Abstract
Objectives
To evaluate risk factors associated with unfavourable outcomes: emergency care, hospitalisation, admission to intensive care unit (ICU), mechanical ventilation and death in patients with immune-mediated rheumatic disease (IMRD) and COVID-19.
Methods
Analysis of the first 8 weeks of observational multicentre prospective cohort study (ReumaCoV Brasil register). Patients with IMRD and COVID-19 according to the Ministry of Health criteria were classified as eligible for the study.
Results
334 participants were enrolled, a majority of them women, with a median age of 45 years; systemic lupus erythematosus (32.9%) was the most frequent IMRD. Emergency care was required in 160 patients, 33.0% were hospitalised, 15.0% were admitted to the ICU and 10.5% underwent mechanical ventilation; 28 patients (8.4%) died. In the multivariate adjustment model for emergency care, diabetes (prevalence ratio, PR 1.38; 95% CI 1.11 to 1.73; p=0.004), kidney disease (PR 1.36; 95% CI 1.05 to 1.77; p=0.020), oral glucocorticoids (GC) (PR 1.49; 95% CI 1.21 to 1.85; p<0.001) and pulse therapy with methylprednisolone (PR 1.38; 95% CI 1.14 to 1.67; p=0.001) remained significant; for hospitalisation, age >50 years (PR 1.89; 95% CI 1.26 to 2.85; p=0.002), no use of tumour necrosis factor inhibitor (TNFi) (PR 2.51;95% CI 1.16 to 5.45; p=0.004) and methylprednisolone pulse therapy (PR 2.50; 95% CI 1.59 to 3.92; p<0.001); for ICU admission, oral GC (PR 2.24; 95% CI 1.36 to 3.71; p<0.001) and pulse therapy with methylprednisolone (PR 1.65; 95% CI 1.00 to 2.68; p<0.043); the two variables associated with death were pulse therapy with methylprednisolone or cyclophosphamide (PR 2.86; 95% CI 1.59 to 5.14; p<0.018).
Conclusions
Age >50 years and immunosuppression with GC and cyclophosphamide were associated with unfavourable outcomes of COVID-19. Treatment with TNFi may have been protective, perhaps leading to the COVID-19 inflammatory process.
Keywords: autoimmune diseases, epidemiology, outcome assessment, health care, therapeutics, outcome and process assessment, health care
Key messages.
What is already known about this subject?
Patients with immune-mediated rheumatic diseases (IMRD) are at increased risk of infections.
There are uncertainties as to whether patients with IMRD are at an increased risk of developing more severe forms of COVID-19.
What does this study add?
Patients with IMRD with COVID-19 did not demonstrate an increased risk of more severe infection in previous studies.
High levels of immunosuppression with methylprednisolone or cyclophosphamide pulse therapy and chronic oral GC were associated with unfavourable outcomes of the SARS-CoV-2 infection.
Tumour necrosis factor inhibitor (TNFi) had an association with a lower prevalence of hospitalisation and need for intensive care unit admission.
How might this impact on clinical practice?
Immunosuppressed patients should be routinely and even more carefully evaluated for SARS-CoV-2 infection, as they may have unfavourable outcomes.
In countries where the COVID-19 epidemic is on the rise, high-grade immunosuppression and GC should be stopped or reduced, as long disease activity allows.
TNFi can be continued, as it appears to protect against severe forms of the disease.
Introduction
Patients with immune-mediated rheumatic diseases (IMRD) are at increased risk of infections, with significant morbidity associated with serious infections, constituting one of the main causes of mortality in these patients.1 Although previously published studies that evaluated patients with IMRD with COVID-19 did not demonstrate an increased risk of more severe infections in these groups when compared with the general population,2–4 these studies do not fully clarify whether patients with IMRD are at an increased risk of developing more severe forms of COVID-19.5 6 Moreover, Brazil is a country of continental dimensions, with important regional differences in relation to socioeconomic status, basic sanitation and access to health, and the evolution of patients with COVID-19 and IMRD may assume a different behaviour from other parts of the world.
The primary aim of this paper was to describe the patients included in the first 8 weeks of the ReumaCoV Brasil register, evaluating the factors associated with the following outcomes: (1) need for emergency care (patients who went to the hospital, except those who were seen in an outpatient clinic), (2) hospitalisation (more than 24 hours of hospital permanence), (3) intensive care unit (ICU) admission, (4) mechanical ventilation and (5) death. Our hypothesis was that patients with IMRD and high-grade immunosuppression could have an unfavourable evolution compared with those with less immunosuppression.
Methods
The complete study methodology was previously published.7 Briefly, the ReumaCoV Brasil is a multicentre, observational, prospective cohort study carried out to monitor adult IMRD patients with COVID-19 diagnosis, using a convenience sample, whose data collection began 20 May 2020, with inclusion scheduled until December 2020, with 43 participating research centres.8 This paper will present the analysis of data for the first 8 weeks of inclusion in the study.
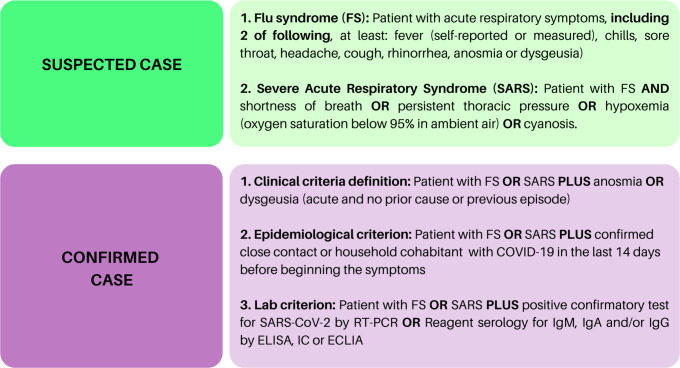
Eligible patients were selected based on the identification of a case of COVID-19 by the researcher, through telephone contact, outpatient consultation or during hospitalisation for COVID-19. Inclusion criteria were: (1) age over 18, (2) COVID-19 diagnosis, according to the Brazilian Health Minister (BMH) (figure 1) and (3) prior diagnosis of IMRD, according to the American College of Rheumatology or the European League against Rheumatism criteria. Exclusion criteria were other immunodeficiency diseases, past organ or bone marrow transplantation, neoplasms within the last 5 years, current chemotherapy, HIV diagnosis and thymus diseases.
Figure 1.
COVID-19 diagnosis established by the Brazilian Ministry of Health during the pandemic period. FS, flu syndrome.
Demographic data such as age, sex, work situation and social isolation during the pandemic, as well diagnosis and treatment of IMRD, comorbidities (https://www.who.int/classifications/icd/icdonlineversions/en/), clinical characteristics, treatment and evolution of COVID-19 were collected using a Research Eletronic Data Capture (REDCap) database (https://www.project-redcap.org/), through telephone call or face-to-face interview, if permitted by local health recommendations. In case of hospitalisation, the data were collected directly with the patient, if possible, or from medical records. In cases where death was notified, data were collected directly from a family member, who authorised the inclusion of the data in the register.
For data analysis, a database was built using the REDCap database, which was exported to the SPSS program, V.21, where the analysis was performed. To characterise the profile of the patients, the percentage of frequencies were calculated, and the frequency distribution of the evaluated factors was constructed. For the quantitative variables, the median and IQR statistics were calculated. In order to verify which factors influenced the outcomes, the contingency table was constructed and the Chi-square test for the independence sample was applied. In cases where the assumptions of the χ2 test were violated, Fisher’s exact test was applied. In addition, prevalence ratios (PR) and the respective CI were calculated. Since the objective of the study was to assess the evolution of the most severe forms of COVID-19 in patients with IMRD, the IUC and death outcomes were analysed only among patients who were hospitalised.
All conclusions were drawn considering the significance level of 5%. The variables that showed statistical significance of up to 20% in the bivariate analysis were included in the Poisson multivariate adjustment. Variables with a 5% significance remained in the final model. The OR was calculated to assess the chance of a COVID-19 symptom occurring in patients with laboratory confirmed disease.
This study was registered at the Brazilian Registry of Clinical Trials—REBEC, RBR-33YTQC. All patients read and signed the informed consent form before inclusion.
Results
Results are reported in accordance with STROBE guidelines. Between 20 May 20 and 24 July 2020, 334 IMRD patients with a diagnosis of COVID-19 were included in the register. The median age was 45 years (IQR=31–57) and 81.4% were female. In regard to their work situation, 186 (55.0%) patients were active at the time of SARS-CoV-2 infection; among the inactive, most were retired or on work leave due to rheumatic disease (69.0%); 126 (37.3%) patients reported a profession that dealt directly with the public (public attendance, health, security, education); 159 (47.0%) patients reported no social isolation during the pandemic; 159 (47.0%) reported close contact with a confirmed case of COVID-19, with 104 (30.8%) events occurring at home. The most common comorbidities were hypertension (35.8%), obesity (15.7%) and diabetes (11.5%); smoking was reported by 4.4% of patients. Regarding rheumatic disease diagnosis, systemic lupus erythematosus (SLE) (32.9%) and rheumatoid arthritis (RA) (28.4%) were the most frequent. Hydroxychloroquine (HCQ) (118/338; 34.9%), oral glucocorticoids (GC) (116/338; 34.2%), tumour necrosis factor inhibitor (TNFi) (75/338; 22.2%) and methotrexate (68/338; 20.1%) were the most common rheumatic disease treatments. All patients included were COVID-19 confirmed cases, according to BMH recommendations (figure 1), most of them classified according to lab criterion (76.8%), mostly through RT-PCR (n=175; 51.8%). Table 1 describes demographic and clinical data of the sample.
Table 1.
Demographic and clinical characteristics of 334 patients with confirmed or suspected COVID-19 and rheumatic diseases
| Variables | n | % |
| Female | 275 | 81.4 |
| Age, median (IQR) | 45 (31–57) | |
| Professions that deal with the public | 126 | 37.3 |
| Active at work | 186 | 55.0 |
| Retired/ work leave due rheumatic disease* | 230 | 69.0 |
| Social isolation | 159 | 47.0 |
| Close contact with a confirmed case of COVID-19 | 159 | 47.0 |
| Comorbidities | ||
| Hypertension | 121 | 35.8 |
| Obesity | 53 | 15.7 |
| Diabetes | 39 | 11.5 |
| Hypothyroidism | 20 | 5.9 |
| Lung disease | 32 | 9.4 |
| Heart disease | 25 | 7.4 |
| Dyslipidaemia | 22 | 6.5 |
| Fibromyalgia | 12 | 3.6 |
| Kidney disease | 21 | 6.2 |
| Smoking | 15 | 4.4 |
| Alcoholism | 8 | 2.4 |
| Depression | 7 | 2.1 |
| Rheumatic diseases diagnostic | ||
| Systemic lupus erythematosus | 110 | 32.9 |
| Rheumatoid arthritis | 95 | 28.4 |
| Axial Spondyloarthritis | 45 | 13.5 |
| Systemic sclerosis | 23 | 6.9 |
| Psoriatic arthritis | 23 | 6.9 |
| Vasculitis | 10 | 3.3 |
| Others | 28 | 8.3 |
| Rheumatic disease treatment | ||
| Hydroxychloroquine | 118 | 34.9 |
| Oral corticosteroids | 116 | 34.3 |
| Methotrexate | 68 | 20.1 |
| Azathioprine | 42 | 12.4 |
| Leflunomide | 23 | 11.8 |
| Mycophenolate mofetil | 21 | 6.2 |
| TNFi | 75 | 22.2 |
| Non-TNFi | 41 | 12.1 |
| Rituximab | 13 | 3.8 |
| Anti-IL-17 | 12 | 3.6 |
| Tocilizumab | 9 | 2.7 |
| Belimumab | 3 | 0.9 |
| Abatacept | 3 | 0.9 |
| Anti-IL12-23 | 1 | 0.3 |
| JAK-inhibitors | 12 | 3.6 |
| Cyclophosphamide (pulse therapy) | 10 | 3.0 |
| Methylprednisolone (pulse therapy) | 8 | 2.4 |
| COVID-19 symptoms | ||
| Cough | 195 | 56.7 |
| Shortness of breath | 160 | 46.5 |
| Headache | 200 | 58.0 |
| Asthenia | 165 | 47.9 |
| Fever | 176 | 51.2 |
| Anosmia | 153 | 44.4 |
| Rhinorrhea | 111 | 32.2 |
| Joint pain | 72 | 21.0 |
| Myalgia | 140 | 40.8 |
| Dysgeusia | 146 | 42.3 |
| COVID-19 lab confirmation | 255 | 76.0 |
| RT-PCR | 175 | 51.8 |
| SARS-CoV-2 Serology (IgM or IgG) | 98 | 29.3 |
| Unknown | 30 | 8.9 |
| Emergency care | 160 | 48.0 |
| Hospitalised | 110 | 33.0 |
| Discharge alive | 66 | 19.8 |
| Intensive unit care | 50 | 15.0 |
| Mechanical ventilation | 35 | 10.5 |
| Death | 28 | 8.4 |
*Among 148 inactive at work
GC, glucocorticoids; IL-17, interleukin 17; JAK, Janus kinase; TNFi, tumour necrosis factor inhibitor.
In regard to COVID-19 symptoms, the most frequent were headache (58.0%), cough (56.5%) and fever (51.2%). Twelve asymptomatic patients (3.6%) were included because they presented a positive RT-PCR for SARS-CoV-2, collected due to contact with a confirmed case of COVID-19.
The median duration of symptoms was 12 days (IQR=10) and 102 patients (30.2%) still had symptoms at study entry. The most common medications used to treat COVID-19 were analgesics (n=166, 49.6%) and azithromycin (n=165, 49.3%), HCQ (n=66, 19.7%) and oral GC (n=71, 20.6%), at a dosage above >30 mg/day in 44.1% of patients. Pulse therapy with GC was used by 14 (4.2%) patients.
Regarding the main outcomes, emergency care was required in 160 patients (48%); 110 (33.0%) patients were hospitalised, 50 (15.0%) were admitted to the ICU, 35 (10.5%) underwent mechanical ventilation and 28 (8.4 %) died. Among the 28 patients who died, 24 (85.7%) were women, and the median age was 53 years (IQR 36–69). The diagnosis was SLE in 11 patients, 4 were RA, 2 axial spondyloarthritis, 5 systemic sclerosis and 6 had other diseases; 5 (17.9%) patients were using pulse therapy with methylprednisolone and 5 (17.9%) patients were using pulse therapy with cyclophosphamide.
Table 2 describes the binary associations between the need for emergency care and explanatory variables only in the COVID-19 lab confirmed group. There was a statistically significant difference in relation to being inactive at work (PR 1.42, 95% CI 1.13 to 1.78; p=0.002), the presence of diabetes (PR 1.49, 95% CI 1.18 to 1.87; p=0.008), and having hypertension (PR 1.30, 95% CI 1.05 to 1.62; p=0.020), hypothyroidism (PR 1.52, 95% CI 1.17 to 1.98; p=0.030), kidney disease (PR 1.49, 95% CI 1.13 to 1.97; p=0.046), using oral corticosteroids (PR1.60, 95% CI 1.30 to 1.97; p<0.001) and methylprednisolone pulse therapy (PR 1.86, 95% CI 1.65 to 2.08; p=0.018). Not using TNFi was associated with an increased prevalence rate for hospitalisation (PR 1.53; 95% CI 1.07 to 2.18; p=0.007). No differences were observed regarding age, gender, social isolation, heart and lung disease, obesity, smoking, HCQ, methotrexate, leflunomide or rheumatic disease diagnosis.
Table 2.
Associations between the need for emergency care with explanatory variables (255) patients with laboratory confirmed COVID-19
| Variables | Emergency care | p-value | PR 95% CI | ||
| Yes (n, %) | No (n, %) | ||||
| Age | |||||
| Up to 50 years | 81 (52.9) | 72 (47.1) | 0.429* | 1.00; | – |
| >50 years | 58 (58.0) | 42 (42.0) | 1.10; | 0.88 to 1.37 | |
| Sex | |||||
| Male | 25 (53.2) | 22 (46.8) | 0.769* | 1.00; | – |
| Female | 115 (55.6) | 92 (44.4) | 1.04; | 0.78 to 1.40 | |
| Work situation | |||||
| Inactive | 73 (65.2) | 39 (34.8) | 0.002* | 1.42; | 1.13 to 1.78 |
| Active | 63 (46.0) | 74 (54.0) | 1.00; | – | |
| Skin colour | |||||
| White | 78 (56.9) | 59 (43.1) | 0.529* | 1.07; | 0.86 to 1.34 |
| Non white | 62 (53.0) | 55 (47.0) | 1.00; | – | |
| Geographic distribution | |||||
| Non-southeast | 58 (49.6) | 59 (50.4) | 0.101* | 1.00; | – |
| Southeast | 82 (59.9) | 55 (40.1) | 1.21; | 0.96 to 1.52 | |
| Hypertension | |||||
| No | 81 (49.7) | 82 (50.3) | 0.020* | 1.00; | – |
| Yes | 59 (64.8) | 32 (35.2) | 1.3 | 1.05 to 1.62 | |
| Obesity | |||||
| No | 113(53,6) | 98(46,4) | 0.267* | 1.00; | – |
| Yes | 27(62,8) | 16(37,2) | 1.17 | 0.90 to 1.52 | |
| Diabetes | |||||
| No | 116 (52.0) | 107 (48.0) | 0.008* | 1.00; | – |
| Yes | 24 (77.4) | 7 (22.6) | 1.49 | 1.18 to 1.87 | |
| Lung disease | |||||
| No | 121 (54.3) | 102 (45.7) | 0.461* | 1.00; | – |
| Yes | 19 (61.3) | 12 (38.7) | 1.13 | 0.83 to 1.53 | |
| Cardiovascular disease | |||||
| No | 124 (53.7) | 107 (46.3) | 0.144* | 1.00; | – |
| Yes | 16 (69.6%) | 7 (30.4) | 1.3 | 0.96 to 1.74 | |
| Dyslipidaemia | |||||
| No | 129 (54.4) | 108 (45.6) | 0.411* | 1.00; | – |
| Yes | 11 (64.7) | 6 (35.3) | 1.19 | 0.82 to 1.72 | |
| Hypothyroidism | |||||
| No | 127 (53.4) | 111 (46.6) | 0.030* | 1.00; | – |
| Yes | 13 (81.3) | 3 (18.8) | 1.52 | 1.17 to 1.98 | |
| Kidney disease | |||||
| No | 128 (53.6) | 111 (46.4) | 0.046* | 1.00; | – |
| Yes | 12 (80.0) | 3 (20.0) | 1.49 | 1.13 to 1.97 | |
| Smoking | |||||
| No | 136 (56.0) | 107 (44.0) | 0.228† | 1.54 | 0.70 to 3.39 |
| Yes | 4 (36.4) | 7 (63.6) | 1.00; | – | |
| Fibromyalgia | |||||
| No | 133 (54.5) | 111 (45.5) | 0.519† | 1.00; | – |
| Yes | 7 (70.0) | 3 (30.0) | 1.28 | 0.84 to 1.96 | |
| Alcoholism | |||||
| No | 137 (55.9) | 108 (44.1) | 0.049† | 3.91 | 0.64 to 24.11 |
| Yes | 1 (14.3) | 6 (85.7) | 1.00; | – | |
| Depression | |||||
| No | 137 (55.2) | 111 (44.8) | 1.000† | 1.1 | 0.49 to 2.48 |
| Yes | 3 (50.0) | 3 (5.,0) | 1.00; | – | |
| TNFi | |||||
| No | 119 (59.5) | 81 (40.5) | 0.007* | 1.53 | 1.07–2.18 |
| Yes | 21 (38.9) | 33 (6.1) | 1.00; | – | |
| HCQ | |||||
| No | 86 (51.2) | 82 (48.8) | 0.079* | 1.00; | – |
| Yes | 54 (62.8) | 32 (37.2) | 1.23 | 0.98 to 1.53 | |
| Oral GC | |||||
| No | 80 (46.2) | 93 (53.8) | <0.001 | 1.00; | – |
| Yes | 60 (74.1) | 21 (25.9) | 1.6 | 1.30 to 1.97 | |
| GC dosage | |||||
| <20 mg/day | 43 (69.4) | 19 (30.6) | 0.133† | 1.00; | – |
| ≥20 mg/day | 17 (89.5) | 2 (10.5) | 1.29 | 1.03 to 1.62 | |
| Intravenous GC | |||||
| No | 133 (53.8) | 114 (46.2) | 0.018† | 1.00; | – |
| Yes | 7 (100.0) | 0 (0,0) | 1.86 | 1.65 to 2.08 | |
| CYC | |||||
| No | 133 (54.3) | 112 (45.7) | 0.193† | 1.00; | – |
| Yes | 7 (77.8) | 2 (22.2) | 1.44 | 1.00 to 2.09 | |
*P value of the χ2 test for independence;
†P value of Fisher’s exact test.
CYC, cyclophosphamide pulse therapy; GC, glucocorticoids; HCQ, hydroxychloroquine; Methyl, methylprednisolone e pulse therapy; PR, prevalence ratio; TNFi, tumour necrosis factor inhibitor.
In the multivariate adjustment using the Poisson model for emergency care, diabetes, kidney disease, use of oral GC and pulse therapy with methylprednisolone remained significant (table 3).
Table 3.
Multivariate adjustment using the Poisson model for emergency care, hospitalisation and intensive unit care admission
| Variables | PR | 95% CI | P value* |
| Emergency care | |||
| Diabetes | |||
| No | 1.00 | – | |
| Yes | 1.38 | 1.11 to 1.73 | 0.004 |
| Kidney disease | |||
| No | 1.00 | – | |
| Yes | 1.36 | 1.05 to 1.77 | 0.020 |
| Oral GC | |||
| No | 1.00 | – | |
| Yes | 1.49 | 1.21 to 1.85 | <0.001 |
| Intravenous GC | |||
| No | 1.00 | – | |
| Yes | 1.38 | 1.14 to 1.67 | 0.001 |
| Hospitalisation | |||
| Age | |||
| Up to 50 | 1.00 | – | – |
| >50 | 1.89 | 1.26 to 2.85 | 0.002 |
| TNFi | |||
| No | 2.51 | 1.16 to 5.45 | 0.020 |
| Yes | 1.00 | – | – |
| Intravenous GC | |||
| No | 1.00 | – | – |
| Yes | 2.50 | 1.59 to 3.92 | <0.001 |
| Intensive care unit admission | |||
| Oral GC | |||
| No | 1.00 | – | – |
| Yes | 2.24 | 1.36 to 3.71 | 0.002 |
| Intravenous GC | |||
| No | 1.00 | – | – |
| Yes | 1.65 | 1.0 to 2.68 | 0.043 |
| SLE | |||
| No | 1.00 | – | |
| Yes | 1.72 | 1.04 to 2.88 | 0.036 |
*P value of the Wald test
PR, prevalence ratio; TNFi, tumour necrosis factor inhibitor; GC, glucocorticoids; SLE, systemic lupus erythematosus.
Table 4 shows the binary associations between the primary outcomes: hospitalisation, ICU, mechanical ventilation, death and explanatory variables. For hospitalisation, a statistically significant association was observed with age >50 years (PR 1.91; 95% CI 1.26 to 2.91; p=0.002), not using TNFi (PR 2.69; 95% CI 1.26 to 2.91; p=0.005), oral GC (PR 1.82; 95% CI 1.1 to 2.74; p=0.005), oral GC dose above 20 mg/day (PR 2.18; 95% CI 1.29 to 3.66; p=0.007) and methylprednisolone pulse therapy to treat rheumatic disease (PR 2.90; 95% CI 1.73 to 4.87; p=0.014). In multivariate analysis using the Poisson model, age >50 years, and not using TNFi and methylprednisolone pulse therapy remained statistically significant (table 3).
Table 4.
Associations between the primary outcomes (hospitalisation, ICU, mechanical ventilation and death) with explanatory variables
| Variables | Hospitalisation | ICU* | Death* | |||||||||
| Yes | No | P value | RP | Yes | No | P value | RP | Yes | No (n,%) | P value | RP; 95% CI | |
| (n, %) | (n, %) | 95% CI | (n, %) | (n, %) | 95% CI | (n, %) | ||||||
| Age | ||||||||||||
| Up to 50 years | 29 | 125 | 0.002 | 1.91; | 23 | 58 | 0.111† | 1.46; | 13 | 33 | 0.949† | 1.02; |
| −18.8 | −81.2 | 1.26 to 2.91 | −28.4 | −71.6 | 0.92 to 2.31 | −28.3 | −71.7 | 0.54 to 1.91 | ||||
| >50 years | 36 | 64 | 24 | 34 | 15 | 37 | ||||||
| −36 | −64 | −41.4 | −58.6 | −28.8 | −71.2 | |||||||
| SLE | ||||||||||||
| No | 46 | 132 | 0.982 | 1.01; | 33 | 55 | 0.200† | 1.39; | 17 | 50 | 0.352† | 1.35; |
| −25.8 | −74.2 | 0.64 to 1.58 | −37.5 | −62.5 | 0.83 to 2.35 | −25.4 | −74.6 | 0.72 to 2.55 | ||||
| Yes | 20 | 57 | 14 | 38 | 11 | 21 | ||||||
| −26 | −74 | −26.9 | −73.1 | −34.4 | −65.6 | |||||||
| Anti-TNF | ||||||||||||
| No | 60 | 141 | 0.005 | 2.69; | 44 | 75 | 0.042† | 2.59; | 26 | 64 | 1.000‡ | 1.30; |
| −29.9 | −70.1 | 1.23 to 5.88 | −37 | −63 | 0.88 to 7.57 | −28.9 | −71.1 | 0.37 to 4.60 | ||||
| Yes | 6 | 48 | 3 | 18 | 2 | 7 | ||||||
| −11.1 | −88.9 | −14.3 | −85.7 | −22.2 | −77.8 | |||||||
| Oral GC | ||||||||||||
| No | 36 | 138 | 0.006 | 1,82; | 18 | 62 | 0.001† | 2.15; | 13 | 37 | 0.610† | 1.18; |
| −20.7 | −79.3 | 1.21 to 2.74 | −22.5 | −77.5 | 1.32 to 3.48 | −26 | −74 | 0.63 to 2.21 | ||||
| Yes | 30 | 51 | 29 | 31 | 15 | 34 | ||||||
| −37 | −63 | −48.3 | −51.7 | −30.6 | −69.4 | |||||||
| Oral GC | ||||||||||||
| <20 mg/day | 18 | 44 | 0.007 | 2.18; | 18 | 25 | 0.111† | 1.55; | 10 | 23 | 1.000‡ | 1.03; |
| (29,0) | −71 | 1.29 to 3.66 | −41.9 | −58.1 | 0.94 to 2.54 | −30.3 | −69.7 | 0.42 to 2.52 | ||||
| ≥20 mg/day | 12 | 7 | 11 | 6 | 5 | 11 | ||||||
| −63.2 | −38.8 | −64.7 | −35.3 | −31.3 | −68.8 | |||||||
| HCQ | ||||||||||||
| No | 46 | 122 | 0.448 | 1.19; | 32 | 54 | 0.250† | 1.34; | 18 | 48 | 0.752† | 1.11; |
| −27.4 | −72.6 | 0.75 to 1.88 | −37.2 | −62.8 | 0.80 to 2.23 | −27.3 | −72.7 | 0.58 to 2.13 | ||||
| Yes | 20 | 67 | 15 | 39 | 10 | 23 | ||||||
| −23 | −77 | −27.8 | −72.2 | −30.3 | −69.7 | |||||||
| CYC | ||||||||||||
| No | 62 | 184 | 0.196 | 1.76; | 42 | 91 | 0.042‡ | 2.26; | 23 | 69 | 0.018‡ | 2.86; |
| −25.2 | −74.8 | 0.82 to 3.78 | −31.6 | −68.4 | 1.33 to 3.85 | −25 | −75 | 1.59 to 5.14 | ||||
| Yes | 4 | 5 | 5 | 2 | 5 | 2 | ||||||
| −44.5 | −55.6 | −71.4 | −28.6 | −71.4 | −28.6 | |||||||
| Methyl | ||||||||||||
| No | 61 | 187 | 0.0142 | 2.90; | 42 | 91 | 0.042‡ | 2.26; | 23 | 69 | 0.018‡ | 2.86; |
| −24.6 | −75.4 | 1.73 to 4.87 | −31.6 | (68,4) | 1.33 to 3.85 | −25 | −75 | 1,59 to 5.14 | ||||
| Yes | 5 | 2 | 5 | 2 | 5 | 2 | ||||||
| −71.4 | −28.6 | −71.4 | −28.6 | −71.4 | −28.6 | |||||||
*Calculated only for hospitalised patients
†P value of the χ2 test for independence;
‡P value of Fisher’s exact test.
anti-TNF, tumour necrose factor inhibitor; CYC, cyclophosphamide pulse therapy; GC, glucocorticoids; HCQ, hydroxychloroquine; ICU, intensive care unit; Methyl, methylprednisolone pulse therapy; RP, prevalence ratio; SLE, systemic lupus erythematosus.
Regarding admission to the ICU, a statistically significant association was observed with oral GC (PR 2.15, 95% CI 1.32 to 3.48; p=0.001), not using TNFi (PR 2.59; 95% CI 0.88 to 7.57), pulse therapy with methylprednisolone or cyclophosphamide for rheumatic disease treatment (PR 2.26, 95% CI 1.33 to 3.85; p=0.042), with these last two also associated with increased risk of death (PR 2.86, 95% CI 1.59 to 5.14; p=0.018) (table 4). In multivariate analysis using the Poisson model, oral CE and pulse therapy with methylprednisolone remained statistically significant SLE was shown to have a possible protective effect for IUC in the multivariate (table 3). None of the tested variables were associated with mechanical ventilation (table 4).
Before including the use of TNFi in the binary analysis, it was tested whether there was an association with the use of biologicals of all classes as a group, and an association was observed with the ICU outcome (p=0.001). However, when we separated the groups into biological TNFi (p=0.006) and non TNFi (p=0.089), the difference remained only for the TNFi group. For this reason, only this group was included in the binary analysis and Poisson model.
Discussion
Brazil is the country with the third highest number of cases of COVID-19 in the world, with the first case confirmed 26 February 2020, and counting 4 123 000 cases and 126 203 deaths through 6 September 2020.9 To the best of our knowledge, ReumaCoV Brasil is the largest cohort of patients with COVID-19 and underlying IMRD from a single country. Our results demonstrate that age over 50, diabetes, kidney disease, use of oral GC, not using TNFi, pulse therapy with methylprednisolone and cyclophosphamide were associated with a higher prevalence of worse outcomes of COVID-19 in patients with IMRD. We did not find any association between the variables and the need for mechanical ventilation.
The first published report regarding COVID-19 in patients with rheumatic diseases suggested that there would be no greater risk in relation to the general population or with other comorbidities.3 10 Since then, some studies addressed the risk and severity of COVID-19 infection in people with IMRD, confirming this initial impression, except for hospitalisation in patients exposed to high GC doses.11 However, evidence on COVID-19 risk and outcome in patients with systemic autoimmune diseases is limited and conflicting and should be interpreted with great caution.
Almost half of the patients in our study required emergency care, being more prevalent among patients with diabetes, kidney disease and chronic users of corticosteroids, either oral or in the form of pulse therapy. Among those who sought emergency care, there was the need for hospitalisation in two thirds of cases, especially in older patients, those who were treated with methylprednisolone pulse therapy and those who did not use TNFi. The prevalence is higher than those reported in most published studies,11–16 and similar to that reported in an Italian cohort by Fredi et al.17 One possible explanation for these differences is the lower social conditions in Brazil, which makes patients more susceptible to more severe conditions, besides the difference in the patient’s disease profile and medications, raising the need for greater concern for patients in developing countries. In accordance with ReumaCov Brazil, most of the studies have found advanced age associated with a higher risk of hospitalisation.13 15–18
Chronic GC use, both oral and pulse therapy, was associated with all outcomes, except mechanical ventilation. Other previous studies describe similar results with oral GC, with doses ranging from 5 to 10 mg11 12 14 19; however, none of these studies described the impact of pulse therapy with methylprednisolone to treat IMRD in COVID-19 outcomes. Although recent studies have shown that the use of GC s in the moderate to severe acute phase of COVID-19 has led to a benefit,20 21 the effect seems to be deleterious in patients on chronic use, probably associated increased risk of infection with higher dose of GC,22 due to impairment of innate immune responses with a reduction in neutrophil recruitment and a delay in viral clearance.23
The result that associated lower prevalence of hospitalisation and ICU admission in patients using TNFi therapy is similar to that described in other studies12–14 and could not be demonstrated to all classes of biologicals. We must also consider that the number of patients using non-TNFi biologicals was lower (12.1%), therefore, the data should be interpreted with caution. However, other studies, including populations with different diseases, have shown similar results, which demonstrates that there must be a biological plausibility for this effect.11 24 25 Gianfrancesco et al also reported that TNFi use was associated with reduced odds of hospitalisation (OR 0.40, 95% CI 0.19 to 0.81), a finding that was not seen with conventional DMARDs alone or in combination with biologics or Janus kinase inhibitors.11
A possible explanation for the TNFi effect on COVID-19 could be inflammation control, based on the evidence that patients with more severe COVID-19 have higher levels of cytokines as TNF and IL-6,26–28 and the TNF inhibition in animal models has led to a protection against SARS-CoV-2 infection,29 induces a rapid decrease of IL-6 and IL-1 concentrations in patients with active RA,30 triggers a reduction of adhesion molecules and vascular endothelial growth factor, which is partly responsible for capillary leak,31 with a consequence of less leucocyte traffic to inflamed tissues.32 A similar effect was also observed in other viral infections, such as Chikungunya fever, where the use of TNFi was associated with better outcomes.33
Twenty-eight patients died, accounting for 8.4% of the total of our series and 17.5% of hospitalised patients, which is quite similar to the data found in other cohorts.11–13 17 19 The factors associated with mortality in these various studies were variable, but the use of oral GC was the common factor for most of them. In our study deaths were associated with pulse therapy with methylprednisolone and cyclophosphamide. The impact of these medications on both hospitalisation and mortality may be due to the greater number of patients with SLE included in our cohort when compared with others, but also the greater number of SLE among the deaths. It is noteworthy that patients treated with these medications have more severe disease, especially in SLE. This fact calls attention to the evaluation of treatment alternatives during the COVID-19 pandemic, with lower doses of GC and other immunosuppressants than cyclophosphamide, once this is possible.
HCQ was not protective against COVID-19. Despite some initial promising in vitro results,34 35 this hypothesis was not supported by our results or by the results of other studies performed in pre-exposed and postexposition prophylaxis using HCQ, as well as more recent randomised clinical trials, including mild-moderate and severe forms of COVID-19.36–39 More recently, Gianfrancesco et al reported no association of antimalarial use (OR 0.94, 95% CI 0.57 to 1.57) with hospitalisation.11
Patients with rheumatic diseases had greater need for ICU hospitalisation and presented over a threefold increased risk of requiring mechanical ventilation.15 Here, we report that 35 out of 50 patients in the ICU required invasive mechanical ventilation, corresponding to 70% of the patients in the ICU. This represents a need for ventilatory assistance in a higher proportion than described in other cohorts of IMRD patients and in the general population.40
Other important points addressed by our study deserve to be highlighted, as they demonstrate a different profile from other data previously published. As in the other series, there was a predominance of females, probably reflecting the higher prevalence of IMRD in women.11 17 However, different from other studies, our patients were younger11 17 19 and most of those who died were women under the age of 60 (median 53 years). Considering the median age of 45 years of patients in our cohort, and the mean age of the patients that died, it suggests that immunosuppression is a relevant factor associated with mortality in COVID-19. The immunosuppressed, younger patients can be more vulnerable, and should be considered as a group for shielding. Although our patients were younger, more than two-thirds were not working, and among those who were active at work, most performed activities involving care or contact with the public, which may have favoured infection by SARS-CoV-2. Less than half reported social isolation, suggesting a lack of confidence in social distancing measures or for being considered as breadwinners. Compared with other cohorts, in which SLE patients comprise 6.5%–19.0%, we have found a higher proportion (32.9%).11–13 15 17 Some cohorts that evaluated only SLE patients demonstrated a higher rate of hospitalisation41 42 with no difference between those who used or did not use HCQ and also with no difference in relation to the need for mechanical ventilation or extracorporeal membrane oxygenation.41 43
Although the most recent systematic review and meta-analysis44 has shown the IMRD patients are more susceptible to the COVID-19, including unfavourable outcomes, when SLE patients are separately analysed, particularly in case–control studies, this finding does not seem to be an absolute true. It probably reflects a selection bias, frequently reported by observational studies, similar to our findings, since most research centres have a great number of SLE patients, with easy access to the researcher and to hospital. In addition, these patients could have been more frequently hospitalised because the clinician may have considered the potential severity of the disease in the COVID-19 scenario. In the multivariate analysis, having a diagnosis of SLE was considered as a possible protective effect for ICU. Nonetheless, it is worth emphasising that SLE patients may have the combination of infection and disease activity in the context of immunosuppression and the rheumatologist needs to individualise the treatment weighing benefits and risks. Interestingly, the current reports have not shown reactivation of underlying IMRD after the COVID-19.45 46 Thus, large and longitudinal studies are necessary to address this relevant issue.
In the 74 Latin American patients with rheumatic diseases and COVID-19 reported from the COVID-19 Global Rheumatology Alliance Physician-Reported Registry there were more RA patients (35%) than SLE patients (22%), while in our sample the proportion of SLE patients (32,9%) was greater than the RA (28,4%).47
Although hypertension and diabetes were the most frequent comorbidities, as described in other cohorts, we observed that diabetes and renal diseases were the two diseases associated with emergency care at the final model. Of interest, we found 15.7% of obesity, which was not frequently described in other cohorts, but almost the same to one multicentric cohort.13 15 18
A strength of our study is that we included patients from different states of Brazil, a continental country, with most of the patients with confirmed diagnosis of COVID-19 based on positive COVID-19 RT-PCR testing. In addition, the 8-week interim analysis is related to the first weeks of community viral transmission, a relevant finding simulating the pandemic epidemiological curve in Brazil.48
As a limitation of the study, many cases may be not included in the cohort because they were not tested or have not been confirmed, usually for presenting a benign evolution of the disease. Since in the Brazilian public health system only hospitalised patients were being tested, this may have become a selection bias, including only the most severely ill patients. Because this is a national register, patients were treated in different services, possibly with different physical and personnel characteristics—a fact that may have interfered with the results. The availability of healthcare in Brazil can be different when it comes to the public or private health system. Regarding treatment, it was not possible to evaluate the association between COVID-19 with the combination use of different immunosuppressants or DMARDs combination.
Another limitation is related to main endpoints, including hospitalisation, need of mechanical ventilation and death, because they could not be adjusted for potential bias, such as access to healthcare systems, availability of hospital beds, strategies to mitigate the community viral transmission, heterogeneous expertise of medical team.49 50 Physicians’ beliefs on the risk of poor outcome in IMRD patients, especially those under immunosuppression, could have driven decision making, such as the need of ICU and medications given earlier. However, it is also important to consider that some patients enrolled in our registry had active and severe underlying IMRD. Therefore, the unfavourable evolution of them could occur itself, regardless of COVID-19.
Brazil is a country with a heterogeneous population, with variations in socioeconomic, cultural, ethnic and health status. The fact that we included representative patients from all Brazilian geographic regions allows our results to be generalised to Brazil and possibly to Latin American countries, with the same population pattern. Future studies comparing the different populations are needed to confirm whether these data occur similarly or not in the rest of the world.
In conclusion, the results of first 8 weeks of the ReumaCoV Brazil registry showed that aspects related to the patients with IMRD (age >50 years), and those related to their treatment (immunosuppression with GC and cyclophosphamide) were associated with unfavourable outcomes of the SARS-CoV-2 infection. Treatment with TNFi, on the other hand, may have been protective, perhaps leading to the control of COVID-19 inflammatory process, but randomised controlled trials to prove this effect are needed.
Acknowledgments
The authors would like to thank the Brazilian Society of Rheumatology for technical support and rapid nationwide mobilisation and all Collaborators on behalf of the ReumaCoV Brasil register
Footnotes
Contributors: All authors have contributed to study conception and design. CDLM, AMK, LMHM, ETR-N and ESP drafted the manuscript, which was critically reviewed by all authors. All authors have read and approved the final version of the manuscript.
Funding: This work is supported by Research Funding of the Brazilian Society of Rheumatology and National Council for Scientific and Technological Development (CNPq).
Competing interests: CDLM reports grants from National Council for Scientific and Technological Development (CNPq) and from Brazilian Society of Rheumatology, personal fees from Janssen, Novartis, Abbvie. AMK reports personal fees from Abbvie, Janssen, Pfizer, Lilly and Roche. MMP reports personal fees from Abbvie, Janssen, UCB, Novartis, Pfizer and Lilly. LMHM reports personal fees from Abbvie, Janssen, Pfizer, Roche, Boehringer Ingelheim, GSK, Libbs and Lilly. CRS reports personal fees from Pfizer, Abbvie and Novartis. AD reports personal fees from Boehringer Ingelheim. RDG reports personal fees from Janssen, Eli Lilly, Roche, Boehringer Ingelheim, Amgen Brasil, Pfizer, Sandoz, Novartis Brasil. LDAV personal fees from Janssen, Novartis and UCB. AKGM reports grants from Brazilian Society of Rheumatology, personal fees from Janssen and UCB. MAY reports personal fees from Novartis, Abbvie, Lilly and, UCB. RAdT reports personal fees from ABBVIE, GSK, JANSSEN, NOVARTIS, LILLY, PFIZER, ROCHE and UCB. RX reports grants and personal fees from Abbvie, Eli-Lilly, Pfizer, Janssen and Roche, personal fees from Novartis, UCB.
Patient consent for publication: Not required.
Ethics approval: This study was approved by Brazilian Committee of Ethics in Human Research (CONEP), on 5 April 2020, CAAE 30186820.2.1001.8807
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article.
References
- 1.Falagas ME, Manta KG, Betsi GI, et al. . Infection-Related morbidity and mortality in patients with connective tissue diseases: a systematic review. Clin Rheumatol 2007;26:663–70. 10.1007/s10067-006-0441-9 [DOI] [PubMed] [Google Scholar]
- 2.Favalli EG, Ingegnoli F, De Lucia O, et al. . COVID-19 infection and rheumatoid arthritis: Faraway, so close! Autoimmun Rev 2020;19:102523. 10.1016/j.autrev.2020.102523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Figueroa-Parra G, Aguirre-Garcia GM, Gamboa-Alonso CM, et al. . Are my patients with rheumatic diseases at higher risk of COVID-19? Ann Rheum Dis 2020;79:839–40. 10.1136/annrheumdis-2020-217322 [DOI] [PubMed] [Google Scholar]
- 4.Lu C, Li S, Liu Y. Role of immunosuppressive therapy in rheumatic diseases concurrent with COVID-19. Ann Rheum Dis 2020;79:737–9. 10.1136/annrheumdis-2020-217460 [DOI] [PubMed] [Google Scholar]
- 5.Marques C, Pinheiro MM, Reis Neto ET. COVID-19 in patients with rheumatic diseases: what is the real mortality risk? Ann Rheum Dis 2020. [DOI] [PubMed] [Google Scholar]
- 6.Messina F, Pampaloni F, Piaserico S. Comment on: recovery from COVID-19 in a patient with spondyloarthritis treated with TNF-alpha inhibitor etanercept. A report on a patient with COVID-19 with psoriatic arthritis receiving ustekinumab. Ann Rheum Dis 2020. 10.1136/annrheumdis-2020-218029. [Epub ahead of print: 18 Aug 2020]. [DOI] [PubMed] [Google Scholar]
- 7.Marques C, Kakehasi AM, Gomides APM. ReumaCoV Brasil registry: a Brazilian cohort of patients with Immuno-mediated chronic inflammatory diseases infected by SARS-CoV-2. JMIR Res Protoc 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.et alMarques CK, Gomides AM, Martins AP. ReumaCoV Brasil registry: Brazilian study of patients with Immuno-mediated chronic inflammatory diseases infected by SARS-CoV-2 nature, 2020. Available: https://protocolexchange.researchsquare.com/article/pex-1104/v1 [Accessed 6 Sep 2020]. [DOI] [PMC free article] [PubMed]
- 9.Saude.gov Brasil Painel Coronavírus: Ministério dA Saúde, 2020. Available: https://covid.saude.gov.br/ [Accessed 06 Sep 2020].
- 10.Monti S, Balduzzi S, Delvino P, et al. . Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis 2020;79:667–8. 10.1136/annrheumdis-2020-217424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. . Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis 2020;79:859–66. 10.1136/annrheumdis-2020-217871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nuno L, Novella Navarro M, Bonilla G. Clinical course, severity and mortality in a cohort of patients with COVID-19 with rheumatic diseases. Ann Rheum Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freites Nuñez DD, Leon L, Mucientes A, et al. . Risk factors for hospital admissions related to COVID-19 in patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis 2020;79:1393–9. 10.1136/annrheumdis-2020-217984 [DOI] [PubMed] [Google Scholar]
- 14.Haberman RH, Castillo R, Chen A, et al. . COVID-19 in patients with inflammatory arthritis: a prospective study on the effects of comorbidities and disease-modifying antirheumatic drugs on clinical outcomes. Arthritis Rheumatol 2020;72:1981–9. 10.1002/art.41456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Silva KM, Serling-Boyd N, Wallwork R, et al. . Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US 'hot spot'. Ann Rheum Dis 2020;79:1156–62. 10.1136/annrheumdis-2020-217888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haberman R, Axelrad J, Chen A, et al. . Covid-19 in Immune-Mediated Inflammatory Diseases - Case Series from New York. N Engl J Med 2020;383:85–8. 10.1056/NEJMc2009567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fredi M, Cavazzana I, Moschetti L, et al. . COVID-19 in patients with rheumatic diseases in northern Italy: a single-centre observational and case-control study. Lancet Rheumatol 2020;2:e549–56. 10.1016/S2665-9913(20)30169-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pablos JL, Galindo M, Carmona L, et al. . Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann Rheum Dis 2020;79:1544–9. 10.1136/annrheumdis-2020-218296 [DOI] [PubMed] [Google Scholar]
- 19.Montero F, Martínez-Barrio J, Serrano-Benavente B, et al. . Coronavirus disease 2019 (COVID-19) in autoimmune and inflammatory conditions: clinical characteristics of poor outcomes. Rheumatol Int 2020;40:1593–8. 10.1007/s00296-020-04676-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomazini BM, Maia IS, Cavalcanti AB, et al. . Effect of dexamethasone on days alive and Ventilator-Free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the Codex randomized clinical trial. JAMA 2020;324:1307. 10.1001/jama.2020.17021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye Z, Wang Y, Colunga-Lozano LE, et al. . Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis. CMAJ 2020;192:E756–67. 10.1503/cmaj.200645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Favalli EG, Desiati F, Atzeni F, et al. . Serious infections during anti-TNFalpha treatment in rheumatoid arthritis patients. Autoimmun Rev 2009;8:266–73. 10.1016/j.autrev.2008.11.002 [DOI] [PubMed] [Google Scholar]
- 23.Hasan SS, Capstick T, Zaidi STR, et al. . Use of corticosteroids in asthma and COPD patients with or without COVID-19. Respir Med 2020;170:106045. 10.1016/j.rmed.2020.106045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brenner EJ, Ungaro RC, Gearry RB, et al. . Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology 2020;159:481–91. 10.1053/j.gastro.2020.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okeke F, Mone A, Swaminath A. The course of SARS-COV2 infection was not severe in a Crohn's patient who administered maintenance anti-TNF therapy overlapping the early pre-symptomatic period of infection. Antibodies 2020;9. 10.3390/antib9030042. [Epub ahead of print: 15 Aug 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leija-Martínez JJ, Huang F, Del-Río-Navarro BE, et al. . Il-17A and TNF-α as potential biomarkers for acute respiratory distress syndrome and mortality in patients with obesity and COVID-19. Med Hypotheses 2020;144:109935. 10.1016/j.mehy.2020.109935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perlin DS, Zafir-Lavie I, Roadcap L, et al. . Levels of the TNF-related cytokine light increase in hospitalized COVID-19 patients with cytokine release syndrome and ARDS. mSphere 2020;5. 10.1128/mSphere.00699-20. [Epub ahead of print: 12 Aug 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McElvaney OJ, McEvoy NL, McElvaney OF, et al. . Characterization of the inflammatory response to severe COVID-19 illness. Am J Respir Crit Care Med 2020;202:812 10.1164/rccm.202005-1583OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDermott JE, Mitchell HD, Gralinski LE, et al. . The effect of inhibition of PP1 and TNFα signaling on pathogenesis of SARS coronavirus. BMC Syst Biol 2016;10:93. 10.1186/s12918-016-0336-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charles P, Elliott MJ, Davis D, et al. . Regulation of cytokines, cytokine inhibitors, and acute-phase proteins following anti-TNF-alpha therapy in rheumatoid arthritis. J Immunol 1999;163:1521–8. [PubMed] [Google Scholar]
- 31.Paleolog EM, Young S, Stark AC, et al. . Modulation of angiogenic vascular endothelial growth factor by tumor necrosis factor alpha and interleukin-1 in rheumatoid arthritis. Arthritis Rheum 1998;41:1258–65. [DOI] [PubMed] [Google Scholar]
- 32.Taylor PC, Peters AM, Paleolog E, et al. . Reduction of chemokine levels and leukocyte traffic to joints by tumor necrosis factor alpha blockade in patients with rheumatoid arthritis. Arthritis Rheum 2000;43:38–47. [DOI] [PubMed] [Google Scholar]
- 33.de Brito CAA, Marques CDL, França RFO, et al. . Reduced duration of Postchikungunya musculoskeletal pain in Rheumatological patients treated with biologicals. J Trop Med 2020;2020:2071325 10.1155/2020/2071325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao X, Ye F, Zhang M, et al. . In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2020;71:732–9. 10.1093/cid/ciaa237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J, Cao R, Xu M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 2020;6:16. 10.1038/s41421-020-0156-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borba MGS, Val FFA, Sampaio VS, et al. . Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open 2020;3:e208857. 10.1001/jamanetworkopen.2020.8857 [DOI] [PubMed] [Google Scholar]
- 37.Cavalcanti AB, Zampieri FG, Rosa RG. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cortegiani A, Ippolito M, Ingoglia G, et al. . Update I. A systematic review on the efficacy and safety of chloroquine/hydroxychloroquine for COVID-19. J Crit Care 2020;59:176–90. 10.1016/j.jcrc.2020.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lahouati M, Mériglier E, Martin L, et al. . COVID-19 infection also occurs in patients taking hydroxychloroquine. J Antimicrob Chemother 2020;75:2014–5. 10.1093/jac/dkaa193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goyal P, Choi JJ, Pinheiro LC, et al. . Clinical characteristics of Covid-19 in New York City. N Engl J Med 2020;382:2372–4. 10.1056/NEJMc2010419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathian A, Mahevas M, Rohmer J, et al. . Clinical course of coronavirus disease 2019 (COVID-19) in a series of 17 patients with systemic lupus erythematosus under long-term treatment with hydroxychloroquine. Ann Rheum Dis 2020;79:837–9. 10.1136/annrheumdis-2020-217566 [DOI] [PubMed] [Google Scholar]
- 42.Fernandez-Ruiz R, Masson M, Kim MY, et al. . Leveraging the United States epicenter to provide insights on COVID-19 in patients with systemic lupus erythematosus. Arthritis Rheumatol 2020;72:1971–80. 10.1002/art.41450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Konig MF, Kim AH, Scheetz MH. Baseline use of hydroxychloroquine in systemic lupus erythematosus does not preclude SARS-CoV-2 infection and severe COVID-19. Ann Rheum Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akiyama S, Hamdeh S, Micic D, et al. . Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis 2020. 10.1136/annrheumdis-2020-218946. [Epub ahead of print: 13 Oct 2020]. [DOI] [PubMed] [Google Scholar]
- 45.Favalli EG, Monti S, Ingegnoli F, et al. . Incidence of COVID-19 in patients with rheumatic diseases treated with targeted immunosuppressive drugs: what can we learn from observational data? Arthritis Rheumatol 2020;72:1600–6. 10.1002/art.41388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salvarani C, Bajocchi G, Mancuso P, et al. . Susceptibility and severity of COVID-19 in patients treated with bDMARDS and tsDMARDs: a population-based study. Ann Rheum Dis 2020;79:986.2–8. 10.1136/annrheumdis-2020-217903 [DOI] [PubMed] [Google Scholar]
- 47.Ugarte-Gil MM C, Alpizar-Rodriguez D. Characteristics associated with Covid-19 in patients with rheumatic disease in Latin America: data from the Covid-19 global rheumatology alliance physician-reported registry: global rheumatology by PANLAR, 2020. Available: https://globalrheumpanlar.org/node/254 [Accessed 09 Nov 2020].
- 48.Candido DS, Claro IM, de Jesus JG, et al. . Evolution and epidemic spread of SARS-CoV-2 in Brazil. Science 2020;369:1255–60. 10.1126/science.abd2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tirupathi R, Muradova V, Shekhar R, et al. . COVID-19 disparity among racial and ethnic minorities in the US: a cross sectional analysis. Travel Med Infect Dis 2020;38:101904. 10.1016/j.tmaid.2020.101904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim A, Gupta N, Lim A, et al. . Description of the effect of patient flow, junior doctor supervision and pandemic preparation on the ability of emergency physicians to provide direct patient care. Aust Health Rev 2020;44:741–7. 10.1071/AH20180 [DOI] [PubMed] [Google Scholar]