Abstract
The severity, course, and outcomes of thrombosis are determined mainly by the size and location of the thrombus, but studying thrombus structure and composition has been an important but challenging task. The substantial progress in determination of thrombus morphology has become possible due to new intravital imaging methodologies in combination with mechanical thrombectomy, which allows extraction of a fresh thrombus from a patient followed by microscopy. Thrombi have been found to contain various structural forms of fibrin along with platelet aggregates, leukocytes, and red blood cells, many of which acquire a polyhedral shape (polyhedrocytes) as a result of intravital platelet‐driven contraction. The relative volume fractions of thrombus components and their structural forms vary substantially, depending on the clinical and pathogenic characteristics. This review summarizes recent research that describes quantitative and qualitative morphologic characteristics of arterial and venous thrombi that are relevant for the pathogenesis, prophylaxis, diagnosis, and treatment of thrombosis.
Keywords: deep vein thrombosis, ischemic stroke, myocardial infarction, pulmonary embolism, thrombectomy, thrombolysis, thrombosis
Essentials.
Thrombus structure and composition relates to formation, embolization, and thrombus resolution.
Morphology of thrombi is studied using intravital imaging or microscopy following thrombectomy.
Content of thrombus components and structural forms varies substantially with clinical features.
Intravital platelet‐driven thrombus contraction forms polyhedral erythrocytes (polyhedrocytes).
1. INTRODUCTION
Because arterial and venous thromboembolic disorders are among the most frequent causes of morbidity and mortality, there has been extensive research on the molecular and cellular mechanisms that are involved. Nevertheless, there has been little description of how the composition of thrombi and emboli depends on their vascular origin and age. We describe here what is now known of the structure and composition of thrombi and thrombotic emboli from both ancient and recent approaches to visualization.
There are at least three major aspects that determine the importance of studying thrombus structure and composition. First, knowledge of cellular and noncellular constituents and their variability helps us to understand the pathophysiology of thrombus formation determined and modulated by general and local conditions, such as hemodynamics, inflammation, and the like. Second, comparing morphologic properties of parental thrombi and thrombotic emboli provides clues to understanding the causes and mechanisms of thrombus rupture and embolization. Third, the structure and composition determine mechanical and lytic resistance of a thrombus and, therefore, have important implications for thrombolysis, thrombectomy, and other methods of thrombus resolution.
The relative proportions and arrangement of platelets, fibrin, and red blood cells (RBCs) and the density and porosity of the thrombi are all important. Specifically, it is now known that clot contraction (retraction) has a major influence on thrombus resolution, as it results in redistribution of platelets and fibrin to the periphery of the clot and RBCs to the interior, where they are compressed to polyhedra or forms intermediate between biconcave and polyhedral, forming a tessellated array of very tightly packed cells. 1 As a result, these so‐called polyhedrocytes are a morphologic sign of clot contraction. Examination of venous and arterial thrombi and pulmonary emboli has shown that polyhedrocytes are a major component of nearly all thrombi and thrombotic emboli. Moreover, the permeability of these tightly packed polyhedrocytes is very low, which has significant implications for the penetration and effectiveness of fibrinolytic compounds or any thrombolytic or thrombectomy treatments.
2. HISTORICAL PERSPECTIVES OF STUDYING THROMBUS MORPHOLOGY
Remarkably, Marcello Malpighi published a paper in 1666 in which he reported the examination by light microscopy of cardiac thrombi obtained by dissection and whole blood clots formed in vitro and described their structures. 2 He compared their structures and concluded that the “polyps” (thrombi) taken from the heart were closely related to the clotted blood and were not fat or derived from the liver, as previously proposed by others. In the clots or thrombi, he observed “red particles” that could be washed away, what we now call RBCs. He also accurately described fibrin as “a fibrous texture, and a network of nerve‐like threads, where small meshes and honeycomb like interstices develop...” and recognized that this network of fibers “contributes the strength of the whole blood clot.” Malpighi also described what we now know as clot contraction or retraction: “the network contains the red atoms compressed by the overlying mass, and so shows a change of substance and color; it becomes limp, through containing the final fringes of the fibres, and dark, from the crowding of the particles contained in it.” He also surmised that clots form when the proper fluid nature of the blood is compromised and that the network is held together by some sort of “hooks” that cause precipitation, a remarkably prescient description of the knobs and holes that actually hold fibrin together. Finally, Malpighi discussed differences in thrombi between the right and left sides of the heart and various diseases in which these structures are found.
It was not until 1788 that Antoine Fourcroy named fibrin as “animal matter that forms clots in blood.” 3 Then, in 1838, Berzelius defined the term protein as an “organic oxide of fibrin and albumin.” 4 In 1847, Virchow introduced the concept of fibrinogen as the term for “a more slowly clottable substance than that which clotted normally.” 5
3. VISUALIZING THROMBI IN VIVO AND EX VIVO
There are numerous noninvasive imaging techniques that are currently used in clinical settings for visualizing thrombi in vivo. The traditional and most often used are radiography, ultrasonography, and computed tomography or magnetic resonance–based angiography. 6 , 7 Most of them have substantial diagnostic limitations because they detect a thrombus based on indirect signs, such as narrowing of a blood vessel lumen, a contrast filling defect in an angiogram, or reduced organ perfusion. 8 For example, vessel narrowing could be due to atherosclerosis or extravascular compression, imaging is affected by the surrounding vascular anatomy, and tests that use contrast may be contraindicated in patients with impaired kidney function. In addition, these imaging modalities cannot help to determine how old the thrombi are or if they are susceptible to thrombolytic therapy. 9
Direct visualization of thrombosis in humans has made huge progress in the past decades through increasing spatial and temporal resolution beyond the levels available with conventional techniques. The new thrombus imaging techniques include single photon emission computed tomography, positron emission tomography, optical methods, and the like, that are based on the use of highly specific radiolabeled molecular probes detected at concentrations as low as 10−12 M to 10−10 M for a wide range of molecular targets. 10 , 11 , 12 , 13 Traditional imaging techniques such as magnetic resonance imaging have also been improved by application of molecular imaging, involving the use of magnetic nanoparticles functionalized with highly specific vector molecules. Ultrasound imaging can now use microbubbles and other contrast agents coated with specific targeting ligand molecules that bind selectively to the site of interest. 14 Thrombus‐targeted imaging technologies use ligands that are specific either for fibrin, 15 activated platelets expressing the integrin αIIbβ3, 16 or prothrombotic components such as factor XIIIa, 17 tissue factor, von Willebrand factor, or exposed collagen. 14 Noninvasive molecular imaging techniques targeting thrombus components have been used both in disease‐state animal models and in clinical applications.
Maturation of a thrombus is associated with its structural reorganization. A fresh clot usually starts with a tangled mesh of activated platelets and fibrin and contains more RBCs, while the maturing clot shrinks in volume and accumulates leukocytes. A corresponding decrease in hemoglobin concentration within the clot is followed by reduced optical transparency, suggesting the use of optical absorbance or photoacoustic imaging. The magnitude of the photoacoustic signal from the thrombus directly depends on the relative content of RBCs and is inversely proportional to the age of the clot. 18 The new imaging modalities not only allow earlier and better diagnosis of thrombosis but lead to a deeper understanding of the pathogenic mechanisms of thrombus formation and fate.
To visualize flowing blood, blood cells, and thrombi, intravital molecular‐based fluorescence imaging has been widely used in animal models of thrombosis. 19 , 20 Various blood components are labeled with different dyes and fluorescent antibodies followed by intravital epifluorescence or fluorescent confocal microscopy that allows time‐lapse imaging to track formation and maturation of a thrombus. 21 Imaging and analysis of the formation of microthrombi in real time provides high‐speed, near‐simultaneous acquisition of images of multiple fluorescent probes at the site of local vessel wall injury. 22 This innovative technology has been used to localize various components during thrombus formation, to reveal defects in thrombus growth in genetically modified mice, to quantify the time course of platelet activation following vascular injury, to visualize leukocyte rolling on arterial thrombi, to generate three‐dimensional models of thrombi, to analyze the effect of antithrombotic agents in vivo, and much more. 23
Despite substantial advances in the in vivo imaging methodology, there are inherent general restrictions in studying thrombus composition due to worse spatial resolution compared to direct pathomorphologic examination of ex vivo thrombi extracted intravitally. The advent of mechanical thrombectomy allows extraction of a thrombus from a patient, including the coronary or cerebral arteries, followed by light microscopy of histologic sections or electron microscopy analysis. 24 , 25 This approach allows the retrieval and visualization of fresh thrombi, thus overcoming the limitations of postmortem studies. Detailed structural characterization and determination of the composition of ex vivo thrombi is one main subject of this review.
There are still existing restrictions to direct structural examination of freshly obtained ex vivo thrombi related to the lack of standardized identification and quantification of different thrombus structures, uncertainty about how to handle analysis of thrombi, and varying nomenclature regarding thrombus components.
4. MORPHOLOGIC SIGNS OF INTRAVITAL CONTRACTION OF THROMBI
Recently, there has been a resurgence of interest in clot contraction (retraction), and some of the basic cellular and molecular mechanisms have been uncovered. Platelets contain all the cytoskeletal machinery necessary for cellular motility and are quite strong cells that can generate up to 30 nN of force. 26 Actin inside platelets is attached by several anchor proteins to αIIbβ3 in the cell membrane, and nonmuscle myosin IIa generates force that is transmitted to fibrin outside of the platelet attached to the extracellular portion of αIIbβ3. Platelets extend filopodia that attach to fibrin fibers and pull, making a kink in the fiber and agglomerating fibrin around the platelets. 27 Additional filopodia attach to the same or different fibers, pulling hand‐over‐hand, clustering platelets in addition to accumulating piles of fibrin around the platelets.
All three main constituent parts of clots and thrombi, that is, RBCs, platelets, and fibrin, undergo significant changes in the process of platelet‐driven volume shrinkage known as contraction or retraction. Thrombin‐activated platelets aggregate and form filopodia that attach to fibrin fibers and pull on them, release secretory granules, decrease in size, and fall apart into fragments. 27 , 28 In addition, activated platelets release membrane‐derived microvesicles that attach to fibrin fibers and modify their structure and properties. 29 Besides the clot shrinkage and compaction, clot contraction is accompanied by spatial redistribution of the components in such a way that the fibrin‐platelet mesh moves to the periphery, while the interior part of the clot accumulates compacted RBCs. 1 Upon transection of a contracted blood clot, electron and confocal microscopy reveals erythrocytes inside that have the shape of polyhedra; hence, they have been called polyhedrocytes 1 (Figure 1). The formation of polyhedrocytes is due to their mechanical compression by the contractile forces generated by platelets and transmitted through the fibrin network. The most obvious physiologic consequences of the compaction of deformed RBCs include densification and impermeability of the clot for pathogens and fibrinolytic enzymes 1 and altered susceptibility to external and internal fibrinolysis. 30 As a matter of fact, the polyhedrocytes are a hitherto unknown natural morphologic variant of RBCs, which have been studied under the microscope since the time of Jan Swammerdam, Marcello Malpighi, and Anthony van Leeuwenhoek.
Figure 1.
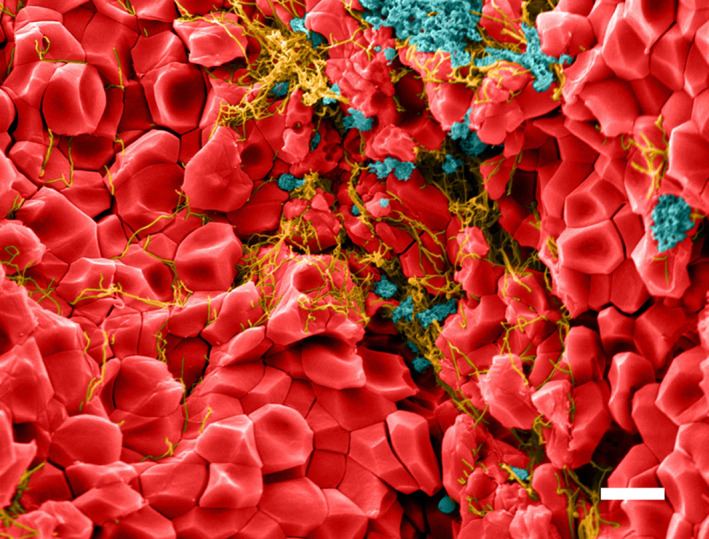
Colorized scanning electron micrograph of contracted whole blood clot. Red blood cells, red; fibrin, yellow; platelets and platelet microvesicles, blue. Red blood cells redistribute to the interior of a contracted whole blood clot or thrombus due to the contractile forced generated by activated platelets pulling on fibrin. As a result of compressive forces, the red blood cells take on a polyhedral shape; hence, they have been named polyhedrocytes (with permission fromBlood, 2014, v. 123, issue 10 – cover page). Magnification bar = 5 µm
Because in vitro contracted blood clots have characteristic structural alterations, namely, formation of polyhedrocytes and accumulation of fibrin and platelets at the periphery of the clot, these features can be considered as morphologic criteria to detect contraction of blood clots and thrombi in vivo. Practically speaking, these structural characteristics can be used as markers to reveal in ex vivo blood clots and thrombi the presence of in vivo contraction. Until recently, there were no systematic studies of intravital contraction of obstructive blood clots and thrombotic emboli. We are aware of one study published in 1971, in which histologic preparations and scanning electron micrographs of thrombi revealed polygonal cells and the accumulation of fibrin on the clot periphery, 31 but these results were not fully understood at that time and remained unnoticed and did not serve as an impetus to the study of clot contraction in thrombosis. Compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin toward the clot periphery were for the first time correlated with contraction in vivo by comparing electron micrographs of in vitro blood clots and aspirated coronary artery thrombi. 1 Although RBCs comprised only about 17% of the thrombus volume, the presence of polyhedrocytes inside a thrombus indicated that the thrombus had undergone mechanical compression or contraction. This observation was later confirmed by further studies of coronary thrombi, most of which contained polyhedrocytes. 32 , 33 An extensive study of the structure and composition of venous and arterial thrombi and pulmonary emboli showed that they all had compressed RBCs and only about 2% biconcave RBCs. 34 , 35 In addition, in ex vivo thrombi there are many partially deformed erythrocytes, having intermediate form between the usual biconcave cells and fully formed polyhedra. Altogether, partially or completely deformed erythrocytes account for 88% of RBCs in the composition of arterial thrombi, 89% in venous thrombi, and 99% in pulmonary emboli. 34
The morphologic signs of intravital contraction have been also found in thrombotic emboli extracted from pulmonary arteries at autopsy. 36 The emboli are characterized by the presence of deformed polyhedral erythrocytes and redistribution of fibrin to the periphery, indicative of intravital compression of the parental venous thrombi and/or secondary thrombotic emboli. The relationship between contraction of a primary thrombus and thrombotic embolus remains open as well as the role of impaired contraction in the propensity for thrombotic embolization. 35
Thus, the aggregate of data available provide strong evidence that contraction of blood clots occurs not only in vitro but also inside blood vessels and in wounds, 37 comprising a real but understudied and underestimated pathophysiological process that affects the structure, composition, and properties of blood clots and thrombi.
There is also now considerable evidence that thrombotic conditions affect the extent and rate of clot contraction. In ischemic stroke, 38 venous thromboembolism, 35 systemic lupus erythematosus, 39 recurrent pregnancy loss, 40 and sickle cell disease, 41 there is a decreased extent and rate of contraction of blood taken from patients with these conditions. Moreover, impaired clot contraction in the early postoperative period is associated with imminent deep vein thrombosis (DVT), suggesting that it is a prothrombotic risk factor and promotional mechanism. 42 These differences arise because the platelets in these patients are partially activated, exhausted and refractory, in addition to changes in the composition of the blood.
5. COMPOSITION AND STRUCTURE OF CORONARY ARTERY THROMBI
To understand the dynamic process of in vivo thrombus formation, the structure and composition of coronary artery thrombi taken from patients with ST‐segment–elevation myocardial infarction coming to the emergency department at different times has been quantified by analysis of high‐resolution scanning electron micrographs 43 (Figure 2). Surprisingly for arterial thrombi, the main component of these thrombi was fibrin (56%) followed by platelets (17%) and erythrocytes (11%), cholesterol crystals (5%), and leukocytes (~1.5%). The strongest correlation between thrombus composition and patient clinical history was related to ischemic time, defined as the time between onset of symptoms and removal of thrombus burden by thromboaspiration in primary percutaneous intervention. There was a twofold increase of fibrin content for every ischemic hour and a 50% reduction in platelets (Figure 3). These changes with time occur because more fibrin is added as a result of the presence of high concentrations of thrombin in the thrombus and because platelets fragment and undergo cell death. 28 These results account for the fact that early treatment is beneficial because thrombolytic drugs will be less effective with increasing amounts of fibrin to digest, while antiplatelet drugs will become less useful with fewer platelets. The thrombotic masses aspirated from a coronary artery of patients with acute ST‐segment–elevation myocardial infarction were analyzed histologically, and thrombus age was classified as fresh (<1 day), lytic thrombus (1‐5 days), and organized thrombus (>5 days). In at least half of these patients, coronary thrombi were days or weeks old, indicating that sudden coronary occlusion is often preceded by a relatively long period of plaque instability and thrombus formation. 44
Figure 2.
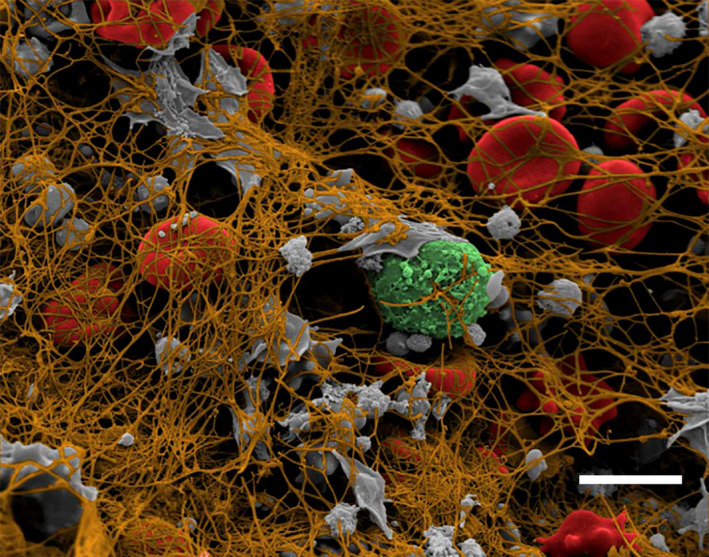
Colorized scanning electron micrograph of a coronary artery thrombus from an ST‐elevation myocardial infarction patient (with permission fromBiophysical Journal, 2017, Volume 112, Issue 4 – cover page). Platelets, gray; fibrin, brown, red blood cells, red; white blood cell, green. Magnification bar = 5 µm
Figure 3.
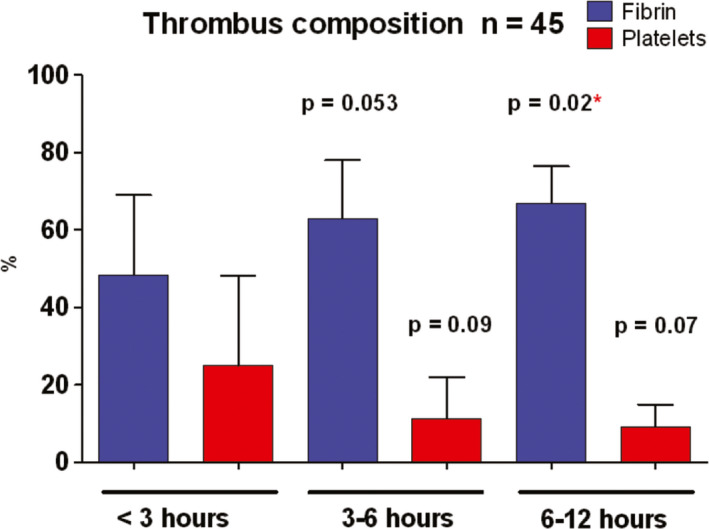
Differences in the proportion of platelets and fibrin in coronary artery thrombi taken by aspiration from ST‐elevation myocardial infarction patients as a function of time between onset of symptoms of heart attack and percutaneous coronary intervention (with permission from J Amer Coll Cardiol. 2011;57:1359)
The Thrombus and Inflammation Study in Sudden Cardiac Death included patients with angiographically proven acute coronary occlusion as a cause of ST‐segment–elevation myocardial infarction complicated by sudden cardiac death or not. 45 This study investigated the possibility that vascular vulnerability related to a specific pattern of abrupt coronary occlusion may be involved. Thrombi were examined by high‐resolution scanning electron microscopy, and their composition was analyzed in the same manner as the study just described, with the result that there was no difference between the groups with sudden cardiac death or not. 46 In all of these patients, there was a similar and striking effect of ischemic time on the composition, with increasing fibrin and decreasing platelets with time, and there was no difference in thrombi between patients receiving or not receiving antiplatelet drugs.
It has been shown that at a liquid‐air interface, fibrin(ogen) can form a biofilm that helps retain blood cells and protect against microbial infections. 47 This biofilm consists of a unique arrangement of fibrin molecules tethered to the fibrin network of fibers. A similar sort of film has been seen on intracoronary artery thrombi extracted from patients with acute ST‐segment–elevation myocardial infarction. 48 Since the detailed structure of these films on thrombi is not known and these thrombi are not formed at a liquid‐air interface, it is not yet clear how they relate to the fibrin biofilm observed in vitro or in skin wounds. 47
6. COMPOSITION AND STRUCTURE OF CEREBRAL ARTERIAL THROMBI
Stroke is a leading global cause of death worldwide, and about 87% strokes are caused by focal cerebral ischemia due to arterial occlusion. 49 The main trigger of ischemic stroke is a thrombus, which forms in a cerebral artery (usually narrowed and altered by atherosclerosis) or travels through the bloodstream to the brain from the heart or another artery. The etiology of ischemic stroke remains unclear in about 40% of patients; hence, the condition is called cryptogenic stroke. 25 , 50 , 51 The composition of cerebral thrombi and its relation to the course and outcomes of ischemic stroke has been described in detail in a number of recent reviews. 52 , 53 Staessens et al 54 discussed explicitly technical aspects of collecting and processing cerebral thrombi for histopathologic examination.
It has been shown that cerebral thrombi, as well as thrombi of other localization, consist of fibrin‐platelet accumulations, erythrocytes, and leukocytes. 55 , 56 , 57 Despite the presence of the same components in all cases, there is a large diversity of their spatial distribution as well as in quantitative proportions of cellular and noncellular structures. Marder et al 56 explained this morphologic diversity by random and chaotic conditions of blood flow, shear, and turbulence at sites of thrombus formation. Since there is heterogeneity of clot composition, traditional distinction between “red” versus “white” clots is not fully applicable. 58 , 59 Some authors 49 think that the composition of clots depends on the embolic source, while others 60 suggest that the differences between the stroke subtypes could not be confirmed on the basis of morphologic characteristics, and the structure of thrombi and thrombotic emboli depends on the underlying local conditions rather than the difference in etiology.
Most researchers classified extracted cerebral thrombi on the basis of the histopathologic examination as RBC or fibrin dominant, or when the content of RBC and fibrin are equal. 24 , 55 Based on the composition, cerebral thrombi could be classified according to the age as fresh (<1 day), lytic (1‐5 days), or organized (>5 days), but the majority of cerebral thrombi are fresh, and no difference has been found in the age of thrombi between stroke subtypes. Thrombi from a large atherosclerotic artery had the highest volume fraction of RBCs, while cardioembolic thrombi and thrombi of unknown origin had the lowest. No relationship has been revealed between stroke subtype and platelets or fibrin content. 61 In contrast, Boeckh‐Behrens et al 62 found that the fibrin‐platelet fraction predominated in the more organized cardioembolic thrombi, whereas RBCs prevailed in noncardioembolic thrombi. Thrombi from patients with cryptogenic stroke showed the same basic composition as cardioembolic thrombi, with higher proportions of fibrin‐platelet component and a smaller fraction of RBCs, which is distinct from thrombi originating from atherosclerotic arteries. Ahn et al 60 also observed that the volume fraction of RBCs was significantly greater in atherosclerotic than in cardioembolic thrombi, whereas amount of fibrin was larger in cardioembolic versus atherosclerotic thrombi. A study by Sporns et al 25 supports the notion that RBC‐rich thrombi are associated with a stroke cause of large artery atherosclerosis, whereas fibrin‐rich thrombi show a correlation with cardioembolic stroke. This finding also correlates with recent work by Simons et al 24 that reports the combination of high fibrin and low RBC proportions in cardioembolic thrombi.
Unlike in the majority of studies, Kim et al 63 found that the clots in patients with cardioembolism had a higher volume fraction of RBCs and a lower proportion of fibrin than in those with large artery atherosclerosis. There were no significant differences in the proportion of platelets and leukocytes between patients with cardioembolism and those with large artery atherosclerosis. It has also been reported that thrombi with a large number of RBCs have a higher risk of separation and embolization. 55
Remarkably, cerebral thrombi often contain leukocytes, both sparse and clustered, which were associated with worse disease outcomes. 64 The presence of neutrophils in cerebral thrombi correlated with the formation of neutrophil extracellular traps (NETs) that can promote thrombosis and substantially reduce the susceptibility of thrombi to pathophysiological fibrinolysis and therapeutic thrombolysis. 65 , 66 , 67 Colocalization of fibrin and NETs has been demonstrated in arterial thrombi extracted from patients with ischemic stroke. 67 , 68 Notably, the higher content of leukocytes in cerebral thrombi was revealed in cerebral thrombi of cardioembolic origin. 50 , 62 Thus, local inflammation can affect the structure and properties of cerebral thrombi and have a strong impact on the course and outcomes of ischemic stroke.
Analyzing the ultrastructure of atherosclerotic thrombi, Ahn et al 60 discovered that RBC masses were located in the center of the thrombi, while the periphery contained platelets covered by a fibrin coating that constituted the outer shells of thrombi; some platelets were aggregated with a mixture of fibrin and activated leukocytes. Such spatial distribution is a morphological signature of the intravital contraction of thrombi. 1 , 34 , 35 In contrast, cardioembolic thrombi tended to show thick fibrin deposition along with clustering of platelets within the thrombus. 60 Although the composition of cerebral thrombi is highly heterogeneous, they often have a remarkable spatial redistribution of the components described above, so that there is an inner core made of compressed polyhedrocytes and an outer shell made of densely compacted platelets or a fibrin‐platelet mesh 64 , 69 (Figure 4). Based on in vitro experiments and in vivo observations, it has been proposed that the thrombus outer shell is responsible for a decreased susceptibility to thrombolysis induced by tissue‐type plasminogen activator (t‐PA). 70 This lytic resistance could be, at least in part, due to the presence of NETs that reduced the rate of thrombus resolution induced by t‐PA. 65
Figure 4.
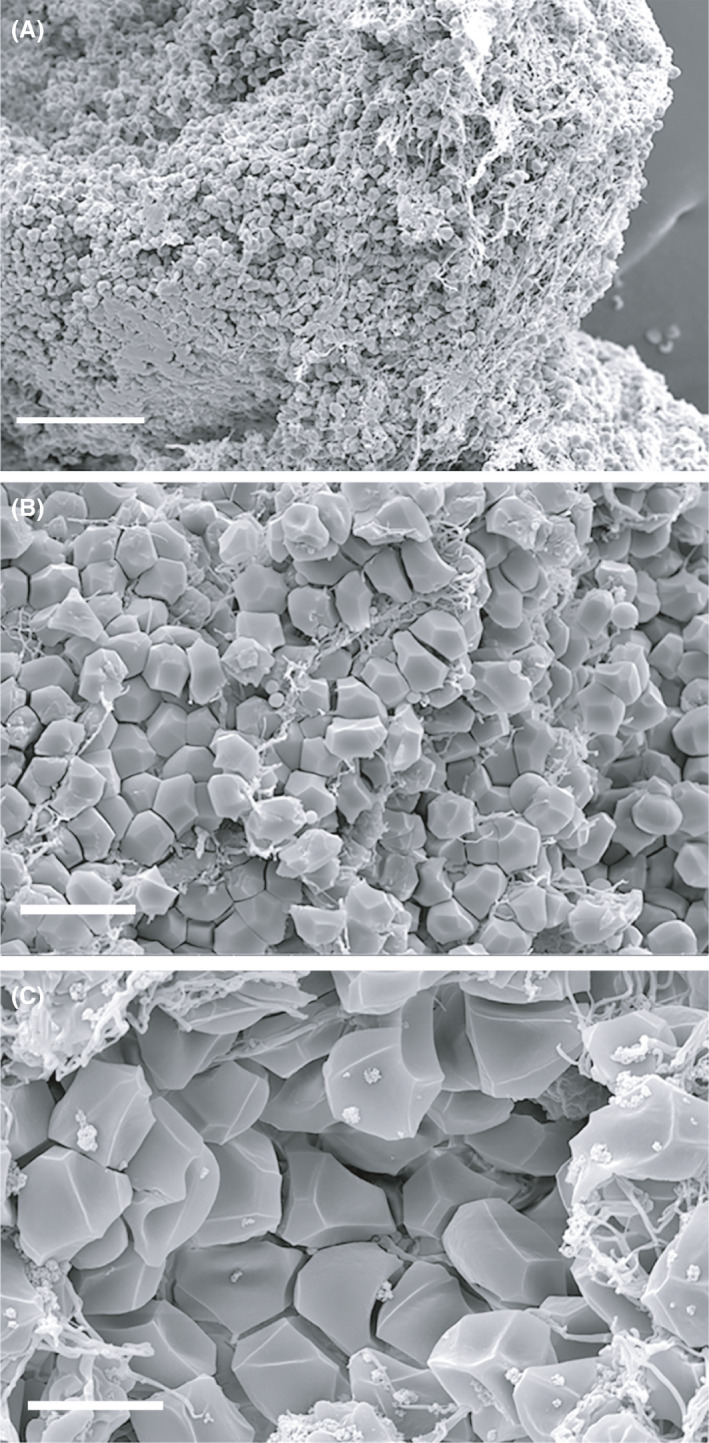
Scanning electron micrographs of ex vivo cerebral arterial thrombi at different magnifications showing morphological signs of intravital contraction: (A) Segregation of RBCs to the core and platelet‐fibrin accumulation on the periphery of the thrombus and (B, C) formation of polyhedral RBCs (polyhedrocytes). Magnification bars = 50 µm (A), 10 µm (B), and 5 µm (C)
In conclusion, thrombi extracted from patients with stroke are highly heterogeneous, with distinct architecture and complex composition. They differ between stroke subtypes and reflect the etiology of disease, but establishing a direct correlation with thrombus content is difficult. Further morphologic investigations with a systematic and quantitative approach can potentially reveal the association between etiology, pathogenesis, and composition of cerebral thrombi to improve the efficacy of stroke management and clinical outcomes. 71
7. COMPARATIVE STRUCTURE AND COMPOSITION OF VENOUS THROMBI AND PULMONARY EMBOLI
Venous thromboembolism (VTE), which includes DVT and pulmonary embolism (PE), is the most common cardiovascular disease after heart attack and ischemic stroke. 72 , 73 The architecture and composition of venous thrombi are determined by relatively low shear rate (10‐100 s‐1), time after formation, 74 and thrombin concentration. 75 Venous thrombi are relatively rich in RBCs and, therefore, have been called traditionally “red” thrombi. The formation of venous thrombi is driven by a combination of hypercoagulability, injured or activated endothelium, and impaired blood flow, altogether known as Virchow's triad. 76 , 77
The overall composition of venous thrombi is distinct from arterial thrombi in that RBCs and fibrin are the predominant components of venous thrombi, while arterial thrombi contain mostly fibrin and platelets 34 , 78 , 79 (Figure 5). RBCs are represented mainly by compressed cells that are present as polyhedrocytes and intermediate‐shaped cells between biconcave and polyhedral; the regular biconcave RBCs comprise only about 2% of the cells in venous thrombi. Venous thrombi often contain echinocytes that likely reflect metabolic changes due to local environmental conditions. Remarkably, fibrin in venous thrombi is structurally heterogeneous, including fibrin fibers, thick fibrin bundles made up of several laterally aggregated fibers, and sponge‐like porous amorphous structures. 80 The platelet content in venous thrombi is much lower than in arterial thrombi; part of the volume fraction is filled with cellular microvesicles and inflammatory white blood cells. 34
Figure 5.
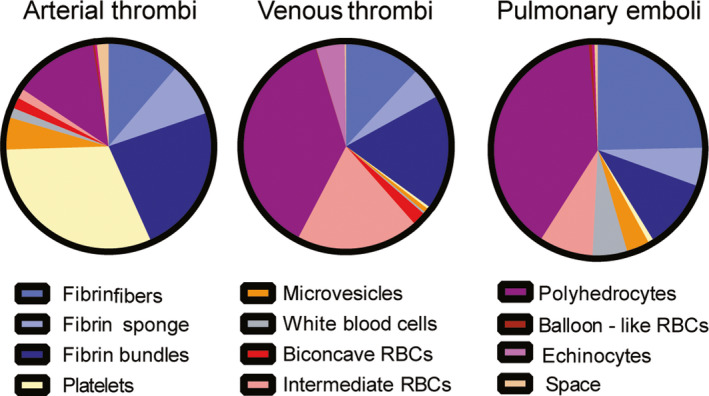
Pie charts showing quantification of structures identified in arterial and venous thrombi and pulmonary emboli (with permission fromSci Rep. 2020;10:5112)
Venous thrombi can undergo time‐dependent changes in structure and composition. Fresh thrombi usually contain alternating layers of platelet‐fibrin and RBC accumulations (lines of Zahn) if they are formed under blood flow. Thrombi formed in conditions of blood stasis have a more homogenous structure. 81 A few days later, the thrombi become infiltrated with inflammatory cells, such as neutrophils, lymphocytes, and monocytes, along with migrating fibroblasts that produce collagen and other components of the connective tissue. The main chemoattractants for inflammatory cells are thrombin and fibrin, with cytokines produced later by monocytes differentiated into macrophages. 82 Maturation of a venous thrombus is followed by a rise in cellular volume fraction and a reduction in fibrin content. 82 , 83 , 84 After weeks, the developing fibrosis and microvascularization result in formation of organized thrombi containing hemosiderin‐loaded macrophages. 81
It is noteworthy that the local inflammatory response associated with venous thrombosis has dual consequences. On the one hand, inflammatory cells that express tissue factor and secrete cytokines contribute to the formation, growth, and maturations of a thrombus. Other potent thrombotic structures are NETs that contain DNA and histones that colocalize with fibrin fibers and increase their lytic stability. 85 On the other hand, the inflammatory cells secrete proteolytic enzymes that, along with fibrinolytic agents, cause thrombus resolution. 86
Thrombotic emboli should potentially retain the structure and composition of the original thrombi from which they originated, but this is not always the case. Thrombotic emboli are similar to parental venous thrombi in that they are both composed primarily of fibrin fibers and RBCs, although the volume fractions of these structures in the emboli are different from the venous thrombi. There are also differences in the prevailing types of fibrin and the RBC content. Pulmonary emboli were shown to contain a higher volume fraction of fibrin, fewer RBCs, and a greater content of WBCs and microvesicles than venous thrombi. Venous thrombi had a much higher content of echinocytes as compared to pulmonary emboli. The content of leukocytes was significantly greater in pulmonary emboli than in venous thrombi. 34 It is hard to say if these differences in composition determined the predisposition to thrombus rupture or developed later after embolization had occurred. One structural feature of a thrombus that can be associated with a tendency to rupture and embolize is the lower extent of clot compaction (and lower strength) as a result of impaired contraction due to platelet dysfunction and/or pathologic changes in blood composition. 35
A clue to the structural relation between a primary thrombus and the secondary embolus comes from the composition of the vessel wall‐attached head, body, and moving tail of the same venous thrombus. 34 These parts of the thrombus are different in age, and the flexible tail is the most likely source of embolization. The tail of this thrombus contained more fibrin and fewer RBCs than the other parts and was similar to the embolus. These results are consistent with time‐dependent changes of the composition of venous thrombi. 87 Over time, the overall content of RBCs in thrombi gradually decreases, while the amount of fibrin increases in the head and tail, with the RBC content remaining highest in the body. Remarkably, the central bodies of thrombi contain mostly fully compressed polyhedrocytes, whereas in the head and tail, the prevailing type of RBCs is intermediate‐shaped or partially compressed RBCs. In agreement with our earlier data, 88 the higher content of fibrin fibers in the clot (namely, in the head and tail) may impair platelet‐induced clot contraction, decreasing formation of polyhedrocytes. The reduced contractility in the tail of venous thrombi in combination with mechanical flexibility could make this region more prone to embolization. Accordingly, impaired contraction of blood clots has been shown to correlate with a higher incidence of PE in patients with DVT. 35
Despite great variability of thrombus structure and composition, there are systematic differences between arterial thrombi and venous thrombi and pulmonary emboli. Namely, the proportion of RBCs in venous thrombi and pulmonary emboli is considerably higher than in arterial thrombi. Polyhedrocytes and intermediate‐shaped forms (both indicative of the intravital compaction of a thrombus mass) are also more frequent in venous thrombi and pulmonary emboli than in arterial thrombi. More fibrin bundles are observed in arterial than in venous thrombi and pulmonary emboli. WBCs are more common in venous thrombi and pulmonary emboli compared with arterial thrombi, while cell‐derived microvesicles are more common in arterial thrombi.
8. THROMBOLYSIS, THROMBECTOMY, AND THROMBUS STRUCTURE
Relationships between clot or thrombus structure and fibrinolysis are complex. In vitro studies have shown a general though not universal conclusion that clots made up of thick fibers are digested more rapidly than clots made up of thinner fibers. 89 , 90 Computer simulations of the fibrinolytic process have provided an explanation for these observations and why the rates of lysis can be reversed at a higher number of t‐PA molecules per surface area. 91 Moreover, many studies have demonstrated a correlation between plasma clots made up of thin fibers in patients with a whole array of (pro)thrombotic conditions, which then accounts for decreased fibrinolysis and a shift of the balance toward clotting in these patients. 92 , 93 , 94 , 95
The presence of platelets and erythrocytes in thrombi also has a major effect on fibrinolysis. Platelets influence fibrinolysis by modulating clot structure, since thrombin is generated on the platelet surface, initiating fibrin fiber formation from platelet aggregates and an abundance of thin fibers. 96 In addition, erythrocytes influence fibrinolysis by their effects on clot structure and their effects on clot permeability. 97 These effects could be related to direct RBC‐fibrin binding because RBCs have been shown to bear fibrinogen‐specific receptor molecules 98 , 99 and the specificity of fibrinogen binding to RBCs can be similar to RBC‐fibrin interactions in blood clots and thrombi. 100
Platelet‐driven clot contraction also has major effects on fibrinolysis. Internal fibrinolysis, in which a plasminogen activator such as t‐PA is added initially before clotting, is a model for physiological fibrinolysis, while external fibrinolysis, where t‐PA is added outside the already formed clot after formation, is a model for clinical thrombolysis. 101 Studies that compared outcomes of t‐PA added to platelet‐rich versus platelet‐poor plasma and clots formed in the presence of increasing numbers of platelets showed that clot contraction enhanced internal fibrinolysis. 102 Studies of clots formed with whole blood demonstrated a similar enhancement of fibrinolysis twofold, probably because the redistribution of erythrocytes and platelets/fibrin that accompanies clot contraction means that fibers are closer together, which enables individual plasmin molecules to move between fibers and cleave fibrin more efficiently. 30 With external fibrinolysis initiated from the clot exterior to simulate therapeutic thrombolysis, most studies showed a slower rate of clot dissolution. 103 , 104 , 105 In contracted whole blood clots, external fibrinolysis was approximately fourfold slower than for uncontracted clots. These results are understandable from the structures of thrombi described in the previous sections, as it is apparent that they are very tightly packed and highly impermeable. This dichotomy in the susceptibility of contracted and uncontracted clots to internal versus external lysis suggests that the rate of lysis is dependent on the interplay between accessibility of fibrin fibers to fibrinolytic agents, including clot permeability, and the spatial proximity of the fibrin fibers that modulate the effects of fibrinolytic enzymes. In addition, the fibrinolytic resistance of compacted fibrin clots can be due to the cross‐linking of α2‐antiplasmin to fibrin by activated factor XIII (FXIIIa), which prevents the plasmin inhibitor from being fully expelled from the clot during contraction. 106 , 107 Understanding how compaction of blood clots influences clot lysis might have important implications for prevention and treatment of thrombotic disorders. In particular, the intravital contraction/compaction of thrombi that impedes external fibrinolysis can explain why intravenous t‐PA, even within a short 3‐ to 4.5‐hour window, has only modest effects on the long‐term outcomes of acute ischemic stroke 108 (Figure 6). There are also side effects and complications of t‐PA infusion like intracranial hemorrhage, which is why lytic agents other than t‐PA and new treatment modalities have been developed, including thrombectomy.
Figure 6.
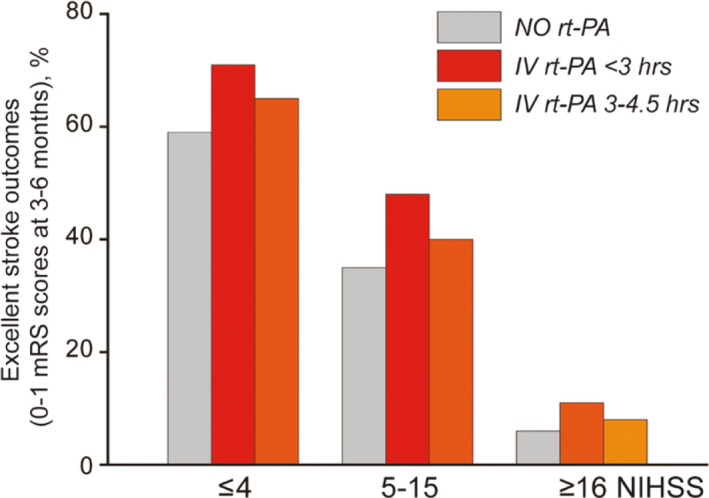
The effects of early thrombolysis on the likelihood of excellent functional outcomes (0‐1 modified Rankin Scale (mRS) scores) at 3‐6 months after stroke depending on the initial stroke severity evaluated by the National Institutes of Health Stroke Scale (NIHSS) scores. These data represent the relatively small difference in the outcomes between patients untreated and treated with intravenous (IV) recombinant tissue plasminogen activator (rt‐PA) at different time periods after the onset of stroke (derived from Lancet Neurol. 2016;15:925‐33)
The unusual mechanical properties of thrombi have implications for understanding their response to compressive forces and for the design and use of thrombectomy or ultrasound devices for removal of these thrombi. 109 Differences seen in the mechanical properties of thrombi and thrombotic emboli with varying fibrin and RBC content can help in understanding what responses to expect when stresses are applied by removal devices to thrombi in vivo and for making such devices work more effectively.
9. INTERNATIONAL SOCIETY ON THROMBOSIS AND HAEMOSTASIS 2020 CONGRESS REPORT
It is a sign of the vitality of this area of research that there were many relevant presentations at the International Society on Thrombosis and Haemostasis 2020 Congress.
Several studies focused on the effects of NETs on fibrinolysis. 110 Another study suggests that the prevalence of von Willebrand factor, NETs, and microcalcifications contribute to thrombectomy resistance. 111 Since NETs intercalate to fibrin and create a dense network that impairs fibrinolysis, circulating markers of NETs might be useful to predict thrombolysis outcomes in patients with acute ischemic stroke. 112 Citrullination of fibrinogen contributes to a clot structure that is lysis resistant because of higher compactness and lower porosity but mechanically vulnerable and consequently more prone to embolization. 113 It was shown that carboxypeptidase U was rapidly generated during thrombolysis and decreased immediately after the end of recombinant t‐PA infusion. 114 Strong correlation of FXIII concentration with the incorporation of C‐terminally intact α2‐antiplasmin into fibrin clot suggests that C‐terminally intact α2‐antiplasmin is the primary substrate of FXIIIa for cross‐linking to fibrin. 115 It was demonstrated that activated platelets initiated not only coagulation but also fibrinolysis via triggering accumulation of plasminogen and t‐PA on their surface, as well as attenuating fibrinolysis through the activation of thrombin‐activatable fibrinolysis inhibitor. 116 Decreased clot‐bound amounts of FXIII with the subsequent reduction in α2‐antiplasmin can be detected in acute PE and show associations with reduced fibrinolytic capacity. 117
There were also some more clinically oriented talks. Platelet‐targeted magnetic nanoparticles can act as an adjuvant to enhance the efficacy of current thrombolytic therapies through the induction of localized magnetic hyperthermia. 118 A reagent has been developed to target fibrinolysis of microthrombi in thrombotic thrombocytopenic purpura. 119 It was shown that pulsed cavitational ultrasound therapy was able to recanalize noninvasively in an in vitro model of acute venous thrombosis. 120 A post hoc analysis of the CAVA (ultrasound‐accelerated catheter‐directed thrombolysis versus anticoagulation for the prevention of post‐thrombotic syndrome) trial of risk for postthrombotic syndrome after early thrombus removal via additional ultrasound‐accelerated, catheter‐directed thrombolysis in patients with acute iliofemoral DVT showed a significant reduction in symptom severity and improvement of generic quality of life. 121 One study of a PE response team investigated the use of catheter‐directed thrombolysis versus systemic intravenous thrombolysis or thrombectomy. 122 A study of patients with acute ischemic stroke undergoing thrombolysis showed that low α2‐plasmin inhibitor antigen levels on admission is an independent predictor of unfavorable long‐term outcomes. 123 An ex vivo study of changes in fibrinolytic factors in an extracorporeal membrane oxygenation circuit showed an elevation in plasmin‐antiplasmin complexes and plasminogen activator inhibitor‐1 and a decrease in t‐PA. 124
10. FUTURE DIRECTIONS
Because of what we now know of thrombus structure, many new approaches are being developed to improve thrombus resolution. Foremost among the methods is the use of thrombectomy for extraction of thrombi from larger vessels. Thrombolysis is still recommended in ischemic stroke for a short treatment window from the onset of occlusion, especially because the risk of side effects of hemorrhage increases with time. Following the success of thromboaspiration and thrombectomy for myocardial infarction, these same methods have been applied successfully to patients with ischemic stroke who have occlusion of large cerebral vessels. Thrombectomy is now recommended for large‐vessel occlusion proven by imaging, as long as a well‐organized and experienced team of specialists is available to carry out the procedure.
Catheter‐directed thrombolysis is also used to place the thrombolytic agent directly to the occlusive thrombus and minimize bleeding side effects. This procedure appears to improve the effectiveness of thrombolytic therapy. Some new technologies use penetration of the thrombus by the catheter and direct infusion of the lytic agent into the interior of the thrombus, which allows delivery to an otherwise impermeable structure. 125 Another future direction in therapeutic thrombolysis and thromboprophylaxis is infusion of RBCs or nanoparticles loaded with fibrinolytic agents that prolong their circulation times and prevent penetration into tissues and existing hemostatic clots, while delivering the agents to the interior of nascent thrombi. 126 , 127
Ultrasound has been used in a variety of forms for recanalizing vessels. Focused ultrasound has been used as a noninvasive therapeutic technology for patients with DVT but is still at the early stages of development. This novel technology is used to focus intense beams of ultrasound energy precisely and accurately on targets deep in the body to ablate a thrombus by thermal destruction without damaging surrounding normal tissue. More commonly, ultrasound can be used to enhance thrombolysis, with ultrasound frequencies and intensities that do not induce thermal damage. 128 Ultrasound causes a decrease in the diameter of the fibers due to tension as a result of vibration, leading to increased binding sites for plasmin(ogen)/t‐PA. 129 The positive feedback of this structural change together with increased mixing/transport of t‐PA/plasmin(ogen) is likely to account for the observed enhancement of fibrinolysis by ultrasound.
Now it is increasingly common for the use of a combination of thrombolysis and thrombectomy. 130 Ultrasound‐assisted thrombolysis together with percutaneous coronary intervention improves recanalization rates and reduces infarct size, resulting in sustained improvements in heart function after ST‐segment–elevation myocardial infarction. 131 For ischemic stroke, it appears that patients treated with both intravenous thrombolysis and thrombectomy show better functional outcomes, probably also with lower mortality, and a higher rate of successful recanalization. 132
The new discoveries of thrombus structure and properties summarized here will undoubtedly lead to other novel strategies for accomplishing more effective thrombus resolution.
AUTHOR CONTRIBUTIONS
Dr Weisel and Dr Litvinov cowrote the paper.
RELATIONSHIP DISCLOSURES
The authors declare no conflicts of interest.
Weisel JW, Litvinov RI. Visualizing thrombosis to improve thrombus resolution. Res Pract Thromb Haemost. 2021;5:38–50. 10.1002/rth2.12469
Handling Editor: Dr Alisa Wolberg.
Funding informationNIH grants HL135254, HL146373, HL148227, and bridge funding from the University of Pennsylvania Perelman School of Medicine.
REFERENCES
- 1. Cines DB, Lebedeva T, Nagaswami C, Hayes V, Massefski W, Litvinov RI, et al. Clot contraction: compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin. Blood. 2014;123:1596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Forrester JM. Malpighi’s De polypo cordis: an annotated translation. Med Hist. 1995;39:477–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carpenter JK. Protein and energy: A study of changing ideas in nutrition. New York: Cambridge University Press; 1994. [Google Scholar]
- 4. Kastritis PL, Bonvin AM. On the binding affinity of macromolecular interactions: daring to ask why proteins interact. J R Soc Interface. 2013;10:20120835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rosenfeld L. Four centuries of clinical chemistry. Boca Raton, Florida: CRC Press; 1999. [Google Scholar]
- 6. Dmytriw AA, Song JSA, Yu E, Poon CS. Cerebral venous thrombosis: state of the art diagnosis and management. Neuroradiology. 2018;60:669–85. [DOI] [PubMed] [Google Scholar]
- 7. van Dam LF, van Walderveen MAA, Kroft LJM, Kruyt ND, Wermer MJH, van Osch MJP, et al. Current imaging modalities for diagnosing cerebral vein thrombosis ‐ a critical review. Thromb Res. 2020;189:132–9. [DOI] [PubMed] [Google Scholar]
- 8. Choudhury RP, Fuster V, Fayad ZA. Molecular, cellular and functional imaging of atherothrombosis. Nat Rev Drug Discov. 2004;3:913–25. [DOI] [PubMed] [Google Scholar]
- 9. Oliveira BL, Caravan P. Peptide‐based fibrin‐targeting probes for thrombus imaging. Dalton Trans. 2017;46:14488–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dronkers CE, Klok FA, Huisman MV. Current and future perspectives in imaging of venous thromboembolism. J Thromb Haemost. 2016;14:1696–710. [DOI] [PubMed] [Google Scholar]
- 11. Hara T, Bhayana B, Thompson B, Kessinger CW, Khatri A, McCarthy JR, et al. Molecular imaging of fibrin deposition in deep vein thrombosis using fibrin‐targeted near‐infrared fluorescence. JACC Cardiovasc Imaging. 2012;5:607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. James ML, Gambhir SS. A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev. 2012;92:897–965. [DOI] [PubMed] [Google Scholar]
- 13. Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kosareva A, Abou‐Elkacem L, Chowdhury S, Lindner JR, Kaufmann BA. Seeing the invisible‐ultrasound molecular imaging. Ultrasound Med Biol. 2020;46:479–97. [DOI] [PubMed] [Google Scholar]
- 15. Blasi F, Oliveira BL, Rietz TA, Rotile NJ, Naha PC, Cormode DP, et al. Multisite thrombus Imaging and fibrin content estimation with a single whole‐body PET scan in rats. Arterioscler Thromb Vasc Biol. 2015;35:2114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang X, Hagemeyer CE, Hohmann JD, Leitner E, Armstrong PC, Jia F, et al. Novel single‐chain antibody‐targeted microbubbles for molecular ultrasound imaging of thrombosis: validation of a unique noninvasive method for rapid and sensitive detection of thrombi and monitoring of success or failure of thrombolysis in mice. Circulation. 2012;125:3117–26. [DOI] [PubMed] [Google Scholar]
- 17. Andrews JPM, Portal C, Walton T, Macaskill MG, Hadoke PWF, Alcaide Corral C, et al. Non‐invasive in vivo imaging of acute thrombosis: development of a novel factor XIIIa radiotracer. Eur Heart J Cardiovasc Imaging. 2020;21:673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karpiouk AB, Aglyamov SR, Mallidi S, Shah J, Scott WG, Rubin JM, et al. Combined ultrasound and photoacoustic imaging to detect and stage deep vein thrombosis: phantom and ex vivo studies. J Biomed Opt. 2008;13:54061. [DOI] [PubMed] [Google Scholar]
- 19. Stalker TJ. Mouse laser injury models: variations on a theme. Platelets. 2020;31:423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Welsh JD, Muthard RW, Stalker TJ, Taliaferro JP, Diamond SL, Brass LF. A systems approach to hemostasis: 4. How hemostatic thrombi limit the loss of plasma‐borne molecules from the microvasculature. Blood. 2016;127:1598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Okano M, Hara T, Nishimori M, Irino Y, Satomi‐Kobayashi S, Shinohara M, et al. In vivo imaging of venous thrombus and pulmonary embolism using novel murine venous thromboembolism model. JACC Basic Transl Sci. 2020;5:344–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Celi A, Merrill‐Skoloff G, Gross P, Falati S, Sim DS, Flaumenhaft R, et al. Thrombus formation: direct real‐time observation and digital analysis of thrombus assembly in a living mouse by confocal and widefield intravital microscopy. J Thromb Haemost. 2003;1:60–8. [DOI] [PubMed] [Google Scholar]
- 23. Bellido‐Martin L, Chen V, Jasuja R, Furie B, Furie BC. Imaging fibrin formation and platelet and endothelial cell activation in vivo. Thromb Haemost. 2011;105:776–82. [DOI] [PubMed] [Google Scholar]
- 24. Simons N, Mitchell P, Dowling R, Gonzales M, Yan B. Thrombus composition in acute ischemic stroke: a histopathological study of thrombus extracted by endovascular retrieval. J Neuroradiol. 2015;42:86–92. [DOI] [PubMed] [Google Scholar]
- 25. Sporns PB, Hanning U, Schwindt W, Velasco A, Minnerup J, Zoubi T, et al. Ischemic stroke: What does the histological composition tell us about the origin of the thrombus? Stroke. 2017;48:2206–10. [DOI] [PubMed] [Google Scholar]
- 26. Lam WA, Chaudhuri O, Crow A, Webster KD, Li TD, Kita A, et al. Mechanics and contraction dynamics of single platelets and implications for clot stiffening. Nat Mater. 2011;10:61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim OV, Litvinov RI, Alber MS, Weisel JW. Quantitative structural mechanobiology of platelet‐driven blood clot contraction. Nat Commun. 2017;8:1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim OV, Nevzorova TA, Mordakhanova ER, Ponomareva AA, Andrianova IA, Le Minh G, et al. Fatal dysfunction and disintegration of thrombin‐stimulated platelets. Haematologica. 2019;104:1866–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zubairova LD, Nabiullina RM, Nagaswami C, Zuev YF, Mustafin IG, Litvinov RI, et al. Circulating microparticles alter formation, structure, and properties of fibrin clots. Sci Rep. 2015;5:17611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tutwiler V, Peshkova AD, Le Minh G, Zaitsev S, Litvinov RI, Cines DB, et al. Blood clot contraction differentially modulates internal and external fibrinolysis. J Thromb Haemost. 2019;17:361–70. [DOI] [PubMed] [Google Scholar]
- 31. Gottlob R, Stockinger L, Potting U, Schattenmann G. Studies on thrombolysis with streptokinase. 3. Morphological examinations of thrombi‐thrombus retraction and secondary swelling and the termination of lysibility because of organization. Thromb Diath Haemorrh. 1971;25:354–78. [PubMed] [Google Scholar]
- 32. Zabczyk M, Sadowski M, Zalewski J, Undas A. Polyhedrocytes in intracoronary thrombi from patients with ST‐elevation myocardial infarction. Int J Cardiol. 2015;179:186–7. [DOI] [PubMed] [Google Scholar]
- 33. Zalewski J, Bogaert J, Sadowski M, Woznicka O, Doulaptsis K, Ntoumpanaki M, et al. Plasma fibrin clot phenotype independently affects intracoronary thrombus ultrastructure in patients with acute myocardial infarction. Thromb Haemost. 2015;113:1258–69. [DOI] [PubMed] [Google Scholar]
- 34. Chernysh IN, Nagaswami C, Kosolapova S, Peshkova AD, Cuker A, Cines DB, et al. The distinctive structure and composition of arterial and venous thrombi and pulmonary emboli. Sci Rep. 2020;10:5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peshkova AD, Malyasyov DV, Bredikhin RA, Le Minh G, Andrianova IA, Tutwiler V, et al. Reduced contraction of blood clots in venous thromboembolism is a potential thrombogenic and embologenic mechanism. TH Open. 2018;2:e104–e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Litvinov RI, Khismatullin RR, Shakirova AZ, Litvinov TR, Nagaswami C, Peshkova AD, et al. Morphological signs of intravital contraction (retraction) of pulmonary thrombotic emboli. BioNanoScience. 2018;8:428–33. [Google Scholar]
- 37. Leong L, Chernysh IN, Xu Y, Sim D, Nagaswami C, de Lange Z, et al. Clot stability as a determinant of effective factor VIII replacement in hemophilia A. Res Pract Thromb Haemost. 2017;1:231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tutwiler V, Peshkova AD, Andrianova IA, Khasanova DR, Weisel JW, Litvinov RI. Contraction of blood clots is impaired in acute ischemic stroke. Arterioscler Thromb Vasc Biol. 2017;37:271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Le Minh G, Peshkova AD, Andrianova IA, Sibgatullin TB, Maksudova AN, Weisel JW, et al. Impaired contraction of blood clots as a novel prothrombotic mechanism in systemic lupus erythematosus. Clin Sci (Lond). 2018;132:243–54. [DOI] [PubMed] [Google Scholar]
- 40. Peshkova AD, Safiullina SI, Evtugina NG, Baras YS, Ataullakhanov FI, Weisel JW, et al. Premorbid hemostasis in women with a history of pregnancy loss. Thromb Haemost. 2019;119:1994–2004. [DOI] [PubMed] [Google Scholar]
- 41. Tutwiler V, Litvinov RI, Protopopova AD, Nagaswami C, Russell JE, Siegel DL, et al. Blood clot contraction is reduced in sickle cell disease due to increased rigidity of erythrocytes. Biophys J. 2018;114:540a–541a. [Google Scholar]
- 42. Evtugina NG, Peshkova AD, Pichugin AA, Weisel JW, Litvinov RI. Impaired contraction of blood clots precedes and predicts postoperative venous thromboembolism. Sci Rep. 2020;10:18261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Silvain J, Collet JP, Nagaswami C, Beygui F, Edmondson KE, Bellemain‐Appaix A, et al. Composition of coronary thrombus in acute myocardial infarction. J Am Coll Cardiol. 2011;57:1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rittersma SZ, van der Wal AC, Koch KT, Piek JJ, Henriques JP, Mulder KJ, et al. Plaque instability frequently occurs days or weeks before occlusive coronary thrombosis: a pathological thrombectomy study in primary percutaneous coronary intervention. Circulation. 2005;111:1160–5. [DOI] [PubMed] [Google Scholar]
- 45. Empana JP, Boulanger CM, Tafflet M, Renard JM, Leroyer AS, Varenne O, et al. Microparticles and sudden cardiac death due to coronary occlusion. The TIDE (Thrombus and Inflammation in sudden DEath) study. Eur Heart J Acute Cardiovasc Care. 2015;4:28–36. [DOI] [PubMed] [Google Scholar]
- 46. Silvain J, Collet JP, Guedeney P, Varenne O, Nagaswami C, Maupain C, et al. Thrombus composition in sudden cardiac death from acute myocardial infarction. Resuscitation. 2017;113:108–14. [DOI] [PubMed] [Google Scholar]
- 47. Macrae FL, Duval C, Papareddy P, Baker SR, Yuldasheva N, Kearney KJ, et al. A fibrin biofilm covers blood clots and protects from microbial invasion. J Clin Invest. 2018;128:3356–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zabczyk M, Natorska J, Zalewski J, Undas A. Fibrin biofilm can be detected on intracoronary thrombi aspirated from patients with acute myocardial infarction. Cardiovasc Res. 2019;115:1026–8. [DOI] [PubMed] [Google Scholar]
- 49. Pikija S, Magdic J, Trkulja V, Unterkreuter P, Mutzenbach JS, Novak HF, et al. Intracranial thrombus morphology and composition undergoes time‐dependent changes in acute ischemic stroke: A CT densitometry study. Int J Mol Sci. 2016;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Boeckh‐Behrens T, Kleine JF, Zimmer C, Neff F, Scheipl F, Pelisek J, et al. Thrombus Histology Suggests Cardioembolic Cause in Cryptogenic Stroke. Stroke. 2016;47:1864–71. [DOI] [PubMed] [Google Scholar]
- 51. Zhang C, Kasner S. Diagnosis, prognosis, and management of cryptogenic stroke. F1000Research. 2016;5:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bacigaluppi M, Semerano A, Gullotta GS, Strambo D. Insights from thrombi retrieved in stroke due to large vessel occlusion. J Cereb Blood Flow Metab. 2019;39:1433–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. De Meyer SF, Andersson T, Baxter B, Bendszus M, Brouwer P, Brinjikji W, et al. Analyses of thrombi in acute ischemic stroke: a consensus statement on current knowledge and future directions. Int J Stroke. 2017;12:606–14. [DOI] [PubMed] [Google Scholar]
- 54. Staessens S, Fitzgerald S, Andersson T, Clarencon F, Denorme F, Gounis MJ, et al. Histological stroke clot analysis after thrombectomy: Technical aspects and recommendations. Int J Stroke. 2020;15:467–76. [DOI] [PubMed] [Google Scholar]
- 55. Maegerlein C, Friedrich B, Berndt M, Lucia KE, Schirmer L, Poppert H, et al. Impact of histological thrombus composition on preinterventional thrombus migration in patients with acute occlusions of the middle cerebral artery. Interv Neuroradiol. 2018;24:70–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Marder VJ, Chute DJ, Starkman S, Abolian AM, Kidwell C, Liebeskind D, et al. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke. 2006;37:2086–93. [DOI] [PubMed] [Google Scholar]
- 57. Sporns PB, Hanning U, Schwindt W, Velasco A, Buerke B, Cnyrim C, et al. Ischemic stroke: histological thrombus composition and pre‐interventional CT attenuation are associated with intervention time and rate of secondary embolism. Cerebrovasc Dis. 2017;44:344–50. [DOI] [PubMed] [Google Scholar]
- 58. Liebeskind DS, Sanossian N, Yong WH, Starkman S, Tsang MP, Moya AL, et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke. 2011;42:1237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Singh P, Kaur R, Kaur A. Clot composition and treatment approach to acute ischemic stroke: the road so far. Ann Indian Acad Neurol. 2013;16:494–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ahn SH, Hong R, Choo IS, Heo JH, Nam HS, Kang HG, et al. Histologic features of acute thrombi retrieved from stroke patients during mechanical reperfusion therapy. Int J Stroke. 2016;11:1036–44. [DOI] [PubMed] [Google Scholar]
- 61. Niesten JM, van der Schaaf IC, van Dam L, Vink A, Vos JA, Schonewille WJ, et al. Histopathologic composition of cerebral thrombi of acute stroke patients is correlated with stroke subtype and thrombus attenuation. PLoS One. 2014;9:e88882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Boeckh‐Behrens T, Schubert M, Forschler A, Prothmann S, Kreiser K, Zimmer C, et al. The impact of histological clot composition in embolic stroke. Clin Neuroradiol. 2016;26:189–97. [DOI] [PubMed] [Google Scholar]
- 63. Kim SK, Yoon W, Kim TS, Kim HS, Heo TW, Park MS. Histologic analysis of retrieved clots in acute ischemic stroke: correlation with stroke etiology and gradient‐echo MRI. AJNR Am J Neuroradiol. 2015;36:1756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Khismatullin RR, Nagaswami C, Shakirova AZ, Vrtková A, Procházka V, Gumulec J, et al. Quantitative morphology of cerebral thrombi related to intravital contraction and clinical features of ischemic stroke. Stroke. 2020;51(12):3640–50. [DOI] [PubMed] [Google Scholar]
- 65. Ducroux C, Di Meglio L, Loyau S, Delbosc S, Boisseau W, Deschildre C, et al. Thrombus neutrophil extracellular traps content impair tPA‐induced thrombolysis in acute ischemic stroke. Stroke. 2018;49:754–7. [DOI] [PubMed] [Google Scholar]
- 66. Heo JH, Nam HS, Kim YD, Choi JK, Kim BM, Kim DJ, et al. Pathophysiologic and therapeutic perspectives based on thrombus histology in stroke. J Stroke. 2020;22:64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Laridan E, Denorme F, Desender L, Francois O, Andersson T, Deckmyn H, et al. Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol. 2017;82:223–32. [DOI] [PubMed] [Google Scholar]
- 68. Oklu R, Albadawi H, Watkins MT, Monestier M, Sillesen M, Wicky S. Detection of extracellular genomic DNA scaffold in human thrombus: implications for the use of deoxyribonuclease enzymes in thrombolysis. J Vasc Interv Radiol. 2012;23:712–8. [DOI] [PubMed] [Google Scholar]
- 69. Staessens S, Denorme F, Francois O, Desender L, Dewaele T, Vanacker P, et al. Structural analysis of ischemic stroke thrombi: histological indications for therapy resistance. Haematologica. 2020;105:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Di Meglio L, Desilles JP, Ollivier V, Nomenjanahary MS, Di Meglio S, Deschildre C, et al. Acute ischemic stroke thrombi have an outer shell that impairs fibrinolysis. Neurology. 2019;93:e1686–e1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Xu RG, Ariens RAS. Insights into the composition of stroke thrombi: heterogeneity and distinct clot areas impact treatment. Haematologica. 2020;105:257–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Heit JA. Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost. 2005;3:1611–7. [DOI] [PubMed] [Google Scholar]
- 74. Campbell RA, Aleman M, Gray LD, Falvo MR, Wolberg AS. Flow profoundly influences fibrin network structure: implications for fibrin formation and clot stability in haemostasis. Thromb Haemost. 2010;104:1281–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Aleman MM, Walton BL, Byrnes JR, Wang JG, Heisler MJ, Machlus KR, et al. Elevated prothrombin promotes venous, but not arterial, thrombosis in mice. Arterioscler Thromb Vasc Biol. 2013;33:1829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kumar DR, Hanlin E, Glurich I, Mazza JJ, Yale SH. Virchow's contribution to the understanding of thrombosis and cellular biology. Clin Med Res. 2010;8:168–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wolberg AS, Aleman MM, Leiderman K, Machlus KR. Procoagulant activity in hemostasis and thrombosis: Virchow's triad revisited. Anesth Analg. 2012;114:275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fuchs TA, Brill A, Wagner DD. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2012;32:1777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Konings J, Govers‐Riemslag JW, Philippou H, Mutch NJ, Borissoff JI, Allan P, et al. Factor XIIa regulates the structure of the fibrin clot independently of thrombin generation through direct interaction with fibrin. Blood. 2011;118:3942–51. [DOI] [PubMed] [Google Scholar]
- 81. Seidman MA, Mitchell RN. Surgical pathology of small‐ and medium‐sized vessels. Surg Pathol Clin. 2012;5:435–51. [DOI] [PubMed] [Google Scholar]
- 82. McGuinness CL, Humphries J, Waltham M, Burnand KG, Collins M, Smith A. Recruitment of labelled monocytes by experimental venous thrombi. Thromb Haemost. 2001;85:1018–24. [PubMed] [Google Scholar]
- 83. Comerota AJ, Oostra C, Fayad Z, Gunning W, Henke P, Luke C, et al. A histological and functional description of the tissue causing chronic postthrombotic venous obstruction. Thromb Res. 2015;135:882–7. [DOI] [PubMed] [Google Scholar]
- 84. Lee YU, Lee AY, Humphrey JD, Rausch MK. Histological and biomechanical changes in a mouse model of venous thrombus remodeling. Biorheology. 2015;52:235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Longstaff C, Varju I, Sotonyi P, Szabo L, Krumrey M, Hoell A, et al. Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. J Biol Chem. 2013;288:6946–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wakefield TW, Strieter RM, Wilke CA, Kadell AM, Wrobleski SK, Burdick MD, et al. Venous thrombosis‐associated inflammation and attenuation with neutralizing antibodies to cytokines and adhesion molecules. Arterioscler Thromb Vasc Biol. 1995;15:258–68. [DOI] [PubMed] [Google Scholar]
- 87. Parsons RE, Sigel B, Feleppa EJ, Golub RM, Kodama I, Loiacono LA, et al. Age determination of experimental venous thrombi by ultrasonic tissue characterization. J Vasc Surg. 1993;17:470–8. [DOI] [PubMed] [Google Scholar]
- 88. Tutwiler V, Litvinov RI, Lozhkin AP, Peshkova AD, Lebedeva T, Ataullakhanov FI, et al. Kinetics and mechanics of clot contraction are governed by the molecular and cellular composition of the blood. Blood. 2016;127:149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Collet JP, Park D, Lesty C, Soria J, Soria C, Montalescot G, et al. Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: dynamic and structural approaches by confocal microscopy. Arterioscler Thromb Vasc Biol. 2000;20:1354–61. [DOI] [PubMed] [Google Scholar]
- 90. Kolev K. Structural basis for variable lytic susceptibility of fibrin bridging structure with function in fibrinolysis. Cardiovasc Hematol Agents Med Chem. 2008;6:159–60. [DOI] [PubMed] [Google Scholar]
- 91. Bannish BE, Chernysh IN, Keener JP, Fogelson AL, Weisel JW. Molecular and physical mechanisms of fibrinolysis and thrombolysis from mathematical modeling and experiments. Sci Rep. 2017;7:6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Collet JP, Allali Y, Lesty C, Tanguy ML, Silvain J, Ankri A, et al. Altered fibrin architecture is associated with hypofibrinolysis and premature coronary atherothrombosis. Arterioscler Thromb Vasc Biol. 2006;26:2567–73. [DOI] [PubMed] [Google Scholar]
- 93. Undas A, Zubkiewicz‐Usnarska L, Helbig G, Woszczyk D, Kozinska J, Dmoszynska A, et al. Altered plasma fibrin clot properties and fibrinolysis in patients with multiple myeloma. Eur J Clin Invest. 2014;44:557–66. [DOI] [PubMed] [Google Scholar]
- 94. Konieczynska M, Fil K, Bazanek M, Undas A. Prolonged duration of type 2 diabetes is associated with increased thrombin generation, prothrombotic fibrin clot phenotype and impaired fibrinolysis. Thromb Haemost. 2014;111:685–93. [DOI] [PubMed] [Google Scholar]
- 95. Owczarek D, Cibor D, Salapa K, Glowacki MK, Mach T, Undas A. Reduced plasma fibrin clot permeability and susceptibility to lysis in patients with inflammatory bowel disease: a novel prothrombotic mechanism. Inflamm Bowel Dis. 2013;19:2616–24. [DOI] [PubMed] [Google Scholar]
- 96. Collet JP, Montalescot G, Lesty C, Weisel JW. A structural and dynamic investigation of the facilitating effect of glycoprotein IIb/IIIa inhibitors in dissolving platelet‐rich clots. Circ Res. 2002;90:428–34. [DOI] [PubMed] [Google Scholar]
- 97. Gersh KC, Nagaswami C, Weisel JW. Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thromb Haemost. 2009;102:1169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Carvalho FA, Connell S, Miltenberger‐Miltenyi G, Pereira SV, Tavares A, Ariens RA, et al. Atomic force microscopy‐based molecular recognition of a fibrinogen receptor on human erythrocytes. ACS Nano. 2010;4:4609–20. [DOI] [PubMed] [Google Scholar]
- 99. De Oliveira S, Vitorino de Almeida V, Calado A, Rosario HS, Saldanha C. Integrin‐associated protein (CD47) is a putative mediator for soluble fibrinogen interaction with human red blood cells membrane. Biochim Biophys Acta. 2012;1818(3):481–490. [DOI] [PubMed] [Google Scholar]
- 100. Weisel JW, Litvinov RI. Red blood cells: the forgotten player in hemostasis and thrombosis. J Thromb Haemost. 2019;17:271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Weisel JW, Litvinov RI. Mechanisms of fibrinolysis and basic principles of management In: Saba HI, Roberts HR, editors. Hemostasis and Thrombosis: Practical Guidelines in Clinical Management. Chichester: Wiley‐Blackwell; 2014. p. 169–85. [Google Scholar]
- 102. Carroll RC, Gerrard JM, Gilliam JM. Clot retraction facilitates clot lysis. Blood. 1981;57:44–8. [PubMed] [Google Scholar]
- 103. Kunitada S, FitzGerald GA, Fitzgerald DJ. Inhibition of clot lysis and decreased binding of tissue‐type plasminogen activator as a consequence of clot retraction. Blood. 1992;79:1420–7. [PubMed] [Google Scholar]
- 104. Carrieri C, Galasso R, Semeraro F, Ammollo CT, Semeraro N, Colucci M. The role of thrombin activatable fibrinolysis inhibitor and factor XI in platelet‐mediated fibrinolysis resistance: a thromboelastographic study in whole blood. J Thromb Haemost. 2011;9:154–62. [DOI] [PubMed] [Google Scholar]
- 105. Sabovic M, Lijnen HR, Keber D, Collen D. Effect of retraction on the lysis of human clots with fibrin specific and non‐fibrin specific plasminogen activators. Thromb Haemost. 1989;62:1083–7. [PubMed] [Google Scholar]
- 106. Fraser SR, Booth NA, Mutch NJ. The antifibrinolytic function of factor XIII is exclusively expressed through alpha(2)‐antiplasmin cross‐linking. Blood. 2011;117:6371–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Rijken DC, Abdul S, Malfliet JJ, Leebeek FW. Uitte de Willige S. Compaction of fibrin clots reveals the antifibrinolytic effect of factor XIII. J Thromb Haemost. 2016;14:1453–61. [DOI] [PubMed] [Google Scholar]
- 108. Catanese L, Tarsia J, Fisher M. Acute ischemic stroke therapy overview. Circ Res. 2017;120:541–58. [DOI] [PubMed] [Google Scholar]
- 109. Chernysh IN, Spiewak R, Cambor CL, Purohit PK, Weisel JW. Structure, mechanical properties, and modeling of cyclically compressed pulmonary emboli. J Mech Behavior Biomed Matls. 2020;105:103699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Locke M, Longstaff C. FXIII catalyses histone‐fibrin crosslinking to inhibit fibrinolysis. OC 02.3. Oral Communication. Abstracts Res Pract Thromb Haemost. 2020;4:1–44. [Google Scholar]
- 111. Staessens S, François O, Desender L, Waele TD, Vanacker P, Sciot R, et al. Histological analysis of a thrombectomy‐resistant thrombus in acute ischemic stroke. PB0090. Poster Abstracts. Res Pract Thromb Haemost. 2020;4:1–1311. [Google Scholar]
- 112. Orbán‐Kálmándi R, Sarkady F, Szegedi I, Fekete I, Fekete K, Csiba L, et al. Elevated levels of circulating markers of neutrophil extracellular traps (NETs) are associated with unfavorable outcomes in acute ischemic stroke patients undergoing intravenous thrombolysis. PB0771. Poster Abstracts. Res Pract Thromb Haemost. 2020;4:1–1311. [Google Scholar]
- 113. Varju I, Sorvillo N, Cherpokova D, Farkas VJ, Farkas AZ, Komorowicz E, et al. Fibrinogen is citrullinated in venous thrombi and forms fragile clots with increased resistance to lysis. OC 02.4. Oral Communication Abstracts. Res Pract Thromb Haemost. 202041–44. [Google Scholar]
- 114. Mertens JC, Blanc‐Guillemaud V, Claesen K, Leenaerts D, Tyl B, Cardona P, et al. Carboxypeptidase U (CPU, TAFIa, CPB2) generation in acute ischemic stroke patients undergoing thrombolysis and endovascular thrombectomy. PB0737. Poster Abstracts. Res Pract Thromb Haemost. 2020;4:1–1311. [Google Scholar]
- 115. Baráth B, Katona É. Different incorporation of apha2‐plasmin inhibitor C‐terminal forms into the fibrin clot. PB0753. Poster Abstracts. Res Pract Thromb Haemost. 2020;4:1–1311. [Google Scholar]
- 116. Suzuki Y, Sano H, Honkura N, Urano T. Modulation of thrombin generation or thrombin activity on activated platelets surface largely affected TAFI‐dependent regulation of fibrinolysis. PB0741. Poster Abstracts. Res Pract Thromb Haemost. 2020;4:1–1311. [Google Scholar]
- 117. Zabczyk M, Natorska J, Bagoly Z, Sarkady F, Baráth B, Katona E, et al. Plasma fibrin clots of acute pulmonary embolism patients present reduced amounts of coagulation factor XIII and α2‐antiplasmin compared to clots generated after 3‐month anticoagulation. PB0729. Poster Abstracts. Res Pract Thromb Haemost. 2020;4:1–1311. [Google Scholar]
- 118. Cabrera D, Sharifabad M, Telling N, Harper A. Platelet‐targeted magnetic nanoparticles enhance the efficacy of thrombolysis through induction of magnetic hyperthermia. OC 08.4. Oral Communication Abstracts. Res Pract Thromb Haemost. 2020;4:1–44. [Google Scholar]
- 119. Maat SD, Clark C, Barendrecht A, Mv M, Lenting P, Vanhoorelbeke K, et al. Microlyse: A VWF‐targeting thrombolytic agent for thrombotic thrombocytopenic purpura. PB0766. Poster Abstracts. Res Pract Thromb Haemost. 2020;4:1–1311. [Google Scholar]
- 120. Khider L, Goudot G, Giudice CD, Mirault T, Galloula A, Bruneval P, et al. Recanalization of venous thrombosis by robotic assisted high frequency pulsed cavitational ultrasound therapy: 2 weeks follow‐up in a swine model. PB2208. Poster Abstracts. Res Pract Thromb Haemost. 2020;4:1–1311. [Google Scholar]
- 121. Notten P, Arnoldussen C, Brans R, deSmet A, Tick L, de Poel MV, et al. The impact of successful ultrasound‐accelerated catheter‐directed thrombolysis on the prevention of post‐thrombotic syndrome: A post‐hoc analysis of the CAVA‐trial. PB2206. Poster Abstracts. Res Pract Thromb Haemost. 2020;4:1–1311. [Google Scholar]
- 122. Kramer A, Mortensen CS, Schultz JG, Giordano N, Zheng H, Andersen A, et al. The use of thrombolytic therapy in a multidisciplinary pulmonary embolism response team. PB2482. Poster Abstracts. Res Pract Thromb Haemost. 2020;4:1–1311. [Google Scholar]
- 123. Orbán‐Kálmándi R, Baráth B, Sarkady F, Székely E, Szegedi I, Czuriga‐Kovács KR, et al. Low α2‐plasmin inhibitor antigen levels on admission predict unfavorable outcomes in acute ischemic stroke patients treated with intravenous thrombolysis. OC 02.5. Oral Communication Abstracts. Res Pract Thromb Haemost. 2020;4:1–44. [Google Scholar]
- 124. Doyle A, Gooby N, Parmar K, Breen KA, Barrett NA, Retter A, et al. Changes in fibrinolytic factors in a simulated extracorporeal membrane oxygenator circuit. PB0749. Poster Abstracts. Res Pract Thromb Haemost. 2020;4:1–1311. [Google Scholar]
- 125. Villalba L, Nguyen T, Feitosa RL Jr, Gunanayagam P, Anning N, Dwight K. Single‐session catheter‐directed lysis using adjunctive power‐pulse spray with AngioJet for the treatment of acute massive and submassive pulmonary embolism. J Vasc Surg. 2019;70:1920–6. [DOI] [PubMed] [Google Scholar]
- 126. Gersh KC, Zaitsev S, Cines DB, Muzykantov V, Weisel JW. Flow‐dependent channel formation in clots by an erythrocyte‐bound fibrinolytic agent. Blood. 2011;117:4964–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Fuentes RE, Zaitsev S, Ahn HS, Hayes V, Kowalska MA, Lambert MP, et al. A chimeric platelet‐targeted urokinase prodrug selectively blocks new thrombus formation. J Clin Invest. 2016;126:483–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Zhu Q, Dong G, Wang Z, Sun L, Gao S, Liu Z. Intra‐clot microbubble‐enhanced ultrasound accelerates catheter‐directed thrombolysis for deep vein thrombosis: A clinical study. Ultrasound Med Biol. 2019;45:2427–33. [DOI] [PubMed] [Google Scholar]
- 129. Chernysh IN, Everbach CE, Purohit PK, Weisel JW. Molecular mechanisms of the effect of ultrasound on the fibrinolysis of clots. J Thromb Haemost. 2015;13:601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Katsanos AH, Tsivgoulis G. Is intravenous thrombolysis still necessary in patients who undergo mechanical thrombectomy? Curr Opin Neurol. 2019;32:3–12. [DOI] [PubMed] [Google Scholar]
- 131. Mathias W Jr, Tsutsui JM, Tavares BG, Fava AM, Aguiar MOD, Borges BC, et al. Sonothrombolysis in ST‐segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol. 2019;73:2832–42. [DOI] [PubMed] [Google Scholar]
- 132. Sairanen T, Ritvonen J. Should we thrombolyse prior to endovascular treatment in acute stroke? Clin Neurol Neurosurg. 2019;177:117–22. [DOI] [PubMed] [Google Scholar]


