Abstract
Background
Venous thromboembolism (VTE) causes morbidity and mortality in the general population. Several events occur after lower limb orthopedic surgery, but the contribution from various types of lower limb surgery is not well known.
Objective
To investigate the postoperative incidence of VTE for all types of lower extremity orthopedic surgery compared with the background population.
Methods
Individual‐level linkage of Danish nationwide register data for all Danish residents with first‐time orthopedic surgery of the lower limb (1996‐2017) and, for each of these, four controls from the general population matched on age, sex, and history of VTE. Adjusted hazard ratios (HR) compared the postoperative risk of VTE to the matched controls.
Results
In total 7203 of the 1 012 823 patients with a first orthopedic procedure had a VTE within 180 days after surgery, corresponding to a postoperative cumulative incidence of 0.71% (95% confidence interval [CI], 0.70‐0.73). The cumulative incidence of VTE among controls was 0.11% (95% CI, 0.11‐0.12). The HR of VTE within the first 30 days after surgery below knee level was 20.5 (95% CI, 17.9‐23.5) compared with matched controls. The HRs of VTE after minor distal procedures (eg, meniscectomy and arthroscopies) were 2.9 (95% CI, 1.9‐4.4) to 7.1 (95% CI, 6.4‐8.0).
Conclusion
All types of lower limb orthopedic surgery including minor distal procedures were associated with higher rates of VTE compared with matched controls, in particular within the first 30 days after surgery.
Keywords: cohort studies, epidemiology, orthopedic procedures, pulmonary embolism, venous thromboembolism
Essentials.
Impact of various types of limb surgery to the burden of venous thromboembolism (VTE) is unknown.
Danish nationwide registries provide individual‐level data from patients and controls.
The proportion of VTE was higher after all types of lower limb surgery compared with controls.
VTE rates were high within the first 30 up to 60 postoperative days.
1. INTRODUCTION
Orthopedic surgical procedures are some of the most commonly provided services in the health care systems worldwide. Venous thromboembolism (VTE) is a collective term encompassing deep vein thrombosis and pulmonary embolism and a well‐known complication to orthopedic surgery affecting both short and long‐term mortality and quality of life. 1 , 2 , 3 More than 700 000 Europeans develop a VTE each year, and another 370 000 deaths annually are attributed to VTE, with only a minor fraction of the patients being diagnosed with and treated for VTE antemortem. 4 More than 60% of all VTEs are associated with hospitalization and occur after discharge from the hospital. 2 , 4 , 5
The risk of VTE after orthopedic surgery is 0.4%‐1.0% despite the use of thromboprophylaxis, including minor orthopedic procedures. 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 These findings indicate a need for improved postoperative preventive strategies for high‐risk patients. In order to clarify if high‐risk patients should be identified in only a few types of orthopedic surgery, we need information about the epidemiology of all types of lower‐limb orthopedic surgery relative to the background population. However, no previous studies compared the postoperative risk of VTE in all the different types of orthopedic surgery to the background population.
The Danish national health care registers provide longitudinal electronic data on diagnoses; procedures; and prescribed, reimbursed medicine at individual patient level with essentially no loss to follow‐up. All Danish residents are offered free‐of‐charge surgical treatment paid through federal and local taxation. Thus, the risk of selection toward surgical options based on socioeconomic status is low in Denmark.
This study’s objective was to investigate the postoperative risk of VTE in all types of lower extremity orthopedic surgery compared with the background population to identify areas needing a more thorough investigation of potential use of thromboprophylaxis. We report 180‐day cumulative incidences and hazard ratios (HRs) of VTE after orthopedic surgery in a matched, retrospective cohort study based on Danish national health care register data.
2. METHODS
2.1. Setting
All Danish residents, currently counting 5.8 million, are registered in the Danish Civil Registration System by a unique civil personal registration (CPR) number at birth or immigration. Vital, immigration, and emigration status have been registered at the personal level in Denmark since 1968. 15 Individual contacts to the Danish health care system and pharmacies are registered by the CPR number. 15 , 16 The CPR number enables linkage of data from all Danish registries at the individual level. Data management and analysis for this study was conducted through Statistics Denmark.
2.2. Data sources
We used data from the Danish National Patient Registry (DNPR), the Danish Central Register, and the Danish Prescription Database in this study. 15 , 16 , 17 Information about types of hospital admissions, procedures, and diagnoses has been collected in the DNPR since 1977 using the International Classification of Diseases (ICD) classification for diagnoses. 15 The ICD, Eighth Revision (ICD‐8) classification for diagnoses was used in the DNPR until 1994; since 1995, the Tenth Revision (ICD‐10) system has been used. Surgical procedures and therapies were registered in the DNPR using the Nordic Medico‐Statistical Committee (NOMESCO) classification from 1996. 15 The positive predictive values of orthopedic surgery codes are among the highest in the DNPR. 15 , 18 Prescribed medicine is to some extent reimbursed in Denmark. The Danish Prescription Registry holds information about redeemed prescription medicine, including the redemption date, pack size, and type of medicine registered by Anatomical Therapeutic Chemical codes. 16
2.3. Study design
We included all Danish residents with a first orthopedic surgical procedure of the lower limb from 1996 to 2017. Four reference subjects with no history of orthopedic surgery were sampled from the general population for each patient. The reference subjects were matched on sex, history of VTE, and birth year to the patient at the date of orthopedic surgery (index date), that is, exposure density sampling of controls. 19 If a control underwent orthopedic surgery of the lower limb during the follow‐up period, he or she entered the study as a patient and four reference subjects were sampled as described. All study subjects were followed from the index date until VTE, death, emigration, or administrative censoring (ie, either 180 days after the index date or December 31, 2017), whichever came first.
2.4. Exposure and patient characteristics
Information about orthopedic surgery procedures was retrieved from the DNPR. 15 We included all patients with a first orthopedic surgical procedure of the pelvis, hip, femur, knee, crus, ankle, or foot. The orthopedic surgical procedures were categorized into seven types based on the NOMESCO code. The NOMESCO classification of surgical procedures is based on three letters (positions 1, 2, and 3) and up to two numerical characters (positions 4 and 5). 20 The first position in the NOMESCO classification denotes the functional anatomic group of the body system; “N” stands for the musculoskeletal system. The second position denotes the functional‐anatomic region within the body system; “E” denotes the pelvis, “F” denotes the hip joint and femur, “G” denotes knee and crus, and “H” denotes ankle and foot. The third position denotes the general method of the surgical procedure. We classified the orthopedic surgical procedures based on the third position in the NOMESCO codes for orthopedic surgical procedures: (a) biopsies, arthroscopies, and other smaller procedures; (b) primary and secondary arthroplasties; (c) repair of fractures; (d) amputations; (e) surgical treatment of tumors or infections; (f) soft‐tissue surgery (eg, meniscectomy, ligament reconstruction); and (g) other surgical procedures (eg, osteotomies and pseudo‐arthrosis surgery) (Table S1). The last available information regarding orthopedic surgery was December 31, 2017.
Information about anticoagulation medication was retrieved from the Danish National Prescription Registry using the Anatomical Therapeutic Chemical codes for warfarin (B01AA03), dabigatran (B01AE07), apixaban (B01AF02), rivaroxaban (B01AF01), tinzaparin (B01AB10), dalteparin (B01AB04), enoxaparin (B01AB05), coumarin/phenprocoumon (B01AA04), edoxaban (B01AF03), and fondaparinux (B01AX05). Study subjects were considered on anticoagulation medicine from the date of prescription drug redemption until the calculated last dose of anticoagulation medicine. The subjects remained in the “anticoagulated” group if a new reimbursement was registered before the calculated last dose and in case of hospitalization during a period of anticoagulation.
We obtained information about VTEs (ICD‐8 codes: 450 and 451 plus ICD‐10 codes: I26 and I80.1‐I80.9) and cancer diagnoses (ICD‐8:140‐209, except 173.x; ICD‐10: CC00‐96, except CC44) preceding the index date from the DNPR.
2.5. Outcome
We retrieved information about VTE after the index date (ICD‐10 codes: I26 and I80.1‐I80.9) from the DNPR. We included VTE discharge diagnoses from hospital wards. VTEs coded in emergency departments were excluded if not subsequently coded in a hospital ward or an outpatient clinic due to low positive predictive value of VTE diagnoses solely coded in the emergency departments. The positive predictive value of a VTE discharge diagnoses in the DNPR is 75%‐84% if restricted to wards. 21 , 22
2.6. Statistics
Descriptive continuous variables were presented as medians and interquartile ranges (25th‐75th percentiles). We depicted the cumulative incidences and associated 95% confidence intervals (CIs) of VTEs using the Aalen‐Johansen estimator; death was treated as competing risk. 23
In Cox proportional hazards regression models, we estimated hazard ratios (HR) and associated 95% CIs of VTE stratified by the matching group and included the following predefined variables: calendar year and a history of cancer as categorical variables, and anticoagulation treatment as a time‐varying variable. Time since surgery/index date was included as time‐varying 30‐day intervals. Statistical analyses were performed using R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
2.7. Ethics
This study was approved by the data responsible institute (Region Hovedstaden – Approval number P‐2019‐404) in accordance with the General Data Protection Regulation. Study data will not be shared due to Danish data protection legislation.
3. RESULTS
3.1. Baseline characteristics
A first orthopedic surgical procedure was registered for 1 012 823 patients between 1996 and 2017. Surgery of the knee and lower leg were most frequent (47.5%) followed by hip joint or femur (31.1%) and ankle or foot (19.7%), while pelvic surgery comprised 1.7% of all orthopedic procedures in the study (Table 1). The most frequent type of surgery was soft‐tissue surgery (eg, meniscectomy and ligament reconstruction) (30.3%), while surgical treatment of tumors or infections comprised only 1.9% but with large variations based on the anatomic site of surgery (Figure 1). The median age of the patients was 54.9 years (25th‐75th percentile; 36.2‐71.6) with considerable variation based on the anatomic site and type of surgery (Figure S1).
Table 1.
Baseline characteristics of the patients with a first orthopedic surgical procedure (N = 1 012 823)
| Age, y, median (25th‐75th percentile) | 54.9 (36.2‐71.6) |
| Sex, n (%) | |
| Men | 472 275 (46.7) |
| Women | 540 548 (53.4) |
| History of VTE before surgery, n (%) | |
| Yes | 23 881 (2.4) |
| No | 991 860 (97.6) |
| Anatomic site of surgery, n (%) | |
| Pelvis | 17 023 (1.7) |
| Hip joint/femur | 314 840 (31.1) |
| Knee/crus | 481 435 (47.5) |
| Ankle/foot | 199 525 (19.7) |
| Surgery type, n (%) | |
| 1: Biopsies, arthroscopies, and other smaller procedures | 148 512 (14.7) |
| 2: Primary arthroplasties | 222 393 (22.0) |
| 3: Repair of fractures | 220 458 (21.8) |
| 4: Amputations | 25 714 (2.5) |
| 5: Surgical treatment of tumors or infections | 18 907 (1.9) |
| 6: Soft‐tissue surgery (eg, meniscectomy, ligament reconstruction) | 306 799 (30.3) |
| 7: Other surgical procedures (eg, osteotomies and pseudo arthrosis surgery) | 70 040 (6.9) |
Figure 1.
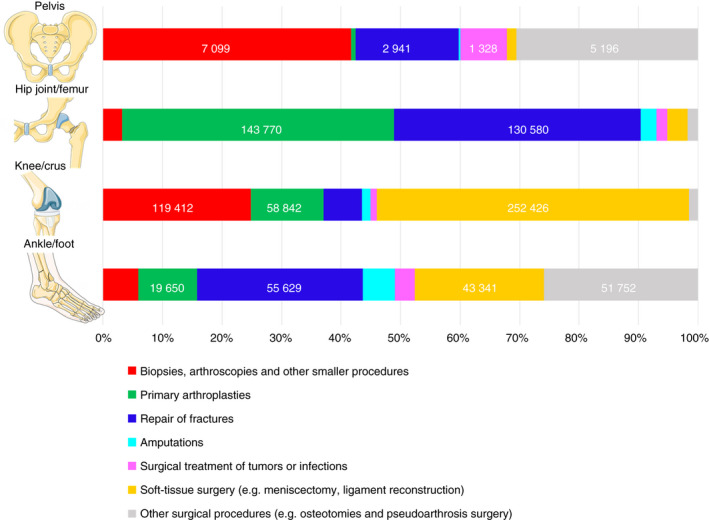
Distribution of types of surgery for each anatomic site of surgery. The figure was illustrated using images from Servier Medical Art Commons Attribution 3.0 Unported License (http://smart.servier.com). Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License
Four reference subjects were identified for 1 012 573 of the orthopedic surgery patients, while three or fewer controls were identified for 71 orthopedic surgery patients. The total number of controls in the study was 4 051 055. Forty‐five percent (1 844 560) of the controls were included only once in the study, while 22.3% (904 363) were included as controls for more than one patient during the study period (ie, in total 1 844 560 + 904 363 = 2 748 923 unique controls). The vast majority of controls included more than once were matched to two or three patients in the study period; 2.6% (83 394) were included as controls for more than three patients. Twelve percent (329 087) of the controls entered the study subsequently as patients.
3.2. Outcome
A total of 7203 VTEs were registered within 6 months after study entry for the orthopedic surgical patients; 154 concurrent pulmonary embolism and deep vein thrombosis, 2314 pulmonary embolisms, and 4735 deep vein thromboses. Among the reference subjects, a total of 4957 VTEs were registered within 180 days from study entry; 161 concurrent pulmonary embolisms and deep vein thromboses, 1990 pulmonary embolisms, and 2806 deep vein thromboses. Within 180 days after orthopedic surgery, 0.71% (95% CI, 0.70‐0.73) of the patients were diagnosed with a VTE. Within the corresponding 180 days after study entry, 0.11% of the controls (95% CI, 0.11‐0.12) were diagnosed with a VTE.
3.3. Anatomic site of surgery and venous thromboembolism
The 180‐day cumulative incidence of VTE was highest after hip joint/femur surgery; 1.15% (95% CI, 1.13‐1.19). Lowest 180‐day cumulative incidence of VTE was seen after foot/ankle surgery; 0.49% (95% CI, 0.42‐0.48). The cumulative of VTE was significantly higher for all anatomic sites of orthopedic surgery than for matched controls (Figure 2).
Figure 2.
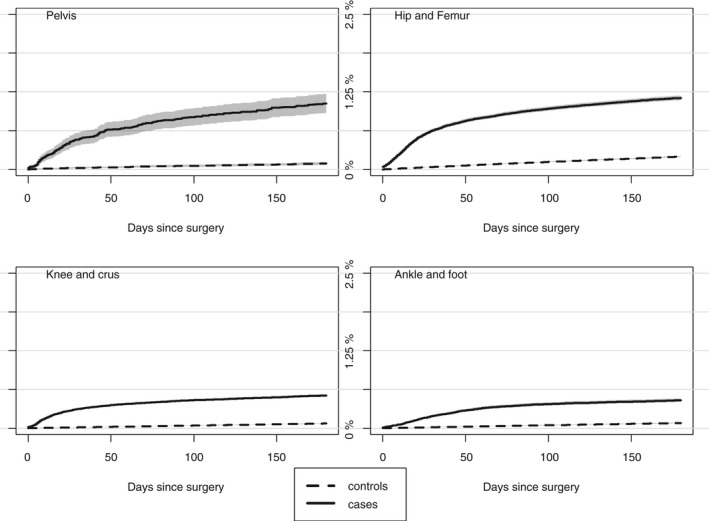
Cumulative incidence of VTE for the four anatomic sites of orthopedic surgery and respective controls. VTE, venous thromboembolism
The 180‐day HR of VTE was highest for patients who underwent pelvic surgery compared with their matched controls (16.7; 95% CI, 10.8‐25.8) in multivariate analysis. The lowest HR of VTE was seen in patients with ankle or foot surgery (5.3; 95% CI, 4.7‐6.0). The HR of VTE for patients who underwent knee/crus surgery was comparable to but actually slightly higher than that of patients with hip joint/femur surgery (Figure 3).
Figure 3.
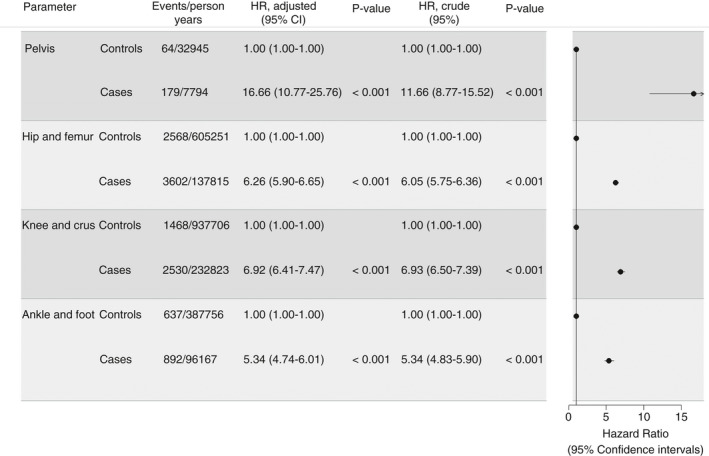
FigCrude and adjusted hazard ratios of VTE within 180 days after surgery for each anatomic surgery site compared with the matched controls. Adjusted estimates are depicted in the forest plot. VTE, venous thromboembolism
The HRs of VTE were highest within the first 30 days after surgery for all anatomic sites of surgery compared with the controls (HRs ranged from 11.1; 95% CI, 9.1‐13.6 for ankle and foot surgery to 23.7; 95% CI, 13.2‐42.7 for pelvic surgery). The HR of VTE within 31‐60 days after ankle and foot surgery was 9.2 (95% CI, 7.3‐11.7), which was higher than the HR of VTE within 31‐60 days after hip/femur surgery (Figure 4).
Figure 4.
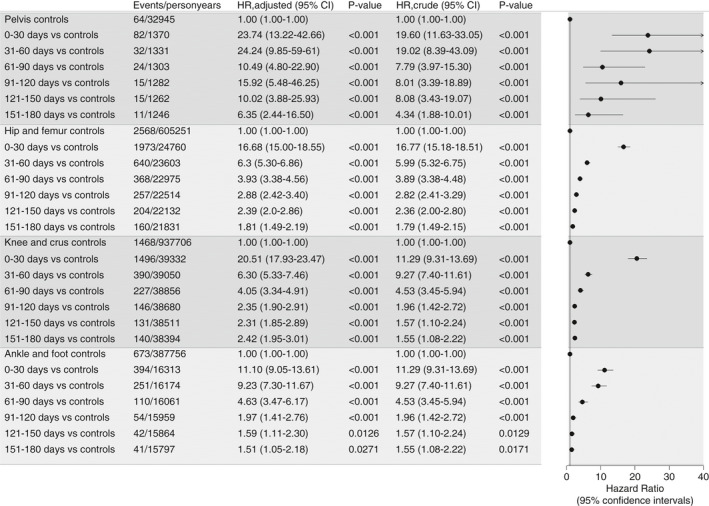
Crude and adjusted hazard ratios of VTE based on anatomic site of surgery by increasing time since surgery compared with matched controls. Adjusted estimates are depicted in the forest plot. VTE, venous thromboembolism
3.4. Type of orthopedic surgery and venous thromboembolism
The cumulative incidence of VTE was higher for all types of surgery than for controls (Figure 5). The cumulative incidence of VTE was highest after arthroplasties, fracture surgery, and amputations; 0.76% (95% CI, 0.74‐0.80), 0.74% (95% CI, 0.72‐0.77), and 0.77% (95% CI, 0.68‐0.50), respectively. The cumulative incidence of VTE after fracture surgery, arthroplasties, and amputations increased relatively steeply until around 50 days after the surgery (Figure 5). The cumulative incidence of VTE after biopsies/arthroscopies, surgical treatment of tumors and infections, and soft‐tissue surgery were at a lower level and inclined steeply for a shorter period after the surgery (Figure 5). The cumulative incidence of VTE was lowest for patients who underwent biopsies (0.32%; 95% CI, 0.24‐0.33).
Figure 5.
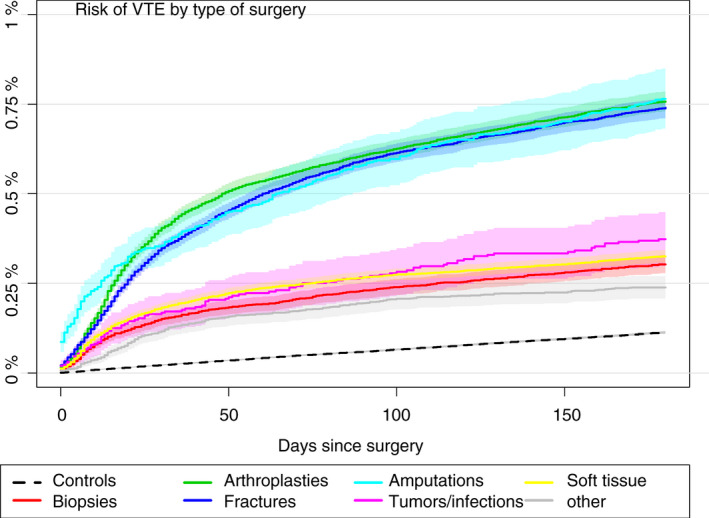
Cumulative incidences of VTE for each of the seven types of surgical procedures and all controls. VTE, venous thromboembolism
Adjusted HRs of VTE for patients with hip joint or knee arthroplasties and surgical treatment of fractures of the hip joint/femur were 4.6 (95% CI, 4.2‐5.0) to 4.9 (95% CI, 4.5‐5.3) in multivariate analysis stratified on anatomic site of surgery, compared with the matched controls. The HR of VTE was higher after surgical treatment of foot/ankle fractures (8.4; 95% CI, 7.1‐10.0) (Figure 6). The adjusted HR of VTE for patients who underwent soft‐tissue surgery of the knee, for example, meniscus operations (7.1; 95% CI, 6.4‐8.0, 4.7), was at least as high as for patients with knee arthroplasties or surgical treatment of fractures of the knee/crus (HRs 4.7; 95% CI, 4.2 to 5.3 and 5.0; 95% CI, 4.3‐5.8, respectively) (Figure 6). The HR of VTE after soft‐tissue surgery of the foot or ankle (eg, Achilles tendon surgery) was 4.8 (95% CI, 3.8‐6.0) compared with matched controls (Figure 6).
Figure 6.
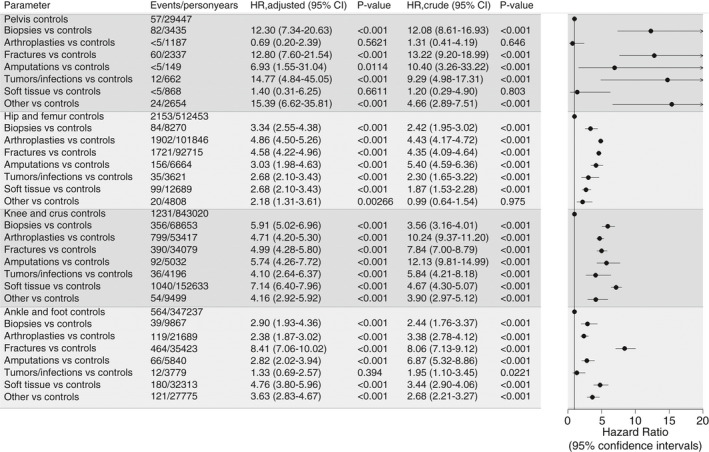
Hazard ratios of VTE based on type of the surgical procedure, stratified on anatomic surgery sites, compared with their matched controls. VTE, venous thromboembolism
3.5. Venous thromboembolism according to age groups, previous VTE, and calendar time
The cumulative incidence of VTE after orthopedic surgery was significantly higher for all age groups compared with the matched controls (Figure 7). The cumulative incidences of postoperative VTE was significantly higher for patients above the age of 40 than younger counterparts. The cumulative incidence of VTE after hip and femur surgery was similar for age groups 41‐60 years and those >80 years. For procedures of the knee, crus, ankle, and foot, the cumulative incidences of VTE for patients between 41 and 60 years of age was significantly lower than for patients >60 years (Figure 7). The 180‐day cumulative incidence of VTE among patients with a history of VTE before orthopedic surgery was 5.87% (95% CI, 5.57‐6.17); for patients without a history of VTE, it was 0.59% (95% CI, 0.58‐0.61). The HR of postoperative VTE in case of a history with VTE before surgery was 7.1 (95% CI, 6.6‐7.5) compared with patients free of previous VTE in multivariate analysis. The risk of VTE was generally lowest from 1996 to 2000 and inclined significantly in the early 2000s, followed by lower cumulative incidences (Figure S2).
Figure 7.
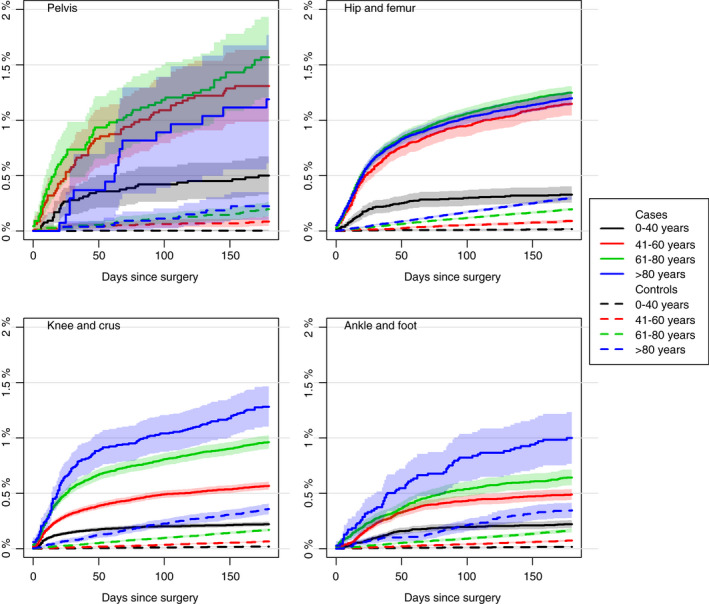
Cumulative incidences of VTE for each anatomical surgery site and controls stratified by age group. VTE, venous thromboembolism
4. DISCUSSION
In this cohort study based on Danish nationwide register data including all first lower limb orthopedic procedures over 3 decades, the rates of VTE were markedly higher after all types of surgery compared with matched controls sampled from the general population. This was particularly evident for patients >40 years of age and for those with a history of VTE before orthopedic surgery.
Our study has three important specific findings. First, the HRs of VTE after hip joint or knee arthroplasty and fracture surgery of the hip/femur were between 4 and 5 compared with matched controls, and the cumulative incidence inclined steeply for at least 30 days after these types of surgery. Second, HRs of VTE following soft‐tissue surgery, arthroscopies, and surgical treatment of fractures from knee level and distal hereto were between 4 and 7 compared with matched controls. Actually, the HR of VTE was 20.5 (95% CI, 18.0‐23.5) within the first 30 days after surgery of the knee and crus compared with matched controls in multivariate analysis. Third, the cumulative incidence of VTE inclined steeply for several weeks, even months, after surgical treatment of fractures and arthroplasties, while the steep incline was limited to the first few weeks after arthroscopies and soft‐tissue surgery.
Our study demonstrates that VTE remains a relatively infrequent, but far from negligible, complication to lower limb orthopedic surgery despite current thromboprophylaxis strategies, including early mobilization after surgery. 24 The rates of postoperative VTE remained higher for several weeks after surgery, as also observed in other studies of postoperative VTE in orthopedic patients. 25 , 26 It is noteworthy that at least one‐third of the VTEs were actually pulmonary embolisms. This is equivalent to the proportion of pulmonary embolisms in other population‐based studies on the VTE incidence. 27 , 28
A recent Danish nationwide study on VTE in association with Achilles tendon rupture found that 1.4% of all patients were diagnosed with VTE within 180 days after the trauma and was highest for those treated nonoperatively. 29 We included only those patients with Achilles tendon rupture who underwent surgical treatment and categorized surgical treatment of Achilles tendon rupture as “soft‐tissue surgery” along with other types of surgery with lower VTE risk. The estimated absolute risk of VTE in this group is thus lower in our study.
A history of VTE before surgery was associated with a markedly higher risk of postoperative VTE compared with patients free of VTE before surgery in our study, as seen in previous studies of postoperative VTE in orthopedic surgery. 14 , 30 In a recent study based on data from the Multiple Environment and Genetic Assessment, Nemeth et al 31 found that the risk of recurrent VTE was increased up to 6 months after surgery compared with no surgery in patients with a history of VTE before surgery.
We observed significantly higher cumulative incidences of postoperative VTE with higher age but with differences based on the anatomic site of surgery. Age has been discussed in previous studies of postoperative VTE in orthopedic surgery, but the results concerning the impact of age on the postoperative risk of VTE are conflicting. 8 , 26 , 32 , 33 The absolute estimates in our study are possibly confounded by comorbidity, and future studies should specifically investigate the impact of age on the risk of postoperative VTE in orthopedic surgery.
As reported by Pedersen et al, 8 we observed a tendency toward higher cumulative incidence of VTE in the latter 2 decades than in the period from 1996 to 2000, possibly partly explained by better diagnostic procedures for VTE and the fact that more patients with higher baseline VTE risk are offered surgery than previously. 34
The estimates in this study are probably rather conservative in general because we excluded VTEs solely registered in the emergency department. It is likely that this excluded valid cases of deep vein thrombosis. We do, however, consider this preferable over inclusion of data of questionable quality.
The most important study limitation is the lack of data concerning use of medical thromboprophylaxis in association with the surgery, as we were able to assess only prescription medication redeemed after discharge from hospital. National health guidelines in Denmark recommend perioperative thromboprophylaxis for hip and knee arthroplasties and for surgical treatment for proximal femoral fractures (major orthopedic surgery). 35 Recommendations for the duration of medical thromboprophylaxis has changed over the study period. Currently, thromboprophylaxis is recommended for a maximum of 5 days for patients undergoing accelerated hip and knee arthroplasties and for a maximum of 10 days for nonaccelerated hip or knee arthroplasties and for patients treated for proximal femoral fractures. 35 The patients having more distal operations received thromboprophylaxis based on personal risk factors, and presumably quite a few of these patients received thromboprophylaxis.
The Danish national guidelines for perioperative thromboprophylaxis relates to about one‐third of all patients in our study (Figure 1). 35 With absolute risks of postoperative VTE between 0.5% and 1.0%, it is clear that perioperative thromboprophylaxis must be individualized based on strong risk factors for VTE. Some risk assessment models for perioperative VTE risk have been made, for example, the Caprini score, 36 but they are not generally used in the clinic, at least in Denmark. Major risk factors for VTE are high age and previous VTE, as also shown in this study, indicating a need for thromboprophylaxis. Unfortunately, we do not have access to information about other relevant risk factors such as obesity, thrombophilia, and immobilization. In a recent systematic review of risk factors for VTE in patients with temporary lower limb immobilization due to injury, Horner et al 37 found varying results in the 15 eligible studies, and age and injury type were the only consistent risk factors. They conclude that there is limited evidence for individual risk factors to guide thromboprophylaxis. On the other hand, it is uncertain if prophylactic medicine can prevent all thromboembolic events. 13 Petersen et al 10 found that a quarter of all VTE events occurred during ongoing prophylaxis, and in randomized trials of VTE after knee arthroscopy and plaster casts of the crus, the prevalence of VTE was unchanged in the groups treated with enoxaparin. We find that several events occur weeks after the operation, even months after foot and ankle surgery. It is uncertain whether the patients really are mobilized when they come home.
Based on the findings in our study, we recommend that orthopedic surgeons consider whether individual patients undergoing surgery are at risk of postoperative VTE (beyond the period where perioperative thromboprophylaxis is recommended) based on both patient factors and type of surgery, also for surgery below knee level. Further studies within the field of postoperative VTE risk for patients undergoing orthopedic surgery should aim at characterization of groups of patients who could benefit from more aggressive and/or longer duration of medical thromboprophylaxis but, importantly, also identification of modifiable risk factors for nonpharmaceutical interventions.
5. CONCLUSION
Major orthopedic surgery of the lower limb was associated with a higher risk of VTE for several weeks postoperatively compared with matched controls. Even distal minor orthopedic procedures (eg, arthroscopies and soft‐tissue surgery) were associated with higher risks of VTE compared with matched controls, although less pronounced and for a shorter postoperative period.
RELATIONSHIP DISCLOSURE
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
ILG and SJR are responsible for the study design, data managing and analysis, interpretation of the results, and manuscript drafts and revisions. SRK conceived the idea of the study. SK contributed with classification of the surgery types. CTP provided access to the study data. SK, MTS, KHK, SRK, and CTP contributed to the study design and interpretation of results, reviewed and commented on the manuscript, and approved the final version. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Supporting information
Fig S1
Fig S2
Table S1‐S2
Gade IL, Kold S, Severinsen MT, et al. Venous thromboembolism after lower extremity orthopedic surgery: A population‐based nationwide cohort study. Res Pract Thromb Haemost.2021;5:148–158. 10.1002/rth2.12449
Handling Editor: Dr Suzanne.
REFERENCES
- 1. Keller K, Tesche C, Gerhold‐Ay A, Nickels S, Klok FA, Rappold L, et al. Quality of life and functional limitations after pulmonary embolism and its prognostic relevance. J Thromb Haemost. 2019;17(11):1923–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gudipati S, Fragkakis EM, Ciriello V, Harrison SJ, Stavrou PZ, Kanakaris NK, et al. A cohort study on the incidence and outcome of pulmonary embolism in trauma and orthopedic patients. BMC Med. 2014;39(12):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ. Predictors of survival after deep vein thrombosis and pulmonary embolism: a population‐based, cohort study. Arch Intern Med. 1999;159(5):445–53. [DOI] [PubMed] [Google Scholar]
- 4. Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98(4):756–64. [DOI] [PubMed] [Google Scholar]
- 5. Stubbs JM, Assareh H, Curnow J, Hitos K, Achat HM. Incidence of in‐hospital and post‐discharge diagnosed hospital‐associated venous thromboembolism using linked administrative data. Intern Med J. 2018;48:157–65. [DOI] [PubMed] [Google Scholar]
- 6. Cote MP, Chen A, Jiang Y, Cheng V, Lieberman JR. Persistent pulmonary embolism rates following total knee arthroplasty even with prophylactic anticoagulants. J Arthroplasty. 2017;32(12):3833–9. [DOI] [PubMed] [Google Scholar]
- 7. Januel JM, Chen G, Ruffieux C, Quan H, Douketis JD, Crowther MA, et al. Symptomatic in‐hospital deep vein thrombosis and pulmonary embolism following hip and knee arthroplasty among patients receiving recommended prophylaxis: A systematic review. JAMA. 2012;307(3):294–303. [DOI] [PubMed] [Google Scholar]
- 8. Pedersen AB, Sorensen HT, Mehnert F, Overgaard S, Johnsen SP. Risk factors for venous thromboembolism in patients undergoing total hip replacement and receiving routine thromboprophylaxis. J Bone Jt Surg. 2010;92(12):2156–64. [DOI] [PubMed] [Google Scholar]
- 9. Pedersen A, Mehnert F, Sorensen H, Emmeluth C, Overgaard S, Johnsen S. The risk of venous thromboembolism, myocardial infarction, stroke, major bleeding and death in patients undergoing total hip and knee replacement: a 15‐year retrospective cohort study of routine clinical practice. Bone Joint J. 2014;96‐B(4):479–85. [DOI] [PubMed] [Google Scholar]
- 10. Petersen PB, Jorgensen CC, Kehlet H. Venous thromboembolism despite ongoing prophylaxis after fast‐track hip and knee arthroplasty: a prospective multicenter study of 34,397 procedures. Thromb Haemost. 2019;119(11):1877–85. [DOI] [PubMed] [Google Scholar]
- 11. Finazzi G. low‐dose aspirin in polycythemia (ECLAP). A prospective analysis of thrombotic events in the European collaboration study on low‐dose aspirin in polycythemia (ECLAP). Pathol Biol (Paris). 2004;52(5):285–8. [DOI] [PubMed] [Google Scholar]
- 12. Lowe JA, Mitchell SM, Agarwal S, Jones CB. The incidence of venous thromboembolism following pelvic and lower extremity trauma despite adherence to modern prophylactic protocols. J Orthop Trauma. 2020;34(8):418–21. [DOI] [PubMed] [Google Scholar]
- 13. Van Adrichem RA, Nemeth B, Algra A, le Cessie S, Rosendaal FR, Schipper IB, et al. Thromboprophylaxis after knee arthroscopy and lower‐leg casting. N Engl J Med. 2017;376:515–25. [DOI] [PubMed] [Google Scholar]
- 14. Nemeth B, Cannegieter SC. Venous thrombosis following lower‐leg cast immobilization and knee arthroscopy: from a population‐based approach to individualized therapy. Thromb Res. 2019;174:62–75. [DOI] [PubMed] [Google Scholar]
- 15. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Heal. 2011;39(7):38–41. [DOI] [PubMed] [Google Scholar]
- 17. Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39(7_suppl):22–5. [DOI] [PubMed] [Google Scholar]
- 18. Lass P, Lilholt J, Thomsen L, Lundbye‐Christensen S, Enevoldsen H, Simonsen OH. The quality of diagnosis and procedure coding in orthopaedic surgery Northern Jutland | Kvaliteten af diagnose‐ og procedurekodning i ortopædkirurgi Nordjylland. Ugeskr Laeger. 2006;168(48):4212–4215. [PubMed] [Google Scholar]
- 19. Ohneberg K, Beyersmann J, Schumacher M. Exposure density sampling: dynamic matching with respect to a time‐dependent exposure. Stat Med. 2019;38:4390–403. [DOI] [PubMed] [Google Scholar]
- 20. Nordic Centre for Classifications in Health Care . NOMESCO Classification of Surgical Procedures. Oslo; 2010. https://norden.diva-portal.org/smash/get/diva2:970547/FULLTEXT01.pdf
- 21. Severinsen MT, Kristensen SR, Overvad K, Dethlefsen C, Tjonneland A, Johnsen SP. Venous thromboembolism discharge diagnoses in the Danish National Patient Registry should be used with caution. J Clin Epidemiol. 2010;63(2):223–8. [DOI] [PubMed] [Google Scholar]
- 22. Gade IL, Riddersholm SJ, Christiansen I, Rewes A, Frederiksen M, Enggaard L, et al. Venous thromboembolism in chronic lymphocytic leukemia: a Danish nationwide cohort study. Blood Adv. 2018;2(21):3025–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coviello V, Boggess M. Cumulative incidence estimation in the presence of competing risks. Stata J. 2004;4(2):103–12. [Google Scholar]
- 24. Falck‐Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 Suppl):e278S–e325S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Caron A, Depas N, Chazard E, Yelnik C, Jeanpierre E, Paris C, et al. Risk of pulmonary embolism more than 6 weeks after surgery among cancer‐free middle‐aged patients. JAMA Surg. 2019;154(12):1126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Adrichem RA, Nelissen RGHH, Schipper IB, Rosendaal FR, Cannegieter SC. Risk of venous thrombosis after arthroscopy of the knee: Results from a large population‐based case‐control study. J Thromb Haemost. 2015;13:1441–8. [DOI] [PubMed] [Google Scholar]
- 27. Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrom J. Incidence and mortality of venous thrombosis: a population‐based study. J Thromb Haemost. 2007;5(4):692–9. [DOI] [PubMed] [Google Scholar]
- 28. Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, Enright P, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117(1):19–25. [DOI] [PubMed] [Google Scholar]
- 29. Pedersen MH, Wahlsten LR, Grønborg H, Gislason GH, Petersen MM, Bonde AN. Symptomatic venous thromboembolism after Achilles tendon rupture: a nationwide Danish cohort study of 28,546 patients with Achilles tendon rupture. Am J Sports Med. 2019;47(13):3229–37. [DOI] [PubMed] [Google Scholar]
- 30. Richey JM, Ritterman Weintraub ML, Schuberth JM. Incidence and risk factors of symptomatic venous thromboembolism following foot and ankle surgery. Foot Ankle Int. 2019;40(1):98–104. [DOI] [PubMed] [Google Scholar]
- 31. Nemeth B, Lijfering WM, Nelissen RGHH, Schipper IB, Rosendaal FR, le Cessie S, et al. Risk and risk factors associated with recurrent venous thromboembolism following surgery in patients with history of venous thromboembolism. JAMA Netw Open. 2019;2(5):e193690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nemeth B, Adrichem RAV, Hylckama Vlieg AV, Bucciarelli P, Martinelli I, Baglin T, et al. Venous thrombosis risk after cast immobilization of the lower extremity: derivation and validation of a clinical prediction score, L‐TRiP(cast), in three population‐based case–control studies. PLoS Med. 2015;12(11):e1001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Risk factors for clinically relevant pulmonary embolism and deep venous thrombosis in patients undergoing primary hip or knee arthroplasty. Anesthesiology. 2003;99(3):552–60. [DOI] [PubMed] [Google Scholar]
- 34. Cram P, Lu X, Kaboli PJ, Vaughan‐Sarrazin MS, Cai X, Wolf BR, et al. Clinical characteristics and outcomes of Medicare patients undergoing total hip arthroplasty, 1991–2008. JAMA. 2011;305(15):1560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. The Danish Council for the Use of Expensive Hospital Medicines . Background note: Thromboprophylaxis for Orthopedic surgical pateints|Baggrundsnotat: Tromboseprofylakse Til Ortopædkirurgiske Patienter; 2014. https://rads.dk/media/2084/210514-baggrundsnotat-tromboseprofylakse.pdf Accessed September 15, 2020
- 36. Caprini JA, Arcelus JI, Hasty JH, Tamhane AC, Fabrega F. Clinical assessment of venous thromboembolic risk in surgical patients. Semin Thromb Hemost. 1991;17(suppl 3):304–12. [PubMed] [Google Scholar]
- 37. Horner D, Pandor A, Goodacre S, Clowes M, Hunt BJ. Individual risk factors predictive of venous thromboembolism in patients with temporary lower limb immobilization due to injury: a systematic review. J Thromb Haemost. 2019;17(2):329–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Table S1‐S2


