Abstract
Introduction
The ST Genesia is a benchtop, fully automated thrombin generation (TG) device. It is completely standardized and ensures a uniform heat distribution throughout the measurement. We aimed to determine reference values and to compare TG in men and women with and without the use of oral contraceptives (OCs).
Materials and Methods
Plasma from 117 healthy donors was measured on the ST Genesia with the available reagent kits: STG‐BleedScreen, STG‐DrugScreen, and STG‐ThromboScreen. All kits include at least two quality controls and a reference plasma to normalize data. STG‐ThromboScreen has a second trigger containing thrombomodulin (TM) to include the effect on the protein C pathway. Means were compared with one‐way analysis of variance and reference ranges were established with 2.5th to 97.5th percentiles on absolute TG parameters.
Results
Mean age of the donors was 35 years (SD ± 12); 49.6% were men, 37.6% women without OCs, and 12.8% women with OCs. Men and women without OCs had, respectively, a mean peak height of 167 nM and 164 nM with STG‐BleedScreen, 335 nM and 351 nM with STG‐DrugScreen, and 192 nM and 198 nM with STG‐ThromboScreen. Women taking OCs had a mean peak height of 263 nM, 473 nM, and 312nM, respectively (P < .05 compared to men/women without OCs). TM decreased endogenous thrombin potential by 54% in men, 47% in women without OCs, and only 25% in women with OCs (P < .05 compared to men/women without OCs).
Conclusions
TG in men and women without OCs was similar; however, women taking OCs had significantly higher TG values, and the effect of TM was also less pronounced in these women.
Keywords: oral contraceptives, protein C, reference values, thrombin, thrombomodulin
Essentials.
The ST Genesia is a new, fully automated thrombin generation (TG) device.
We determined reference values in men and women with and without oral contraceptives (OCs) usage.
Women using OCs demonstrated significantly higher TG values compared to men/women without OCs.
Thrombomodulin influenced TG significantly less in women on OCs compared to men/women without OCs.
1. INTRODUCTION
The thrombin generation (TG) assay is an established research tool in the research field of thrombosis and hemostasis. 1 It mirrors a significant part of the overall function of the blood‐clotting system. The assay has been used since the early 1950s, but back then it was performed by subsampling, which was very labor intensive. 2 , 3 , 4 Nowadays, more (semi)automated assays are commercially available such as the Calibrated Automated Thrombinography (CAT) assay (Diagnostica Stago SAS, Asnières sur Seine, France), the Technothrombin (Technoclone, Wien, Austria) and the Innovance ETP assay (Siemens Healthineers, Erlangen, Germany). Numerous studies have already demonstrated the added value of measuring TG in a patient sample 2 , 5 , 6 , 7 , 8 , for example, in patients with rare inherited coagulation disorders, 9 patients with hypercoagulability, 10 or patients with hemophilia 11 , 12 . The TG assay was revealed to be instrumental in the elucidation of the coagulopathy of chronic liver diseases. 13 , 14 , 15 , 16 Moreover, TG can also be used to monitor or predict the outcome of blood product transfusion. 17 , 18 Unfortunately, the assay is still not available in the clinic due to a lack of standardization (eg, differences in protocol or reagents), which makes it difficult to compare data between laboratories, as well as a lack of clear cutoff values for clinical treatment decisions. 19 , 20 , 21
The newly developed ST Genesia (Diagnostica Stago) is a benchtop, fully automated TG assay, related to the previous CAT assay. The ST Genesia method is more standardized, has specific kits for specific experimental aims, and ensures a stable and uniform heat distribution throughout the measurement and the measuring cuvette. Other differences include the presence of a reference plasma and quality controls in the reagent kits and a different calibration method. The necessary quality controls for clinical use of the ST Genesia are available and comply with local legislation or guidelines, which recommend the use of at least two levels of controls. 3 The use of a reference plasma to normalize the data is specifically advantageous, as plasma samples do not have to be measured all at once anymore and the need for a single batch of reagents also becomes unnecessary. In addition, it enables comparison of data between laboratories, and it reduces the interlaboratory variation. 22 , 23 , 24 , 25 The ST Genesia is easy to use; as it measures TG in a fully automated manner, the only action to be done in the laboratory is to reconstitute some of the reagents with distilled water and subsequently to load the reagents and samples into the device. The following TG parameters can be obtained: lag time (time needed to produce the first traces of thrombin), endogenous thrombin potential (ETP; area under the curve that stands for the total amount of active thrombin formed during the whole experiment), peak height (maximal active thrombin formed), time to peak (time needed to achieve the peak height), start tail (time needed until all thrombin formation ceased), and velocity index (velocity of active thrombin formation).
The goal of this study was to determine the reference values and interindividual variation of 117 healthy donors measured with the three commercially available reagents on the ST Genesia. These values can be useful for laboratories that are implementing the measurement of TG in plasma samples with the ST Genesia. Additionally, a comparison between men and women with and without the use of oral contraceptives (OCs) was evaluated in the same population of healthy donors.
2. MATERIALS AND METHODS
2.1. Samples
Personnel of the university campus of Maastricht were approached and asked to participate to our study. In total, 120 healthy donors volunteered to participate. The study was approved by the Medical Ethical Committee of the Maastricht University Medical Centre and conducted according to the Declaration of Helsinki (2013). Blood samples were taken after the informed consents were signed. Exclusion criteria included the use of drugs interfering with coagulation, known coagulation disorders, and/or being younger than age 18 years or older than age 65 years. Twenty‐seven milliliters of blood was drawn aseptically in vacuum blood collection tubes (Greiner Bio‐One, Kremsmünster, Upper Austria) containing 3.2% sodium citrate (in a 9:1 ratio), from the antecubital vein of healthy subjects. Directly after blood drawing, platelet‐poor plasma was prepared by centrifuging the blood for 10 minutes at 2630 g at room temperature. Immediately thereafter, the supernatant was collected and centrifuged for 10 minutes at 2630 g at room temperature. The platelet‐poor plasma samples were stored anonymously at −80°C until further use. The samples were measured at the latest 11 months after blood collection.
2.2. Thrombin generation
TG was measured on the ST Genesia (Diagnostica Stago SAS). The first step was to run a calibration curve. This calibration curve was performed with the STG‐Cal&Fluo kit, which contained STG‐ThrombiCal, STG‐FluoStart, and STG‐FluoSet. The STG‐ThrombiCal (lyophilized powder) contained the calibrator and had to be reconstituted with 2 mL of distilled water. The STG‐FluoStart (liquid) contained the substrate and calcium, while the STG‐FluoSet (liquid) contained a fluorophore product that was used for calibration of the samples. All reagents had to be kept for 30 minutes on the bench at room temperature before use.
Once the calibration was performed successfully, the reference plasma and quality controls could be measured. Three reagent kits that were commercially available for the ST Genesia were used for this study: STG‐BleedScreen, STG‐ThromboScreen and STG‐DrugScreen. The tissue factor (TF) concentrations of the aforementioned reagent kits were manufacturer proprietary information; however, they ranged from low to medium and high, respectively. Each reagent kit contained its own reference plasma that was used to normalize the sample TG data, as well as two quality controls for the STG‐BleedScreen and STG‐DrugScreen (nomal and low) and three quality controls for the STG‐ThromboScreen (high, normal, and low). The STG‐ThromboScreen reagent kit contained two activators with the same TF concentration and phospholipid content, but one of the two also contained rabbit‐derived thrombomodulin (TM). All reagents were lyophilized powders and needed to be reconstituted with 1 mL of distilled water and were left on the bench for 30 minutes at room temperature before use. First, the quality controls and reference plasmas were run during this study. After successful completion of these runs, the plasma samples of the healthy donors were thawed. Thawing occurred by putting the small plasma tubes into a warm water bath at 37°C for 5 minutes. Immediately thereafter, the plasma tubes were gently mixed and TG was measured. The fluorescence measurement occurred every 15 seconds at an absorption and emission wavelength of 377 and 450 nm, respectively. The samples were run in duplicate, and the ST Genesia embedded software analyzed the data automatically and gave a mean result. For each run and each trigger, a specific freeze‐dried normal human plasma, STG‐RefPlasma, was run in parallel to samples, as also described earlier. 3 This reference plasma came with specific assigned ranges provided on a barcoded flyer and helped to normalize results. 3 , 24 Normalized results for each sample were calculated by applying the formula [Patient sample result / Reference plasma result * Activity assigned for the particular lot and parameter of this reference plasma]. This normalization calculation was done automatically by the software of the analyzer.
2.3. Statistical Analysis
Prism version 8.4.2 (GraphPad Software, San Diego, CA, USA) was used to determine the statistical significance of the results. The results are presented as mean ± standard deviation (SD) or median ± interquartile range (IQR) and interindividual variation as indicated. Reference ranges are shown as 2.5th to 97.5th percentile according to the clinical and Laboratory Standards Institute EP28‐A3c guidelines. 26 Data were checked for normality with the Shapiro‐Wilk test. As not all groups passed normality, the nonparametrical Dunn’s multiple comparisons test was used for statistical comparison. Outliers were established by the ROUT method (combination of Robust regression and Outlier removal). P < .05 was considered statistically significant.
3. RESULTS
3.1. Study population characteristics
TG was measured with the ST Genesia in 117 of 120 healthy donors, as 3 samples had to be discarded due to very low plasma volume. One sample did not contain enough plasma for measuring TG with all triggers (STG‐ThromboScreen with TM was annulled). Table 1 shows the number of healthy donors per subgroup: men (49.6%), women without OCs (women –OCs, 37.6%) and women taking OCs (women + OCs, 12.8%). The mean age of the whole population was 35 ± 12 years (mean ± SD), and the median age was 30 years (IQR, 25‐42). The female group taking OCs was statistically younger than the men or women without OCs (P = .029 and P < .00, respectively). No statistical difference in age was observed between men and women without the use of OCs.
Table 1.
Patient characteristics
| Men | Women –OCs | Women + OCs | Total | |
|---|---|---|---|---|
| Number (%) | 58 (49.6) | 44 (37.6) | 15 (12.8) | 117 (100) |
| Mean age, y (SD) | 33 (12) | 40 (13) | 25 (4) | 35 (12) |
| Median age, y (IQR) | 29 (25‐39) | 38 (27‐52) | 25 (21‐27) | 30 (25‐42) |
Data are expressed as indicated.
Abbreviations: IQR, interquartile range; OCs, oral contraceptives; SD, standard deviation.
3.2. Thrombin generation measured with the STG‐BleedScreen, STG‐DrugScreen, and STG‐ThromboScreen
Tables 2, 3, and 4 show the data of all TG parameters measured with STG‐BleedScreen, STG‐DrugScreen, and STG‐ThromboScreen, respectively. The absolute data are shown in the upper panels (N = 116‐117), while the normalized data are in the lower or middle panels (N = 93‐102). The reason for the lower number of subjects in the normalized data panel is that normalization did not occur in all samples due to human error. Of all data sets, median with IQRs, as well as mean (SD) and interindividual variation are given. Overall, the lowest interindividual variation was for the start tail, while the highest variation was for velocity index. The variation for peak height decreased with increasing TF concentration in the reagent, while the variation in ETP did not vary. The interindividual variation of the normalized data was similar to the absolute data but somewhat lower. Tables [Link], [Link]‐S3 contain the same data excluding the values of women taking OCs. Irrespective of the trigger used, TG parameters did not differ significantly when these women were omitted from the data set.
Table 2.
Absolute and normalized TG parameter values and interindividual variation measured with STG‐BleedScreen
| Absolute data (N = 117) | Median | IQR | Mean (SD) | Interindividual variation (%) |
|---|---|---|---|---|
| Lag time, min | 2.9 | 2.6‐3.4 | 3.0 (0.7) | 23.3 |
| Peak, nM | 167 | 140‐204 | 178 (57.9) | 32.5 |
| Time to peak, min | 6.4 | 5.8‐7.3 | 6.5 (1.2) | 18.1 |
| ETP, nM/min | 1128 | 938‐1325 | 1141 (266) | 23.3 |
| Velocity index, nM/min | 60.2 | 45.2‐81.6 | 70.9 (37.6) | 53.0 |
| Start tail, min | 19.6 | 16.6‐21.6 | 19.6 (3.5) | 18.0 |
| Normalized data (N = 102) | Median | IQR | Mean (SD) | Interindividual variation (%) |
|---|---|---|---|---|
| Lag time, ratio | 1.0 | 0.9‐1.2 | 1.1 (0.2) | 20.1 |
| Peak, % | 131 | 105‐155 | 136 (43.8) | 32.1 |
| Time to peak, ratio | 1.1 | 0.9‐1.2 | 1.1 (0.2) | 17.8 |
| ETP, % | 115 | 95.8‐129 | 115 (25.2) | 22.0 |
| Velocity index, % | 115 | 86.8‐156 | 136 (73.2) | 53.9 |
| Start tail, ratio | 0.8 | 0.8‐1.0 | 0.9 (0.2) | 17.8 |
Data are expressed as indicated.
Abbreviations: ETP, endogenous thrombin potential; IQR, interquartile range; SD, standard deviation; TG, thrombin generation.
Table 3.
Absolute and normalized TG parameters values and interindividual variation measured with STG‐DrugScreen
| Absolute data (N = 117) | Median | IQR | Mean (SD) | Interindividual variation (%) |
|---|---|---|---|---|
| Lag time, min | 1.3 | 1.2‐1.5 | 1.4 (0.3) | 18.7 |
| Peak, nM | 349 | 301‐409 | 359 (85.6) | 23.8 |
| Time to peak, min | 2.8 | 2.5‐3.2 | 2.9 (0.6) | 20.9 |
| ETP, nM/min | 1225 | 1089‐1432 | 1288 (299) | 23.2 |
| Velocity index, nM/min | 329 | 242‐423 | 350 (163) | 46.5 |
| Start tail, min | 11.2 | 10.3‐12.3 | 11.5 (1.5) | 13.1 |
| Normalized data (N = 93) | Median | IQR | Mean (SD) | Interindividual variation (%) |
|---|---|---|---|---|
| Lag time, ratio | 1.0 | 0.9‐1.1 | 1.0 (0.2) | 17.4 |
| Peak, % | 76.1 | 66.7‐88.3 | 77.8 (18.2) | 23.4 |
| Time to peak, ratio | 1.1 | 1.0‐1.3 | 1.2 (0.2) | 20.3 |
| ETP, % | 70.1 | 63.0‐81.0 | 73.0 (16.2) | 22.2 |
| Velocity index, % | 55.1 | 40.2‐67.2 | 58.1 (26.3) | 45.2 |
| Start tail, ratio | 0.9 | 0.8‐0.9 | 0.9 (0.1) | 13.2 |
Data are expressed as indicated.
Abbreviations: ETP, endogenous thrombin potential; IQR, interquartile range; SD, standard deviation; TG, thrombin generation.
Table 4.
Absolute and normalized TG parameters values and interindividual variation measured with STG‐ThromboScreen
| Absolute data –TM (N = 117) | Median | IQR | Mean (SD) | Interindividual variation (%) |
|---|---|---|---|---|
| Lag time, min | 2.3 | 2.1‐2.6 | 2.4 (0.5) | 19.6 |
| Peak, nM | 200 | 169‐238 | 210 (64.3) | 30.6 |
| Time to peak, min | 5.4 | 4.6‐6.2 | 5.5 (1.1) | 19.6 |
| ETP, nM/min) | 1131 | 996‐1388 | 1192 (282.6) | 23.7 |
| Velocity index, nM/min | 84.5 | 62.9‐116 | 98.0 (56.2) | 57.3 |
| Start tail, min | 16.5 | 14.7‐19.0 | 17.1 (2.9) | 17.1 |
| Normalized data (N = 102) | Median | IQR | Mean (SD) | Interindividual variation (%) |
|---|---|---|---|---|
| Lag time, ratio | 1.1 | 1.0‐1.3 | 1.1 (0.2) | 18.0 |
| Peak, % | 83.2 | 68.6‐99.9 | 86.8 (26.8) | 30.9 |
| Time to peak, ratio | 1.2 | 1.0‐1.4 | 1.2 (0.3) | 20.5 |
| ETP, % | 82.7 | 72.1‐98.2 | 85.6 (19.8) | 23.1 |
| Velocity index, % | 63.0 | 45.4‐93.5 | 75.9 (43.5) | 57.3 |
| Start tail, ratio | 1.0 | 0.8‐1.1 | 1.0 (0.2) | 18.2 |
| Absolute data + TM (N = 116) | Median | IQR | Mean (SD) | Interindividual variation (%) |
|---|---|---|---|---|
| Lag time, ratio | 2.2 | 2.1‐2.6 | 2.4 (0.5) | 21.7 |
| Peak, % | 127 | 92.7‐181 | 145 (75.3) | 52.0 |
| Time to peak, ratio | 4.5 | 4.1‐5.1 | 4.6 (0.7) | 15.6 |
| ETP, % | 579 | 396‐799 | 635 (316) | 49.8 |
| Velocity index, % | 72.8 | 52.2‐118 | 91.6 (62.5) | 68.3 |
| Start tail, ratio | 13.4 | 12.6‐14.6 | 13.6 (1.5) | 10.9 |
| ETP inhibition, % | 47.7 | 34.9‐64.2 | 48.3 (17.8) | 36.9 |
Data are expressed as indicated.
Abbreviations: ETP, endogenous thrombin potential; IQR, interquartile range; SD, standard deviation; TG, thrombin generation; TM, thrombomodulin.
3.3. The reference ranges for the three reagent kits
The reference ranges (2.5th to 97.5th percentiles) of the TG parameters for the three reagent kits are depicted in Table 5. Overall, the lag time, time to peak, and start tail results shortened with increasing TF activity in the trigger, while peak height, ETP, and velocity index increased. In particular, the reference ranges for the peak height values were 92‐320 nM for STG‐BleedScreen, 205‐620 nM for STG DrugScreen, 108‐372 nM for STG ThromboScreen without TM, and 51‐319 nM for STG‐Thromboscreen with TM. The reference ranges for the ETP were 706‐1767 nM/min for STG‐BleedScreen, 759‐2032 nM/min for STG DrugScreen, 657‐1806 nM/min for STG ThromboScreen without TM, and 209‐1437 nM/min for STG‐ThromboScreen with TM. The number of subjects varied throughout the table, as outliers were excluded from the data set.
Table 5.
Reference ranges for the ST Genesia
| Reference ranges, absolute data (%) | Lag time (min) | Peak height (nM) | Time to peak (min) | ETP (nM/min) | Velocity index (nM/min) | Start tail (min) | ETP% | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.5‐97.5 | 2.5‐97.5 | 2.5‐97.5 | 2.5‐97.5 | 2.5‐97.5 | 2.5‐97.5 | 2.5 97.5 | ||||||||
| STG‐BleedScreen (N = 111‐117) | 1.9 | 4.4 | 92 | 320 | 4.2 | 9.0 | 706 | 1767 | 26 | 132 | 13.5 | 26.6 | ||
| STG‐DrugScreen (N = 115‐117) | 1.0 | 2.0 | 205 | 620 | 2.0 | 4.3 | 759 | 2032 | 111 | 788 | 9.1 | 15.0 | ||
| STG‐ThromboScreen (N = 110‐117) | 1.6 | 3.3 | 108 | 372 | 3.5 | 7.5 | 657 | 1806 | 37 | 170 | 12.7 | 25.0 | ||
| STG‐ThromboScreen + TM (N = 109‐116) | 1.5 | 3.4 | 51 | 319 | 3.3 | 6.2 | 209 | 1437 | 23 | 171 | 10.9 | 16.7 | 13.4 | 77.1 |
| Reference ranges, normalized data | Lag time (min) | Peak height (nM) | Time to peak (min) | ETP (nM/min) | Velocity index (nM/min) | Start tail (min) | ETP% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.5‐97.5 | 2.5‐97.5 | 2.5‐97.5 | 2.5‐97.5 | 2.5‐97.5 | 2.5‐97.5 | 2.5‐97.5 | |||||||
| STG‐Bleedscreen (N = 96‐102) | 0.7 | 1.5 | 75 | 255 | 0.7 | 1.5 | 75 | 160 | 51 | 264 | 0.6 | 1.2 | |
| STG‐Drugscreen (N = 90‐93) | 0.8 | 1.4 | 44 | 106 | 0.9 | 1.8 | 43 | 102 | 17 | 115 | 0.7 | 1.2 | |
| STG‐Thromboscreen (N = 97‐102) | 0.8 | 1.6 | 48 | 163 | 0.8 | 1.7 | 47 | 128 | 29 | 165 | 0.7 | 1.5 | |
The reference ranges are obtained from the whole population of healthy donors excluding outliers. Data are expressed as raw and normalized values of the 2.5% ‐ 97.5% range for all three reagents.
Abbreviations: ETP, endogenous thrombin potential; TM, thrombomodulin.
3.4. The effect of the use of OCs on thrombin generation
The effect of OC usage on TG parameters was also investigated. Table 6 shows the TG parameters obtained with the three reagent kits for the three groups of healthy donors: men, women without OCs, and women with OCs. TG parameters (except for lag time) did not differ significantly between men and women without the use of OCs. Lag times were significantly longer in men compared to women without the use of OCs for all reagents. Peak height, ETP, and velocity index values were statistically higher in women taking OCs compared to the other groups (men, women without OCs, and the total population of healthy donors). This observation was irrespective of the reagent used to activate the samples. The only exception here is the ETP measured with STG‐DrugScreen of women without OCs versus women with OCs. The ETP% inhibition was also significantly lower in women with OCs (25%) compared to men (54.2%) and women without OCs (47.4%). Women taking OCs had shorter lag time, time to peak, and start tail compared to men and to women without the use of OCs. However, statistically, women with OCs had only significantly shorter lag times compared to men for all reagents. Time to peak was also significantly shorter in women taking OCs compared to the other groups (except between women with and without OCs activated with STG‐DrugScreen and STG‐ThromboScreen + TM. Start tail was significantly shorter in women with OCs compared to men and women without OCs when activating with STG‐BleedScreen and STG‐ThromboScreen –TM but not with STG‐DrugScreen and STG‐ThromboScreen + TM. The ratios of the three groups versus the total population for peak height values and ETP% inhibition are illustrated in Figure 1.
Table 6.
TG parameter comparison between men and women with and without the use of oral contraceptives
| Men (N = 58) | Women –OCs (N = 44) | Women + OCs (N = 13‐15) | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | M/W– | M/W+ | W–/W+ | |
| STG‐BleedScreen | |||||||||
| Lag time, min | 3.1 | 2.8‐3.6 | 2.7 | 2.5‐3.2 | 2.5 | 2.1‐2.6 | .0082 | <.0001 | NS |
| Peak height, nM | 162 | 140‐194 | 164 | 120‐194 | 271 | 223‐312 | NS | <.0001 | <.0001 |
| Time to peak, min | 6.8 | 6.1‐7.6 | 6.3 | 5.8‐7.2 | 5.2 | 4.3‐5.9 | NS | <.0001 | .0102 |
| ETP, Nm/min | 1092 | 937‐1229 | 1070 | 906‐1312 | 1374 | 1147‐1543 | NS | .0030 | .0037 |
| Velocity index, nM/min | 58.4 | 42.1‐74.8 | 56.9 | 43.3‐77.3 | 130 | 89.4‐157 | NS | <.0001 | <.0001 |
| Start tail, min | 19.8 | 17.8‐21.9 | 20.1 | 16.7‐22.7 | 16.0 | 14.6‐18.9 | NS | .0062 | .0057 |
| STG‐DrugScreen | |||||||||
| Lag time, min | 1.4 | 1.3‐1.6 | 1.3 | 1.2‐1.4 | 1.2 | 1.1‐1.3 | .0181 | .0002 | NS |
| Peak height, nM | 334 | 296‐378 | 338 | 301‐402 | 483 | 410‐539 | NS | <.0001 | .0006 |
| Time to peak, min | 3.1 | 2.6‐3.4 | 2.7 | 2.4‐3.0 | 2.4 | 2.1‐2.5 | .0246 | <.0001 | NS |
| ETP, nM/min | 1185 | 1081‐1402 | 1224 | 1059‐1413 | 1409 | 1183‐1851 | NS | .0492 | NS |
| Velocity index, nM/min | 299.0 | 206‐367 | 338.0 | 277‐425 | 585 | 418‐782 | NS | <.0001 | .0004 |
| Start tail, min | 11.4 | 10.6‐12.8 | 11.3 | 10.2‐12.3 | 10.9 | 10.1‐11.7 | NS | NS | NS |
| STG‐ThromboScreen –TM | |||||||||
| Lag time, min | 2.5 | 2.2‐2.8 | 2.2 | 2.0‐2.5 | 2.0 | 1.8‐2.2 | .0390 | .0002 | NS |
| Peak height, nM | 190 | 169‐219 | 194 | 161 ‐229 | 280 | 240‐376 | NS | <.0001 | <.0001 |
| Time to peak, min | 5.7 | 5.1‐6.4 | 5.1 | 4.7‐6.1 | 4.4 | 3.6‐4.7 | NS | <.0001 | .0097 |
| ETP, nM/min | 1130 | 974‐1362 | 1100 | 969‐1332 | 1414 | 1154‐1774 | NS | .0109 | .0098 |
| Velocity index, nM/min | 71.9 | 59.6‐95.2 | 83.7 | 63.1‐112 | 191 | 118‐259 | NS | <.0001 | <.0001 |
| Start tail, min | 17.6 | 14.9‐19.3 | 16.8 | 15.2‐19 | 14.6 | 13.6‐15.4 | NS | .0045 | .0153 |
| STG‐ThromboScreen + TM | |||||||||
| Lag time, min | 2.5 | 2.2‐2.8 | 2.1 | 2.0‐2.5 | 2.0 | 1.8‐2.2 | .0435 | .0004 | NS |
| Peak height, nM | 114 | 83‐137 | 133 | 91‐180 | 309 | 197‐339 | NS | <.0001 | <.0001 |
| Time to peak, min | 4.8 | 4.3‐5.1 | 4.4 | 4.1‐4.8 | 3.9 | 3.4‐4.2 | NS | .0003 | NS |
| ETP (nM/min) | 499 | 373‐643 | 594 | 425‐799 | 1124 | 900‐1397 | NS | <.0001 | .0001 |
| Velocity index, nM/min | 64.7 | 45.7‐78.9 | 78.9 | 50.5‐118 | 217 | 123‐295 | NS | <.0001 | <.0001 |
| Start tail, min | 13.3 | 12.6‐14.8 | 13.5 | 12.8‐14.1 | 13.2 | 12.1‐14.3 | NS | NS | NS |
| ETP inhibition, % | 57 | 43‐66 | 45 | 35‐63 | 25.4 | 19‐31 | NS | <.0001 | .0005 |
Data are shown as median values with interquartile ranges (IQRs).
Abbreviations: ETP, endogenous thrombin potential; M, men; NS, not significant; OCs, oral contraceptives; TM, thrombomodulin; W–, women without OCs; W+, women with OCs.
Figure 1.
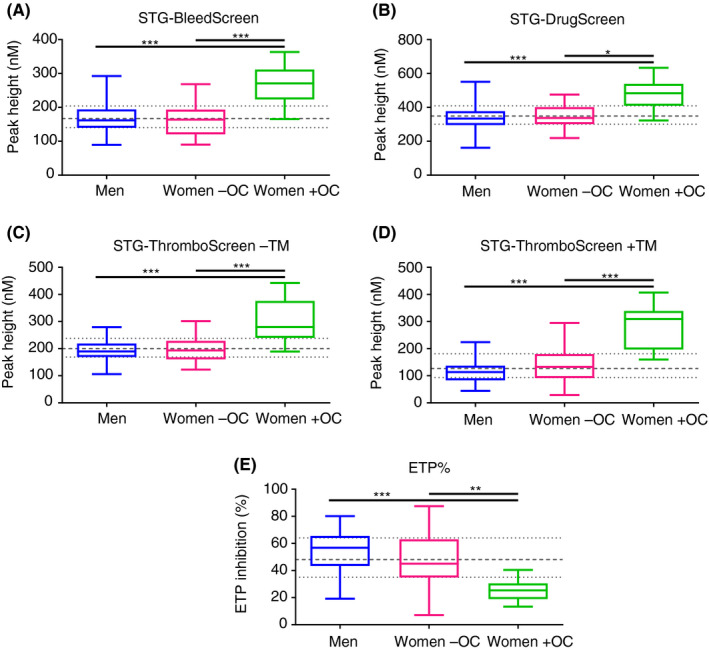
Peak height and ETP inhibition of men, women without OCs and women with OCs and total population of healthy donors. Peak height values obtained by activating the plasma samples with (A) STG‐BleedScreen (N = 117), (B) STG‐DrugScreen (N = 117), (C) STG‐ThromboScreen without thrombomodulin (TM; N = 117), and (D) STG‐ThromboScreen with TM (N = 116). (E) The ETP inhibition by the addition of TM (N = 115). Box plots represent medians with interquartile ranges and minimum and maximum values. The gray interrupted line and dotted line are median and interquartile ranges of the whole population, respectively. ETP, endogenous thrombin potential; TM, thrombomodulin; Women –OC, women without the use of oral contraceptives; Women + OC, women with the use of oral contraceptives. *P = .0006; **P = .0005; ***P < .0001
4. DISCUSSION
The ST Genesia is a fully automated benchtop device that measures TG in a standardized manner and requires only very limited laboratory handling. The goal of this study was to establish the reference values for the ST Genesia of the STG‐BleedScreen, STG‐DrugScreen, and STG‐ThromboScreen reagent kits. The activator of the STG‐BleedScreen reagent kit contains TF at a low picomolar level that is balanced for sensitivity to factor deficiencies (eg, hemophilia A and B), while the STG‐DrugScreen activator contains TF at a high picomolar level to assess the anticoagulant effect at prophylactic and therapeutic doses (eg, direct oral anticoagulants, heparin, vitamin K antagonists). The activator of the STG‐ThromboScreen reagent contains TF at a medium picomolar level balanced for sensitivity to deficiencies in natural anticoagulants (eg, protein C). To date, this is the first study that used the three reagent kits to simultaneously measure TG in a large population of healthy donors.
TG interindividual variation is similar to the one obtained by the CAT‐TG assay (ie, 23% for ETP). 27 The interindividual variation of the peak height and velocity index decreased with increasing TF concentration of the reagent kit, but the one of ETP remained stable. This is probably due to the ETP being less dependent on the TF concentration but more dependent on the overall coagulation potential of the sample. On the contrary, peak height and velocity index are highly dependent of the TF concentration as the TF will affect the rate at which thrombin is being formed and therefore also the propagation of TG. 27 , 28
One of the advantages of ST Genesia kits is the use of a reference plasma to normalize the data and the use of quality controls to ensure qualitative results. The use of normalized data makes it possible to compare data between laboratories and reduces interlaboratory variation. 24 , 29 One of the ST Genesia reagent kits, the STG‐ThromboScreen, also includes an activator that contains TM. The addition of TM activates the protein C pathway and in that way also involves this major coagulation regulation loop. The data of the samples measured with TM are not normalized, but they do reveal the percentage of ETP inhibition in comparison to the measurement without TM. The chosen TM concentration in this reagent kit is based on the TM concentration needed to inhibit 50% of the ETP in a pooled normal plasma. 3 , 22 If we then compare this ETP% inhibition to the data obtained in this study, we indeed observed an overall ETP inhibition of 48.3%.
The ST Genesia is based on its precursor the CAT assay. They both use the same thrombin‐sensitive substrate, but one of the major differences between the CAT assay and the ST Genesia is the method used for calibrating the samples. For the CAT assay, a thrombin calibrator is used (α2‐macroglobulin–thrombin complex) that is added to every sample. Calibration of samples on the ST Genesia occurs in two steps: (i) Once daily a calibration curve is obtained using purified human thrombin in buffer with the fluorogenic substrate and also the activity of a fluorophore product is measured in the same buffer; and (ii) every plasma sample is spiked with a fluorophore product and measured. By taking the ratio between the fluorophore product measured in the plasma sample and in the buffer system, the plasma sample can be calibrated correctly. The CAT assay remains a very important tool in the hemostatic research field, as it can be modified to investigate a specific part of the coagulation cascade, for example, by using a specific activator or by including the presence of the platelets. 30 , 31 The ST Genesia also offers the possibility of using an in‐house activator, although this could result in the same problems as with the other TG assays: no standardization of the reagent and therefore the data will not be comparable between laboratories.
Even though the number of women taking OCs was low in this study (n = 15), and their inclusion did not alter TG parameters significantly, we did observe significantly higher TG values between those women and men and women without the use of OCs. This finding, also demonstrated by Calzavarini et al, 22 was seen with the three ST Genesia reagents and for almost every TG parameter. From these data an important question arises whether women taking OCs should be included when reference values are being generated. It is a well‐known phenomenon that the use of OCs affects coagulation and increases the activated protein C resistance in women. 32 , 33 , 34 Indeed, the ETP inhibition was 54.2% for men, 47.4% for women without OCs, and only 25% for women with OCs. However, these women are mostly young adults who are part of the adult healthy population. Therefore, the impasse will remain whether these women should be included in the reference values of healthy donors. Interestingly, no differences were observed between men and women without the use of OCs. Calzavarini et al showed the same findings, though only by two of the available reagents (STG‐BleedScreen and STG‐ThromboScreen). 22 Therefore, we can exclude the need of separate reference values for men and women, which prevents the need for 240 healthy donors to achieve the 90% confidence limit of the upper and lower reference limits when using nonparametric statistics. 35
Some limitations of our study are that the data come from only one research center and only one batch of reagents was used to measure all samples. A similar study should be performed by another research lab to confirm our data, but the batch‐to‐batch variability can be solved by using the normalized data of this study. Additionally, differences in ethnicity should also be investigated with the ST Genesia, as they could result in differences in TG. Currently, contradicting data are published, whereby Tan et al 36 did not find differences in TG, while other research groups did. 37 , 38 From our previous TG survey, we have learned that not all research groups store their plasma samples at −80°C but also at −20°C. 19 Therefore, it could be interesting for future studies to investigate the effect of long‐term sample storage at −80°C and −20°C, as well as the freeze‐thaw effects on the reproducibility of the ST Genesia. 22 Douxfils et al 3 tested the effect of long‐term sample storage at −70°C with the STG‐DrugScreen and concluded that samples were stable up to at least 11 months. As this was not tested with the other reagents, it thus generates another study limitation.
To conclude, we determined the reference ranges of the ST Genesia for the reagent kits available at the moment (STG‐BleedScreen, STG‐DrugScreen, and STG‐ThromboScreen). These reference ranges can be applied by other laboratories using this assay. The ST Genesia finally brings the TG assay into the clinic in a standardized and qualitative manner. However, clinical validation studies will be needed in the near future to establish cutoff values that could be used in the clinic for therapeutic decisions.
RELATIONSHIP DISCLOSURE
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
BL and AC designed the study. RLK collected the samples. MN performed the experiments and wrote the first draft of the manuscript. RLK, AC, and BL critically revised the manuscript.
Supporting information
Supplementary Material
Table S1‐S3
ACKNOWLEDGEMENTS
A. Carlo is an employee of Diagnostica Stago SAS and provided the reagents for this study.
Ninivaggi M, de Laat‐Kremers RMW, Carlo A, de Laat B. ST Genesia reference values of 117 healthy donors measured with STG‐BleedScreen, STG‐DrugScreen and STG‐ThromboScreen reagents. Res Pract Thromb Haemost.2021;5:187–196. 10.1002/rth2.12455
Handling Editor: Dr Pantep Angchaisuksiri
REFERENCES
- 1. Duarte RCF, Rios DRA, Rezende SM, Jardim LL, Ferreira CN, Carvalho MDG. Standardization and evaluation of the performance of the thrombin generation test under hypo‐ and hypercoagulability conditions. Hematol Transfus Cell Ther. 2019;41(3):244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hemker HC, Giesen P, AlDieri R, Regnault V, de Smed E, Wagenvoord R, et al. The calibrated automated thrombogram (CAT): a universal routine test for hyper‐ and hypocoagulability. Pathophysiol Haemost Thromb. 2002;32(5–6):249–53. [DOI] [PubMed] [Google Scholar]
- 3. Douxfils J, Morimont L, Bouvy C, de Saint‐Hubert M, Devalet B, Devroye C, et al. Assessment of the analytical performances and sample stability on ST Genesia system using the STG‐DrugScreen application. J Thromb Haemost. 2019;17(8):1273–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Macfarlane RG, Biggs R. A thrombin generation test; the application in haemophilia and thrombocytopenia. J Clin Pathol. 1953;6(1):3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campo G, Pavasini R, Pollina A, Fileti L, Marchesini J, Tebaldi M, et al. Thrombin generation assay: a new tool to predict and optimize clinical outcome in cardiovascular patients? Blood Coagul Fibrinolysis. 2012;23(8):680–7. [DOI] [PubMed] [Google Scholar]
- 6. Castoldi E, Duckers C, Radu C, Spiezia L, Rossetto V, Tagariello G, et al. Homozygous F5 deep‐intronic splicing mutation resulting in severe factor V deficiency and undetectable thrombin generation in platelet‐rich plasma. J Thromb Haemost. 2011;9(5):959–68. [DOI] [PubMed] [Google Scholar]
- 7. Liestol S, Sandset PM, Mowinckel MC, Wisloff F. Activated protein C resistance determined with a thrombin generation‐based test is associated with thrombotic events in patients with lupus anticoagulants. J Thromb Haemost. 2007;5(11):2204–10. [DOI] [PubMed] [Google Scholar]
- 8. Marchetti M, Castoldi E, Spronk HM, van Oerle R, Balducci D, Barbui T, et al. Thrombin generation and activated protein C resistance in patients with essential thrombocythemia and polycythemia vera. Blood. 2008;112(10):4061–8. [DOI] [PubMed] [Google Scholar]
- 9. Al Dieri R, Peyvandi F, Santagostino E, Giansily M, Mannucci PM, Schved JF, et al. The thrombogram in rare inherited coagulation disorders: its relation to clinical bleeding. Thromb Haemost. 2002;88(4):576–82. [PubMed] [Google Scholar]
- 10. Regnault V, Hemker HC, Wahl D, Lecompte T. Phenotyping the haemostatic system by thrombography–potential for the estimation of thrombotic risk. Thromb Res. 2004;114(5–6):539–45. [DOI] [PubMed] [Google Scholar]
- 11. Dargaud Y, Beguin S, Lienhart A, Al Dieri R, Trzeciak C, Bordet JC, et al. Evaluation of thrombin generating capacity in plasma from patients with haemophilia A and B. Thromb Haemost. 2005;93(3):475–80. [DOI] [PubMed] [Google Scholar]
- 12. Siegemund T, Petros S, Siegemund A, Scholz U, Engelmann L. Thrombin generation in severe haemophilia A and B: the endogenous thrombin potential in platelet‐rich plasma. Thromb Haemost. 2003;90(5):781–6. [DOI] [PubMed] [Google Scholar]
- 13. Kremers RMW, Kleinegris MC, Ninivaggi M, de Laat B, Ten Cate H, Koek GH, et al. Decreased prothrombin conversion and reduced thrombin inactivation explain rebalanced thrombin generation in liver cirrhosis. PLoS One. 2017;12(5):e0177020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tripodi A, Chantarangkul V, Primignani M, Clerici M, Dell'era A, Aghemo A, et al. Thrombin generation in plasma from patients with cirrhosis supplemented with normal plasma: considerations on the efficacy of treatment with fresh‐frozen plasma. Intern Emerg Med. 2012;7(2):139–44. [DOI] [PubMed] [Google Scholar]
- 15. Tripodi A, Primignani M, Chantarangkul V, Clerici M, Dell'Era A, Fabris F, et al. Thrombin generation in patients with cirrhosis: the role of platelets. Hepatology. 2006;44(2):440–5. [DOI] [PubMed] [Google Scholar]
- 16. Tripodi A, Primignani M, Lemma L, Chantarangkul V, Mannucci PM. Evidence that low protein C contributes to the procoagulant imbalance in cirrhosis. J Hepatol. 2013;59(2):265–70. [DOI] [PubMed] [Google Scholar]
- 17. Schols SE, Heemskerk JW, van Pampus EC. Correction of coagulation in dilutional coagulopathy: use of kinetic and capacitive coagulation assays to improve hemostasis. Transfus Med Rev. 2010;24(1):44–52. [DOI] [PubMed] [Google Scholar]
- 18. Schols SE, van der Meijden PE, van Oerle R, Curvers J, Heemskerk JW, van Pampus EC. Increased thrombin generation and fibrinogen level after therapeutic plasma transfusion: relation to bleeding. Thromb Haemost. 2008;99(1):64–70. [DOI] [PubMed] [Google Scholar]
- 19. de Laat‐Kremers RMW, Ninivaggi M, Devreese KMJ, de Laat B. Towards standardization of thrombin generation assays: Inventory of thrombin generation methods based on results of an International Society of Thrombosis and Haemostasis Scientific Standardization Committee survey. J Thromb Haemost. 2020;18(8):1893–9. [DOI] [PubMed] [Google Scholar]
- 20. Spronk HM, Cannegieter S, Morange P, Hackeng T, Huisman M, Nagler M, et al. Theme 2: epidemiology, biomarkers, and imaging of venous thromboembolism (and postthrombotic syndrome). Thromb Res. 2015;136(suppl 1):S8–S12. [DOI] [PubMed] [Google Scholar]
- 21. Dargaud Y, Wolberg AS, Gray E, Negrier C, Hemker HC, Subcommittee on Factor Viii FIX , et al. Proposal for standardized preanalytical and analytical conditions for measuring thrombin generation in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2017;15(8):1704–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Calzavarini S, Brodard J, Quarroz C, Maire L, Nutzi R, Jankovic J, et al. Thrombin generation measurement using the ST Genesia Thrombin Generation System in a cohort of healthy adults: normal values and variability. Res Pract Thromb Haemost. 2019;3(4):758–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Siguret V, Abdoul J, Delavenne X, Curis E, Carlo A, Blanchard A, et al. Rivaroxaban pharmacodynamics in healthy volunteers evaluated with thrombin generation and the active protein C system: modeling and assessing interindividual variability. J Thromb Haemost. 2019;17(10):1670–82. [DOI] [PubMed] [Google Scholar]
- 24. Perrin J, Depasse F, Lecompte T, De Raucourt E, Planche V, Ajzenberg N, et al. Large external quality assessment survey on thrombin generation with CAT: further evidence for the usefulness of normalisation with an external reference plasma. Thromb Res. 2015;136(1):125–30. [DOI] [PubMed] [Google Scholar]
- 25. Dargaud Y, Prevost C, Lienhart A, Claude Bordet J, Negrier C. Evaluation of the overall haemostatic effect of recombinant factor VIIa by measuring thrombin generation and stability of fibrin clots. Haemophilia. 2011;17(6):957–61. [DOI] [PubMed] [Google Scholar]
- 26. Clinical and Laboratory Standards Institute. Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline. CLSI Document C28‐A3 (3rd Edition). 2008.
- 27. Gerotziafas GT, Depasse F, Busson J, Leflem L, Elalamy I, Samama MM. Towards a standardization of thrombin generation assessment: the influence of tissue factor, platelets and phospholipids concentration on the normal values of thrombogram‐thrombinoscope assay. Thromb J. 2005;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kremers RM, Wagenvoord RJ, Hemker HC. The effect of fibrin(ogen) on thrombin generation and decay. Thromb Haemost. 2014;112(3):486–94. [DOI] [PubMed] [Google Scholar]
- 29. Dargaud Y, Wolberg AS, Luddington R, Regnault V, Spronk H, Baglin T, et al. Evaluation of a standardized protocol for thrombin generation measurement using the calibrated automated thrombogram: an international multicentre study. Thromb Res. 2012;130(6):929–34. [DOI] [PubMed] [Google Scholar]
- 30. Ninivaggi M, Dargaud Y, van Oerle R, de Laat B, Hemker HC, Lindhout T. Thrombin generation assay using factor IXa as a trigger to quantify accurately factor VIII levels in haemophilia A. J Thromb Haemost. 2011;9(8):1549–55. [DOI] [PubMed] [Google Scholar]
- 31. Duckers C, Simioni P, Spiezia L, Radu C, Dabrilli P, Gavasso S, et al. Residual platelet factor V ensures thrombin generation in patients with severe congenital factor V deficiency and mild bleeding symptoms. Blood. 2010;115(4):879–86. [DOI] [PubMed] [Google Scholar]
- 32. Douxfils J, Morimont L, Delvigne AS, Devel P, Masereel B, Haguet H, et al. Validation and standardization of the ETP‐based activated protein C resistance test for the clinical investigation of steroid contraceptives in women: an unmet clinical and regulatory need. Clin Chem Lab Med. 2020;58(2):294–305. [DOI] [PubMed] [Google Scholar]
- 33. Sonnevi K, Tchaikovski SN, Holmstrom M, Rosing J, Bremme K, Larfars G. Thrombin generation and activated protein C resistance in the absence of factor V Leiden correlates with the risk of recurrent venous thromboembolism in women aged 18–65 years. Thromb Haemost. 2011;106(5):901–7. [DOI] [PubMed] [Google Scholar]
- 34. Mohamed ABO, Kelchtermans H, Konings J, van Daal J, Al Marzouki A, Harakeh S, et al. The effects of oral contraceptive usage on thrombin generation and activated protein C resistance in Saudi women, with a possible impact of the body mass index. PLoS One. 2018;13(10):e0206376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jones G, Barker A. Reference intervals. Clin Biochem Rev. 2008;29(suppl 1):S93–97. [PMC free article] [PubMed] [Google Scholar]
- 36. Tan CW, Wong WH, Tan CK, Chan YH, Kaur H, Lee LH, et al. The influence of race on plasma thrombin generation in healthy subjects in Singapore. Clin Appl Thromb Hemost. 2018;24(7):1144–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ho P, Ng C, Rigano J, Tacey M, Smith C, Donnan G, et al. Significant age, race and gender differences in global coagulation assays parameters in the normal population. Thromb Res. 2017;154:80–3. [DOI] [PubMed] [Google Scholar]
- 38. Roberts LN, Patel RK, Chitongo P, Bonner L, Arya R. African‐Caribbean ethnicity is associated with a hypercoagulable state as measured by thrombin generation. Blood Coagul Fibrinolysis. 2013;24(1):40–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Table S1‐S3


