Abstract
We previously identified novel S100A8/A9 receptors, extracellular matrix metalloproteinase inducer (EMMPRIN), melanoma cell adhesion molecule (MCAM), activated leukocyte cell adhesion molecule (ALCAM), and neuroplastin (NPTN) β, that are critically involved in S100A8/A9-mediated cancer metastasis and inflammation when expressed at high levels. However, little is known about the presence of any cancer-specific mechanism(s) that modifies these receptors, further inducing upregulation at protein levels without any transcriptional regulation. Expression levels of glycosyltransferase-encoding genes were examined by a PCR-based profiling array followed by confirmation with quantitative real-time PCR. Cell migration and invasion were assessed using a Boyden chamber. Western blotting was used to examine the protein level, and the RNA level was examined by Northern blotting. Immunohistochemistry was used to examine the expression pattern of β-1,3-galactosyl-O-glycosyl-glycoprotein β-1,6-N-acetylglucosaminyltransferase 3 (GCNT3) and MCAM in melanoma tissue. We found that GCNT3 is overexpressed in highly metastatic melanomas. Silencing and functional inhibition of GCNT3 greatly suppressed migration and invasion of melanoma cells, resulting in the loss of S100A8/A9 responsiveness. Among the novel S100A8/A9 receptors, GCNT3 favorably glycosylates the MCAM receptor, extending its half-life and leading to further elevation of S100A8/A9-mediated cellular motility in melanoma cells. GCNT3 expression is positively correlated to MCAM expression in patients with high-grade melanomas. Collectively, our results showed that GCNT3 is an upstream regulator of MCAM protein and indicate the possibility of a potential molecular target in melanoma therapeutics through abrogation of the S100A8/A9–MCAM axis.
Key words: S100A8/A9, GCNT3, Glycosylation, Receptor, Metastasis
INTRODUCTION
A single glycosyltransferase is able to induce glycosylation of many different proteins, especially in integrated membrane proteins and secreted proteins, through an endoplasmic reticulum (ER)/Golgi pathway1–4. Therefore, a simple change of enzymes at expression levels can affect the function of many different glycoproteins, which in turn will significantly influence cell responsiveness1,4, metabolism1,4, growth1,5, and motility1,6, leading to the development and progression of several diseases1–6. Accumulating evidence indicates that glycosylation changes are involved in tumor progression1–11. One notable involvement is in tumor cell dissemination3,8,9, since interactive communication between cells or between cells and surrounding tissue is strongly regulated by the relationship between ligand and receptor molecules, most of which are glycoproteins.
S100A8 and S100A9 are small EF-hand calcium-binding proteins belonging to the S100 family and have been shown to be highly expressed in and secreted by keratinocytes, myeloid cells, and neutrophils under inflammatory conditions in vitro and in vivo12,13. They physiologically form a heterodimer complex, simply termed S100A8/A9 or calprotectin13,14, a major functional form reported to be in close association with cancer metastasis13,15–19. The secreted extracellular S100A8/A9 functions as ligands to the canonical receptor Toll-like receptor 4 (TLR4)15,20 and the receptor for advanced glycation end products (RAGE)16,21,22 on the surfaces of cells, triggering cancer cell dissemination. We have been studying this interesting heterodimer protein for a long time to determine its biological significance in cancer progression18,19. Our search for other unknown receptors for S100A8/A9 resulted in the discovery of novel S100A8/A9 receptors in cancer cells, extracellular matrix metalloproteinase inducer (EMMPRIN)18, melanoma cell adhesion molecule (MCAM), and activated leukocyte cell adhesion molecule (ALCAM)19, which play a critical role in cancer metastasis. Together, we termed these receptor proteins “S100 soil sensor receptors” or simply SSSRs19. These receptors are highly glycosylated, owing to their cell surface location as receptors utilizing the ER/Golgi pathway. In a study by Bubka et al., glycosylation of MCAM by mannosyl (β-1,4-)-glycoprotein β-1,4-N-acetyl-glucosaminyltransferase (MGAT3) or mannosyl (α-1,6-)-glycoprotein β-1,6-N-acetyl-glucosaminyltransferase (MGAT5) did not result in significant differences in the viability of cancer cells and the capability of cancer cells to migrate through the endothelial layer23. Even so, it is still not known whether there is any cancer-specific glycosylation of SSSRs and whether these modifications have any functionally important properties.
In this study, we found that the β-1,3-galactosyl-O-glycosyl-glycoprotein β-1,6-N-acetylglucosaminyltransferase 3 (GCNT3) was efficiently upregulated in malignant melanoma cells, lung cancer cells, and mesothelioma cells compared to their nonmalignant immortalized cell counterparts. GCNT3 induced a marked increase in S100A8/A9-mediated cell migration and invasion through the functional activation of MCAM, but other SSSRs, EMMPRIN, RAGE, and ALCAM, did not. Interestingly, we also found that GCNT3-mediated glycosylation of MCAM is elevated in cancer cells that are linked to an increase in stability between the engagement of S100A8/A9 and MCAM. These novel findings provide insights into the pivotal role of GCNT3-mediated MCAM glycosylation in S100A8/A9-mediated cancer progression.
MATERIALS AND METHODS
Cell Culture and Chemicals
The following two human melanoma cell lines established from the same patient were used: WM-115 (derived from the primary tumor; ATCC, Rockville, MD, USA) and WM-266-4 (derived from a metastatic site; ATCC). These cell lines were cultivated in D/F medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Intergen, Purchase, NY, USA). Talniflumate, a novel and specific inhibitor of GCNT37, and cycloheximide, an inhibitor of protein translation, were purchased from commercial sources, Tokyo Chemical Industry (Tokyo, Japan) and Sigma-Aldrich (St. Louis, MO, USA), respectively.
Plasmid Constructs
For temporal expression, we used the pIDT-SMART (C-TSC) vector, also named pCMViR-TSC24. A series of cDNAs was inserted into pIDT-SMART (C-TSC). Human cDNAs encoding full-length RAGE25, EMMPRIN18, MCAM, and ALCAM19 were designed for expression as C-terminal 3xHA-6His-tagged forms. Human cDNAs encoding full-length polypeptide N-acetylgalactosaminyltransferase 12 (GALNT12), GCNT3, and mannosyl (α-1,3-)-glycoprotein β-1,4-N-acetylglucosaminyltransferase, isozyme A (MGAT4A) were designed for expression as C-terminal 3xMyc-6His-tagged forms. A catalytic dead GCNT3 (mutGCNT3) cDNA was designed for expression as C-terminal deletion form (lacking the region from 323 aa through 438 aa) with the same epitope, 3xMyc-6His, at its C-terminal end. Cells were transiently transfected with the plasmid vectors as described above using FuGENE HD (Promega BioSciences, San Luis Obispo, CA, USA).
We established stable clones, which showed indefinitely stable expression of the aberrant GCNT3-Myc with much higher efficiency. The clones were established by a convenient electroporation gene delivery method using an improved plasmid construct based on the pIDT-SMART (C-TSC) vector24.
RNA Interference
Predesigned siRNAs for human GCNT3 (#I, ID No. s17676; #II, ID No. s17677; #III, ID No. s17678) and control siRNA (silencer negative control 2 siRNA) were purchased from Ambion (Austin, TX, USA). siRNAs were transfected using Lipofectamin RNAiMAX reagent (Invitrogen).
S100A8/A9 Recombinant Protein
S100A8/A9 was prepared as reported previously19. In brief, human S100A8/A9 recombinant protein was expressed using the FreeStyle 293 Expression System (Invitrogen), which enabled the production of a large amount of secreted S100A8/A9 in the culture medium. The secreted S100A8/A9 was then purified by Talon® Metal Affinity Resin (Takara Bio, Shiga, Japan).
Western Blotting
Western blot analysis was performed under conventional conditions. The following antibodies were used: rabbit anti-human GCNT3 antibody (GeneTex, Irvine, CA, USA), rabbit anti-MCAM antibody (Epitomics Abcam, Cambridge, MA, USA), mouse anti-Myc tag antibody (clone 9B11; Cell Signaling Technology, Beverly, MA, USA), mouse anti-human β-actin (Sigma-Aldrich), and mouse anti-human tubulin antibody (Sigma-Aldrich). The rabbit anti-MCAM antibody (Epitomics Abcam) was biotinylated using a Biotin Labeling Kit-SH (Dojindo Molecular Technologies, Rockville, MD, USA) to recover antibody-free samples after immunoprecipitation using streptavidin–agarose (Invitrogen). N-acetyl-d-glucosamine (GlcNAc) bound to the precipitated MCAM protein was detected using HRP-conjugated wheat germ agglutinin (WGA; Vector Laboratories, Burlingame, CA, USA).
Northern Blotting
Ten micrograms of total RNA isolated by the acid guanidinium thiocyanate/phenol–chloroform method was electrophoresed in a 1% agarose gel and transferred to a Nytran Plus nylon membrane (GE Healthcare Bio-Sciences, Piscataway, NJ, USA). Partial coding regions of human MCAM (480 bp) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 450 bp) genes were used as biotin probes for Northern blot analysis.
Quantitative RT-PCR
Quantitative RT-PCR was performed on a LightCycler rapid thermal cycler system (ABI 7900HT; Applied Biosystems, Foster City, CA, USA) using LightCycler 480 SYBR Green I Master (Roche Diagnostics, Indianapolis, IN, USA) according to the manufacturer’s instructions. Forward and reverse primer pairs used (5′ to 3′) are listed in Supplementary Table 1 (available at https://www.dropbox.com/s/mnve32hwx0h8n64/2017-07-12%20Sumardika%20et%20al-SuppleData%20Rev.pptx?dl=0). GAPDH was used as a reference gene. The amounts of mRNA were normalized to those of GAPDH and are presented as ratios to those of the untreated control.
Invasion and Migration Assay
Cells were starved by serum-free D/F medium for 24 h prior to an invasion and migration assay. Cell invasion or migration was assayed using the Boyden chamber method with filter inserts (pore size, 8 μm) precoated (for invasion assay) or not precoated with Matrigel (for migration assay) in 24-well plates (BD Biosciences, Franklin Lakes, NJ, USA). Cells (3 × 104 cells/insert) were seeded on the top chamber. The top chamber was filled with serum-free D/F medium, and the bottom chamber was filled with 10% FBS D/F medium. The recombinant S100A8/A9 was then set in the bottom chamber at a final concentration of 100 ng/ml. After incubation for 12 h in the case of WM-266-4 cells and for 24 h in the case of WM-115 cells, cells that passed through the filter and were attached to the lower surface of the filter were counted by staining with 0.01% crystal violet in 25% methanol. Cells attached to the lower surface of the filter membrane were then quantified by cell counting in five nonoverlapping fields at 100× magnification and are presented as the average from three independent experiments.
Immunohistochemistry
The studies using patient-derived tissue sections were approved by the research ethics committees of Niigata University Medical and Dental Hospital. Written informed consent was obtained from each patient for use of the materials. Paraffin-embedded tissue specimens were collected through the donation of patient-derived tissue samples from Niigata University Medical and Dental Hospital. Immunostaining was carried out on human malignant melanoma and benign nevus tissues. The staining method was the same as that previously reported19. Briefly, immunostaining was carried out using primary antibodies to rabbit anti-MCAM antibody (Epitomics Abcam) and rabbit anti-human GCNT3 antibody (GeneTex). Final detection was performed with the AEC reagent (HISTOFINE Simple Stain AEC Solution; Nichirei Biosciences, Tokyo, Japan).
Statistical Analysis
Results are expressed as means ± SD unless otherwise indicated. Statistical analysis was performed by the Mann–Whitney U-test. Values of p ≤ 0.05 were considered statistically significant.
RESULTS
Glycosyltransferases, GALNT12, GCNT3, and MGAT4A, Are Overexpressed in Various Cancer Cells
To identify a glycosyltransferase(s) that is associated with metastatic signatures via S100A8/A9–SSSRs signaling, we first compared the expression levels of key glycosyltransferase-encoding genes in nonmetastatic WM-115 melanoma cells at the primary site and in highly metastatic WM-266-4 cells that were derived from the same patient26,27. For serial analysis, we used a PCR-based profiling array (data not shown) and subsequent confirmation by quantitative real-time PCR analysis using primers that were different to those used in the profiling array. The analysis revealed eight genes [GALNT3 (polypeptide N-acetylgalactosaminyltransferase 3), GALNT12, GCNT3, MAN1C1 (mannosidase a class 1C member 1), MGAT4A, MGAT4C (mannosyl (α-1,3-)-glycoprotein β-1,4-N-acetylglucosaminyltransferase, isozyme C), NEU3 (neuraminidase 3 (membrane sialidase)), and ST8SIA6 (ST8 α-N-acetyl-neuraminide α-2,8-sialyltransferase 6)] that were significantly upregulated in WM-266-4 cells by threefold compared to the levels in WM-115 cells (Fig. S1; supplemental figures available at https://www.dropbox.com/s/mnve32hwx0h8n64/2017-07-12%20Sumardika%20et%20al-SuppleData%20Rev.pptx?dl=0). We further analyzed the expression levels of the eight genes in other metastatic melanoma cell lines (MMAc, MeWo, A2082, and SK-Mel2) in comparison to the levels in WM-115 cells and normal human melanocytes (NMC). The results showed three genes (GALNT12, GCNT3, and MGAT4A) that outstandingly displayed high expression levels consistently in at least three metastatic cells lines compared to the levels in nonmetastatic cells, WM-115 and NMC cells (Fig. S2). We also found that these genes showed relatively high expression levels with some variations among different cancer species compared to the levels in nonmetastatic immortalized cells (Fig. S3). GALNT12 was upregulated in lung cancer cells and mesothelioma cells. GCNT3 was elevated in skin and mesothelioma cells and in some lung and breast cancer cells. MGAT4A expression level was higher in skin cancer cells and some lung cancer cells. The results suggest that these three glycosyltransferases may have a critical role in cancer progression in a manner specific to their expression in different cancer species.
GCNT3 Plays a Key Role in S100A8/A9-Mediated Cancer Motility
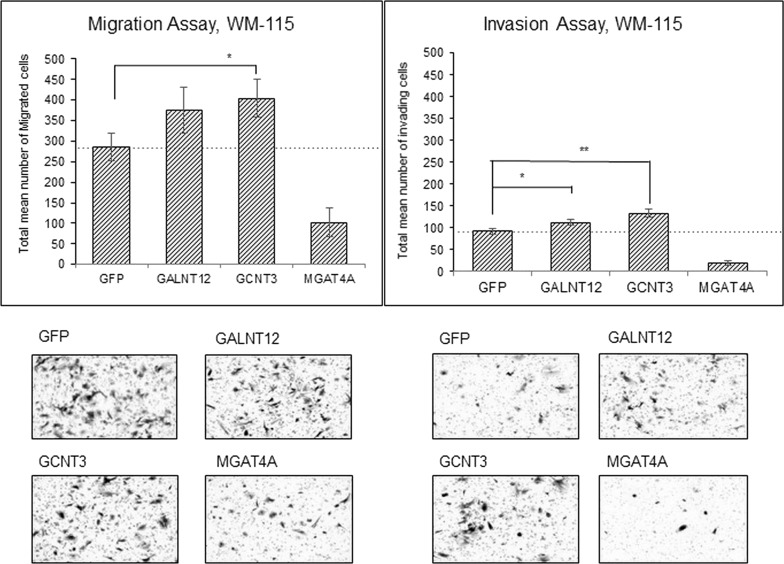
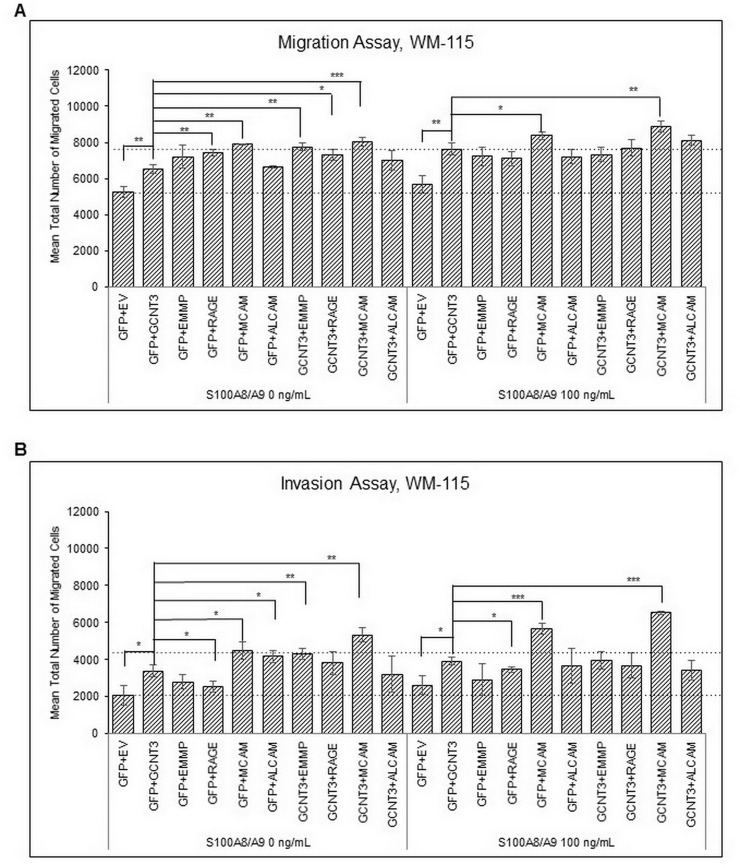
We next examined whether these three candidate genes show any correlation to S100A8/A9-mediated cancer metastasis. The Boyden chamber technique is a convenient and powerful tool for monitoring cancer motility and invasion, and we therefore used this assay system to assess cancer behavior. WM-115 cells that were ectopically overexpressed with the three candidate genes were set on the top chamber and then stimulated by the presence of S100A8/A9 in the bottom chamber. Interestingly, GALNT12 and GCNT3, but not MGAT4A, induced an increase in S100A8/A9-mediated migration as well as invasion. The highest activity was provided by the forced expression of GCNT3 (Fig. 1). A similar tendency was also observed in the assessment of the lung immortalized cell line BEAS-2B (Fig. S4). BEAS-2B cells require extracellular S100A8/A9 for their receptors to function, and we therefore examined whether overexpression of GCNT3 affects the currently identified S100A8/A9 receptors including RAGE, MCAM, ALCAM, and EMMPRIN in terms of their abilities for cellular motility upon S100A8/A9 stimulation. Through this approach, we found that MCAM was the only protein required for not only acceleration of S100A8/A9-mediated migration (Fig. 2A) but also invasion in WM-115 cells (Fig. 2B). This was more obvious in the invasion assessment. Furthermore, in BEAS-2B cells, MCAM responsiveness to S100A8/A9 during an invasion assay (Fig. S5B) was significantly increased by GCNT3 combination rather than that at migration assay (Fig. S5A). Taken together, these results indicate that GCNT3 has a role for enhancing S100A8/A9–MCAM responsiveness as a metastatic signature.
Figure 1.
Effects of glycosyltransferases on S100A8/A9-induced migration and invasion. Migration (left) and invasion (right) of WM-115 cells transfected with indicated genes [green fluorescence protein (GFP) as a control] were assessed by the Boyden chamber method. The transfected WM-115 cells were placed in the insert chamber, and purified recombinant S100A8/A9 (final concentration of 100 ng/ml) was added to the bottom well. The quantified results were viewed from the upper surface of the insert chamber membrane, and representative images of migrating or invading cells were obtained using crystal violet staining at the lower side. Data are shown as means ± SD. *p < 0.05 and **p < 0.01.
Figure 2.
Effects of GCNT3 (β-1,3-galactosyl-O-glycosyl-glycoprotein β-1,6-N-acetylglucosaminyltransferase 3) combined with each S100 soil sensor receptors (SSSR) on S100A8/A9-induced migration and invasion. Migration (A) and invasion (B) of WM-115 cells transfected with indicated combinations of the genes [empty vector (EV) and GFP used as controls] were assessed by the Boyden chamber method. The transfected WM-115 cells in the top chamber were stimulated or not stimulated with the purified recombinant S100A8/A9 (final concentration of 100 ng/ml). Data are shown as means ± SD. *p < 0.05, **p < 0.01, and ***p < 0.001.
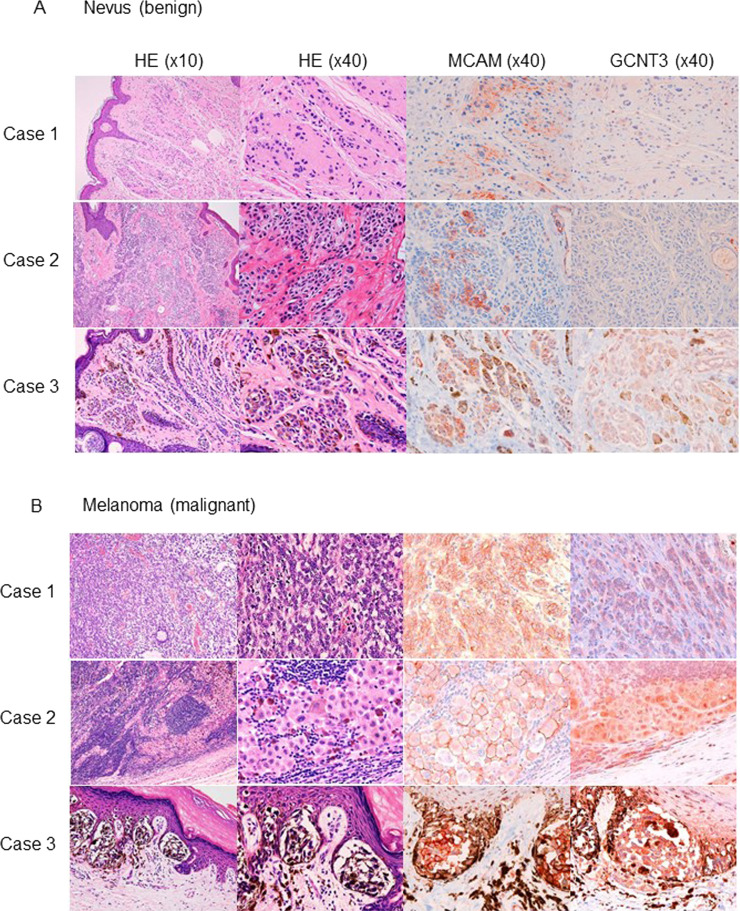
GCNT3 Expression Is Overlapped by MCAM Expression in Melanoma Tissue
We examined the clinical relevance of expression patterns of GCNT3 and MCAM using tissues obtained from melanoma patients. Because of the melanin-based brown color, we stained the melanoma tissues using red stain. As shown in Figure 3A, in nevus cases, MCAM was stained in some small parts with weak signals, and GCNT3 was hardly detected in any of the cases. On the other hand, in melanoma tissues, MCAM was strongly stained with membrane localization, and GCNT3 was also clearly positive in the intracellular area of melanoma regions (Fig. 3B). These results indicate that GCNT3 expression is positively correlated with MCAM expression in melanoma tissue.
Figure 3.
Immunohistochemistry analysis of melanoma cell adhesion molecule (MCAM) and GCNT3 in melanoma tissues. MCAM and GCNT3 were immunostained using red stain (right) in nevus (A) and melanoma (B). The images on the left are hematoxylin–eosin (H&E)-stained images corresponding to the MCAM- and GCNT3-stained images on the right.
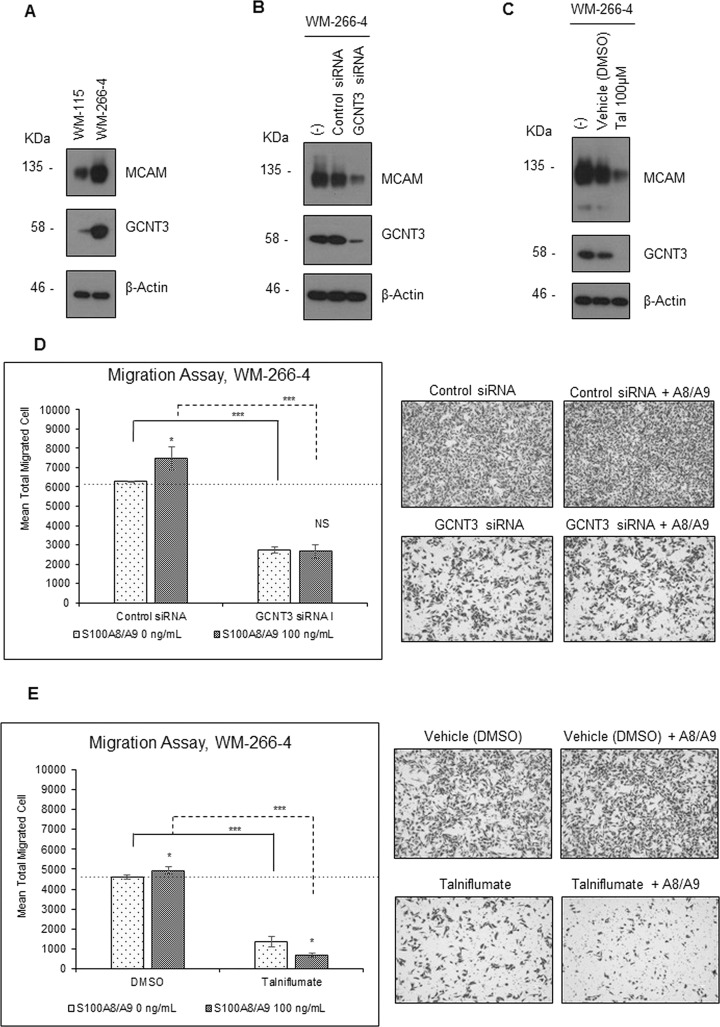
GCNT3 Regulates MCAM Content at the Protein Level
We next investigated the physiological role of GCNT3 in S100A8/A9–MCAM axis-mediated cancer motility using siRNA in WM-266-4 cells. Higher expression of GCNT3 was confirmed in WM-266-4 cells than in WM-115 cells at the protein level (Fig. 4A). We also found that MCAM was upregulated in WM-266-4 cells. Interestingly, we found that the level of MCAM was significantly decreased by the downregulation of GCNT3 using the most effective siRNA, GCNT3 siRNA I (Figs. S6 and 4B). Similarly, downregulation of MCAM was also associated with the suppression of GCNT3 by talniflumate (Fig. 4C). Talniflumate was identified as a novel selective inhibitor of GCNT3, which decreased the GCNT3 expression level7. The GCNT3 siRNA and talniflumate had no appreciable effect on MCAM expression at the mRNA level in WM-266-4 cells (Fig. S7), indicating that MCAM was critically regulated by GCNT3 at the protein level. In addition, we found that both GCNT3 siRNA-mediated GCNT3 suppression (Fig. 4D) and talniflumate-mediated GCNT3 suppression (Fig. 4E) significantly attenuated the basal ability of in vitro migration of WM-266-4 cells. Under these suppressive conditions, S100A8/A9 cellular migration never increased.
Figure 4.
Effect of inhibition of intrinsic GCNT3 on S100A8/A9-induced migration and invasion. (A) Protein levels of MCAM and GCNT3 were compared between WM-115 and WM-266-4 cells by Western blotting. Protein levels of MCAM were determine by Western blotting after treatment of WM-266-4 cells with the indicated siRNAs (final concentration of 20 nM) (B) and with talniflumate (Tal) (final concentration of 100 μM) (C). After treatment of WM-266-4 cells with the indicated siRNAs (final concentration of 20 nM) (D) or Tal (final concentration of 100 μM) (E), migration of the treated cells was assessed by the Boyden chamber method. The treated cells in the top chamber were stimulated or not stimulated with purified recombinant S100A8/A9 (final concentration of 100 ng/ml). The quantified results are displayed on the left, and representative images of migrating cells detected by crystal violet staining are shown on the right. Data are shown as means ± SD. *p < 0.05, ***p < 0.001.
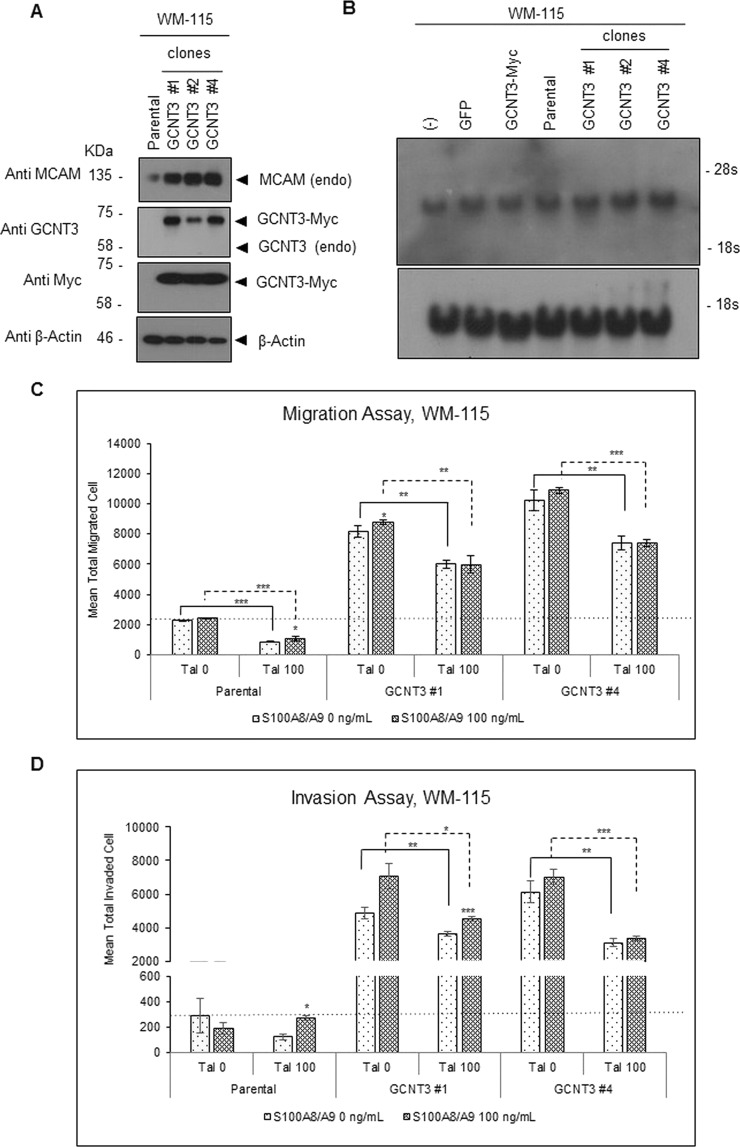
To evaluate deeply the results obtained by the GCNT3 suppression, we further studied the role of GCNT3 in cellular motility by performing an overexpression experiment. A total of 17 stable clones from WM-115 cells that expressed GCNT3 at significant levels were established beforehand (Fig. S8). Among the clones, we chose those showing particularly high expression levels compared to the others in a random manner and analyzed the protein levels of intrinsic MCAM in the selected clones. Using this approach, we found that MCAM was upregulated commonly at protein levels (Fig. 5A) but not at mRNA levels (Fig. 5B) in selected GCNT3 clones in comparison to the levels in parental WM-115 cells. In vitro migration (Fig. 5C) and invasion (Fig. 5D) assays of individual clones showed clear upregulation of basal ability as well as further enhancement in S100A8/A9 responsiveness among the GCNT3-overexpressed clones (#1 and #4). These elevations were effectively impaired by talniflumate treatment. Collectively, the results indicate that GCNT3 plays a pivotal role in the maintenance of MCAM protein at a high level, resulting in the acquisition of strong responsiveness to S100A8/A9 that is linked to increased cellular migration and invasion.
Figure 5.
Effect of aberrant overexpression of GCNT3 on S100A8/A9-induced migration and invasion. (A) Protein levels of MCAM expression were examined by Western blotting in GCNT3-stable clones established from WM-115 cells. (B) mRNA levels of MCAM expression were examined by Northern blotting. (−), nontransfection; GFP and GCNT3-Myc, transgene expression with the indicated genes; parental, equal to (−); GCNT3#1, 2, 4, stable clones for GCNT3 overexpression. All specimens were prepared from WM-115 cells. Migration (C) and invasion (D) of WM-115-derived GCNT3 stable clones were assessed by the Boyden chamber method. The cells in the top chamber were stimulated or not stimulated with the purified recombinant S100A8/A9 (final concentration of 100 ng/ml). Data are shown as means ± SD. *p < 0.05, **p < 0.01 and ***p < 0.001.
GCNT3 Regulates MCAM Stability
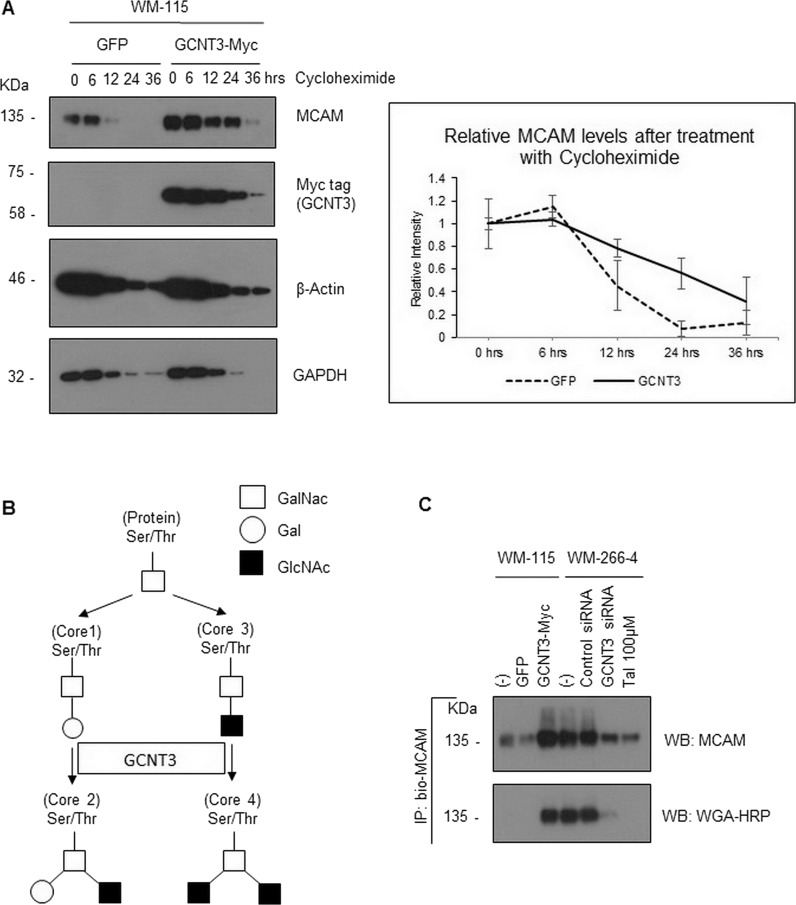
To gain an insight into the mechanisms by which GCNT3 maintains MCAM protein at a high level, we examined the half-life of intrinsic MCAM protein in WM-115 cells using cycloheximide, a general inhibitor of protein translation. Interestingly, we found that MCAM degradation was significantly delayed in cells transfected with GCNT3 but not in cells transfected with green fluorescence protein (GFP) (Fig. 6A). The difference was obvious at 12 and 24 h after treatment with cycloheximide. To determine how GCNT3 was able to have a prolonged effect on MCAM stability, we attempted to detect specific glycosylations on MCAM, since GCNT3 is a glycosyltransferase that transfers GlcNAc to N-acetylgalactosamine (GalNAc) of the core 1 acceptor structure to form the core 2 branch in the β-1,6 linkage28. In addition to the formation of the core 2 structure, GCNT3 also functions to form the core 4 structure (Fig. 6B)28. We therefore immunoprecipitated endogenous MCAM using a biotin-conjugated MCAM antibody with streptavidin beads. This method is useful to avoid detection of the precipitated antibody that may hide the MCAM band. With the aim of detecting specific glycosylations within the MCAM band, we also used 400 mM NaCl to wash out the precipitate in the hope of removing proteins that interact with MCAM and also with the beads. WGA–HRP was used to detect specific GlcNac modifications. As a result, we found that the immunoprecipitated MCAM showed higher levels of GlcNac when WM-115 cells were transfected with aberrant GCNT3. Conversely, both siRNA-mediated silencing and talniflumate-mediated inhibition of endogenous GCNT3 resulted in a significant reduction of GlcNac modification on the endogenous MCAM protein. These levels of GlcNac modification positively matched the precipitated MCAM levels (Fig. 6C).
Figure 6.
Effect of GCNT3 on MCAM stability. (A) WM-115 cells were transiently expressed with aberrant GCNT3 or GFP as a control for 24 h and then treated with cycloheximide (final concentration of 10 μM). Following the indicated time intervals, cells were collected and analyzed for their intrinsic MCAM protein levels (left). The results from three independent experiments using the same protocols for MCAM are displayed by declined curves after normalization of the MCAM bands using their matched tubulin bands and subsequently adjusting the starting points to be at 0 h and 1.0 as standards (right). (B) Proposed pathway of GCNT3-mediated glycosylation is shown schematically. (C) GlcNAc levels of MCAM protein from the prepared cell samples were determined by immunoprecipitation and following detection with HRP-conjugated wheat germ agglutinin (WGA). WM-115 cells were transiently transfected or not transfected (−) with the indicated plasmids, GFP and GCNT3-Myc. WM-266-4 cells were treated or not treated (−) with the indicated siRNAs or Tal.
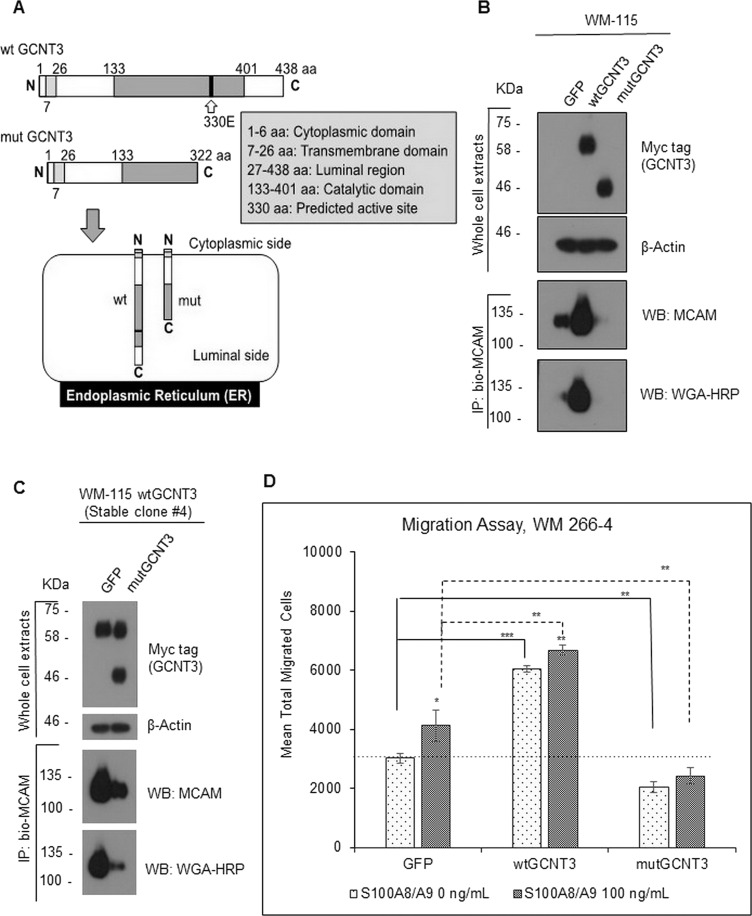
In order to confirm this, that is, to clarify the essential role of catalytic activity of GCNT3 in glycosylation on MCAM stability, we last tried to make a catalytic dead GCNT3 and assess the effect of its forced expression on the intrinsic MCAM level and on the S100A8/A9-mediated cellular migration. It has not been reported, so far, to create the catalytic dead GCNT3, so we were first in searching the catalytic domain of GCNT3 using the protein Pfam program in the EMBL-EBI public database (database http://pfam.xfam.org/protein/O95395) and structural information written in a previous report28, resulting in finding of the catalytic region (133–401 aa) and the presence of a predicted active site at 330 aa (330E). As a reference of this information, we designed to construct the expression vector to produce catalytic dead mutant of GCNT3 (mutGCNT3) that stands for partial deletion of the catalytic domain of the protein (lacking the region from 323 aa through the C-terminal end). The deleted region includes the predicted active amino acid, 330E (Fig. 7A).
Figure 7.
Effect of catalytic dead GCNT3 (mutGCNT3) on MCAM stability and on S100A8/A9-induced migration. (A) Schematic of protein compositions of wtGCNT3 and mutGCNT3. The mutGCNT3 was designed to produce C-terminal deletion mutant of GCNT3, which lacks the catalytic domain in part but does not lose the endoplasmic reticulum (ER) localization. (B) WM-115 cells were transiently expressed with aberrant wtGCNT3, mutGCNT3, or GFP as a control for 48 h. The expressed foreign GCNT3 (wt and mut) was detected by myc antibody. After immunoprecipitation of the confirmed extracts, we used 400 mM NaCl to wash out the precipitate in the hope of removing proteins that interact with MCAM and also with the beads. The endogenous MCAM proteins and their GlcNAc levels from the prepared cell samples were determined by immunoprecipitation and following detection with either MCAM antibody or HRP-conjugated WGA. (C) The wtGCNT3-overexpressed WM-115 clone #4 with stable mode was transiently transfected with either GFP or mutGCNT3 for 48 h. The foreign expression of the GCNT3 with stable wild and temporal mutant was detected simultaneously by myc antibody, and the following immunoprecipitation and detection of the intrinsic MCAM and its GlcNAc level were carried out by a similar method as shown in (B). (D) Migration activity of WM-266-4 cells was assessed by the Boyden chamber method. The transfected cells (GFP, wtGCNT3, and mutGCNT3) in the top chamber were stimulated or not stimulated with purified recombinant S100A8/A9 (final concentration of 100 ng/ml). The quantified results are displayed. Data are shown as means ± SD. *p < 0.05, **p < 0.01, and ***p < 0.001.
We then transfected the mutGCNT3 to WM-115 cells. After immunoprecipitation of the endogenous MCAM using a biotin-conjugated MCAM antibody with streptavidin beads, we found that MCAM in the mutGCNT3-overexpressed cells appeared at a significantly lower level than that of the control GFP transfectant. On the other hand, endogenous MCAM in the wtGCNT3-overexpressed cells was present at the highest level in protein among those in all of the temporal transfectants (Fig. 7B). At this time, we confirmed that GlcNAc modification level was greatly attenuated by the expression of the catalytic dead mutGCNT3 but not wtGCNT3. A similar phenomenon was also observed in the WM-115-derived clone #4, which stably expressed the wild type of GCNT3 at a significant level, after forced expression of the mutGCNT3 to the stable clone. The upregulation of endogenous MCAM protein in the clone #4 was mitigated by the temporal overexpression of the foreign mutGCNT3 (Fig. 7C). Downregulation of the GlcNAc modification in the precipitated intrinsic MCAM was also followed by this event with mutGCNT3 overexpression. Finally, we found that forced expression of mutGCNT3 significantly downregulated migration activity of WM-266-4 cells under either the presence or absence of S100A8/A9 in culture (Fig. 7D).
Taken as a whole, our results indicate that GCNT3 controls MCAM stability by its catalytic activity-mediated glycosyl modification that correlates with a greater ability for cancer cell motility and invasion in response to extracellular S100A8/A9.
DISCUSSION
Prevention of cancer metastasis is important since the majority of cancer deaths are related to this process. We need a better understanding of the mechanism involved in metastasis for the prevention of metastasis. It is well known that some organs are more prone than others to metastasis from certain types of cancer. This phenomenon was first discussed by Paget as the “seed and soil” theory over a century ago in 188929. For example, melanomas tend to metastasize to the lungs, prostate cancer tends to metastasize to bones, and colon cancer tends to spread to the liver19,30–32. Hiratsuka et al. reported that S100A8/A9 proteins in the lung area, induced by primary tumors, attract tumor cells from their primary region to travel to the lung, where TLR4 expressed on these tumor cells will function as a key sensor to catch the S100A8/A9 signal30. RAGE expressed on melanoma cells also has the same function19. In addition to these receptors, we have identified novel S100A8/A9 receptors, EMMPRIN, MCAM, ALCAM, and NPTNβ, and clarified their importance in cancer metastasis18,19,33 and inflammatory atopic dermatitis34. We therefore termed these important receptors “S100 soil sensor receptors” or simply as SSSRs.
Cell surface proteins including receptors are commonly glycosylated, and such glycosylation patterns are usually altered during the process of cancer formation and its subsequent progression. The altered glycosylation often influences interaction between cells and between cells and the extracellular environment, linking to cancer growth, adhesion, invasion, and metastasis1–11. However, the glycosyltransferases that regulate the glycosyl modification of cell surface proteins are still not well understood in cancer metastasis involved in the S100A8/A9–SSSRs axis. To our knowledge, this is the first report showing that GCNT3, a glycosyltransferase dominantly expressed in aggressive melanoma, increased the stability of only MCAM among the SSSRs via GlcNac modification that leads to an enhanced responsiveness to S100A8/A9, resulting in greater migration and invasion.
Very recently, Rao and colleagues reported interesting data regarding GCNT3 and pancreatic cancer, and they suggested clinical relevance between GCNT3 overexpression and malignancy in pancreatic cancers7. Through an in vitro approach, they found that forced expression of GCNT3 was associated with cancer aggressiveness and that GCNT3-KO as well as GCNT3 inhibition with talniflumate, a small compound they newly identified, significantly reduced cell viability and spheroid formation7. These findings corresponded well to our results for melanoma with the possible inclusion of other cancers, such as skin, lung, and breast cancers and mesotheliomas, that showed higher expression levels of GCNT3 consistently or partially (Fig. S3). Furthermore, we found that GCNT3 also significantly increased the ability for migration and invasion of lung immortalized BEAS-2B cells (Fig. S4). However, the positive relevance of GCNT3 overexpression and tumor malignancy still remains controversial. Huang et al. reported that GCNT3 was frequently downregulated in colorectal cancers and showed a suppressive effect on colon cancer growth35. In addition, Gonzalez-Vallinas et al. reported that the risk of relapse in colon cancers patients with a low expression level of GCNT3 was significantly higher than that in patients with high expression levels of GCNT336. One reason for this might be cancer type-dependent differences in intracellular machineries involving the GCNT3 pathways that either lead to cancer progression or regression.
Next, we focused on how GCNT3 is involved in increasing the half-life of MCAM. We showed that MCAM was glycosylated by GCNT3 through its catalytic activity, resulting in extra attachments of GlcNAc (Fig. 6C). It has been reported that the addition of β1-6 GlcNAc branching on integrin β1 caused a more fully glycosylated and mature form of integrin β1. The addition of β1-6 GlcNAc branching also inhibited the β1 protein degradation in human hepatocellular carcinoma cells, which was linked to the promotion of integrin-dependent migration and invasion6. This process was mediated by another glycosyltransferase, MGAT5. It is reasonable to assume that a similar process may apply to MCAM. In regard to this, it should be pointed out that the increased ratio of basal ability in nonmetastatic WM-115 cells was more noticeable compared to that of its responsiveness to S100A8/A9 (Figs. 4D and E, and 5C and D). This may be due to the presence of another GCNT3-mediated pathway(s), since many proteins that are involved in cancer progression, including β1 as previously described, may be affected by GCNT3 like MGAT5.
In conclusion, we demonstrated in this study that GCNT3 has a critical role in cancer migration and invasion through positive regulation of MCAM stability. Further analysis of this new axis between GCNT3 and MCAM may provide a better understanding of cancer metastasis that is affected by S100A8/A9. The result of this study may also be useful for designing a promising strategy for therapy of various cancer types.
ACKNOWLEDGMENTS
This research is supported in part by the Project for Cancer Research And Therapeutic Evolution (P-CREATE) from the Japan Agency for Medical Research and Development to M.S., Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Nos. 26290039 and 15K14382 to M.S, and JSPS KAKENHI Grant No. JP15K10201 to A.Y. The studies using patient-derived tissue sections for immunohistochemistry were approved by the research ethics committees of Niigata University Medical and Dental Hospital. Written informed consent was obtained from each patient for use of the materials.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Dennis JW, Nabi IR, Demetriou M. Metabolism, cell surface organization, and disease. Cell 2009;139:1229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferreira JA, Magalhaes A, Gomes J, Peixoto A, Gaiteiro C, Fernandes E, Santos LL, Reis CA. Protein glycosylation in gastric and colorectal cancers: Toward cancer detection and targeted therapeutic. Cancer Lett. 2017;387:32–45. [DOI] [PubMed] [Google Scholar]
- 3. Gill DJ, Tham KM, Chia J, Wang SC, Steentoft C, Clausen H, Bard-Chapeau EA, Bard FA. Initiation of GalNac-type O-glycosilation in the endoplasmic reticulum promotes cancer cell invasiveness. Proc Natl Acad Sci USA 2013;110(34):E3152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Freitas Junior JC, Morgado-Diaz JA. The role of N-glycans in colorectal cancer progression: Potential biomarker and therapeutic applications. Oncotarget 2016;7(15):19395–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chou CH, Huang MJ, Chen CH, Shyu MK, Huang J, Hung JS, Huang CS, Huang MC. Up-regulation of C1GALT1 promotes breast cancer cell growth through MUC1-C signaling pathway. Oncotarget 2015;6(8):6123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang L, Liang Y, Li Z, Cai X, Zhang W, Wu G, Jin J, Fang Z, Zha X. Increase in β1-6 GlcNac branching caused by N-acetylglucosaminyltransferase V directs integrin β1 stability in human hepatocellular carcinoma cell line SMMC-7721. J Cell Biochem. 2007;100:230–41. [DOI] [PubMed] [Google Scholar]
- 7. Rao CV, Janakiram NB, Madka V, Kumar G, Scott EJ, Pathuri G, Bryant T, Kutche H, Zhang Y, Biddick L, Gali H, Zhao YD, Lightfoot S, Mohammed A. Small-molecule inhibition of GCNT3 disrupts mucin biosynthesis and malignant cellular behaviors in pancreatic cancer. Cancer Res. 2016;76(7):1965–74. [DOI] [PubMed] [Google Scholar]
- 8. Wang ZQ, Bachvarova M, Morin C, Plante M, Gregoire J, Renaud MC, Sebastianelli A, Bachvarov D. Role of the polypeptide N-acetylgalactosaminyltransferase 3 in ovarian cancer progression: Possible implications in abnormal mucin o-glycosylation. Oncotarget 2014;5(2):544–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hung JS, Huang J, Lin YC, Huang MJ, Lee PH, Lai HS, Liang JT, Huang MC. C1GALT1 overexpression promotes the invasive behavior of colon cancer cells through modifying o-glycosylation of FGFR2. Oncotarget 2014;5(8):2096–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheray M, Petit D, Forestier L, Karayan-Tapon L, Maftah A, Jauberteau MO, Battu S, Gallet FP, Lalloue F. Glycosylation-related gene expression is linked to differentiation status in glioblastomas undifferentiated cells. Cancer Lett. 2011;312:24–32. [DOI] [PubMed] [Google Scholar]
- 11. Wu YM, Liu CH, Huang MJ, Lai HS, Lee PH, Hu RH, Huang MC. C1GALT1 enhances proliferation of hepatocellular carcinoma cells via modulating MET glycosylation and dimerization. Cancer Res. 2013;73(17):5580–90. [DOI] [PubMed] [Google Scholar]
- 12. Ghavami S, Chitayat S, Hashemi M, Eshraghi M, Chazin WJ, Halayko AJ, Kerkhoff C. S100A8/A9: A janus-faced molecule in cancer therapy and tumorgenesis. Eur J Pharmacol. 2009;625:73–83. [DOI] [PubMed] [Google Scholar]
- 13. De Ponti A, Weichert L, Schneller D, Pusteria T, Longerich T, Hogg N, Vogel A, Schirmacher P, Hess J, Angel P. A pro-tumorigenic function of S100A8/A9 in carcinogen-induced hepatocellular carcinoma. Cancer Lett. 2015;369:396–404. [DOI] [PubMed] [Google Scholar]
- 14. Edgeworth J, Gorman M, Bennett, Freemont P, Hogg N. Identification of p8, 14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J Biol Chem. 1991;266(120):7706–13. [PubMed] [Google Scholar]
- 15. Vogl T, Tebrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen AD, Nacken W, Foell D, van der Poll T, Sorg C, Roth J. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13(9):1042–9. [DOI] [PubMed] [Google Scholar]
- 16. Ichikawa M, Williams R, Wang L, Vogl T, Srikrishna G. S100A8/A9 activate key genes and pathways in colon tumor progression. Mol Cancer Res. 2011;9(2):133–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova-Todorova K, Leversha M, Hogg N, Seshan VE, Norton L, Brogi E, Massague J. A CXCL1 paracrine network link cancer chemoresistance and metastasis. Cell 2012;150:165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hibino T, Sakaguchi M, Miyamoto S, Yamamoto M, Motoyama A, Hosoi J, Shimokata T, Ito T, Tsuboi R, Huh N. S100A9 is a novel ligand of EMMPRIN that promotes melanoma metastasis. Cancer Res. 2012;73(1):172–83. [DOI] [PubMed] [Google Scholar]
- 19. Ruma IM, Putranto EW, Kondo E, Murata H, Watanabe M, Huang P, Kinoshita R, Futami J, Inoue Y, Yamauchi A, Sumardika IW, Youyi C, Yamamoto K, Nasu Y, Nishibori M, Hibino T, Sakaguchi M. MCAM, as a novel receptor for S100A8/A9, mediates progression of malignant melanoma through prominent activation of NF-κB and ROS formation upon ligand binding. Clin Exp Metastasis 2016;33(6):609–27. [DOI] [PubMed] [Google Scholar]
- 20. Sakaguchi M, Murata H, Yamamoto K-I, Ono T, Sakaguchi Y, Motoyama A, Hibino T, Kataoka K, Huh N-h. TIRAP an adaptor protein for TLR2/4, transduces a signal from RAGE phosphorylated upon ligand binding. PloS One 2011;6(8):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ghavami S, Rashedi I, Dattilo BM, Eshraghi M, Chazin WJ, Hashemi M, Wesselborg S, Kerkhoff C, Los M. S100A8/A9 at low concentration promotes tumor cell growth via RAGE ligation and MAP kinase-dependent pathway. J Leukoc Biol. 2008;83:1484–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Turovskaya O, Foell Dirk, Sinha P, Vogl T, Newlin R, Nayak J, Nguyen M, Olsson A, Nawroth PP, Bierhaus A, Varki N, Kronenberg M, Freeze HH, Srikrishna G. RAGE, carboxylated glycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis 2008;29(10):2035–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bubka M, Link Lenczowski P, Janik M, Pochec E, Litynska A. Overexpression of N-acetylglucosaminyltransferases III and V in human melanoma cells. Implications for MCAM N-glycosylation. Biochimie 2014;103:37–49. [DOI] [PubMed] [Google Scholar]
- 24. Sakaguchi M, Watanabe M, Kinoshita R, Kaku H, Ueki H, Futami J, Murata H, Inoue Y, Li S-a, Huang P, Putranto EW, Ruma IM, Nasu Y, Kumon H, Huh N-h. Dramatic increase in expression of a transgene by insertion of promoters downstream of the cargo gene. Mol Biotechnol. 2014;56:621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sakaguchi M, Murata H, Aoyama Y, Hibino T, Putranto EW, Ruma IM, Inoue Y, Sakaguchi Y, Yamamoto K-i, Kinoshita R, Futami J, Kataoka K, Iwatsuki K, Huh N-h. DNAX-activating protein 10 (DAP10) membrane adaptor associates with receptor for advanced glycation end products (RAGE) and modulates the RAGE-triggered signaling pathway in human keratinocytes. J Biol Chem. 2014;289(34):23389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bobrowska J, Moffat J, Awsiuk K, Pabijan J, Rysz J, Budkowski A, Reading M, Lekka M. Comparing surface properties of melanoma cells using time of flight secondary ions mass spectrometry. Analyst 2016;141(22):6217–25. [DOI] [PubMed] [Google Scholar]
- 27. Serini S, Fasano E, Piccioni E, Monego G, Cittadini ARM, Celleno L, Ranelletti FO, Calviello G. DHA induces apoptosis and differentiation in human melanoma cells in vitro: Involvement of HuR-mediated COX-2 mRNA stabilization and β-catenin nuclear translocation. Carcinogenesis 2012;33(1):164–73. [DOI] [PubMed] [Google Scholar]
- 28. Yeh JC, Ong E, Fukuda M. Molecular cloning and expression of a novel β-1, 6-N-acetylglucosaminyltransferase that forms core 2, core 4, and I branches. J Biol Chem. 1999;274:3215–21. [DOI] [PubMed] [Google Scholar]
- 29. Paget S. The distribution of secondary growths in cancer of the breast. Lancet 1889;571–3. [PubMed] [Google Scholar]
- 30. Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predeterrmines lung metastasis. Nat Cell Biol. 2006;8(12):1369–5. [DOI] [PubMed] [Google Scholar]
- 31. Dupaul-Chicoine J, Arabzadeh A, Dagenais M, Douglas T, Champagne C, Morizot A, Rodrigue-Gervais IG, Breton V, Colpitts SL, Beauchemin, Saleh M. The Nlrp3 inflammasome suppresses colorectal cancer metastatic growth in the liver by promoting natural killer cell tumoricidal activity. Immunity 2015;43:751–63. [DOI] [PubMed] [Google Scholar]
- 32. Patel LR, Camascho DF, Shiozawa Y, Pienta KJ, Taichman RS. Mechanisms of cancer cell metastasis to the bone: A multistep process. Future Oncol. 2011;7(11):1285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sakaguchi M, Yamamoto M, Miyai M, Maeda T, Hiruma J, Murata H, Kinoshita R, Ruma IM W, Putranto EW, Inoue Y, Morizane S, Huh N, Tsuboi R, Hibino T. Identification of an S100A8 receptor neuroplastin-β and its heterodimer formation with EMMPRIN. J Invest Dermatol. 2016;136(11):2240–50. [DOI] [PubMed] [Google Scholar]
- 34. Aochi S, Tsuji K, Sakaguchi M, Huh N, Tsuda T, Yamanishi K, Komine M, Iwatsuki K. Markedly elevated serum level of calcium-binding S100A8/A9 proteins in psoriatic arthritis are due to activated monocytes/macrophages. J Am Acad Dermatol. 2011;64(5):879–87. [DOI] [PubMed] [Google Scholar]
- 35. Huang M-C, Chen H-Y, Huang H-C, Huang J, Liang J-T, Shen T-L, Lin N-Y, Ho C-C, Cho I-M, Hsu S-M. C2GnT-M is downregulated in colorectal cancer and its re-expression causes growth inhibition of colon cancer cells. Oncogene 2006;25:3267–76. [DOI] [PubMed] [Google Scholar]
- 36. Gonzales-Vallinas M, Vargas T, Moreno-Rubio J, Molina S, Herranz J, Cejas P, Burgos E, Aquayo C, Custodio A, Reglero G, Feliu J, Ramirez de Molina A. Clinical relevance of the differential expression of the glycosyltransferase gene GCNT3 in colon cancer. Eur J Cancer 2015;51(1):1–8. [DOI] [PubMed] [Google Scholar]