-type (Wt) Notch1 3′-UTR containing the binding sequences of miR-708 and a mutant-type (Mut) Notch1 3′-UTR containing the mutated binding sequences of miR-708 were chemically synthesized by Shanghai GenePharma Co., Ltd. and subsequently subcloned into a pMIR-REPORT™ luciferase plasmid (Thermo Fisher Scientific, Inc.), and named as pMIR-Notch1-3′-UTR-Wt and pMIR-Notch1-3′-UTR-Mut, respectively. For dual-luciferase reporter assay, cells were plated into 24-well plates at a density of 1.5 × 105 cells/well and then cotransfected with pMIR-Notch1-3′-UTR-Wt or pMIR-Notch1-3′-UTR-Mut and miR-708 mimics or miR-NC using Lipofectamine™ 2000 following the manufacturer’s instructions. Luciferase activities were measured using a Dual-Luciferase Reporter Assay Kit (Promega, Madison, WI, USA) at 48 h posttransfection in accordance with the manufacturer’s instruction. The luciferase activity was normalized with Renilla luciferase activity.
Western Blot Analysis
Tissue samples or cells were solubilized in ice-cold radioimmunoprecipitation assay lysis buffer (Tiangen) supplemented with protease inhibitor (Sigma-Aldrich, St. Louis, MO, USA). Total protein concentration was analyzed using a bicinchoninic acid protein quantitation kit (Beyotime Institute of Biotechnology) according to the manufacturer’s instruction. Equative proteins were separated using 10% SDS-PAGE and electroblotted onto a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). After blocking in nonfat milk for 2 h at room temperature, the membranes were incubated at 4°C overnight with primary antibodies: mouse anti-human monoclonal Notch1 antibody (dilution, 1:1,000; Cat. No. sc-373891) and mouse anti-human monoclonal GAPDH antibody (dilution, 1:1,000; Cat. No. sc-32233; both from Santa Cruz Biotechnology, Inc., Dallas, TX, USA). After three washes with Tris-buffered saline/Tween 20, the membranes were incubated with corresponding secondary antibody conjugated with horseradish peroxidase (dilution, 1:5,000; Cat. No. sc-2005; Santa Cruz Biotechnology, Inc.), followed by visualization with BeyoECL Plus Enhanced Chemiluminescence Plus Reagent (Beyotime Institute of Biotechnology). Relative protein expression was analyzed with Quantity One software (Bio-Rad) and represented as a density ratio compared with that of GAPDH.
Statistical Analysis
Statistical Package for the Social Sciences (SPSS) version 18.0 (SPSS Inc., Chicago, IL, USA) was utilized for statistical analysis. All data were represented as mean ± standard deviation (SD) and analyzed with Student’s t-test and one-way analysis of variance (ANOVA). Student–Newman–Keuls test was employed as a post hoc test following ANOVA. Spearman’s correlation analysis was applied to evaluate the association between miR-708 and Notch1 mRNA in gastric cancer tissues. A value of p < 0.05 was considered to indicate statistical significance.
RESULTS
miR-708 Expression Is Downregulated in Gastric Cancer Tissue and Cell Lines
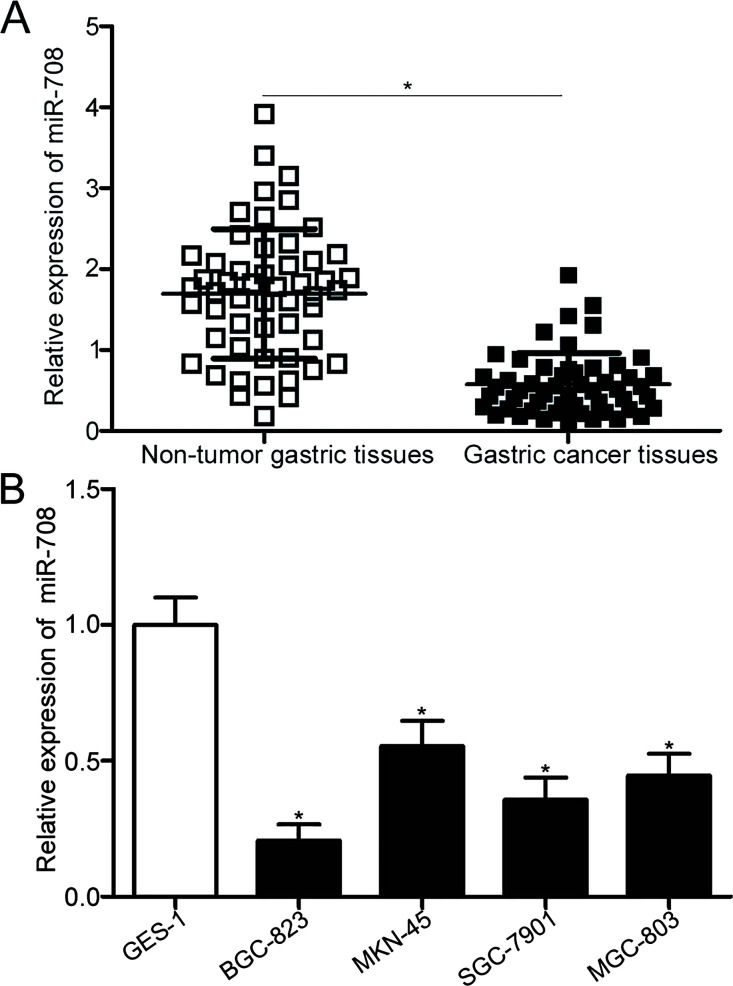
The expression level of miR-708 was detected in the 53 paired tissues using RT-qPCR to determine whether miR-708 was involved in the onset and development of gastric cancer. The data clearly showed that miR-708 was downregulated in gastric cancer tissues compared to adjacent nontumor gastric tissues (p < 0.05) (Fig. 1A). Subsequently, the association between miR-708 expression and clinicopathological features was investigated to determine the clinical values of miR-708. All gastric cancer patients were divided into two groups, namely, miR-708 low-expression group (n = 27) and miR-708 high-expression group (n = 26), on the basis of the median expression level of miR-708. As shown in Table 1, the downregulated miR-708 expression was significantly associated with lymphatic metastasis (p = 0.040), invasive depth (p = 0.019), and TNM stage (p = 0.038). However, no association was found between miR-708 and other clinicopathological factors, including age, sex, tumor size, and differentiation (all p > 0.05). Moreover, RT-qPCR was performed to measure miR-708 expression in four gastric cancer cell lines (BGC-823, MKN-45, SGC-7901, and MGC-803). Compared with the human gastric epithelial cell line GES-1, the expression level of miR-708 was lower in all detected gastric cancer cell lines (p < 0.05) (Fig. 1B). These results suggest that miR-708 may be associated with gastric cancer initiation and progression.
Figure 1.
Relative expression of microRNA-708 (miR-708) in gastric cancer tissues and cell lines. (A) Expression levels of miR-708 in 53 paired gastric cancer tissues and adjacent nontumor gastric tissues were determined using reverse transcription quantitative polymerase chain reaction (RT-qPCR). *p < 0.05 compared with nontumor gastric tissues. (B) RT-qPCR analysis was conducted to detect miR-708 expression in four gastric cancer cell lines (BGC-823, MKN-45, SGC-7901, and MGC-803) and a human gastric epithelial cell line, GES-1. *p < 0.05 compared with GES-1.
Table 1.
The Association Between MicroRNA-708 Expression and Clinicopathological Characteristics of Patients With Gastric Cancer
| Characteristics | No. | MicroRNA-708 Expression | p | |
|---|---|---|---|---|
| Low | High | |||
| Age | 0.288 | |||
| <55 years | 18 | 11 | 7 | |
| ≥55 years | 35 | 16 | 19 | |
| Sex | 0.968 | |||
| Male | 31 | 13 | 18 | |
| Female | 22 | 14 | 8 | |
| Tumor size | 0.341 | |||
| <4 cm | 23 | 10 | 13 | |
| ≥4 cm | 30 | 17 | 13 | |
| Differentiation | 0.335 | |||
| Well and moderate | 27 | 12 | 15 | |
| Poor and signet | 26 | 15 | 11 | |
| Lymphatic metastasis | 0.040 | |||
| Absent | 25 | 9 | 16 | |
| Present | 28 | 18 | 10 | |
| Invasive depth | 0.019 | |||
| T1 + T2 | 28 | 10 | 18 | |
| T3 + T4 | 25 | 17 | 8 | |
| TNM stage | 0.038 | |||
| I–II | 21 | 7 | 14 | |
| III–IV | 33 | 20 | 12 | |
miR-708 Inhibits Cell Proliferation and Invasion in Gastric Cancer
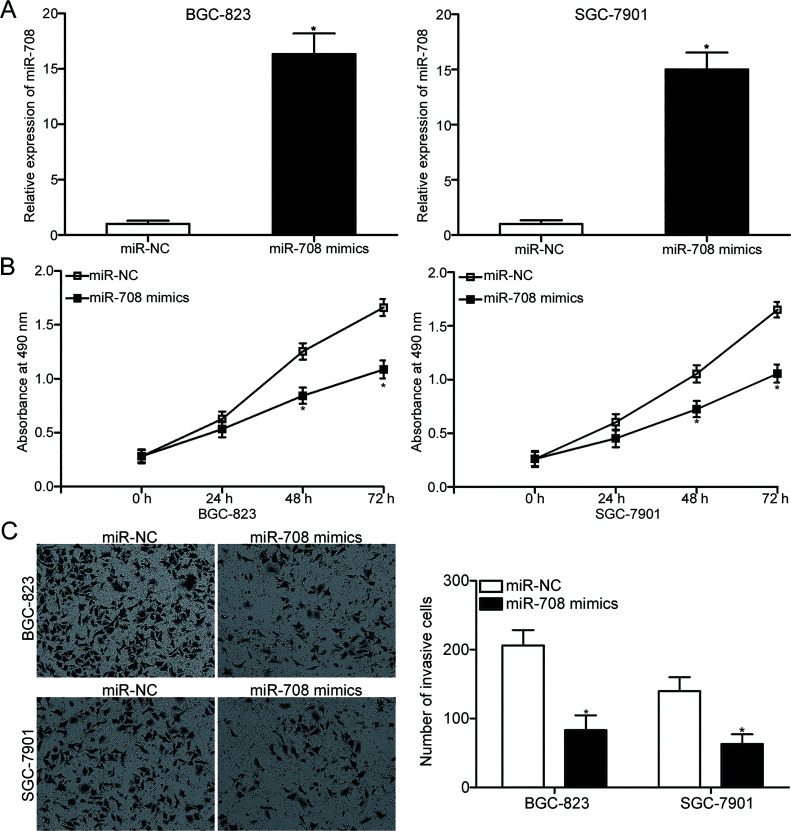
BGC-823 and SGC-7901 cells were transfected with miR-708 mimic or miR-NC to explore the biological roles of miR-708 in gastric cancer. The RT-qPCR results showed that miR-708 was markedly overexpressed in BGC-823 and SGC-7901 cells transfected with miR-708 mimic compared to cells transfected with miR-NC (p < 0.05) (Fig. 2A). MTT assay was performed to evaluate the effect of miR-708 overexpression on gastric cancer cell proliferation. Figure 2B shows that the upregulated expression of miR-708 attenuated the proliferative abilities of BGC-823 and SGC-7901 cells (p < 0.05). We then determined whether resumption of expression of miR-708 affected the invasion capacities of gastric cancer cell lines. The results of the Matrigel invasion assay revealed that the enforced expression of miR-708 decreased the invasion of BGC-823 and SGC-7901 cells (p < 0.05) (Fig. 2C). These results suggest that miR-708 may serve tumor-suppressive roles in gastric cancer growth and metastasis.
Figure 2.
miR-708 overexpression attenuates the proliferation and invasion of BGC-823 and SGC-7901 cells. (A) BGC-823 and SGC-7901 cells were transfected with miR-708 mimic or miRNA mimic negative control (miR-NC). After transfection for 48 h, total RNA was isolated and then subjected to RT-qPCR to detect the miR-708 expression. *p < 0.05 compared with miR-NC. (B) MTT assay was employed to study the effect of miR-708 overexpression on the proliferation of BGC-823 and SGC-7901 cells. *p < 0.05 compared with miR-NC. (C) Cell invasion ability was evaluated in BGC-823 and SGC-7901 cells transfected with miR-708 mimics or miR-NC using Matrigel invasion assay. *p < 0.05 compared with miR-NC.
Notch1 Is a Target of miR-708 in Gastric Cancer
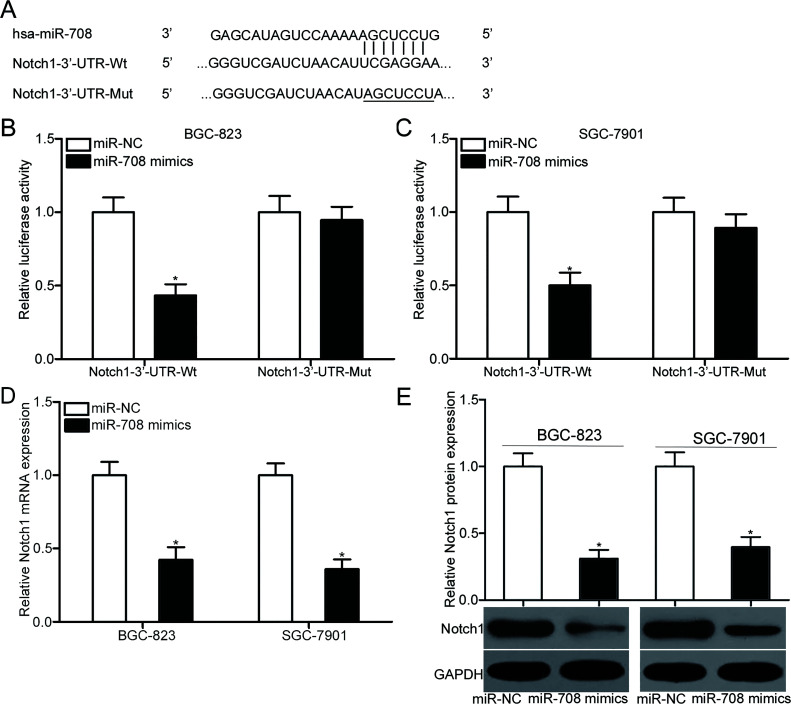
Bioinformatics analysis was conducted to search for the potential targets of miR-708 to elucidate the mechanisms by which miR-708 suppressed gastric cancer cell proliferation and invasion. Notch1 (Fig. 3A) was predicted as a candidate target of miR-708 and selected for further confirmation because this gene was found to contribute to the regulation of gastric carcinogenesis and progression21–24. This prediction was confirmed by transfecting the luciferase reporter plasmid containing the Wt or Mut putative binding sites in the 3′-UTR of Notch1, along with miR-708 mimic or miR-NC, into BGC-823 and SGC-7901 cells. The results of the dual-luciferase reporter assays showed that luciferase activities were significantly reduced in BGC-823 and SGC-7901 cells cotransfected with pMIR-Notch1-3′-UTR-Wt and miR-708 mimics relative to cells cotransfected with pMIR-Notch1-3′-UTR-Wt and miR-NC (p < 0.05). However, luciferase activities were unaffected in BGC-823 and SGC-7901 cells cotransfected with the pMIR-Notch1-3′-UTR-Mut and miR-708 mimics (Fig. 3B and C), suggesting that miR-708 directly targeted the 3′-UTR of Notch1. Furthermore, RT-qPCR and Western blot analysis were performed to assess the effect of miR-708 overexpression on endogenous Notch1 expression. Figure 3D and E show that the mRNA (p < 0.05) and protein (p < 0.05) expression of Notch1 was significantly downregulated in BGC-823 and SGC-7901 cells after transfection with miR-708 mimic. Thus, Notch1 is a direct target gene of miR-708 in gastric cancer.
Figure 3.
Notch1 is a direct target of miR-708 in gastric cancer. (A) The putative wild-type (Wt) and mutant (Mut) miR-708 binding sequences in the 3′-untranslated region (3′-UTR) of Notch1 are shown. (B, C) BGC-823 and SGC-7901 cells were cotransfected with miR-708 mimics or miR-NC and luciferase reporter plasmid carrying Wt or Mut binding sites for miR-708 in the 3′-UTR of Notch1. Then 48 h posttransfection, cells were harvested and subjected to the analysis of luciferase activity using dual-luciferase reporter assays. *p < 0.05 compared with miR-NC. (D, E) RT-qPCR and Western blot analysis were conducted to measure the expression of Notch1 at the mRNA and protein levels, respectively, in BGC-823 and SGC-7901 cells transfected with miR-708 mimics or miR-NC. *p < 0.05 compared with miR-NC.
miR-708 and Notch1 Are Clinically Relevant in Gastric Cancer Tissues
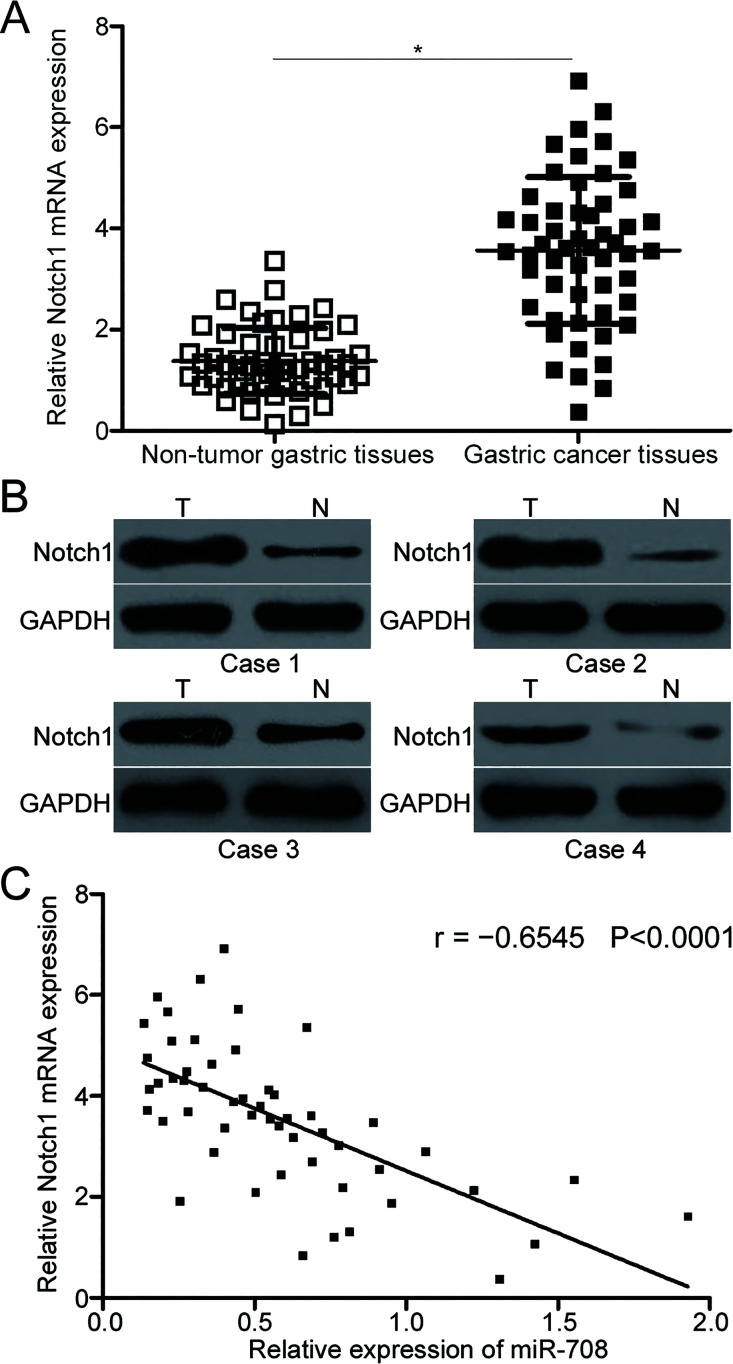
RT-qPCR was conducted to detect Notch1 expression in the 53 paired tissues to further illustrate the association between miR-708 and Notch1 in gastric cancer. The results indicated that the expression level of Notch1 mRNA was significantly upregulated in the gastric cancer tissues compared to the adjacent nontumor gastric tissues (p < 0.05) (Fig. 4A). Additionally, Western blot analysis confirmed that Notch1 protein was also highly expressed in gastric cancer tissues relative to that in the adjacent nontumor gastric tissues (Fig. 4B). Furthermore, a negative association was found between the miR-708 and Notch1 mRNA expression levels in the gastric cancer tissues (r = −0.6545, p < 0.0001) (Fig. 4C). These results further demonstrated that Notch1 is a direct target gene of miR-708 in gastric cancer.
Figure 4.
Notch1 is overexpressed in gastric cancer tissues and inversely correlated with miR-708 level. (A) Notch1 mRNA expression was measured in 53 paired gastric cancer tissues and adjacent nontumor gastric tissues by RT-qPCR. *p < 0.05 compared with nontumor gastric tissues. (B) The expression levels of Notch1 protein in the gastric cancer tissues and adjacent nontumor gastric tissues were determined by Western blot analysis. (C) Spearman’s correlation analysis was applied to evaluate the association between miR-708 and Notch1 mRNA in gastric cancer tissues. r = −0.6545, p < 0.0001.
Reintroduction of Notch1 Counteracts the Tumor-Suppressive Effects of miR-708 Overexpression in Gastric Cancer
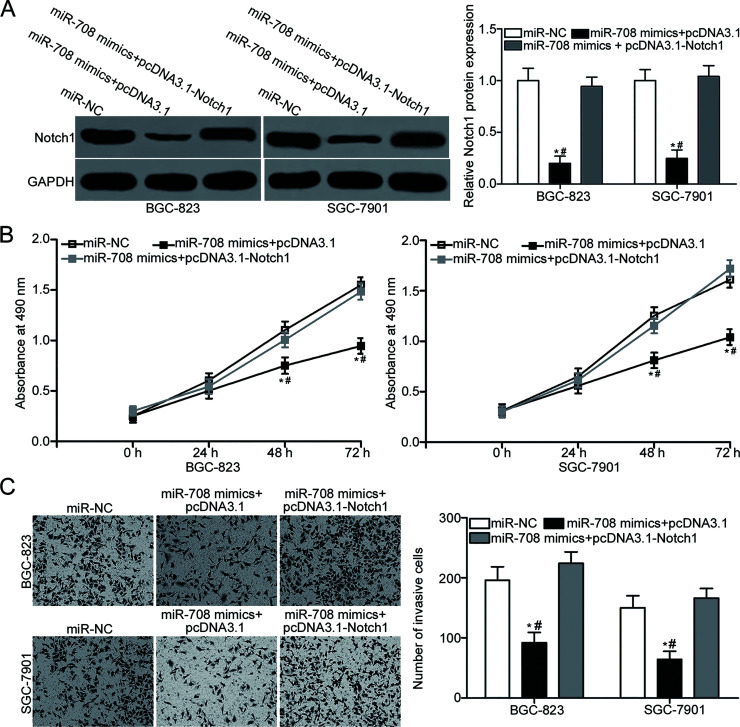
A series of rescue experiments were performed to investigate whether Notch1 was responsible for the suppressive roles of miR-708 in gastric cancer. miR-708 mimic and Notch1 overexpression plasmid pcDNA3. 1-Notch1 or empty pcDNA3.1 plasmid were introduced in BGC-823 and SGC-7901 cells. Then 72 h after transfection, Western blot analysis was performed, and the results indicated that cotransfection of pcDNA3.1-Notch1 partially abrogated the miR-708-mediated downregulation of Notch1 in BGC-823 and SGC-7901 cells (p < 0.05) (Fig. 5A). Subsequent MTT and Matrigel invasion assays indicated that reintroduction of Notch1 rescued the inhibitory effects on BGC-823 and SGC-7901 cell proliferation (p < 0.05) (Fig. 5B) and invasion (p < 0.05) (Fig. 5C) caused by miR-708 overexpression. These results clearly suggested that miR-708 may play tumor-suppressive roles in gastric cancer, at least in part, by inhibiting Notch1.
Figure 5.
Restored Notch1 expression rescues the suppressive effects of miR-708 on gastric cancer cell proliferation and invasion. BGC-823 and SGC-7901 cells were transfected with miR-708 mimics together with pcDNA3.1 or pcDNA3.1-Notch1. (A) After transfection for 72 h, Western blot analysis was conducted to detect Notch1 protein expression. *p < 0.05 compared with miR-NC. #p < 0.05 compared with miR-708 mimics + pcDNA3.1-Notch1. (B) MTT and (C) Matrigel invasion assays were employed to determine cell proliferation and invasion in the above different treated cells. *p < 0.05 compared with miR-NC. #p < 0.05 compared with miR-708 mimics + pcDNA3.1-Notch1.
DISCUSSION
Growing evidence demonstrated that numerous miRNAs may participate in the regulation of gastric carcinogenesis and progression. This phenomenon suggests that gastric cancer-related miRNAs can be identified as effective therapeutic targets for this disease25–27. In this study, miR-708 was underexpressed in gastric cancer tissues and cell lines. Downregulated miR-708 expression was significantly associated with lymphatic metastasis, invasive depth, and TNM stage. Functional assays revealed that resumption of miR-708 expression restricted cell proliferation and invasion in gastric cancer. In addition, Notch1 was identified as a direct target of miR-708 in gastric cancer. Notch1 was upregulated in the gastric cancer tissues and inversely correlated with the miR-708 expression level. Moreover, restored Notch1 expression reversed the tumor-suppressive effects of miR-708 overexpression in gastric cancer. These findings suggest that miR-708 can be developed as an anticancer drug for patients with gastric cancer.
miR-708 has been widely reported to be dysregulated in multiple types of human cancer. For example, miR-708 was downregulated in hepatocellular carcinoma tissues, and the expression was obviously correlated with the Edmondson–Steiner grading and TNM stage15,16. Downregulation of miR-708 was also observed in glioblastoma17, breast cancer18, renal cancer19, and melanoma28. However, miR-708 was upregulated in lung adenocarcinoma, and this expression was associated with age, sex, and tumor stage29. Highly expressed miR-708 was also found in colorectal cancer30, bladder cancer31, and acute lymphoblastic leukemia32. These conflicting findings indicate that there is a tissue specificity of miR-708 expression in human malignancy, and miR-708 may be investigated as a novel marker of diagnosis in these specific cancer types.
miR-708 is considered to play key roles in the formation and progression of human cancers. For instance, restoration expression of miR-708 inhibited cell proliferation, migration, and invasion of hepatocellular carcinoma15,16. Guo et al. showed that ectopic expression of miR-708 attenuated cell proliferation and invasion, as well as promoted apoptosis, in glioblastoma17. Ma et al. indicated that miR-708 overexpression prohibited breast cancer cell proliferation and invasion18. Kim et al. demonstrated that enforced expression of miR-708 decreased cell proliferation, colony formation ability, and metastasis, as well as promoted apoptosis and increased the cell sensitivity to anticancer drugs in renal cancer and reduced tumor growth in vivo19. In a study of melanoma, Song et al. revealed that miR-708 reexpression suppressed cell growth, metastasis, and epithelial–mesenchymal transition, as well as induced cell apoptosis28. Nevertheless, miR-708 served as an oncogene in lung adenocarcinoma by regulating cell growth and metastasis29. Lei et al. found that upregulation of miR-708 increased cell proliferation and invasion in colorectal cancer30. Song et al. revealed that suppression of miR-708 restricted cell proliferation and induced apoptosis in bladder cancer31. Zhang et al. reported that inhibition of miR-708 repressed cell proliferation, promoted apoptosis, and induced cell cycle arrest of acute lymphoblastic leukemia32. These findings suggest that the biological roles of miR-708 in tumorigenesis and development have tissue specificity, and miR-708 may be developed as an efficient therapeutic target for treatment of patients with these cancer types.
Various targets have been validated, including mothers against decapentaplegic 3 (SMAD3)15 in hepatocellular carcinoma, lysine demethylase 1 (LSD1)18 in breast cancer, long form of the cellular FLICE-like inhibitory protein (c-FLIPL)19 in renal cancer, lymphoid enhancer-binding factor 1 (LEF1)28 in melanoma, transmembrane protein 88 (TMEM88)29 in lung adenocarcinoma, cyclin-dependent kinase inhibitor 2B (CDKN2B)30 in colorectal cancer, caspase 231 in bladder cancer, and Dickkopf homolog 3 (DKK3)32 in acute lymphoblastic leukemia. In our study, Notch1, a member of the Notch receptors, was identified as a direct target of miR-708 in gastric cancer. This receptor was previously reported to be overexpressed in numerous types of human cancer, such as breast cancer33, ovarian cancer34, colorectal cancer35, bladder cancer36, renal cell carcinoma37, and lung cancer38. In gastric cancer, the expression level of Notch1 was higher in gastric cancer tissues and strongly associated with tumor size, differentiation grade, depth of invasion, and vessel invasion. Gastric cancer patients with high Notch1 expression had shorter 3-year survival rate than those with low Notch1 level. Moreover, Notch1 expression was confirmed as an independent prognostic factor of patients with gastric cancer21. Additionally, Notch1 participates in the onset and progression of gastric cancer by regulating proliferation, apoptosis, colony formation, and metastasis22–24. Hence, targeting Notch1 may be an effective therapeutic strategy for gastric cancer.
In conclusion, this study demonstrated that miR-708 was significantly reduced in gastric cancer, and this dysregulation was correlated with lymphatic metastasis, invasive depth, and TNM stage. In vitro studies revealed that miR-708 overexpression inhibited the proliferation and invasion of gastric cancer cells. Notch1 was mechanistically identified as a direct target gene of miR-708 in gastric cancer, and miR-708 might be a novel effective target for treatment of patients with this disease.
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- 2. Majeed W, Iftikhar A, Khaliq T, Aslam B, Muzaffar H, Atta K, Mahmood A, Waris S. Gastric carcinoma: Recent trends in diagnostic biomarkers and molecular targeted therapies. Asian Pac J Cancer Prev. 2016;17:3053–60. [PubMed] [Google Scholar]
- 3. Cheng XJ, Lin JC, Tu SP. Etiology and prevention of gastric cancer. Gastrointest Tumors 2016;3:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Compare D, Rocco A, Nardone G. Risk factors in gastric cancer. Eur Rev Med Pharmacol Sci. 2010;14:302–8. [PubMed] [Google Scholar]
- 5. Kim SJ, Wang YG, Lee HW, Kang HG, La SH, Choi IJ, Irimura T, Ro JY, Bresalier RS, Chun KH. Up-regulation of neogenin-1 increases cell proliferation and motility in gastric cancer. Oncotarget 2014;5:3386–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Terry MB, Gaudet MM, Gammon MD. The epidemiology of gastric cancer. Semin Radiat Oncol. 2002;12:111–27. [DOI] [PubMed] [Google Scholar]
- 7. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 8. Kota J, Chivukula RR, O’Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR, Mendell JR, Mendell JT. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell 2009;137:1005–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farazi TA, Spitzer JI, Morozov P, Tuschl T. miRNAs in human cancer. J Pathol. 2011;223:102–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell 2009;136:215–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Assumpcao MB, Moreira FC, Hamoy IG, Magalhaes L, Vidal A, Pereira A, Burbano R, Khayat A, Silva A, Santos S, Demachki S, Ribeiro-Dos-Santos A, Assumpcao P. High-throughput miRNA sequencing reveals a field effect in gastric cancer and suggests an epigenetic network mechanism. Bioinform Biol Insights 2015;9:111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell 2005;122:6–7. [DOI] [PubMed] [Google Scholar]
- 13. Winter J, Diederichs S. MicroRNA biogenesis and cancer. Methods Mol Biol. 2011;676:3–22. [DOI] [PubMed] [Google Scholar]
- 14. Calin GA, Croce CM. MicroRNA-cancer connection: The beginning of a new tale. Cancer Res. 2006;66:7390–4. [DOI] [PubMed] [Google Scholar]
- 15. Li Q, Li S, Wu Y, Gao F. miRNA-708 functions as a tumour suppressor in hepatocellular carcinoma by targeting SMAD3. Oncol Lett. 2017;14:2552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li G, Yang F, Xu H, Yue Z, Fang X, Liu J. MicroRNA-708 is downregulated in hepatocellular carcinoma and suppresses tumor invasion and migration. Biomed Pharmacother. 2015;73:154–9. [DOI] [PubMed] [Google Scholar]
- 17. Guo P, Lan J, Ge J, Nie Q, Mao Q, Qiu Y. miR-708 acts as a tumor suppressor in human glioblastoma cells. Oncol Rep. 2013;30:870–6. [DOI] [PubMed] [Google Scholar]
- 18. Ma L, Ma S, Zhao G, Yang L, Zhang P, Yi Q, Cheng S. miR-708/LSD1 axis regulates the proliferation and invasion of breast cancer cells. Cancer Med. 2016;5:684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim EA, Kim SW, Nam J, Sung EG, Song IH, Kim JY, Kwon TK, Lee TJ. Inhibition of c-FLIPL expression by miRNA-708 increases the sensitivity of renal cancer cells to anti-cancer drugs. Oncotarget 2016;7:31832–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 21. Li DW, Wu Q, Peng ZH, Yang ZR, Wang Y. [Expression and significance of Notch1 and PTEN in gastric cancer]. Ai Zheng 2007;26:1183–7. [PubMed] [Google Scholar]
- 22. Zhang XS, Hu YH, Gao HY, Lan XW, Xue YW. Downregulation of Notch1 inhibits the invasion and metastasis of human gastric cancer cells SGC7901 and MKN74 in vitro through PTEN activation and dephosphorylation of Akt and FAK. Mol Med Rep. 2017;16:2318–24. [DOI] [PubMed] [Google Scholar]
- 23. Yeh TS, Wu CW, Hsu KW, Liao WJ, Yang MC, Li AF, Wang AM, Kuo ML, Chi CW. The activated Notch1 signal pathway is associated with gastric cancer progression through cyclooxygenase-2. Cancer Res. 2009;69:5039–48. [DOI] [PubMed] [Google Scholar]
- 24. Hsu KW, Hsieh RH, Huang KH, Fen-Yau Li A, Chi CW, Wang TY, Tseng MJ, Wu KJ, Yeh TS. Activation of the Notch1/STAT3/Twist signaling axis promotes gastric cancer progression. Carcinogenesis 2012;33:1459–67. [DOI] [PubMed] [Google Scholar]
- 25. Shrestha S, Hsu SD, Huang WY, Huang HY, Chen W, Weng SL, Huang HD. A systematic review of microRNA expression profiling studies in human gastric cancer. Cancer Med. 2014;3:878–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhu X, Lv M, Wang H, Guan W. Identification of circulating microRNAs as novel potential biomarkers for gastric cancer detection: A systematic review and meta-analysis. Dig Dis Sci. 2014;59:911–9. [DOI] [PubMed] [Google Scholar]
- 27. Rao M, Zhu Y, Zhou Y, Cong X, Feng L. MicroRNA-122 inhibits proliferation and invasion in gastric cancer by targeting CREB1. Am J Cancer Res. 2017;7:323–33. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28. Song XF, Wang QH, Huo R. Effects of microRNA-708 on epithelial-mesenchymal transition, cell proliferation and apoptosis in melanoma cells by targeting LEF1 through the wnt signaling pathway. Pathol Oncol Res. 2017. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29. Jang JS, Jeon HS, Sun Z, Aubry MC, Tang H, Park CH, Rakhshan F, Schultz DA, Kolbert CP, Lupu R, Park JY, Harris CC, Yang P, Jen J. Increased miR-708 expression in NSCLC and its association with poor survival in lung adenocarcinoma from never smokers. Clin Cancer Res. 2012;18:3658–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lei SL, Zhao H, Yao HL, Chen Y, Lei ZD, Liu KJ, Yang Q. Regulatory roles of microRNA-708 and microRNA-31 in proliferation, apoptosis and invasion of colorectal cancer cells. Oncol Lett. 2014;8:1768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Song T, Zhang X, Zhang L, Dong J, Cai W, Gao J, Hong B. miR-708 promotes the development of bladder carcinoma via direct repression of Caspase-2. J Cancer Res Clin Oncol. 2013;139:1189–98. [DOI] [PubMed] [Google Scholar]
- 32. Zhang Y, Li H, Cao R, Sun L, Wang Y, Fan S, Zhao Y, Kong D, Cui L, Lin L, Wang K, Li Y, Zhou J. Suppression of miR-708 inhibits the Wnt/beta-catenin signaling pathway by activating DKK3 in adult B-all. Oncotarget 2017;8:64114–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhong Y, Shen S, Zhou Y, Mao F, Lin Y, Guan J, Xu Y, Zhang S, Liu X, Sun Q. NOTCH1 is a poor prognostic factor for breast cancer and is associated with breast cancer stem cells. Onco Targets Ther. 2016;9:6865–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alniaimi AN, Demorest-Hayes K, Alexander VM, Seo S, Yang D, Rose S. Increased Notch1 expression is associated with poor overall survival in patients with ovarian cancer. Int J Gynecol Cancer 2015;25:208–13. [DOI] [PubMed] [Google Scholar]
- 35. Chu D, Zhou Y, Zhang Z, Li Y, Li J, Zheng J, Zhang H, Zhao Q, Wang W, Wang R, Ji G. Notch1 expression, which is related to p65 status, is an independent predictor of prognosis in colorectal cancer. Clin Cancer Res. 2011;17:5686–94. [DOI] [PubMed] [Google Scholar]
- 36. Sima J, Zhu MY, Ai Q, Zhang C, Yao ZY, Huang QB, Zhang X. [Expression analysis of NOTCH1/HES1/PTEN signaling pathway in invasive bladder transitional cell carcinoma]. Zhonghua Yi Xue Za Zhi. 2012;92:964–7. [PubMed] [Google Scholar]
- 37. Ai Q, Ma X, Huang Q, Liu S, Shi T, Zhang C, Zhu M, Zhang Y, Wang B, Ni D, Li H, Zheng T, Zhang X. High-level expression of Notch1 increased the risk of metastasis in T1 stage clear cell renal cell carcinoma. PLoS One 2012;7:e35022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kikuchi H, Sakakibara-Konishi J, Furuta M, Yokouchi H, Nishihara H, Yamazaki S, Uramoto H, Tanaka F, Harada M, Akie K, Sugaya F, Fujita Y, Takamura K, Kojima T, Harada T, Higuchi M, Honjo O, Minami Y, Watanabe N, Oizumi S, Suzuki H, Ishida T, Dosaka-Akita H, Isobe H, Munakata M, Nishimura M. Expression of Notch1 and Numb in small cell lung cancer. Oncotarget 2017;8:10348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]