Abstract
Background
The optimal therapy of patients with acute subsegmental pulmonary embolism (PE) is controversial.
Methods
We used the RIETE (Registro Informatizado Enfermedad TromboEmbólica) database to compare the rate of symptomatic PE recurrences during anticoagulation in patients with subsegmental, segmental, or more central PEs.
Results
Among 15 963 patients with a first episode of symptomatic PE, 834 (5.2%) had subsegmental PE, 3797 (24%) segmental, and 11 332 (71%) more central PE. Most patients in all subgroups received initial therapy with low‐molecular‐weight heparin, and then most switched to vitamin K antagonists. Median duration of therapy was 179, 185, and 204 days, respectively. During anticoagulation, 183 patients developed PE recurrences, 131 developed deep vein thrombosis (DVT), 543 bled, and 1718 died (fatal PE, 135). The rate of PE recurrences was twofold higher in patients with subsegmental PE than in those with segmental (hazard ratio [HR], 2.13; 95% confidence interval [CI], 1.16‐3.85) or more central PE (HR, 1.89; 95% CI, 1.12‐3.13). On multivariable analysis, patients with subsegmental PE had a higher risk for PE recurrences than those with central PE (adjusted HR, 1.75; 95% CI, 1.02‐3.03). After stratifying patients with subsegmental PE according to ultrasound imaging in the lower limbs, the rate of PE recurrences was similar in patients with DVT, in patients without DVT, and in those with no ultrasound imaging.
Conclusions
Our study reveals that the risk for PE recurrences in patients with segmental PE is not lower than in those with more central PE, thus suggesting that the risk of PE recurrences is not influenced by the anatomic location of PE.
Keywords: anticoagulant, deep vein thrombosis, outcomes, pulmonary embolism, subsegmental
Essentials.
The optimal therapy of patients with acute subsegmental pulmonary embolism is controversial.
Computed tomography scan has increased how often subsegmental pulmonary embolism (PE) is diagnosed in clinical practice.
Patients with subsegmental PE had a higher risk for PE recurrences during therapy.
Persistent risk factors for recurrent PE are better predictors than the location of the PE.
1. INTRODUCTION
The optimal therapy of patients presenting with acute symptomatic subsegmental pulmonary embolism (PE) is controversial. 1 , 2 , 3 , 4 , 5 , 6 Subsegmental PE refers to PE that is confined to the subsegmental pulmonary arteries. 7 Improvements in computed tomography (CT) pulmonary angiography have increased how often subsegmental PE is diagnosed in clinical practice, 8 , 9 , 10 , 11 but since the abnormalities are usually small, a diagnosis of subsegmental PE may be a false‐positive finding. 12 , 13 , 14 , 15 , 16 , 17 Current guidelines from the American College of Chest Physicians recommend ultrasound imaging of the deep veins of both legs to exclude proximal deep vein thrombosis (DVT). 18 , 19 In patients with proximal DVT, anticoagulant therapy is recommended. In those without DVT, current guidelines suggest clinical surveillance if there is a low risk for recurrent venous thromboembolism (VTE) and anticoagulation if there is a high risk for recurrent VTE. 18 , 19 However, a recent Cochrane systematic review concluded that there is no evidence to support any recommendation in these patients. 20
The RIETE (Registro Informatizado de Enfermedad TromboEmbólica) Registry is an ongoing, multicenter, international, observational registry of consecutive patients with objectively confirmed acute VTE (ClinicalTrials.gov identifier: NCT02832245). Data from this registry have been used to evaluate outcomes after acute VTE, such as the frequency of recurrent VTE, bleeding, and mortality and risk factors for these outcomes. 21 , 22 , 23 , 24 The rationale and methodology of RIETE have been previously reported elsewhere. 25 In the current study, we aimed to compare the rate of acute symptomatic PE recurrences, major bleeding, or death appearing during the course of anticoagulant therapy in patients with acute symptomatic PE in the subsegmental, segmental, or more proximal pulmonary arteries.
2. METHODS
2.1. Data source
This is an analysis of prospectively collected data in the RIETE Registry, from 179 hospitals in 24 countries. RIETE included consecutive patients with acute DVT or PE, confirmed by objective tests (compression ultrasonography or contrast venography for suspected DVT; pulmonary angiography, ventilation‐perfusion lung scan, or helical CT scan for suspected PE) since March 2001. Exclusion criteria were a current enrollment in a therapeutic clinical trial with a blinded therapy. Informed consent was obtained from participants in accordance with local ethics committee requirements. All institutional review board committee names and project approval numbers for all study centers can be found in the Supplementary Material.
2.2. Study design
We included consecutive patients with acute symptomatic PE confirmed by CT scan. Patients with incidentally found PE were excluded. The primary outcome of the study was acute symptomatic, recurrent PE appearing during the course of anticoagulant therapy in patients with subsegmental PE, segmental PE, or PE in more proximal pulmonary arteries. Each episode of clinically suspected recurrent PE was investigated by repeat helical CT scan. Secondary outcomes were fatal PE, major bleeding, and all‐cause death. PE localization is a variable included in RIETE only since 2009. From 2001 to 2008 this information was not documented. Hence, all RIETE participants with a first episode of acute, symptomatic PE diagnosed by helical CT scan from January 2009 to January 2019 were considered for the study. We excluded participants with prior VTE (n = 2577). Fatal PE, in the absence of autopsy, was defined as any death appearing within 10 days after symptomatic PE diagnosis (either the index PE or recurrent PE), in the absence of any alternative cause of death. Bleeding events were classified as “major” if they were overt and required transfusion of two units or more of blood, or were retroperitoneal, spinal, or intracranial or when they were fatal. 25 Fatal bleeding was defined as any death occurring within 10 days of a major bleeding episode in the absence of an alternative cause of death. 25
Incident PEs were categorized as subsegmental only, segmental (with or without subsegmental arteries), and central PE (lobar or pulmonary arteries, with or without segmental or subsegmental arteries), based on the largest arteries involved on radiological images. Characteristics of the incident PE episode and of participants (demographics, comorbidities, medications) were recorded at baseline. Active cancer included cancer diagnosed within the 3 months before the incident PE, metastatic cancer, or cancer with current therapy (surgery, chemotherapy, radiotherapy, hormonal or support therapy). PE was considered as secondary to surgery if appearing within 2 months of the procedure, and secondary to immobilization if within 2 months of confinement to bed with bathroom privileges for ≥ 4 days.
Patients were managed according to each participating hospital clinical practice and there was no standardization or recommendation of treatment. All participants were followed up for at least 3 months in the outpatient clinic. Further, in case of participants’ approval, the follow‐up could be indefinitely prolonged. All data were recorded electronically using standardized case report forms, by trained coordinators at each participating center. All episodes of clinically suspected symptomatic PE recurrences during anticoagulation were investigated by repeat helical CT pulmonary angiography or pulmonary angiography.
2.3. Statistical analysis
Continuous variables were reported as mean and standard deviation (or median with interquartile range, if nonnormally distributed). Categorical variables were reported as frequency counts and percentages. We used the chi‐squared test for comparison of proportions between groups. We analyzed primary and secondary outcomes with time‐to‐event methods. The primary analysis compared the rates of symptomatic, objectively proven PE recurrences in three subgroups of patients, according to their initial presentation as (i) subsegmental PE, (ii) segmental PE, or (iii) more proximal PE. The incidence rates of PE recurrences were calculated as number of events per 100 patient‐years and compared using the hazard ratios (HRs) and 95% confidence intervals (CIs). Further, we compared the outcomes in the subgroup of patients with subsegmental PE according to ultrasound imaging into three subgroups of patients: those with DVT confirmed, those with DVT ruled out, and those with no ultrasound imaging. Mortality was assessed with the Cox proportional hazard model. Because we anticipated different mortality risks between patients with subsegmental PE and those with more central PEs, the risk for PE recurrences was assessed using competing risk models (Fine‐Gray hazard models), with mortality (not due to recurrent PE) as the competing event.
For the analysis during the period of anticoagulation, time zero was the date of the incident PE, and censoring occurred at the time of last follow‐up or cessation of anticoagulation. We selected the following covariates in regression models for adjustment: sex; age; body weight; chronic lung disease; chronic heart disease; atrial fibrillation; renal function; leg varicosities; provoking factors for incident PE (cancer vs other provoked vs unprovoked); systolic blood pressure levels at baseline; heart rate at baseline; and initial therapy with either low‐molecular‐weight heparin (LMWH), unfractionated heparin, or thrombolytics. For the multivariable analysis, we included only those variables showing a P value < .1 on univariable analyses. All statistical tests were two‐sided, with a significance level of < 0.05. SPSS software (version 20, SPSS Inc, Chicago, IL, USA) was used for all analyses.
3. RESULTS
3.1. Clinical characteristics
Among 15 963 patients with a first episode of acute symptomatic PE, 834 (5.2%) had subsegmental PE, 3797 (24%) had segmental PE, and 11 332 (71%) had central PE. Their mean age was 64, 65, and 67 years, respectively, and 48% were men. Patients with subsegmental or segmental PE were slightly more likely to have recent surgery or active cancer than those with more central PE but less likely to have recent immobility (Table 1). At baseline, patients with subsegmental or segmental PE were less likely to initially present with dyspnea or syncope or have signs of severe PE (hypotension, tachycardia, hypoxemia) or abnormalities on biological markers (troponin or d‐dimer levels) or echocardiographic findings (raised pulmonary artery pressure levels) than those with more central PE. Interestingly, the proportion of patients with concomitant DVT in the lower limbs was also lower among those with subsegmental (45%) or segmental PE (50%) than in those with central PE (65%).
Table 1.
Clinical characteristics of 15 963 patients with a first episode of acute PE, according to the location of the emboli
| Subsegmental | Segmental | Central | |
|---|---|---|---|
| Patients, N | 834 | 3797 | 11 332 |
| Clinical characteristics | |||
| Male sex, n (%) | 398 (48) | 1840 (48) | 5385 (48) |
| Age, mean y ± SD | 64 ± 18 | 65 ± 18 | 67 ± 17*** |
| Body weight (mean kg ± SD) | 76 ± 18 | 76 ± 169 | 78 ± 17* |
| Inpatients, n (%) | 312 (39) | 1360 (37) | 3379 (31)*** |
| Risk factors for PE, n (%) | |||
| Recent surgery | 122 (15) | 575 (15) | 1239 (11)** |
| Recent immobilization ≥ 4 d | 140 (17) | 749 (20) | 2412 (21)** |
| Cancer | 205 (25) | 907 (24) | 2444 (22)* |
| Pregnancy or postpartum | 10 (1.2) | 41 (1.1) | 67 (0.6)* |
| Estrogen use | 61 (7.3) | 259 (6.8) | 818 (7.2) |
| None of the above (unprovoked) | 386 (46) | 1599 (42)* | 5210 (46) |
| Leg varicosities | 124 (17) | 540 (16) | 1878 (18) |
| Underlying conditions, n (%) | |||
| Chronic lung disease | 147 (18) | 627 (17) | 1518 (13)*** |
| Chronic heart failure | 93 (11) | 364 (9.6) | 906 (8.0)** |
| Atrial fibrillation | 74 (8.9) | 356 (9.4) | 943 (8.3) |
| CrCl levels < 30 mL/min | 34 (4.1) | 147 (3.9) | 508 (4.5) |
| CrCl levels 30‐60 mL/min | 198 (24) | 989 (26) | 3248 (29)** |
| Symptoms and signs, n (%) | |||
| Dyspnea | 589 (71) | 2731 (72) | 9430 (83)*** |
| Chest pain | 405 (49) | 1827 (48) | 5061 (45)* |
| Syncope | 88 (11) | 394 (10) | 1924 (17)*** |
| SBP levels < 100 mm Hg | 44 (5.3) | 241 (6.4) | 1070 (9.4)*** |
| Heart rate > 110 bpm | 95 (12) | 471 (13) | 2121 (19)*** |
| Objective tests, n (%) | |||
| Sat O2 levels < 90% (N = 8360) | 81 (22) | 404 (23) | 1947 (31)*** |
| PAP levels > 45 mm Hg (N = 4502) | 40 (27) | 190 (25) | 1633 (46)*** |
| Raised troponin levels (N = 9011) | 84 (21) | 491 (26)* | 3202 (48)*** |
| Positive d‐dimer levels (N = 12 163) | 576 (97) | 2689 (98) | 8762 (99)*** |
| sPESI score < 1 points | 322 (39) | 1454 (38) | 3743 (33)** |
| Compression ultrasonography, n (%) | |||
| Yes | 440 | 2111 | 7315 |
| Symptomatic, confirmed DVT | 135 (31) | 707 (33) | 3047 (42)*** |
| Asymptomatic, confirmed DVT | 63 (14) | 339 (16) | 1675 (23)*** |
| Asymptomatic, excluded DVT | 242 (55) | 1065 (50) | 2593 (35)*** |
Abbreviations: CrCl, creatinine clearance; DVT, deep vein thrombosis; PAP, pulmonary artery pressure; PE, pulmonary embolism; SBP, systolic blood pressure; SD, standard deviation; sPESI, simplified pulmonary embolism severity index.
Comparisons between patients with subsegmental PE versus other subgroups:
P < .05;
P < .01;
P < .001.
3.2. Therapeutic strategies
The vast majority of patients in all subgroups received anticoagulant therapy, but 15 patients (0.09%) did not receive anticoagulation at all, and 72 (0.45%) received only initial or long‐term therapy (Table 2). Patients not receiving anticoagulation were not included in this analysis. Among patients receiving anticoagulation, there were no differences in the duration of therapy (median, 179, 185, and 204 days, respectively). Most patients in all subgroups (88%, 88%, and 85%, respectively) received initial therapy with LMWH at similar daily doses, and then most switched to vitamin K antagonists (54%, 54%, and 59%) or persisted on LMWH (27%, 27% and 25%), with a marginal use of direct oral anticoagulants (14%, 15%, and 12%), as these were approved only toward the end of study period. There was a higher use of thrombolysis or vena cava filter in patients with central PE than in those with subsegmental or segmental PE.
Table 2.
Treatments administered
| Subsegmental | Segmental | Central | |
|---|---|---|---|
| Patients, N | 834 | 3797 | 11 332 |
| Outpatients | 522 | 2437 | 7953 |
| Outpatients treated in hospital, n (%) | 452 (87) | 2179 (89) | 7460 (94)*** |
| Initial therapy (first 7‐10 days), | |||
| LMWH, n (%) | 738 (88) | 3353 (88) | 9604 (85)** |
| Mean LMWH doses (IU/kg/day) | 176 ± 43 | 176 ± 42 | 178 ± 41 |
| Unfractionated heparin, n (%) | 36 (4.3) | 142 (3.7) | 757 (6.7)** |
| Fondaparinux, n (%) | 14 (1.7) | 87 (2.3) | 159 (1.4) |
| DOACs, n (%) | 30 (3.6) | 165 (4.3) | 283 (2.5) |
| Thrombolytics, n (%) | 1 (0.12) | 10 (0.26) | 456 (4.0)*** |
| Inferior vena cava filter, n (%) | 14 (1.7) | 81 (2.1) | 356 (3.1)* |
| Long‐term therapy (beyond days 7‐10), | |||
| Vitamin K antagonists, n (%) | 454 (54) | 2062 (54) | 6723 (59)** |
| LMWH, n (%) | 226 (27) | 1014 (27) | 2794 (25) |
| Mean LMWH doses (IU/kg/day) | 154 ± 46 | 151 ± 44 | 154 ± 44 |
| DOACs, n (%) | 119 (14) | 570 (15) | 1391 (12) |
| Patients without anticoagulation, n (%) | |||
| No initial therapy (long‐term only) | 3 (0.36) | 4 (0.11) | 7 (0.06)* |
| No long‐term therapy (initial only) | 7 (0.84) | 12 (0.32) | 24 (0.21)** |
| No therapy at all | 2 (0.24) | 2 (0.05) | 11 (0.10) |
| Inferior vena cava filter | 1 (0.12) | 3 (0.08) | 12 (0.11) |
Abbreviations: DOACs, direct oral anticoagulants; IU, international units; LMWH, low‐molecular‐weight heparin.
Comparisons between patients with subsegmental PE versus other subgroups:
P < .05;
P < .01;
P < .001.
3.3. Outcomes
During the course of anticoagulant therapy, 183 patients developed symptomatic PE recurrences, 131 had recurrent DVT, 543 had major bleeding, and 1718 died (114 died of the initial PE, 21 died of recurrent PE, and 83 died of bleeding). Patients with subsegmental PE had a significantly higher rate of PE recurrences than those with segmental PE (HR, 2.13; 95% CI, 1.16‐3.85) or central PE (HR, 1.89; 95% CI, 1.12‐3.13), as shown in Table 3. Interestingly, half of the PE recurrences (7/15; 47%) in patients with segmental PE appeared during the first 30 days of therapy, as compared with 23% and 33%, respectively, in those with segmental or more central PE (Figure 1). The higher rate of PE recurrences in patients with subsegmental PE persisted after patients with active cancer were excluded (Table 3). The rates of DVT recurrences, major bleeding, all‐cause death, or fatal PE (including the index PE and recurrent PE) were similar in all three subgroups (Table 3 and Figure 2).
Table 3.
Clinical outcomes during anticoagulation, according to the location of the emboli at baseline
| Subsegmental PE | Segmental PE | Central PE | ||||
|---|---|---|---|---|---|---|
| N | Events per 100 | N | Events per 100 | N | Events per 100 | |
| patient‐years | patient‐years | patient‐years | ||||
| All patients, N | 832 | 3795 | 11 321 | |||
| Duration of therapy, | ||||||
| Mean days ± SD | 276 ± 339 | 283 ± 367 | 352 ± 473*** | |||
| Median days (IQR) | 179 (101‐327) | 185 (101‐326) | 204 (111‐380)*** | |||
| Events | ||||||
| PE recurrences | 16 | 2.58 (1.52‐4.09) | 35 | 1.20 (0.85‐1.65)* | 132 | 1.23 (1.03‐1.45)* |
| DVT recurrences | 4 | 0.64 (0.20‐1.53) | 22 | 0.75 (0.48‐1.12) | 105 | 0.97 (0.80‐1.17) |
| Major bleeding | 30 | 4.83 (3.32‐6.82) | 125 | 4.32 (3.61‐5.13) | 388 | 3.62 (3.27‐3.99) |
| Death | 76 | 12.1 (9.57‐15.0) | 412 | 14.0 (12.7‐15.4) | 1230 | 11.3 (10.7‐11.9) |
| Causes of death, | ||||||
| Pulmonary embolism | 3 | 0.48 (0.12‐1.30) | 21 | 0.71 (0.45‐1.07) | 111 | 1.02 (0.84‐1.22) |
| Initial PE | 2 | 0.32 (0.05‐1.05) | 17 | 0.58 (0.35‐0.91) | 95 | 0.87 (0.71‐1.06) |
| Fatal PE recurrences | 1 | 0.16 (0.01‐0.78) | 4 | 0.14 (0.04‐0.33) | 16 | 0.15 (0.09‐0.23) |
| Bleeding | 5 | 0.79 (0.29‐1.76) | 16 | 0.54 (0.32‐0.87) | 62 | 0.57 (0.44‐0.72) |
| Patients without cancer, N | 629 | 2890 | 8888 | |||
| Duration of therapy, | ||||||
| Mean days ± SD | 298 ± 368 | 291 ± 363 | 368 ± 480*** | |||
| Median days (IQR) | 185 (105‐341) | 189 (105‐342) | 212 (121‐389)*** | |||
| Events, | ||||||
| PE recurrences | 7 | 1.38 (0.60‐2.72) | 17 | 0.75 (0.45‐1.17) | 93 | 0.75 (0.45‐1.17) |
| DVT recurrences | 1 | 0.20 (0.01‐0.96) | 8 | 0.35 (0.16‐0.66) | 56 | 0.35 (0.16‐0.66) |
| Major bleeding | 14 | 2.75 (1.56‐4.50) | 74 | 3.26 (2.58‐4.08) | 255 | 3.26 (2.58‐4.08) |
| Death | 21 | 4.10 (2.61‐6.16) | 157 | 6.83 (5.83‐7.97)* | 509 | 6.83 (5.83‐7.97)* |
| Causes of death | ||||||
| Pulmonary embolism | 0 | … | 10 | 0.44 (0.22‐0.78) | 64 | 0.44 (0.22‐0.78) |
| Initial PE | 0 | … | 8 | 0.35 (0.16‐0.66) | 57 | 0.35 (0.16‐0.66) |
| Fatal PE recurrences | 0 | … | 2 | 0.09 (0.01‐0.29) | 7 | 0.09 (0.01‐0.29) |
| Bleeding | 2 | 0.39 (0.07‐1.29) | 7 | 0.30 (0.13‐0.60) | 37 | 0.30 (0.13‐0.60) |
Abbreviations: DVT, deep vein thrombosis; IQR, interquartile range; PE, pulmonary embolism; SD, standard deviation.
Comparisons between patients with subsegmental PE versus other subgroups:
P < .05;
P < .01;
P < .001.
Figure 1.
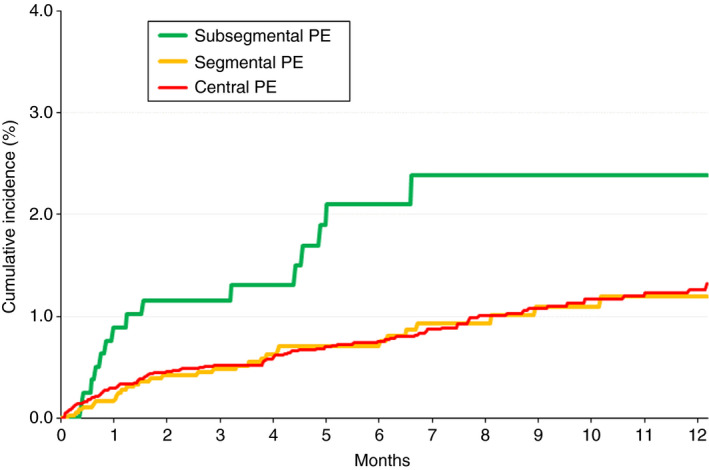
Cumulative rates of PE recurrences from baseline, according to the location of the emboli. PE, pulmonary embolism
Figure 2.
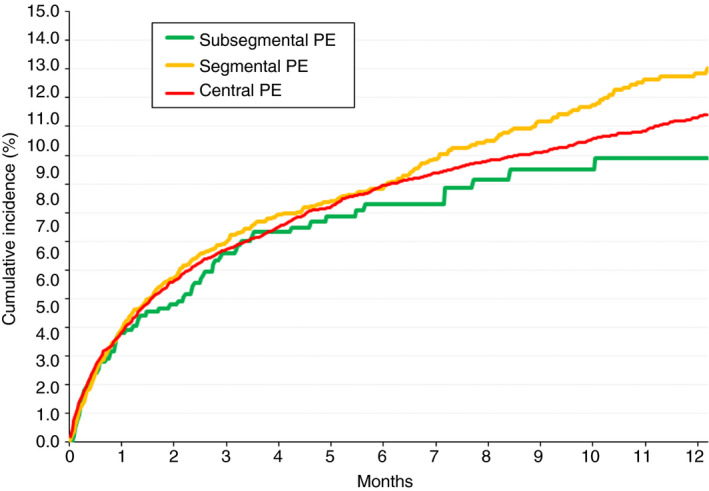
Cumulative mortality rates during the course of anticoagulant therapy, according to the location of the emboli at baseline. PE, pulmonary embolism
When patients with subsegmental PE were stratified into three categories according to ultrasound imaging in the lower limbs, the rate of PE recurrences during the course of anticoagulant therapy was similar in all three subgroups: 2.54 (95% CI, 0.81‐6.12) events per 100 patient‐years in patients with confirmed DVT, 3.73 (95% CI, 1.63‐7.37) in those with no DVT, and 1.81 (95% CI, 0.66‐4.02) in those with no ultrasound imaging (Table 4). On multivariable analysis, patients with subsegmental PE had a higher risk for PE recurrences than those with segmental or more central PE (adjusted HR, 1.75; 95% CI, 1.02‐3.03) (Table 5).
Table 4.
Clinical outcomes during anticoagulation in patients with subsegmental PE, according to the presence or absence of DVT on lower limb ultrasonography
| Concomitant DVT | No DVT | Not performed | ||||
|---|---|---|---|---|---|---|
| N | Events per 100 | N | Events per 100 | N | Events per 100 | |
| patient‐years | patient‐years | patient‐years | ||||
| Patients, N | 198 | 242 | 392 | |||
| PE recurrences | 4 | 2.54 (0.81‐6.12) | 7 | 3.73 (1.63‐7.37) | 5 | 1.81 (0.66‐4.02) |
| DVT recurrences | 2 | 1.25 (0.21‐4.14) | 1 | 0.52 (0.03‐2.57) | 1 | 0.36 (0.02‐1.78) |
| Major bleeding | 6 | 3.83 (1.55‐7.97) | 6 | 3.15 (1.28‐6.55) | 18 | 6.58 (4.02‐10.2) |
| Death | 14 | 8.74 (4.98‐14.3) | 14 | 7.29 (4.15‐11.9) | 48 | 17.3 (12.9‐22.7)* |
| Fatal PE | 1 | 0.62 (0.03‐3.08) | 0 | … | 2 | 0.72 (0.12‐2.38) |
| Fatal bleeding | 0 | … | 0 | … | 5 | 1.80 (0.66‐3.99) |
Abbreviations: DVT, deep vein thrombosis IQR, interquartile range; PE, pulmonary embolism; SD, standard deviation.
Comparisons between patients with concomitant DVT versus other subgroups:
P < .05.
Table 5.
Uni‐ and multivariable analyses for the risk of symptomatic PE recurrences
| Univariable analysis | P value | Multivariable analysis | P value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Male sex | 1.11 (0.83‐1.49) | .47 | … | |
| Age ≥ 70 y | 0.71 (0.53‐0.95) | .02 | 0.76 (0.56‐1.04) | .09 |
| Body weight ≥ 76 kg | 0.81 (0.60‐1.07) | .13 | … | |
| Concomitant disorders | ||||
| Chronic lung disease | 1.60 (1.12‐2.28) | .01 | 1.65 (1.13‐2.41) | .01 |
| Chronic heart failure | 0.94 (0.55‐1.59) | .82 | … | |
| Atrial fibrillation | 1.09 (0.69‐1.72) | .72 | … | |
| CrCl levels (>60 mL/min) | Ref. | |||
| CrCl levels (<30 mL/min) | 0.87 (0.41‐1.83) | .71 | … | |
| CrCl levels (30‐60 mL/min) | 0.97 (0.71‐1.33) | .83 | … | |
| Leg varicosities | 0.53 (0.33‐0.85) | .01 | 0.51 (0.30‐0.84) | .01 |
| Risk factors for PE | ||||
| Unprovoked | Ref. | Ref. | ||
| Cancer | 1.94 (1.43‐2.63) | .00 | 1.85 (1.32‐2.61) | .00 |
| Transient risk factors | 0.74 (0.53‐1.04) | .08 | 0.92 (0.63‐1.36) | .69 |
| PE signs at baseline | ||||
| SBP levels < 100 mm Hg | 0.69 (0.37‐1.26) | .23 | ||
| Heart rate > 110 bpm | 1.48 (1.05‐2.09) | .03 | 1.37 (0.95‐1.97) | .09 |
| Initial therapy | ||||
| Low‐molecular‐weight heparin | Ref. | Ref. | ||
| Unfractionated heparin | 1.72 (1.01‐2.92) | .04 | 1.68 (0.99‐2.86) | .06 |
| Thrombolytics | 1.03 (0.46‐2.33) | .94 | 1.01 (0.44‐2.32) | .98 |
| PE location at baseline | ||||
| Central PE | Ref. | Ref. | ||
| Subsegmental PE | 1.93 (1.16‐3.23) | .01 | 1.75 (1.02‐3.03) | .04 |
| Segmental PE | 0.83 (0.57‐1.20) | .33 | 0.88 (0.60‐1.29) | .51 |
Multivariable analysis used competing‐risk analysis. Results are expressed as hazard ratio and 95% confidence intervals.
Abbreviations: bpm, beats per minute; CrCl, creatinine clearance; PE, pulmonary embolism; SBP, systolic blood pressure levels.
4. DISCUSSION
Patients with acute PE must receive anticoagulant therapy in order to prevent PE recurrences and death. 18 However, whether patients with “only” subsegmental PE should receive the same anticoagulant therapy as those with more central PE is controversial 26 , 27 , 28 , 29 , 30 , 31 for several reasons. First, since the abnormalities are small, their diagnosis may be a false‐positive finding. 12 , 13 , 14 , 15 , 16 , 17 Second, because a true subsegmental PE is likely to have arisen from a small DVT, the risk for PE recurrences without anticoagulation may be expected to be lower than in patients with larger PEs. 1 , 5 , 32 , 33 Our findings, obtained from a large series of consecutive patients with acute PE, reveal that in real‐life 1 in every 20 such patients (834/15 963; 5.2%) had subsegmental PE. The vast majority received anticoagulant therapy at similar doses (and similar duration) than those with more central PEs, and their rate of symptomatic PE recurrences was higher than in those with more proximal PE. This increased risk for subsequent PE recurrences was confirmed after adjusting for a number of potential confounders and was highest during the first 30 days of anticoagulation. Potential explanations for our finding, which was unexpected, include that international normalized ratio (INR) control was poorer among patients with subsegmental PE (we did not gather INR information in RIETE), misclassification of PE recurrences, or residual confounding.
There were a number of differences in the clinical characteristics and risk factors for PE among patients in the three subgroups. Patients with subsegmental or segmental PE were slightly more likely to have recent surgery or active cancer than those with more central PE, but less likely to have recent immobility. They were less likely to have dyspnea, hypotension, hypoxemia, or tachycardia at baseline than those with more central PE. Finally, they also were less likely to have concomitant DVT in the lower limbs or raised laboratory (and echocardiographic) markers at baseline. Interestingly, however, patients with subsegmental PE had a mortality rate similar to those with more central PEs. These findings compare well with those obtained in a previous study by our group, where central PE was associated with worse survival at 15 days but not at 3 months. 34 They are also consistent with another study on 748 patients with acute PE, where those with subsegmental PE (n = 116) had similar outcomes at 3 months than those with more central PE. 35 However, the proportion of patients with subsegmental PE in that study was threefold higher than in our cohort, possibly because they did not exclude patients with prior PE. In contrast, in a multicenter prospective cohort on 579 patients with hemodynamically stable PE, an increased risk for all‐cause death, or clinical deterioration was observed in patients with central PE compared to those with more peripheral PE. 36
We failed to find different outcomes when patients with subsegmental PE were stratified according to the presence or absence of cancer or according to ultrasound findings in the lower limbs (confirmed DVT, DVT ruled out or not explored). An ongoing prospective cohort study on patients with subsegmental PE and negative lower extremity ultrasound is currently investigating the safety of withholding anticoagulation in these patients (NCT01455818). 37 Unfortunately, the number of patients who did not receive anticoagulation in our cohort was insufficient to draw any conclusions. Unexpectedly, the only patient with a recurrence had a normal ultrasonography at baseline.
Our findings have to be interpreted in view of some limitations. The first limitation is the risk for overdiagnosis in patients categorized as having subsegmental PE. At time of restrained resources, we should be aware of the rise of disease mongering because of the potential impact of an unjustified anticoagulant therapy. 38 , 39 In the setting of a registry (with no central adjudication of CT scans), we cannot be reassured about potential misclassification. Second, there is no centralized quality control in RIETE regarding accuracy of PE diagnosis. For technical reasons, it is not possible to review all the imaging tests from over 200 participating centers in 26 countries. This is an important limitation, since the interrater agreement for diagnosing subsegmental PE is poor and, as a consequence, the risk of misclassification is high. Third, most patients with subsegmental PE who are not being treated are most likely not being entered into RIETE. Thus, there is an important selection bias that may invalidate the conclusions as they are currently presented. Fourth, it was impossible to better characterize PE recurrences, in particular with regard to their localizations (central vs noncentral). Case fatality rates, however, do not suggest different prognoses of recurrent VTE after incident subsegmental vs nonsubsegmental PE. Fifth, the sample size of patients not receiving anticoagulation was insufficient to draw any conclusions. Our study also has definite strengths, in particular the large sample size allowing precision of our results and the objective documentation of both incident and recurrent PE.
In conclusion, in contrast to the common belief that subsegmental PE represents a benign subset of PE, our study suggests that the risk of PE recurrences is not influenced by the anatomic location of PE. It seems more likely that persistent risk factors for recurrent PE are better predictors than the location of the PE.
AUTHOR CONTRIBUTIONS
CFC, ARC, and MM designed the study and had final responsibility for study supervision and data analysis. All authors participated in the data collection. DJ and FM revised the intellectual content. Each author critically reviewed and participated in revising the manuscript, and each author approved the final version.
RELATIONSHIP DISCLOSURE
Dr Moustafa has served as an advisor or consultant for Bayer HealthCare Pharmaceuticals and Sanofi; has served as a speaker for Bayer HealthCare Pharmaceuticals, Boehringer Ingelheim, Daiichi‐Sankyo, and Sanofi; and has received grants from Sanofi, Bayer HealthCare, and LFB. Dr Monreal has served as an advisor or consultant for Bayer HealthCare Pharmaceuticals, Leo Pharma, and Sanofi; has served as a speaker or a member of a speaker's bureau for Bayer HealthCare Pharmaceuticals, Daiichi‐Sankyo, Leo Pharma, and Sanofi; and has received grants for clinical research from Sanofi and Bayer. All other authors declare no conflicts of interest. Coordinator of the RIETE Registry: Manuel Monreal. RIETE Steering Committee Members: Dominique Farge Bancel, Paolo Prandoni and Benjamin Brenner. RIETE National Coordinators: Raquel Barba (Spain), Pierpaolo Di Micco (Italy), Laurent Bertoletti (France), Inna Tzoran (Israel), Abilio Reis (Portugal), Marijan Bosevski (R. Macedonia), Henri Bounameaux (Switzerland), Radovan Malý (Czech Republic), Philip Wells (Canada), and Peter Verhamme (Belgium). RIETE Registry Coordinating Center: S & H Medical Science Service.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Sanofi Spain for supporting this registry with an unrestricted educational grant and Bayer Pharma AG for supporting this registry. Bayer Pharma AG's support was limited to the part of RIETE outside Spain, which accounts for 24.05% of the total patients included in the RIETE Registry. The authors also thank the RIETE Registry Coordinating Center, S&H Medical Science Service, for their quality control data and logistic and administrative support; and Prof. Salvador Ortiz, Universidad Autónoma de Madrid, and Silvia Galindo, both statistical advisors in S&H Medical Science Service for the statistical analysis of the data presented in this article.
Members of RIETE
SPAIN: María Dolores Adarraga, Miguel Ángel Aibar, María Alfonsa, Juan Ignacio Arcelus, Pedro Azcarate‐Agüero, Aitor Ballaz, Pilar Baños, Raquel Barba, Manuel Barrón, Belén Barrón‐Andrés, José Bascuñana, Ángeles Blanco‐Molina, Ana María Camón, Leyre Chasco, Antonio Javier Cruz, Roberto del Pozo, Javier de Miguel, Jorge del Toro, María Carmen Díaz‐Pedroche, José Antonio Díaz‐Peromingo, José Carlos Escribano, Conxita Falgá, Cleofé Fernández‐Aracil, Carmen Fernández‐Capitán, María Ángeles Fidalgo, Carme Font, Llorenç Font, Maria Angelina García, Ferrán García‐Bragado, Marcial García‐Morillo, Aránzazu García‐Raso, Ana Isabel García‐Sánchez, Olga Gavín‐Sebastián, Ignacio Gaya, Covadonga Gómez‐Cuervo, Vicente Gómez, José González, Enric Grau, Ricardo Guijarro, Javier Gutiérrez, Genoveva Hernández‐Comes, Luis Hernández‐Blasco, Elena Hernando, Luis Jara‐Palomares, María Jesús Jaras, David Jiménez, María Dolores Joya, Jorge Lima, Pilar Llamas, José Luis Lobo, Luciano López‐Jiménez, Raquel López‐Reyes, Juan Bosco López‐Sáez, Manuel Alejandro Lorente, Alicia Lorenzo, Marina Lumbierres, Olga Madridano, Ana Maestre, Pablo Javier Marchena, Francisco Martín‐Martos, Miguel Martín‐Romero, Manuel Monreal, María Valle Morales, José Antonio Nieto, Santiago Nieto, Ana Núñez, Manuel Jesús Núñez, Mónica Odriozola, María Carmen Olivares, Sonia Otalora, Remedios Otero, José María Pedrajas, Galadriel Pellejero, Cristina Pérez‐Ductor, María Luisa Peris, Isaac Pons, José Antonio Porras, Laura Ramírez, Oscar Reig, Antoni Riera‐Mestre, David Riesco, Agustina Rivas, María Ángeles Rodríguez‐Dávila, Vladimir Rosa, Pedro Ruiz‐Artacho, Joan Carles Sahuquillo, Maria Carmen Sala‐Sainz, Ángel Sampériz, Rosario Sánchez‐Martínez, Silvia Soler, Bernardo Sopeña, Josep María Suriñach, Carles Tolosa, María Isabel Torres, Jesús Troya, Javier Trujillo‐Santos, Fernando Uresandi, Esther Usandizaga, Beatriz Valero, Reina Valle, Jerónimo Vela, Laura Vela, Gemma Vidal, Aurora Villalobos, BELGIUM: Thomas Vanassche, Christophe Vandenbriele, Peter Verhamme, BRAZIL: Hugo Hyung Bok Yoo, CANADA: Phil Wells, CZECH REPUBLIC: Jana Hirmerova, Radovan Malý, ECUADOR: Estuardo Salgado, FRANCE: Laurent Bertoletti, Alessandra Bura‐Riviere, Nicolas Falvo, Dominique Farge‐Bancel, Adrian Hij, Isabelle Mahé, Farès Moustafa, Isabelle Quere, ISRAEL: Andrei Braester, BenjaminBrenner, Martin Ellis, Inna Tzoran, ITALY: Giancarlo Antonucci, Giovanni Barillari, Franca Bilora, Cristiano Bortoluzzi, Eugenio Bucherini, Antonio Camerota, Chiara Cattabiani, Maurizio Ciammaichella, Francesco Dentali, Pierpaolo Di Micco, Rita Duce, Matteo Giorgi‐Pierfranceschi, Elvira Grandone, Egidio Imbalzano, Gianfranco Lessiani, Rosa Maida, Daniela Mastroiacovo, Federica Pace, Raffaele Pesavento, Mauro Pesavento, Renzo Poggio, Paolo Prandoni, Roberto Quintavalla, Anna Rocci, Carmine Siniscalchi, Eros Tiraferri, Diego Tonello, Adriana Visonà, Beniamino Zalunardo, LATVIA: Valdis Gibietis, Andris Skride, Barbara Vitola, SWITZERLAND: Adriano Alatri, Henri Bounameaux, Luca Calanca, Lucia Mazzolai.
Fernández‐Capitán C, Cobo AR, Jiménez D, et al. Symptomatic subsegmental versus more central pulmonary embolism: Clinical outcomes during anticoagulation. Res Pract Thromb Haemost.2021;5:168–178. 10.1002/rth2.12446
A full list of the RIETE investigators is given in the Appendix.
Contributor Information
David Jiménez, @DJC6998.
Olga Madridano, @OMadridano.
Remedios Otero, @OteroRemedios.
Pierpaolo Di Micco, @pdimicco.
Farès Moustafa, Email: fmoustafa@chu-clermontferrand.fr.
Manuel Monreal, @mmonrealriete.
The RIETE Investigators:
M. D. Adarraga, M. A. Aibar, M. Alfonsa, J. I. Arcelus, P. Azcarate‐Agüero, A. Ballaz, P. Baños, R. Barba, M. Barrón, B. Barrón‐Andrés, J. Bascuñana, A. Blanco‐Molina, A. M. Camón, L. Chasco, A. J Cruz, R. del Pozo, J. de Miguel, J. del Toro, M. C. Díaz‐Pedroche, J. A. Díaz‐Peromingo, J. C. Escribano, C. Falgá, C. Fernández‐Aracil, M. A. Fidalgo, C. Font, L. Font, M. A. García, F. García‐Bragado, M. García‐Morillo, A. García‐Raso, A. I. García‐Sánchez, O. Gavín, I. Gaya, C. Gómez, V. Gómez, J. González, E. Grau, R. Guijarro, J. Gutiérrez, G. Hernández‐Comes, L. Hernández‐Blasco, E. Hernando, L. Jara‐Palomares, M. J. Jaras, D. Jiménez, M. D. Joya, J. Lima, P. Llamas, J. L. Lobo, R. López‐Reyes, J. B. López‐Sáez, M. A. Lorente, A. Lorenzo, M. Lumbierres, A. Maestre, P. J. Marchena, F. Martín‐Martos, M. Martín‐Romero, M. V Morales, J. A. Nieto, S. Nieto, A. Núñez, M. J. Núñez, M. Odriozola, M. C. Olivares, S. Otalora, J. M. Pedrajas, G. Pellejero, C. Pérez‐Ductor, M. L Peris, I. Pons, J. A. Porras, L. Ramírez, O. Reig, A. Riera‐Mestre, D. Riesco, A. Rivas, M. A. Rodríguez‐Dávila, V. Rosa, P. Ruiz‐Artacho, J. C. Sahuquillo, M. C. Sala‐Sainz, A. Sampériz, R. Sánchez‐Martínez, S. Soler, B. Sopeña, J. M Suriñach, C. Tolosa, M. I Torres, J. Troya, J. Trujillo‐Santos, F. Uresandi, B. Valero, R. Valle, J. Vela, L. Vela, G. Vidal, A. Villalobos, T. Vanassche, C. Vandenbriele, P. Verhamme, H. H. B. Yoo, P. Wells, J. Hirmerova, R. Malý, E. Salgado, L. Bertoletti, A. Bura‐Riviere, N. Falvo, D. Farge‐Bancel, A. Hij, I. Mahé, I. Quere, A. Braester, B. Brenner, M. Ellis, I. Tzoran, G. Antonucci, G. Barillari, F. Bilora, C. Bortoluzzi, E. Bucherini, A. Camerota, C. Cattabiani, F. Dentali, R. Duce, M. Giorgi‐Pierfranceschi, E. Grandone, E. Imbalzano, G. Lessiani, R. Maida, D. Mastroiacovo, F. Pace, R. Pesavento, M. Pesavento, R. Poggio, P. Prandoni, R. Quintavalla, A. Rocci, C. Siniscalchi, E. Tiraferri, D. Tonello, A. Visonà, B. Zalunardo, V. Gibietis, A. Skride, B. Vitola, A. Alatri, H. Bounameaux, L. Calanca, and L. Mazzolai
REFERENCES
- 1. Le Gal G, Righini M, Parent F, van Strijen M, Couturaud F. Diagnosis and management of subsegmental pulmonary embolism. J Thromb Haemost. 2006;4:724–31. [DOI] [PubMed] [Google Scholar]
- 2. Wiener RS, Schwartz LM, Woloshin S. When a test is too good: how CT pulmonary angiograms find pulmonary emboli that do not need to be found. BMJ. 2013;347:f3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carrier M, Righini M, Wells PS, Perrier A, Anderson DR, Rodger MA, et al. Subsegmental pulmonary embolism diagnosed by computed tomography: incidence and clinical implications. A systematic review and meta‐analysis of the management outcome studies. J Thromb Haemost. 2010;8:1716–22. [DOI] [PubMed] [Google Scholar]
- 4. Carrier M, Righini M, Le Gal G. Symptomatic subsegmental pulmonary embolism: what is the next step? J Thromb Haemost. 2012;10:1486–90. [DOI] [PubMed] [Google Scholar]
- 5. Stein PD, Goodman LR, Hull RD, Dalen JE, Matta F. Diagnosis and management of isolated subsegmental pulmonary embolism: review and assessment of the options. Clin Appl Thromb. 2012;18:20–6. [DOI] [PubMed] [Google Scholar]
- 6. Fernandes A, Connors JM, Carrier M. Anticoagulation for subsegmental pulmonary embolism. N Engl J Med. 2019;381:1171–4. [DOI] [PubMed] [Google Scholar]
- 7. White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107:I4–8. [DOI] [PubMed] [Google Scholar]
- 8. Goy J, Lee J, Levine O, Chaudhry S, Crowther M. Sub‐segmental pulmonary embolism in three academic teaching hospitals: a review of management and outcomes. J Thromb Haemost. 2015;13:214–8. [DOI] [PubMed] [Google Scholar]
- 9. DeMonaco NA, Dang Q, Kapoor WN. Ragni MV. Pulmonary embolism incidence is increasing with use of spiral computed tomography. Am J Med. 2008;121:611–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mountain D, Keijzers G, Chu K, Joseph A, Read C, Blecher G, et al. RESPECT‐ED: Rates of Pulmonary Emboli (PE) and Sub‐Segmental PE with Modern Computed Tomographic Pulmonary Angiograms in Emergency Departments: a multi‐center observational study finds significant yield variation, uncorrelated with use or small PE rates. Leroyer C, editor. PLoS One. 2016;11:e0166483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Auer RC, Schulman AR, Tuorto S, Gönen M, Gonsalves J, Schwartz L, et al. Use of helical CT is associated with an increased incidence of postoperative pulmonary emboli in cancer patients with no change in the number of fatal pulmonary emboli. J Am Coll Surg J Am Coll Surg. 2009;208:871–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Costantino G, Norsa AH, Amadori R, Ippolito S, Resta F, Bianco R, et al. Interobserver agreement in the interpretation of computed tomography in acute pulmonary embolism. Am J Emerg Med. 2009;27:1109–11. [DOI] [PubMed] [Google Scholar]
- 13. Lucassen WAM, Beenen LFM, Büller HR, Erkens PMG, Schaefer‐Prokop CM, van den Berk IAH, et al. Concerns in using multi‐detector computed tomography for diagnosing pulmonary embolism in daily practice. A cross‐sectional analysis using expert opinion as reference standard. Thromb Res. 2013;131:145–9. [DOI] [PubMed] [Google Scholar]
- 14. Stein PD, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354:2317–27. [DOI] [PubMed] [Google Scholar]
- 15. Courtney DM, Miller C, Smithline H, Klekowski N, Hogg M, Kline JA. Prospective multicenter assessment of interobserver agreement for radiologist interpretation of multidetector computerized tomographic angiography for pulmonary embolism. J Thromb Haemost. 2010;8:533–9. [DOI] [PubMed] [Google Scholar]
- 16. Pena E, Kimpton M, Dennie C, Peterson R, Gal LEG, Carrier M. Difference in interpretation of computed tomography pulmonary angiography diagnosis of subsegmental thrombosis in patients with suspected pulmonary embolism. J Thromb Haemost. 2012;10:496–8. [DOI] [PubMed] [Google Scholar]
- 17. Hutchinson BD, Navin P, Marom EM, Truong MT, Bruzzi JF. Overdiagnosis of pulmonary embolism by pulmonary CT angiography. Am J Roentgenol. 2015;205:271–7. [DOI] [PubMed] [Google Scholar]
- 18. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–52. [DOI] [PubMed] [Google Scholar]
- 19. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing G‐J, Harjola V‐P, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2019;1–61. [DOI] [PubMed] [Google Scholar]
- 20. Yoo HHB, Queluz THAT, El Dib R. Anticoagulant treatment for subsegmental pulmonary embolism (Review). Cochrane Database Syst Rev. 2016;1:CD010222. [DOI] [PubMed] [Google Scholar]
- 21. Monreal M, Falgá C, Valdés M, Suárez C, Gabriel F, Tolosa C, et al. Fatal pulmonary embolism and fatal bleeding in cancer patients with venous thromboembolism: findings from the RIETE registry. J Thromb Haemost. 2006;4:1950–6. [DOI] [PubMed] [Google Scholar]
- 22. Ruíz‐Giménez N, Suárez C, González R, Nieto JA, Todolí JA, Samperiz AL, et al. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE Registry. Thromb Haemost. 2008;100:26–31. [DOI] [PubMed] [Google Scholar]
- 23. Nieto JA, Solano R, Ruiz‐Ribo MD, Ruiz‐Gimenez N, Prandoni P, Kearon C, et al. Fatal bleeding in patients receiving anticoagulant therapy for venous thromboembolism: findings from the RIETE registry. J Thromb Haemost. 2010;8:1216–22. [DOI] [PubMed] [Google Scholar]
- 24. Laporte S, Mismetti P, Décousus H, Uresandi F, Otero R, Lobo JL, et al. Clinical predictors for fatal pulmonary embolism in 15,520 patients with venous thromboembolism: findings from the Registro Informatizado de la Enfermedad TromboEmbolica venosa (RIETE) Registry. Circulation. 2008;117:1711–6. [DOI] [PubMed] [Google Scholar]
- 25. Bikdeli B, Jimenez D, Hawkins M, Ortíz S, Prandoni P, Brenner B, et al. Rationale, design and methodology of the computerized registry of patients with venous thromboembolism (RIETE). Thromb Haemost. 2018;118:214–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Konstantinides SV, Barco S, Lankeit M, Meyer G. Management of pulmonary embolism: an update. J Am Coll Cardiol. 2016;67:976–90. [DOI] [PubMed] [Google Scholar]
- 27. Long B, Koyfman A. Best clinical practice: current controversies in pulmonary embolism imaging and treatment of subsegmental thromboembolic disease. J Emerg Med. 2016:1–10. [DOI] [PubMed] [Google Scholar]
- 28. Peiman S, Abbasi M, Allameh SF, Asadi Gharabaghi M, Abtahi H, Safavi E. Subsegmental pulmonary embolism: a narrative review. Thromb Res. 2015;138:55–60. [DOI] [PubMed] [Google Scholar]
- 29. Ikesaka R, Carrier M. Clinical significance and management of subsegmental pulmonary embolism. J Thromb Thrombolysis. 2015;39:311–4. [DOI] [PubMed] [Google Scholar]
- 30. Stoller N, Limacher A, Méan M, Baumgartner C, Tritschler T, Righini M, et al. Clinical presentation and outcomes in elderly patients with symptomatic isolated subsegmental pulmonary embolism. Thromb Res. 2019;184:24–30. [DOI] [PubMed] [Google Scholar]
- 31. Donato AA, Khoche S, Santora J, Wagner B. Clinical outcomes in patients with isolated subsegmental pulmonary emboli diagnosed by multidetector CT pulmonary angiography. Thromb Res. 2010;126. [DOI] [PubMed] [Google Scholar]
- 32. Carrier M, Righini M, Le Gal G. Symptomatic subsegmental pulmonary embolism: what is the next step? J Thromb Haemost. 2012;10:1486–90. [DOI] [PubMed] [Google Scholar]
- 33. Le Gal G, Righini M, Sanchez O, Roy P‐M, Baba‐Ahmed M, Perrier A, et al. A positive compression ultrasonography of the lower limb veins is highly predictive of pulmonary embolism on computed tomography in suspected patients. Thromb Haemost. 2006;95:963–6. [DOI] [PubMed] [Google Scholar]
- 34. Gouin B, Blondon M, Jiménez D, Fernández‐Capitán C, Bounameaux H, Soler S, et al. Clinical prognosis of non‐massive central and non‐central pulmonary embolism: a registry‐based cohort study. Chest. 2017;151(4):829–837. [DOI] [PubMed] [Google Scholar]
- 35. den Exter PL, van Es J, Klok FA, Kroft LJ, Kruip MJHA, Kamphuisen PW, et al. Risk profile and clinical outcome of symptomatic subsegmental acute pulmonary embolism. Blood. 2013;122:1144–9. quiz 1329. [DOI] [PubMed] [Google Scholar]
- 36. Vedovati MC, Becattini C, Agnelli G, Kamphuisen PW, Masotti L, Pruszczyk P, et al. Multidetector CT scan for acute pulmonary embolism: embolic burden and clinical outcome. Chest. 2012;142:1417–24. [DOI] [PubMed] [Google Scholar]
- 37. Carrier M.A Study to Evaluate the Safety of Withholding Anticoagulation in Patients With Subsegmental PE Who Have a Negative Serial Bilateral Lower Extremity Ultrasound ‐ Full Text View ‐ ClinicalTrials.gov.
- 38. Moynihan R, Heath I, Henry D. Selling sickness: The pharmaceutical industry and disease mongering. Br Med J. 2002;324:886–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Frappé P, Haller DM, Roméas A, Bertoletti L, François M, Robert‐Ebadi H, et al. Avoiding disease mongering: a checklist for vascular physicians and researchers. Thromb Res. 2019:120–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


