Abstract
Background
Recent evidence suggests that generation of neutrophil extracellular traps (NETosis), one of the components of immunothrombosis, is associated with the pathogenesis of both venous thromboembolism and sickle cell disease (SCD). NETosis is a complex process regulated by several proteins such as peptidyl arginine deaminase 4 (PADI4), neutrophil elastase (ELANE), and myeloperoxidase (MPO). Among these regulators, PADI4 is responsible of histone citrullination, an essential step for NETosis. Accordingly, its inhibition has been recently cited as a promising therapeutic strategy for diseases such as SCD. Although attractive, this strategy requires supportive evidence of its role in the pathogenesis of SCD.
Patients and Methods
Patients from two independent cohorts were enrolled in this study. Samples were obtained at steady state (53 patients) or during acute episodes of vaso‐occlusive crisis (VOC; 28 patients) in patients from cohort 1. mRNA was extracted from granulocytes to analyze PADI4, ELANE, and MPO expression by qPCR. Furthermore, plasma activity of PADI4 was assessed from an independent cohort in 15 patients, within 24 hours from admission for VOC. Race‐matched healthy individuals from the same geographic regions were used as controls for each cohort.
Results and Conclusions
Higher levels of gene expression of PADI4 and ELANE were observed during VOC. Furthermore, plasma activity of PADI4 was higher in acute VOC when compared to healthy individuals. These results demonstrate that NETosis regulators are modulated during acute VOC, and pave the way for studies of PADI4 inhibition as a therapeutic strategy for acute VOC in SCD.
Keywords: elastase, neutrophil extracellular traps, peptidylarginine deiminase 4, sickle cell disease, vaso‐occlusive crisis
Essentials.
Generation of neutrophil extracellular traps (NETosis) is a relevant trigger of the pathogenesis in sickle cell disease (SCD).
Expression and activity of NETosis regulators were evaluated in two cohorts of SCD.
Gene expression of PADI4, ELANE, and MPO was increased during SCD vaso‐occlusive crisis.
PADI4 enzymatic activity was also increased during SCD vaso‐occlusive crisis.
1. INTRODUCTION
Sickle cell disease (SCD) is a chronic hemolytic and multisystemic disorder characterized by chronic inflammation and a natural propensity to thrombotic events. 1 , 2 , 3 , 4 Recent findings suggest that hemostatic activation is a relevant mechanism in the pathogenesis of this disease. 5 , 6 , 7 , 8 This activation is part of a broader response to tissue injury involving innate immunity, hemostasis, and several proinflammatory processes that have been recently grouped under the umbrella term immunothrombosis. 9 This complex response, triggered by both pathogen‐associated molecular patterns and danger‐associated molecular patterns, is believed to contribute to an effective immune response to pathogens or tissue damage, by providing transient molecular cues for the recruitment of phagocytes into injured tissues. 7 , 10 However, if activated in a deregulated fashion, these same cellular programs are believed to support secondary tissue damage involving micro‐ and macrovascular thrombotic manifestations in inflammatory diseases. 9 , 11 , 12 Interestingly, neutrophils, monocytes, and platelets, which are critical in the pathogenesis of vaso‐occlusive crisis (VOC) in SCD, are also key elements in immunothrombosis. 13 , 14
One of the main elements of immunothrombosis is the release of neutrophil extracellular traps (NETs) by activated neutrophils, a process that has been associated with the pathogenesis of SCD in both animal models 15 and in humans, 16 with SCD patients presenting higher levels of markers of NET generation (NETosis) during acute VOC. More recently, it has also been demonstrated that proinflammatory cytokines are associated with NETosis during VOC crisis but not in steady state. 13 NETs are composed of DNA, histones, and antimicrobial components such as neutrophil elastase (ELANE) and contribute to pathogen clearance from the host. However, these components can also trigger thrombosis and inflammation in poorly regulated responses. 17 Accordingly, NETosis is a finely regulated process that involves the participation of several proteins such as peptidyl arginine deaminase 4 (PADI4), ELANE, and myeloperoxidase (MPO).
Among these regulators, PADI4 is responsible for histone citrullination, an essential step and initiator of NETosis. PADI4 knockout model animals have been shown to be protected from NETosis and from some thrombotic complications. 18 , 19 However, little is known about the role of PADI4 deregulation or its genetic modifications in SCD. 20 Upon activation, neutrophils undergo dynamic alteration of their cellular compartments and release reactive oxygen species (ROS) that promote membrane degradation and liberation of granule components such as ELANE and MPO. Before NET formation, ELANE released from azurophilic granules degrades F‐actin to arrest actin dynamics 21 and is subsequently translocated to the nucleus, 22 where it degrades histone H1 linkers. By targeting histone H1, ELANE promotes chromatin decondensation, 22 facilitating PADI4 enzymatic action and nuclear component release. Evidence suggests that MPO is required for ELANE translocation to the nucleus, 21 contributing to chromatin partial degradation. 23
Based on its importance in NETosis regulation, PADI4 inhibition has been recently cited as a potential therapeutic strategy for diseases in which NETosis could play a relevant pathogenic role. 19 , 24 Although attractive, the use of PADI4 inhibitors in SCD requires direct evidence of its role in the pathogenesis of this disease. Herein, we used two independent cohorts of patients to investigate the expression of NETosis regulators (PADI4, ELANE, and MPO) in SCD at both steady state and during acute VOC, and to assess whether PADI4 activity is increased during acute VOC.
2. MATERIALS AND METHODS
2.1. Patients and healthy volunteers
This was a cross‐sectional study that included patients with SCD recruited in two independent centers in Brazil. Patients from cohort 1 were recruited at the Hematology and Hemotherapy Foundation of Pernambuco (HEMOPE) in either steady state (ie, at least 3 months from the last acute VOC), or within 24 hours from admission for an acute VOC. These patients provided samples for gene expression studies. Patients from cohort 2 were recruited at Hematology and Hemotherapy Foundation of Amazonas State (HEMOAM) within 24 hours from admission for acute VOC, as part of a previous study that obtained plasma samples to measure total heme levels during acute VOC. Accordingly, only plasma samples were available from patients from cohort 2. Race‐matched ethnic healthy individuals were recruited independently for each study, from the same geographic region of each cohort. All of the healthy donors were homozygous for hemoglobin genotype and did not present any previous history of arterial or venous thrombotic diseases. Furthermore, they were free of inflammatory or infectious diseases before sampling. The study was performed in accordance with the Declaration of Helsinki and approved by the Ethics Committees of State University of Campinas (CAAE: 46 853 115.4.0000.5404), HEMOPE (CEP: 13 175‐667) and HEMOAM (CAAE: 71 147 817.3.0000.0009). Each participant provided written informed consent form before sample collection. Samples were immediately preprocessed at each site of sample collection, and only retro‐transcribed cDNA (cohort 1) or plasma (cohorts 1 and 2) were transported to University of Campinas in dry ice.
2.2. Quantitative PCR of NETosis regulators
Whole blood samples were obtained from healthy volunteers and patients from cohort 1 in tubes containing EDTA anticoagulant. Granulocytes were then isolated by two Ficoll gradients (Histopaque®‐1.119, Histopaque®‐1.077; Sigma‐Aldrich, St. Louis, MO, USA). Briefly, Histopaque gradient was obtained in a centrifuge tube containing a layer of 3 mL of each Histopaque media. Then, 6 mL of diluted blood samples (dilution v/v with phosphate buffered saline 1X) were carefully layered onto the Histopaque and were centrifuged at 700 g for 30 minutes at 20°C with the brake turned off. After separation, granulocytes were collected, washed, and RNA extraction was then performed using the Trizol method; 1 μg of total RNA was used to perform cDNA transcription. Gene expression of PADI4, ELANE, and MPO were measured by quantitative PCR (qPCR). Cq values were obtained and normalized using the geometric mean of ACTB and GAPDH as reference genes. 25
2.3. PADI4 activity
Whole blood samples were collected in sodium citrate anticoagulant containing tubes from patients and healthy volunteers of cohort 2. Platelet‐poor plasma was obtained after centrifugation (2000 g for 20 minutes) and used to measure PADI4 activity using a commercial kit (PADI4, PAD4 Inhibitor Screening Assay Kit; Cayman Chemical Co., Ann Arbor, MI, USA).
2.4. d‐dimer and von Willebrand factor plasma levels
d‐dimer was measured in platelet‐poor plasma obtained as describe above, from blood samples collected in 0.109M sodium citrate–containing tubes. The assay was performed using VIDAS d‐dimer Exclusion kit (bioMérieux, Marcy l'Etoile, France) which is based on an automated two‐step fluorescence test following the fabricant recommendations. Von Willebrand factor (VWF) antigen levels were determined in plasma by an in‐house ELISA, as previously described. 26
2.5. Statistical Analysis
Results are presented as the median of each variable. Statistical analysis was performed using Prism 6 (GraphPad Software Inc., San Diego, California, USA). Mann‐Whitney U tests were used to compare patients during VOC with healthy controls. Kruskal‐Wallis tests were performed with Dunn postcomparison correction to compare gene expression among three groups. We also carried out a multivariable linear regression analysis of NETosis modulator expression and PADI4 plasma activity adjusting for white blood cell (WBC) count and absolute neutrophil count (ANC), respectively, using R, version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria). A P value < .05 was considered statistically significant.
3. RESULTS AND DISCUSSION
In total, 53 patients in steady‐state (51 with genotype SS and 2 SC), 28 in acute crisis (22 with genotype SS and 6 SC), and 40 healthy volunteers were recruited from cohort 1. In addition, 15 patients with genotype SS and 20 healthy volunteers were recruited from cohort 2. Clinical and laboratory characteristics of these patients are described in Table 1. Blood samples were obtained at admission before any treatment or blood transfusion.
Table 1.
Clinical and laboratory characteristics of the study population
| Cohort 1 | |||||
|---|---|---|---|---|---|
|
Healthy volunteers (n = 40) |
Steady state (n = 53) |
P b |
Acute VOC (n = 28) |
P c | |
| Age, y | 25 (23‐30) | 31 (25‐38) | .004 | 28 (24‐37) | .1 |
| Sex, male/female a | 19/21 | 29/24 | .77 | 14 | .83 |
| Hemoglobin, g/dL | 13.9 (12.7‐15.6) | 7.6 (6.9‐8.8) | < .0001 | 8.1 (7.1‐9.3) | < .0001 |
| HbF % | ‐ | 6.5 (4.0‐11.9) | ‐ | 6.5 (5.2‐11.5) | .7 d |
| Platelets, ×109/L | 252 (210‐274) | 338 (274‐436) | < .0001 | 398 (311‐508) | < .0001 |
| ANC, ×109/L | 3.3 (2.11‐4.65) | N/D | ‐ | 6.4 (5.0‐9.6) | < .0001 |
| WBC, ×109/L | 6 (4.7‐8.0) | 11.1 (9.4‐14.5) | < .0001 | 13.9 (11.1‐18.7) | < .0001 |
| Cohort 2 | |||
|---|---|---|---|
|
Healthy volunteers (n = 20) |
VOC admission (n = 15) |
P | |
| Age, y | 29.5 (25.25‐38.75) | 27.0 (15‐34) | .17 |
| Sex, male/female a | 7/13 | 7/8 | .48 |
| Hemoglobin, g/dL | 13.5 (13.03‐15.43) | 7.6 (6.5‐8.5) | < .0001 |
| Platelet count, ×109/L | 277.5 (218.3‐314.8) | 411.0 (377‐567) | < .0001 |
| ANC, ×109/L | 4 (3.32‐4.7) | 7.4 (5.46‐9.6) | .0002 |
| WBC, ×109/L | 6.5 (5.77‐7.39) | 12.3 (9.15‐16.1) | < .0001 |
Variables are presented as median (IQR).
Abbreviations: ANC, absolute neutrophil count; HbF, fetal hemoglobin; IQR, interquartile range; N/D, not available; VOC, vaso‐occlusive crisis; WBC, white blood cell.
Chi‐square test.
Comparison healthy volunteers versus steady state.
Comparison healthy volunteers versus acute VOC.
Comparison of HbF steady state versus acute VOC.
qPCR analysis showed that the patients expressed higher levels of PADI4 during VOC when compared to both healthy volunteers and patients in steady state (Figure 1A). No significant difference was observed between healthy volunteers and patients in steady‐state (Figure 1A). To our knowledge, this is the first report addressing PADI4 expression in a cohort of SCD. Our results suggest that PADI4 could be involved in the pathogenesis of SCD during acute VOC and are in agreement with a recent study showing that NET production is higher when healthy neutrophils are incubated with plasma from SCD patients during VOC when compared to plasma obtained at steady state or from healthy volunteers. 13 Our group recently demonstrated, in a different cohort of patients with SCD, an association between single‐nucleotide polymorphisms in the PADI4 gene, which modify the expression of this gene, with the risk of acute chest syndrome. 20 Accordingly, even though our present results do not represent a direct demonstration of the contribution of PADI4 to NET release in SCD, we believe that they provide additional support to the concept that the upregulation of PADI4 gene expression could be involved in the pathogenesis of SCD.
Figure 1.
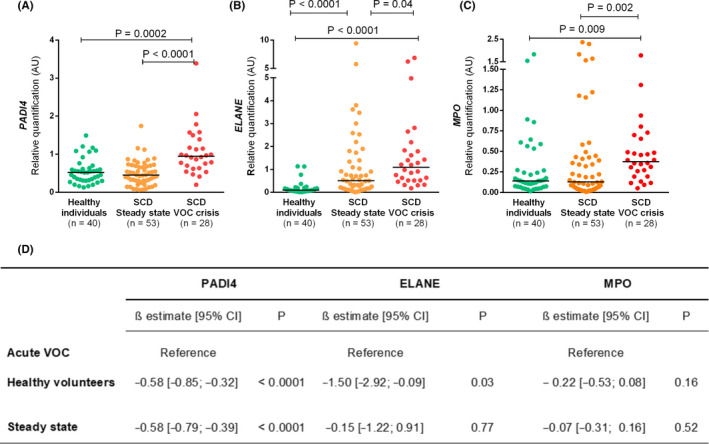
Gene expression of NETosis regulators PADI4, ELANE, and MPO in granulocytes from patients with SCD and healthy individuals. Results are presented as median relative quantifications of gene expression normalized by ACTB and GAPDH as reference genes. Both PADI4 (A) and MPO (C) mRNA expression increased only during acute VOC when compared with healthy volunteers. In contrast ELANE (B) mRNA expression increased in both VOC and steady state when compared with healthy volunteers. Kruskal‐Wallis test was performed with Dunn postcomparison correction. The numbers of patients studied are indicated in each panel. (D) Regression coefficients from multivariable linear regression analysis of the association of patient group with gene expression of each NETosis regulator, adjusted for white blood cell count. CI, confidence interval; ELANE, neutrophil elastase; MPO, myeloperoxidase; PADI4, peptidyl arginine deaminase 4; SCD, sickle cell disease; VOC, vaso‐occlusive crisis
We also analyzed the expression of other genes involved in NETosis, namely, MPO and ELANE. As shown in Figure 1B and C, both were increased during acute VOC when compared to steady‐state and healthy individuals (Figure 1 B and C). Evidence of increased neutrophil activation during acute VOC has been previously demonstrated, as patients in this condition present increased plasma level of elastase‐α1‐antitrypsin complexes. Interestingly, levels of these complexes were positively correlated with nucleosome levels. 16 In addition, both the expression 27 , 28 and the activity 29 of MPO, whose upregulation is crucial to the oxidative burst of neutrophils during inflammation, have been described in SCD. Accordingly, our results provide additional evidence of neutrophil activation in acute VOC in SCD. To account for the potential influence of differences in WBC counts between healthy individuals and patients with SCD (Table 1) in our gene expression assays, we carried out a multivariate analysis to adjust for this parameter. Of note, PADI4 expression remained significantly elevated during VOC when compared to both steady‐state and healthy individuals, supporting the relevance of this finding. Upregulation of ELANE was also confirmed during VOC when compared to healthy individuals, but not when compared to steady state. In contrast, no significant difference was observed in MPO expression between patients and healthy individuals after adjustment for WBC count.
We also analyzed the correlation between mRNA expressions of these three NETosis regulators with biomarkers of hemostatic activation in steady‐state patients, since NET release promotes coagulation and endothelial activation as part of the immunothrombotic process in several inflammatory conditions, including SCD. 9 , 30 Of note, no association could be observed between PADI4 expression and these biomarkers, while only weak/moderate correlations were observed for ELANE and MPO, which were correlated with d‐dimer (R = 0.4, P = .004; and R = 0.32, P = .004, respectively), and with VWF levels (R = 0.27, P = .04; and R = 0.30, P = .04, respectively). These results support a modest relationship between ELANE, MPO, and immunothrombosis during steady state, and are in accordance with our observation that PADI4 mRNA expression is increased only during VOC, and with other studies demonstrating higher levels of NET biomarkers only during VOC in SCD. 16 Additional studies are warranted to explore the association between PADI4 expression and coagulation and endothelial activation during acute VOC in SCD.
We then investigated whether PADI4 activity was also increased during acute VOC, in plasma samples from a different cohort. As shown in Figure 2, PADI4 activity increased during acute VOC when compared to healthy individuals (Figure 2). These results were also adjusted for ANC, and there was no association between ANC and PADI4 activity (ß estimate = 0.001; P = .1).
Figure 2.
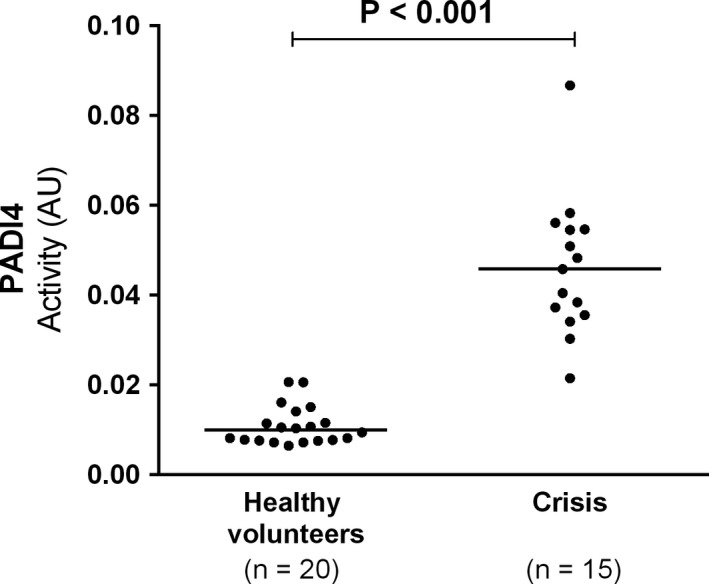
PADI4 plasma activity at admission for an acute vaso‐occlusive crisis was compared with healthy volunteers. Horizontal bars indicate median PADI4 activity. Mann‐Whitney test was performed. AU, arbitrary unit; PADI4, peptidyl arginine deaminase 4
While limited by a relatively small sample size, our results represent, to our knowledge, the first direct evidence that PADI4 levels are modulated during acute VOC in SCD. Modulation of PADI4 expression and/or activity might contribute to immunothrombosis in SCD by promoting NET release. 31 Moreover, if NET release in SCD is confirmed to be PADI4 dependent, PADI4 inhibition can be explored as a therapeutic target for this condition. 24 However, it should be noted that NETosis regulation also involves PADI4‐independent pathways in some specific settings (eg, specific pathogens), which include the production of reactive oxygen species (ROS) by mitochondria or nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. 32 , 33 While SCD environment is a potent trigger of NADPH oxidase–mediated ROS production, there is no evidence that SCD is a condition in which NET release is independent of PADI4 activation. Moreover, NET release has already been shown to be PADI4 dependent in several other inflammatory diseases such as diabetes 17 and can also involve both pathways. 34 Experiments using PADI4 knockout and SCD mice would be important to answer this question. Finally, since infections are very common in patients with SCD, and NET release is an important element of innate immunity, 35 studies involving PADI4 inhibition in the context of SCD should also investigate whether this strategy increases risk of infections. This concern was addressed in a recent study that reported that PADI4 deficiency did not increase host vulnerability to bacterial infections. 36
This study has limitations that need to be considered. First, there was significant heterogeneity between the two cohorts in regard to hydroxyurea use, which can have complex and heterogeneous effects in inflammation in SCD, including a decrease in ROS generation and free heme levels, 37 and hence influence NETosis. While all patients from cohort 2 were under hydroxyurea treatment, only a subgroup of patients from cohort 1 were hydroxyurea users (13/28 patients). Accordingly, we performed a subgroup analysis of patients with acute VOC from cohort 1 and demonstrated that they were similar in regard to baseline factors (hemoglobin, ANC, WBC, and platelets), and that no significant difference could be observed in gene expression levels of PADI4, ELANE and MPO between these two subgroups (Table 2). However, this subgroup comparison should be interpreted with caution considering the small sample size, low power to demonstrate significant differences, and the fact that variations in dose and adherence to hydroxyurea therapy could also have influenced these results.
Table 2.
Subgroups of patients with acute VOC from cohort 1 stratified by hydroxyurea use
|
No hydroxyurea (n = 15) |
Hydroxyurea users (n = 13) |
P | |
|---|---|---|---|
| Hemoglobin, g/dL | 8.2 (7.6‐9.9) | 8.1 (6.65‐9.05) | .84 |
| HbF % | 6.7 (5.3‐9.7) | 6.3 (3.25‐12.5) | .93 |
| ANC, ×109/L | 6.3 (4.99‐9.95) | 6.4 (4.33‐9.46) | .77 |
| WBC, ×109/L | 14.4 (11.6‐16.6) | 12.1 (11.1‐18.75) | .6 |
| PADI4 mRNA, AU | 0.97 (0.62‐1.15) | 0.91 (0.72‐1.68) | .7 |
| ELANE mRNA, AU | 1.28 (0.63‐2.65) | 0.75 (0.38‐1.50) | .1 |
| MPO mRNA, AU | 0.36 (0.27‐0.65) | 0.39 (0.16‐0.61) | .8 |
Variables are presented as median (IQR).
Abbreviations: ANC, absolute neutrophil count; AU, arbitrary unit; HbF, fetal hemoglobin; IQR, interquartile range; WBC, white blood cell.
A second limitation is the fact that gene expression and functional studies were not measured in both cohorts. While the use of different methods strengthens our conclusions about PADI4 modulation during acute VOC, an independent validation of each of these observations is lacking. Of note, an analysis of a public database (GSE139912) of RNA sequencing data from patients with SCD during acute VOC also demonstrated an increase in PADI4 expression (Figure S1), although these data were obtained from whole blood RNA, while in our study RNA was obtained from neutrophils. Third, our data do not exclude the possibility that upregulation of PADI4 activity is not specific to acute VOC and could be observed in other forms of acute illness. Studies exploring PADI4 modulation in other acute conditions are warranted to address this question. Finally, data on the association of NETosis regulators with a more extensive panel of markers of inflammatory and hemostatic activation, also including samples from acute VOC, could provide additional insights into the role of PADI4 in immunothrombosis in SCD.
In summary, we have demonstrated that the mRNA expression of NETosis regulators, including PADI4 is increased in SCD during acute VOC but not in steady state. PADI4 protein activity was also increased in an independent cohort during acute VOC, supporting the concept that PADI4 inhibition could be explored as a therapeutic target for SCD during acute VOC. Additional studies are warranted to confirm these results in independent populations and further elucidate their clinical implications.
RELATIONSHIP DISCLOSURE
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
BWH and FC performed experiments; BWH analyzed and interpreted data and drafted the manuscript; IFD, MJVCS, ASA, ARLANAF, MCB, and MNNS recruited and preprocessed patients from the SCD cohort 1; ECC, PVSN, AM, and NAF recruited and preprocessed patients from the SCD cohort 2, FFC contributed with reagents and laboratory infrastructure; EV designed the study, reviewed and analyzed data, and drafted the manuscript. All authors revised and approved all submitted versions of the manuscript.
Supporting information
Fig S1
Hounkpe BW, Chenou F, Domingos IDF, et al. Neutrophil extracellular trap regulators in sickle cell disease: Modulation of gene expression of PADI4, neutrophil elastase, and myeloperoxidase during vaso‐occlusive crisis. Res Pract Thromb Haemost.2021;5:204–210. 10.1002/rth2.12463
Handling Editor: Dr Pantep Angchaisuksiri.
Funding informationThis study was financially supported by the Sao Paulo Research Foundation. grants # 2014/00984‐3; 2015/24666‐3 and 2016/14172‐6; CNPq Brazil, grant # 309317/2016; and Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior – Brasil. (CAPES) ‐ Finance Code 001.
REFERENCES
- 1. Colella MP, De Paula EV, Conran N, et al. Hydroxyurea is associated with reductions in hypercoagulability markers in sickle cell anemia. J Thromb Haemost. 2012;10:1967–70. [DOI] [PubMed] [Google Scholar]
- 2. Hebbel RP, Vercellotti G, Nath KA. A systems biology consideration of the vasculopathy of sickle cell anemia: the need for multi‐modality chemo‐prophylaxsis. Cardiovasc Hematol Disord Drug Targets. 2009;9:271–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Noubouossie D, Key NS, Ataga KI. Coagulation abnormalities of sickle cell disease: relationship with clinical outcomes and the effect of disease modifying therapies. Blood Rev. 2015;30(4):245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Naik RP, Streiff MB, Lanzkron S. Sickle cell disease and venous thromboembolism: what the anticoagulation expert needs to know. J Thromb Thrombolysis. 2013;35(3):352–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jackson SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019;906–18. [DOI] [PubMed] [Google Scholar]
- 6. Arumugam PI, Mullins ES, Shanmukhappa SK, et al. Genetic diminution of circulating prothrombin ameliorates multiorgan pathologies in sickle cell disease mice. Blood. 2015;126:1844–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang D, Xu C, Manwani D, et al. Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology. Blood. 2016;801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hounkpe BW, Fiusa MML, Colella MP, et al. Role of innate immunity‐triggered pathways in the pathogenesis of sickle cell disease: a meta‐analysis of gene expression studies. Sci Rep. 2015;5:17822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. [DOI] [PubMed] [Google Scholar]
- 10. Grover SP, Neutrophils MN. NETs, and immunothrombosis. Blood. 2018;1360–1. [DOI] [PubMed] [Google Scholar]
- 11. Gollomp K, Kim M, Johnston I, et al. Neutrophil accumulation and NET release contribute to thrombosis in HIT. JCI Insight. 2018;3:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fuchs TA, Brill A, Wagner DD. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2012;32:1777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barbu EA, Mendelsohn L, Samsel L, et al. Pro‐inflammatory cytokines associate with NETosis during sickle cell vaso‐occlusive crises. Cytokine Academic Press. 2020;127:154933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bennewitz MF, Jimenez MA, Vats R, et al. Lung vaso‐occlusion in sickle cell disease mediated by arteriolar neutrophil‐platelet microemboli. JCI Insight. 2017;2:e89761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen G, Zhang D, Fuchs TA, et al. Heme‐induced neutrophil extracellular traps contribute to the pathogenesis of sickle cell disease. Blood. 2014;123(24):3818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schimmel M, Nur E, Biemond BJ, et al. Nucleosomes and neutrophil activation in sickle cell disease painful crisis. Haematologica. 2013;98:1797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong SL, Demers M, Martinod K, et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med. 2015;21:815–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li P, Li M, Lindberg MR, et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207:1853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martinod K, Demers M, Fuchs TA, et al. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A. 2013;110:8674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chenou F, Hounkpe BW, de Albuquerque DM, et al. PADI4 gene polymorphism as a risk factor for acute chest syndrome in sickle cell anemia patients. Blood. 2017;130:954.28818981 [Google Scholar]
- 21. Metzler KD, Goosmann C, Lubojemska A, et al. Myeloperoxidase‐containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 2014;8(3):883–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Papayannopoulos V, Metzler KD, Hakkim A, et al. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med. 2017;23(3):279–87. [DOI] [PubMed] [Google Scholar]
- 24. Lewis HD, Liddle J, Coote JE, et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem Biol. 2015;11:189–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real‐time quantitative RT‐PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chenou F, Albuquerque DM, Leonardo DP, et al. Endothelial nitric oxide synthase (eNOS) gene polymorphisms and markers of hemolysis, inflammation and endothelial dysfunction in Brazilian sickle cell anemia patients. Biochem Gen. 2020;58:580–94. [DOI] [PubMed] [Google Scholar]
- 27. Yalcinkaya A, Unal S, Oztas Y. Altered HDL particle in sickle cell disease: decreased cholesterol content is associated with hemolysis, whereas decreased Apolipoprotein A1 is linked to inflammation. Lipids Health Dis. 2019;18(225):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Castilhos LG, de Oliveira JS, Adefegha SA, et al. Increased oxidative stress alters nucleosides metabolite levels in sickle cell anemia. Redox Rep. 2017;22(6):451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barbosa MC, de Jesus dos Santos TE, dos Santos TN, et al. The effect of a selective inhibitor of phosphodiesterase‐9 on oxidative stress, inflammation and cytotoxicity in neutrophils from patients with sickle cell anaemia. Basic Clin Pharmacol Toxicol. 2016;118(4):271–8. [DOI] [PubMed] [Google Scholar]
- 30. Conran N, De Paula EV. Thromboinflammatory mechanisms in sickle cell disease‐challenging the hemostatic balance. Haematologica. 2020;105(10):2380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Noubouossie DF, Reeves BN, Strahl BD, et al. Neutrophils: back in the thrombosis spotlight. Blood. 2019;133(20):2186–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guiducci E, Lemberg C, Küng N, et al. Candida albicans–induced NETosis is independent of peptidylarginine deiminase 4. Front Immunol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kenny EF, Herzig A, Krüger R, et al. Diverse stimuli engage different neutrophil extracellular trap pathways. eLife. 2017;6:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Muraro SP, De Souza GF, Gallo SW, et al. Respiratory Syncytial Virus induces the classical ROS‐dependent NETosis through PAD‐4 and necroptosis pathways activation. Sci Rep. 2018;8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science (New York, NY). 2004;303:1532–5. [DOI] [PubMed] [Google Scholar]
- 36. Martinod K, Fuchs TA, Zitomersky NL, et al. PAD4‐deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood. 2015;125(12):1948–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Almeida CB, Franco‐Penteado C, Saad STO, et al. Sickle cell disease serum induces NADPH enzyme subunit expression and oxidant production in leukocytes. Hematology. 2010;15(6):422–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1


