Abstract
Background
Thrombotic thrombocytopenic purpura (TTP) is a life‐threatening thrombotic microangiopathy (TMA) caused by a severe functional deficiency in ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type I repeats‐13), the specific von Willebrand factor (VWF) cleaving protease. ADAMTS13 activity is essential to diagnose TTP but remains challenging to assess, as reference ADAMTS13 activity assays are manual and time consuming. Current techniques also lack robustness in low detectable ADAMTS13 activity range, which could prove problematic for therapy‐driven monitoring.
Objectives
The HemosIL AcuStar ADAMTS13 activity assay is a fast, automated chemiluminescent assay, the performance of which remains to be evaluated prospectively on very large cohorts of patients with TMA and in real‐life conditions.
Patients and Methods
Our study was conducted over two successive sequences: a retrospective evaluation followed by a “real‐life” prospective evaluation. Overall, we evaluated the HemosIL AcuStar ADAMTS13 activity assay on 539 citrated plasma samples. We extensively studied linearity, limit of detection, contamination, intra‐assay and interassay precisions with a specific focus on levels < 25 IU/dL. Diagnostic performances for the detection of < 10 IU/dL ADAMTS13 activity and overall method comparison were conducted with the fluorescence resonance energy transfer (FRETS)‐VWF73 assay as the reference method.
Results
Technical performance proved excellent. Robustness in low detectable ADAMTS13 activity range was good, potentially qualifying this assay for therapy‐driven monitoring. Comparison with the FRETS‐VWF73 assay was satisfactory (r 2 = .83, P < .0001) as were the diagnostic performances for acute‐phase TTP (specificity, 99.7%; positive predictive value, 99.2%).
Conclusion
The HemosIL AcuStar ADAMTS13 activity assay is a fast, reliable, automated technique well adapted as a first‐line ADAMTS13 activity assay for TTP diagnosis and follow‐up.
Keywords: ADAMTS13 protein, biological monitoring, chemiluminescent assay, diagnosis, thrombotic thrombocytopenic purpura
Essentials.
ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type I repeats‐13) measurement is crucial for diagnosis and follow‐up of thrombotic thrombocytopenic purpura.
The HemosIL AcuStar ADAMTS13 activity assay is a fully automated rapid quantitative immunoassay.
The HemosIL AcuStar ADAMTS13 activity assay was compared to FRETS‐VWF73 using 539 plasma samples.
Analytical and diagnostic performances of the HemosIL AcuStar ADAMTS13 activity were excellent.
1. INTRODUCTION
Thrombotic thrombocytopenic purpura (TTP) is a rare life‐threatening thrombotic microangiopathy (TMA) defined by peripheral thrombocytopenia, mechanical hemolytic anaemia, and visceral ischemia caused by a severe functional deficiency (<10 IU/dL) in ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type I repeats‐13), the specific von Willebrand factor (VWF) cleaving protease. 1 , 2 TTP is mostly an adult acquired disease (acquired TTP [aTTP]) mainly mediated by anti‐ADAMTS13 autoantibodies. 3 , 4 Much less frequently, a severe functional deficiency in ADAMTS13 can also be inherited through mutations in the ADAMTS13 gene leading to congenital TTP, also called Upshaw‐Schulman syndrome (USS) 5 . In more than half of the cases, aTTP is an idiopathic disease, but it can also be associated with underlying situations such as infections, autoimmune disorders, malignancies, pregnancy, or grafts. 3 , 6 , 7 , 8 , 9 TTP is a chronic disease with acute phases interspersed with periods of clinical remissions that can vary widely in duration and marked by the risk of unpredictable relapses. 10 , 11 Patients with TTP must therefore undergo a lifetime of surveillance.
The cornerstone of TTP treatment is ADAMTS13 replacement therapy through plasma exchange (PEX) 12 in association with immunomodulating drugs such as corticosteroids 13 , 14 and rituximab. 15 , 16 Caplacizumab is an anti‐VWF A1 domain nanobody targeting specifically the interaction between VWF A1 domain and platelet glycoprotein Ibα 17 and blocking the formation of new microthrombi. It recently emerged as a novel strategy in association with PEX and immunomodulating agents for the treatment of acute‐phase TTP. Results from the multicenter studies TITAN 18 and HERCULES 19 evaluating its efficacy showed a clear superiority compared to placebo in terms of reduction of exacerbations, and caplacizumab could join PEX and immunomodulating agents as the first‐line treatment for TTP. While TTP is a chronic disease, no long‐term treatment is currently recommended, and long‐term management is based on surveillance.
ADAMTS13 activity is a key parameter that drives both diagnosis and therapeutic course of action. Differential diagnosis of TTP with other TMAs can be uncertain, and the diagnosis of acute‐phase TTP can be ascertained only by documenting an undetectable (<10 IU/dL) ADAMTS13 activity, a specific feature that TTP does not share with other TMAs. 20 ADAMTS13 activity monitoring is also recommended during initial management to demonstrate the recovery of ADAMTS13 activity defining biological remission. During follow‐up, TTP patients in remission must be regularly monitored to detect any downslope of ADAMTS13 activity 21 , 22 , 23 . It is generally accepted that an ADAMTS13 activity < 10 IU/dL indicates need for preemptive therapy to prevent clinical relapses with a favorable risk‐benefit balance. 11 Finally, during the acute phase, caplacizumab‐driven therapy guidelines call for frequent ADAMTS13 activity monitoring 19 , 24 . ADAMTS13 activity is therefore a crucial tool for both initial diagnosis and follow‐up of TTP.
Ever since the unraveling of TTP pathophysiology, several ADAMTS13 activity assays have been reported. They all share a similar principle, using an exogenous substrate (either full‐length VWF or VWF peptide comprising the cleavage site by ADAMTS13) whose degradation products after cleavage by ADAMTS13 are secondarily detected and measured by different methods 25 , 26 , 27 : These include SDS agarose electrophoresis and immunoblotting or western blot, 28 , 29 collagen binding, 30 ristocetin cofactor activity, 31 immunoradiometric assay, 32 chromogenic assay, 33 , 34 , 35 or mass spectrometry. 36 Kokame et al 37 described in 2005 the fluorescence resonance energy transfer (FRETS)‐VWF73 assay, using fluorescence as a means of detection of ADAMTS13 degradation products, which is now the gold standard for the measurement of ADAMTS13 activity. Most methods share the common drawbacks of being manual and time consuming with, for all techniques except chromogenic assays, the added difficulty of requiring extensive technical expertise, which does not allow for wide around‐the‐clock availability. Chromogenic assays are flawed by unexplained discrepancies that prevent them from becoming reference methods. 33 , 34 , 35 Moreover, analytical interferences and a lack of robustness within the low detectable ADAMTS13 activity range (< 25 IU/dL) further complicates adequate ADAMTS13 deficiency diagnosis and follow‐up.
A new fully automated fast ADAMTS13 activity assay could allow for more rapid adequate diagnosis. The HemosIL AcuStar ADAMTS13 activity assay (Instrumentation Laboratory, Bedford, MA, USA) is a novel, fully automated chemiluminescent technique recently developed. 38 Plasma samples are incubated with magnetic particles coated with VWF73, the minimal fragment of VWF required for ADAMTS13 binding and cleavage. After magnetic separation and wash, an isoluminol‐tagged monoclonal antibody 39 specifically binds to the cleaved peptide produced by ADAMTS13 and a second incubation occurs. After a final magnetic separation and wash, the chemiluminescent reaction is triggered by specific reagents, and the emitted light is measured in relative light units by the ACL AcuStar analyzer (Instrumentation Laboratory). In this chemiluminescent assay, ADAMTS13 activity is proportional to the emitted light. The HemosIL AcuStar ADAMTS13 activity assay has been successfully evaluated by other teams 40 , 41 , 42 on 176, 24, and 38 samples. We sought to further evaluate this new method by comparing its diagnostic performances compared to our in‐house FRETS‐VWF73 assay on a larger cohort of patients with TMA tested both retrospectively from a biobank and prospectively in “real‐life” conditions. We also performed a specific focus on the low detectable range of ADAMTS13 activity (0‐25 IU/dL) to evaluate the performance of the HemosIL AcuStar ADAMTS13 activity assay in the therapy‐driven monitoring during follow‐up.
2. MATERIALS AND METHODS
2.1. HemosIL AcuStar ADAMTS13 activity assay analytic performances
Limit of detection and linearity were determined by serial dilutions of a known TTP plasma sample (ADAMTS13 activity < 10 IU/dL with FRETS‐VWF73 assay) in normal plasma previously heated at 56°C for 2 hours and therefore devoid of ADAMTS13 activity (tested < 0.2 IU/dL).
Inter‐ and intra‐assay precisions were calculated using internal quality controls (IQCs) and selected patient samples at critical levels. Interassay precision was determined using IQC targeting 90 IU/dL and 30 IU/dL of ADAMTS13 activity provided by the manufacturer (Instrumentation Laboratory), tested over 51 runs, and using a known TTP plasma sample (ADAMTS13 activity < 10 IU/dL with FRETS‐VWF73 assay) tested over 16 runs. Intra‐assay precision was assessed by repeating 10 times the assay using CRYOCHECK IQC (Abnormal and Normal Reference Controls CRYOCHECK, Cryopep, Montpellier, France) targeting 49 IU/dL and 102 IU/dL of ADAMTS13 activity, and by repeating four times 18 distinct plasma samples from the TMA Biobank (see below) chosen to represent the set of values between 0 and 25 IU/dL of ADAMTS13 activity. Inter‐ and intra‐assay coefficients of variability (CVs) were calculated for each set of values by dividing the standard deviation by the mean.
Contamination was checked by testing a normal sample (pretested; ADAMTS13 activity was 100 IU/dL) and a TTP sample (pretested; ADAMTS13 activity was < 0.2 IU/dL): The TTP sample testing was immediately followed by a repetition of three testings of the normal sample, which was immediately followed by a repetition of two testings of the TTP sample.
Also, to study potential analytical interferences, we studied 11 samples autofluorescent with the FRETS‐VWF73 assay: Samples are autofluorescent with FRETS‐VWF73 when a very strong fluorescent signal compared to the calibrant is picked from the very start of the reading, which makes ADAMTS13 activity uninterpretable. Finally, 8 hemolyzed samples, 3 icteric samples, 5 lactescent samples, 1 hemolyzed and lactescent sample, and 1 icteric and lactescent sample were also tested.
2.2. Study design and blood samples
The study was designed in two successive sequences to evaluate the HemosIL AcuStar ADAMTS13 activity assay on a total of 539 citrated plasma samples. First, we performed a retrospective evaluation using 316 samples stored at −80°C between January 1, 2015, and December 31, 2019, in the biobank of the French National Reference Center for TMA (CRB‐LRB, biobank BB‐0033‐00 064 certified NFS 96‐900, Lariboisière Hospital, APHP.Nord, Paris, France). As a reminder, since 2000, all patients with a presumptive diagnosis of TMA (defined as the presence of microangiopathic hemolytic anemia with hemoglobin level < 12 g/dL and thrombocytopenia < 150 × 109/L with or without ischemic manifestations) were prospectively enrolled in the registry of the National Reference Center for TMA (Health Ministry National Plan for Rare Diseases) and investigated for ADAMTS13 in the central French reference laboratory for ADAMTS13 for diagnostic purposes. 3 Second, we performed a prospective evaluation of 223 consecutive samples (either fresh or frozen at −80°C for a maximum of 3 days) tested in “real‐life” conditions from January 1 to April 30, 2020.
In addition to this study performed exclusively on plasma samples, an ancillary study was conducted with plasma and serum couples obtained from the same blood collection of 100 distinct patients: 49 from the retrospective evaluation and 51 from the prospective evaluation.
2.3. Blood collection and ethics
Venous blood was either collected into 1:10 final volume of 3.8% sodium citrate or into a serum tube without clot activator, centrifuged at 3200 g for 20 minutes. Plasma or serum was then aliquoted and frozen at −80°C and thawed 5 minutes at 37°C before testing.
Written informed consent was obtained from all patients according to the Declaration of Helsinki. The study was approved by the Ethics Committee of Hospital Pitié‐Salpêtrière (Paris. France). The study is registered at www.clinicaltrials.gov as number NCT00426686, at the Health Authority and by the French Ministry of Health as number P051064/PHRC AOM05012.
2.4. ADAMTS13 activity assays
Reference ADAMTS13 activity was assessed in the context of care using our in‐house FRETS‐VWF73 assay 3 , 43 adapted from Kokame et al 37 using commercial recombinant FRETS‐VWF73 peptide (Peptide Institute, Ibaraki, Japan) and standardized against the World Health Organization International Standard. 44 Normal range is between 50 IU/dL and 150 IU/dL.
An interference known as autofluorescence can prevent ADAMTS13 measurement by FRETS‐VWF73 assay: for those samples, ADAMTS13 activity was assessed using the chromogenic assay Technozym ADAMTS13 Activity ELISA assay (Chr‐VWF73, Technoclone, Vienna, Austria) according to the manufacturer’s instructions.
ADAMTS13 activity was assessed for each sample using the HemosIL AcuStar ADAMTS13 activity assay (Instrumentation Laboratory). Calibration was carried out using calibrators supplied by the manufacturers. Samples and IQC were tested following the manufacturer’s instructions.
2.5. Statistical analysis
Linear regression was performed using Pearson’s formula. ADAMTS13 activity levels obtained with HemosIL AcuStar ADAMTS13 activity and FRETS‐VWF73 assays were compared using Bland‐Altmann representation. Our in‐house FRETS‐VWF73 assay has a limit of quantification of 10 IU/dL that can be lowered to 5 IU/dL when ADAMTS13 activity is undetectable if the relative fluorescence units (RFU) are below the limit of detection. We therefore arbitrarily decided for the statistical analysis to respectively set to 5 IU/dL and 0 IU/dL an ADAMTS13 activity that was determined < 10 IU/dL with a detectable RFU and < 5 IU/dL.
Continuous variables were compared with the nonparametric Mann‐Whitney U test. The means of the differences between in‐house FRETS‐VWF73 and HemosIL AcuStar ADAMTS13 Activity assays between the retrospective and the prospective sequences were compared with a Mann Whitney U test. Agreement between methods regarding ADAMTS13 activity < 10 IU/dL threshold for the diagnosis of TTP, was looked into, with FRETS‐VWF73 assay as gold standard. Patients with TTP were defined on clinical presentation of TTP associated with an ADAMTS13 activity < 10 IU/dL with our in‐house FRETS‐VWF73 assay. True‐positive (TP) patients were defined as patients with TTP whose ADAMTS13 activity was < 10 IU/dL with the HemosIL AcuStar ADAMTS13 activity assay. False‐negative (FN) patients were defined as patients with TTP whose ADAMTS13 activity was > 10 IU/dL with the HemosIL AcuStar ADAMTS13 activity assay. True‐negative (TN) patients were defined as patients without TTP whose ADAMTS13 activity was > 10 IU/dL with the HemosIL AcuStar ADAMTS13 activity assay. False‐positive (FP) patients were defined as patients without TTP whose ADAMTS13 activity was < 10 IU/dL with the HemosIL AcuStar ADAMTS13 activity assay.
Sensitivity was calculated as the percentage of correctly detected TTP patients by HemosIL AcuStar ADAMTS13 activity assay, using the formula: sensitivity (%) = (TP × 100)/(TP + FN). Specificity was calculated as the percentage of correctly detected patients without TTP by the HemosIL AcuStar ADAMTS13 activity assay, using the formula: specificity (%) = (TN × 100)/(TN + FP). Positive predictive value (PPV) was estimated as the percentage of patients with TTP among the subjects whose ADAMTS13 activity was < 10 IU/dL, using the formula: PPV = (TP × 100)/(TP + FP). Negative predictive value (NPV) was estimated as the percentage of patients without TTP among the subjects whose ADAMTS13 activity was > 10 IU/dL, using the formula: NPV = (TN × 100)/(TN + FN).
Observed proportionate agreement (Po) was calculated using the formula: Po = (TP + TN)/(TP + FP +TN + FN). The probability of random agreement (Pe) was calculated using the formula: Pe = (TP + FN) × (TP + FP)/(TP + FP +TN + FN)2 + (TN + FN) × (TN + FP)/( TP + FP +TN + FN)2. Cohen’s kappa coefficient was calculated to measure the agreement between our in‐house FRETS‐VWF73 and the HemosIL AcuStar ADAMTS13 activity assays for detecting an < 10 IU/dL ADAMTS13 activity using the formula kappa = (Po – Pe)/(1 – Pe).
Statistical significance was set at P < .05. Statistical analysis was performed using Prism version 7.00 (GraphPad Software, La Jolla, CA, USA).
3. RESULTS
3.1. Analytic performances of the HemosIL Actustar ADAMTS13 activity assay
The limit of detection was determined at 0.2 IU/dL, matching the manufacturer’s claim (Figure S1). No background signal was detected when assessing the ADAMTS13 activity of the heated plasma. Linearity, assessed by serial dilutions of one aTTP plasma sample, proved excellent: Pearson’s correlation coefficient r 2 was 1.00 (Appendix S1: Figure S1). Interassay precisions assessed over 51 runs for the 90 IU/dL and 30 IU/dL ADAMTS13 activity IQC targets, respectively, were 9.11% and 9.64%. Interassay precision assessed over 16 runs for the known TTP plasma (ranging from < 0.2 to 0.5 IU/dL) was 12.9%. Intra‐assay precisions assessed with CRYOCHECK IQC repeated 10 times were 5.5% for the 102 IU/dL ADAMTS13 activity target and 3.3% for the 49 IU/dL ADAMTS13 activity target.
Figure 1.
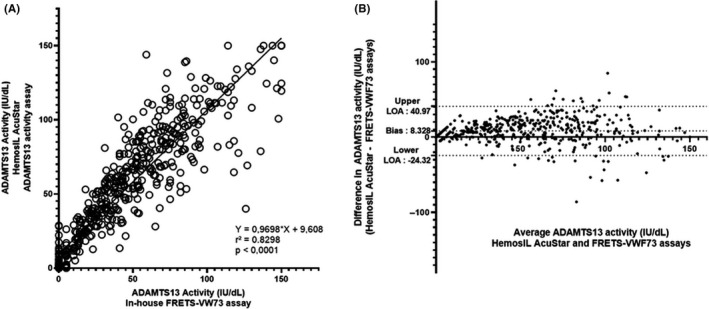
Scatter diagram (A) and Bland‐Altman plot (B) illustrating the comparison of in‐house FRETS‐VWF73 assay (reference method) and HemosIL AcuStar ADAMTS13 activity assay (evaluated method) for the measurement of ADAMTS13 activity in 539 plasma samples. With our in‐house FRETS‐VWF73 assay, 142 patients had an undetectable (<10 IU/dL) ADAMTS13 activity. Among those patients, 14 were found ≥ 10 IU/dL with the HemosIL AcuStar ADAMTS13 activity (false negative). Conversely, 397 patients had a detectable ADAMTS13 activity (≥10 IU/dL) with our in‐house FRETS‐VWF73, and only 1 had undetectable ADAMTS13 activity (< 10 IU/dL) as measured by the HemosIL AcuStar ADAMTS13 activity assay (false positive). For the statistical analysis, values were respectively set to 5 IU/dL and 0 IU/dL if ADAMTS13 activity was determined < 10 IU/dL with a detectable RFU and < 5 IU/dL with our in‐house FRETS‐VWF73. Each sample is represented as a circle. Pearson’s correlation coefficient (r 2) was found at .8298 (P < .0001), and fitted regression line was Y = 0.9698X + 9.608. The Bland‐Altman plot illustrates the comparison of the HemosIL AcuStar ADAMTS13 Activity assay to our in‐house FRETS‐VWF73. Each sample is represented by a dot. The Bland‐Altman plot reveals a mean bias at 8.328 IU/dL and quite large limits of agreement (−24.32 IU/dL; +40.97 IU/dL), indicating (i) a variability between both assays for the measurement of ADAMTS13 activity and (ii) an overall tendency of the HemosIL AcuStar ADAMTS13 Activity assay to overestimate results compared to our in‐house FRETS‐VWF73 assay
Specific attention was given to the intra‐assay precisions of the HemosIL AcuStar in the 0‐25 IU/dL ADAMTS13 activity bracket, critical for adequate monitoring during follow‐up. Assays run in 16 out of the 18 samples ranging between 0 and 25 IU/dL of ADAMTS13 activity showed very satisfactory intra‐assay precisions, with CV ranging from 0% to 5.6%; the 2 remaining assays showed an intra‐assay CV of 22.2% in both cases (Table 1). No contamination was detected. The overall analytic performances of the HemosIL AcuStar ADAMTS13 activity assay determined in our study and compared to FRETS‐VWF73 are reported in Table 2.
Table 1.
Intra‐assay precisions of the HemosIL AcuStar ADAMTS13 Activity assay in the low ADAMTS13 activity range (0‐25 IU/dL)
| Sample N° | ADAMTS13 activity (IU/dL) (HemosIL AcuStar ADAMTS13 activity assay) | CV (%) |
|---|---|---|
| 1 | <0.2 | N/A a |
| 2 | 1.3 | 5.6 |
| 3 | 1.6 | N/A a |
| 4 | 2.3 | 5.0 |
| 5 | 3.6 | 4.0 |
| 6 | 6.2 | 2.8 |
| 7 | 7.2 | 0.0 |
| 8 | 8.8 | 2.5 |
| 9 | 11.4 | 22.2 |
| 10 | 12.8 | 3.2 |
| 11 | 13.8 | 2.8 |
| 12 | 16.8 | 22.2 |
| 13 | 17.2 | 3.0 |
| 14 | 17.3 | 1.1 |
| 15 | 18.2 | 4.4 |
| 16 | 20.7 | 2.7 |
| 17 | 21.9 | 5.5 |
| 18 | 24.7 | 2.9 |
Every intra‐assay precision was determined on 4 consecutive testings. Internal quality controls (IQC 102 IU/dL and ICQ 49 IU/dL) were previously checked valid for each run.
Abbreviations: CV, coefficient of variation; IQC, internal quality control; N/A, not applicable.
All values were < 0.2 IU/dL.
Table 2.
Comparison of analytic performances of in‐house FRETS‐VWF73 and HemosIL AcuStar ADAMTS13 Activity assays
| FRETS‐VWF73 assay | HemosIL AcuStar ADAMTS13 Activity assay | |
|---|---|---|
| Level of automation | Highly manual | Fully automated |
| Substrate | FRETS‐VWF73 | GST‐VWF73 |
| Detection principle | Fluorescence | Chemiluminescence |
| Limit of detection | 10 IU/dL | 0.2 IU/dL |
| Contamination | Not applicable | None |
| Intra‐assay precision (CV) | 6.9% | 5.6% |
| Interassay precision (CV) | 10.2% | 9.7% |
| Assay length a | 1 hour | 33 minutes b |
Abbreviations: CV, coefficient of variation.
This duration does not include sample preparation, calibration and data analysis.
This is the time that the HemosIL AcuStar ADAMTS13 activity assay needs to assess the first sample; each subsequent sample will take an extra minute.
Eleven autofluorescent plasma samples with FRETS‐VWF73 assay and thus assessed by Technozym ADAMTS13 activity ELISA for reference, were tested using the HemosIL AcuStar ADAMTS13. No particular technical difficulty was met, and the ADAMTS13 activity values obtained with the HemosIL AcuStar ADAMTS13 activity assay appear consistent with clinical context and the Technozym ADAMTS13 activity ELISA. The specific features of these 11 patients are provided in Appendix S1, Table S1. Eighteen samples with classical interferences (hemolyzed, icteric, and/or lactescent samples) were tested (data not shown). Only two slight discrepancies were reported for one icteric sample and one icteric and lactescent sample: ADAMTS13 activity assessed by our in‐house FRETS‐VWF73 assay was < 10 IU/dL, while ADAMTS13 activity was measured at 10.5 and 11.3 IU/dL, respectively, with the HemosIL AcuStar ADAMTS13 activity assay.
3.2. Comparisons of HemosIL AcuStar ADAMTS13 activity assay and FRETS‐VWF73 assay (reference method) on plasma samples
3.2.1. Comparison of results obtained from the retrospective and the prospective evaluations
We first compared the data obtained from both sequences of the study to detect any discrepancy related to the length of storage at −80°C, which could bias our study. No significant difference was observed in the comparisons of ADAMTS13 activity between the HemosIL AcuStar ADAMTS13 activity and FRETS‐VWF73 assays in the retrospective sequence (316 plasma samples) and in the prospective sequence (223 plasma samples) of the study (Appendix S1, Figure S2). Specific scatter diagrams and Bland‐Altmann diagrams for each sequence were also analyzed. Pearson’s correlation coefficient was found at 0.90 and 0.78 for the retrospective evaluation and for the prospective evaluation, respectively, and was statistically significant in both cases (P < .0001) (Appendix S1, Figure S3). Overall, we tested 539 samples, consisting of 142 samples in the < 10 IU/dL range, 68 samples in the 10‐25 IU/dL range, 118 samples in the 25‐50 IU/dL range, 167 samples in the 50‐100 IU/dL range, 44 samples in the 100‐150 IU/dL range (Table 3). Fifteen diagnostic discrepancies were found in the overall analysis including 14 samples overestimated by the HemosIL AcusStar ADAMTS 13 activity assay: 7 were detected during the retrospective evaluation and 8 during the prospective evaluation (Table 4). These results allowed us to meld all 539 patients for a global comparison of methods.
Table 3.
Distribution of assessed plasma samples according to diagnosis (1.a) and ADAMTS13 activity using in‐house FRETS‐VWF73 assay (1.b)
| Retrospective sequence | Prospective sequence | Total | |
|---|---|---|---|
| 1.a Diagnosis | |||
| Normal subjects | 25 | 12 | 37 |
| Congenital TTP (USS) | 11 | 7 | 18 |
| aTTP at initial acute phase | 69 | 23 | 92 |
| aTTP during follow‐up (in remission or relapse) | 122 | 114 | 236 |
| Miscellaneous TMAs | 89 | 67 | 156 |
| Total | 316 | 223 | 539 |
| 1.b ADAMTS13 activity (in‐house FRETS‐VWF73 assay (IU/dL)) | |||
| <10 | 96 | 46 | 142 |
| 10‐25 | 47 | 21 | 68 |
| 25‐50 | 67 | 51 | 118 |
| 50‐100 | 85 | 82 | 167 |
| 100‐150 | 21 | 23 | 44 |
| Total | 316 | 223 | 539 |
Abbreviations: aTTP, acquired thrombotic thrombocytopenic purpura; TTP, thrombotic thrombocytopenic purpura; TMA, thrombotic microangiopathy; USS, Upshaw‐Schulman syndrome.
Table 4.
Diagnostic discrepancies between HemosIL AcuStar ADAMTS13 activity assay and in‐house FRETS‐VWF73 assessing < 10 IU/dL ADAMTS13 activity in 15 plasma samples
| Patient | ADAMTS13 activity by in‐house FRETS‐VWF73 assay (IU/dL) | ADAMTS13 activity by HemosIL AcuStar assay (IU/dL) | Anti‐ADAMTS13 IgG titers (ELISA, U/mL) (N < 15 U/mL) | Clinical context and complementary ADAMTS13 investigation |
|---|---|---|---|---|
| TMA | 11 | 5.1 | 9 | Pregnancy and systemic lupus erythematosus |
| aTTP – acute phase | <10 | 12 | 11 | Liver transplantation |
| aTTP – acute phase | <5 | 17 | 24 | Hepatitis B, hepatocellular carcinoma, and kidney transplantation |
| aTTP – relapse | <10 | 13 | 7 | None |
| Congenital TTP (USS) | <5 | 13 | 10 | Exon 3: c.262G > C (p.Val88Leu) Exon 24: c.3178C > T (p.Arg1060Trp) |
| aTTP – acute phase | <5 | 22 | 5 | Pregnancy |
| aTTP – acute phase | <10 | 17 | 6 | Pregnancy |
| aTTP – acute phase | <10 | 26 | 10 | None |
| aTTP – relapse | <5 | 29 | 12 | None |
| aTTP – acute phase | <10 | 16 | 16 | None |
| TMA a (icteric and lactescent sample) | <10 | 11 | 1 | Hematopoietic stem cell transplantation and Escherichia coli O157:H7 infection |
| aTTP – relapse | <5 | 13 | 7 | None |
| aTTP – relapse | <5 | 11 | 27 | Pregnancy |
| TMA* | <5 | 28 | 21 | Tuberculosis |
| TMA a (icteric sample) | <10 | 11 | 2 | Heart failure with congestive hepatopathy |
Abbreviations: aTTP, acquired thrombotic thrombocytopenic purpura, TMA, thrombotic microangiopathy; TTP, thrombotic thrombocytopenic purpura; USS, Upshaw‐Schulman syndrome.
Although ADAMTS13 activity was < 10 IU/dL with FRETS‐VWF73 assay, clinical context led to a diagnosis of TMA with a specific severe functional deficiency in ADAMTS13, that is, TTP‐like syndrome.
3.2.2. Global comparison of methods
Data from both sequences were melded, leading to a total of 539 plasma samples. The distribution of tested samples according to ADAMTS13 activity assessed by the reference method is provided in Table 3 and shows that our cohort represents extensively all values between < 10 and 150 IU/dL of ADAMTS13 activity. The comparison is represented (i) with a linear regression curve and (ii) with a Bland‐Altmann graphic, which are both shown in Figure 1. Pearson’s correlation coefficient was calculated at 0.83 (P < .0001), which is very satisfactory. Bland‐Altmann plot show a bias at 8.3, indicating a tendency to overestimate ADAMTS13 activity compared to FRETS‐VWF73.
Similarly, as for analytic performances shown above, specific attention was given to the comparison of both assays in the critical 0‐25 IU/dL ADAMTS13 activity bracket, involving 210 samples (Figure 2). Pearson’s correlation coefficient proved again to be satisfactory at 0.78 (P < .0001). Interestingly, the Bland‐Altman plot shows a bias of 4.8 IU/dL. Since the comparison with fixed values of 5 IU/dL and 0 IU/dL for < 10 IU/dL ADAMTS13 activity assessed by FRETS‐VWF73 could induce a bias, we also performed the statistical analysis by excluding values of ADAMTS13 activity < 10 IU/dL (data not shown). Indeed, when excluding < 10 IU/dL ADAMTS13 activity values assessed by FRETS‐VWF73, r 2 was found at .71 (P < .0001) and when excluding < 10 IU/dL ADAMTS13 activity values assessed by HemosIL AcuStar ADAMTS13 activity assay, r 2 was found at .73 (P < .0001).
Figure 2.
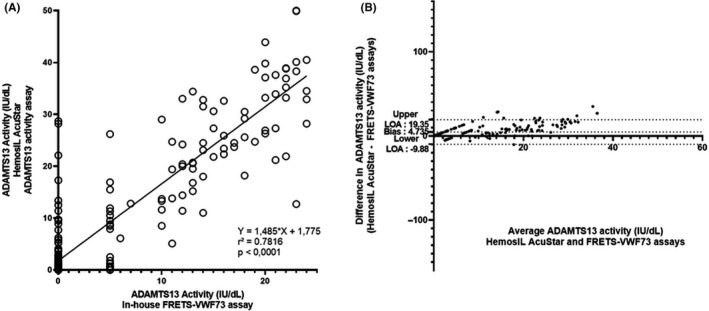
Scatter diagram (A) and Bland‐Altman plot (B) illustrating the comparison of in‐house FRETS‐VWF73 assay (reference method) and HemosIL AcuStar ADAMTS13 activity assay (evaluated method) for the measurement of ADAMTS13 activity for 210 citrated plasma samples with ADAMTS13 activity < 25 IU/dL as assessed by in‐house FRETS‐VWF73 assay. For the statistical analysis, values were respectively set to 5 IU/dL and 0 IU/dL if ADAMTS13 activity was determined < 10 IU/dL with a detectable RFU and < 5 IU/dL with our in‐house FRETS‐VWF73 assay. Each sample is represented as a circle Pearson’s correlation coefficient (r 2) was found at .7816 (P < .0001), and fitted regression line was Y = 1.485X + 1.775. The Bland‐Altmann plot illustrates the comparison of the HemosIL AcuStar ADAMTS13 activity assay to our in‐house FRETS‐VWF73. Each sample is represented by a dot. The Bland‐Altman plot specifically targeting citrated plasmas with ADAMTS13 activity < 25 IU/dL assessed by our in‐house FRETS‐VWF73 assay reveals a lower mean bias, at 4.735 IU/dL, and narrower limits of agreement (−9.88 IU/dL; +19.35 IU/dL) compared to the analysis conducted on all plasmas (0‐150 IU/dL). This result suggests that (i) the variability is far less important between both assays in the measurement of ADAMTS13 activity in the 0‐25 IU/dL range; (ii) the HemosIL AcuStar ADAMTS13 Activity assay tends to overestimate results less compared to our in‐house FRETS‐VWF73 assay in the 0‐25 IU/dL range
The diagnostic value of the HemosIL AcuStar ADAMTS13 activity for acute TTP proved excellent, with a sensitivity of 90.1%, a specificity of 99.7%, a PPV of 99.2%, an NPV of 96.6%, and a kappa coefficient of 0.93 (Table 5). Fifteen discrepancies were detected: 14 FN and 1 FP (Table 4). The distribution of samples tested as a function of ADAMTS13 activity assessed by HemosIL AcuStar ADAMTS13 activity assay and of clinical context is provided in Appendix S1, Figure S4.
Table 5.
Diagnostic performance for acute phase of TTP of the HemosIL AcuStar ADAMTS13 Activity assay in 539 plasma samples
| FRETS‐VWF73 | |||
|---|---|---|---|
| <10 IU/dL (n = 142) | ≥ 10 IU/dL (n = 397) | ||
| HemosIL AcuStar ADAMTS13 Activity assay | <10 IU/dL (n = 129) | 128 | 1 |
| ≥ 10 IU/dL (n = 410) | 14 | 396 | |
| Sensitivity (%) | 90.1 | ||
| Specificity (%) | 99.7 | ||
| Positive predictive value (%) | 99.2 | ||
| Negative predictive value (%) | 96.6 | ||
| Cohen’s kappa coefficient | 0.93 | ||
3.3. Comparison of citrated plasma versus serum samples on HemosIL AcuStar ADAMTS13 activity assay
While the manufacturer validated the HemosIL AcuStar ADAMTS13 activity assay only on plasma, we sought to investigate whether this assay could be performed on serum. An ancillary study was conducted on 100 plasma and serum couples drawn from the same blood collection in 100 patients (Figure 3). Pearson’s correlation coefficient was calculated at 0.79 (P < .0001). The bias calculated using Bland‐Atmann plot was found at 4.3, indicating a mild tendency of overestimation when assessing on serum compared to citrated plasma. This result seems consistent with the 1/10 dilution of plasma sample given that citrated plasma tubes contain a liquid anticoagulant. Thirteen citrated plasma samples had an ADAMTS13 activity < 10 IU/dL assessed by HemosIL AcuStar ADAMTS13 activity assay: agreement regarding < 10 IU/dL between plasma and serum samples was perfect. Additionally, 24 citrated plasma samples had an ADAMTS13 activity < 25 IU/dL; after correlation with their serum counterparts, Pearson’s correlation coefficient was found at 0.73 (P < .0001) (data not shown).
Figure 3.
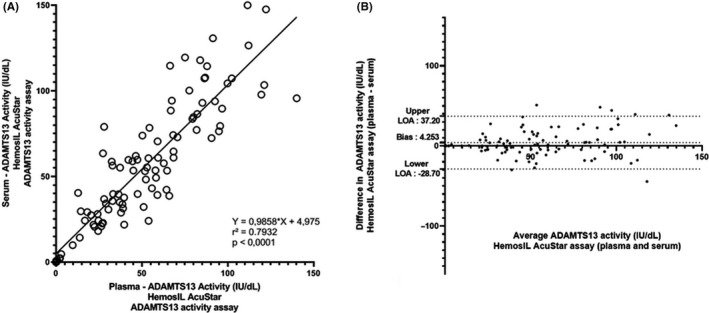
Scatter diagram (A) and Bland‐Altman (B) plot illustrating the comparison of ADAMTS13 activity measured in 100 plasma and serum couples using HemosIL AcuStar ADAMTS13 activity assay. One hundred citrated plasma and serum couples drawn from the same blood collection from 100 disctinct patients were tested with the HemosIL ADAMTS13 activity assay and compared, with citrated plasma as the reference. Thirteen patients had undetectable (<10 IU/dL) ADAMTS13 activity on plasma samples. Agreement with serum counterparts for evidencing an undetectable (<10 IU/dL) ADAMTS13 activity was perfect. Each sample is represented as a circle on the scattered diagram. Pearson's correlation coefficient (r 2) was found at .7932 (P < .0001), and fitted regression line was Y = 0.98588X + 4.975. The Bland‐Altman plot illustrates the comparison of citrated plasma and serum on the measurement of ADAMTS13 activity assessed by the HemosIL® AcuStar ADAMTS13 activity assay. Each sample is represented by a dot. The Bland‐Altman plot reveals a mean bias of 4.253 IU/dL and quite large limits of agreement (−28.70 IU/dL; +37.20 IU/dL), indicating an overall tendency of the HemosIL AcuStar ADAMTS13 Activity assay to slightly overestimate results on serum compared to citrated plasma
4. DISCUSSION
In the current study, we evaluated the HemosIL AcuStar ADAMTS13 activity assay, a new automated, fast chemiluminescent assay for the measurement of ADAMTS13 activity. Analytic performances, robustness in the low detectable range of ADAMTS13 activity (0‐25 IU/dL) including the assessment of undetectable levels (< 10 IU/dL), and global comparison with our referent in‐house FRETS‐VWF73 assay conducted on 539 citrated plasma sample showed very good results. An ancillary study using 100 plasma‐serum couples, also showed that this assay, calling for citrated plasma according to the manufacturer’s instructions, could also be performed on serum.
Three previous studies evaluating the HemosIL AcuStar ADAMTS13 activity assay were published. The first one, by Favresse et al, 42 was a retrospective study comparing the HemosIL AcuStar ADAMTS13 activity assay to the Technozym ADAMTS13 activity ELISA on 38 citrated plasmas samples (including eight patients with TTP) and concluded favorably, highlighting the good diagnostic performances and benefits from automation of the HemosIL AcuStar ADAMTS13 activity assay. Valsecchi et al 40 conducted a thorough evaluation and compared, through a design similar to ours with a retrospective and prospective sequence, the HemosIL AcuStar ADAMTS13 activity assay to the Technozym ADAMTS13 activity ELISA and to their in‐house FRETS‐VWF73 on a total of 176 citrated plasma samples (including 110 TTP samples). This study, benefiting from a large cohort, also concluded very positively on the analytic and diagnostic performances of the HemosIL AcuStar ADAMTS13 activity, notably reporting a kappa coefficient of 0.97 and only two discrepancies in terms of assessing undetectable (< 10 IU/dL) ADAMTS13 activity compared to their in‐house FRETS‐VWF73. Finally, Stratmann et al 41 published a third study comparing the HemosIL AcuStar ADAMTS13 activity assay to the Technozym ADAMTS13 activity ELISA on a total of 24 citrated plasma samples, confirming the practicability of automation for the measurement of ADAMTS13 activity and the good diagnostic performances of this assay.
Our results on the diagnostic performances of the HemosIL AcuStar ADAMTS13 activity assay are largely consistent with these previous findings, although our findings, based on a very large cohort of 539 citrated plasma samples, show more discrepancies than the previous studies. 40 , 41 , 42 While most parameters appear very satisfactory (specificity, PPV and NPV, and kappa coefficient), the sensitivity of the HemosIL AcuStar ADAMTS13 activity assay for acute‐phase TTP was found at 90.1%, indicating the possibility of FN results. Our study reports 14 patients misdiagnosed out of a total of 142 patients (9.8%) with undetectable ADAMTS13 activity, 11 of which were acute‐phase TTP, including 1 congenital TTP (Table 4). This apparent discrepancy compared to the other studies can be partly explained by the very large cohort of samples we studied. Moreover, our reference method was FRETS‐VWF73 assay and not the Technozym ADAMTS13 activity ELISA, an assay previously evaluated by our team and found to provide FN results in 12% of patients with acute TTP 33 . It is also noteworthy that among the 14 cases in which the HemosIL AcuStar ADAMTS13 activity assay is discrepant with FRETS‐VWF73 assay for identifying undetectable (<10 IU/dL) ADAMTS13 activity, 3 were complex TMAs with unspecific severe functional deficiency in ADAMTS13, or TTP‐like syndrome. One of those samples was slightly icteric and another was slightly icteric and lactescent, which is of interest given that icteric samples can lead to inaccurate results with FRETS‐VWF73. 45 However, whether these interferences significantly affected the measurement of ADAMTS13 activity with the HemosIL AcuStar ADAMTS13 Activity assay is uncertain, as other samples with interferences did not appear to be significantly impacted. Diagnostic performances for acute‐phase TTP are very satisfactory in terms of specificity (99.7%) and PPV (99.2%), indicating that the HemosIL AcuStar ADAMTS13 assay is very unlikely to provide a FP result (only one patient in our study, a complex TMA in a pregnant patient with systemic lupus erythematosus). While our study includes a relatively high number of samples, further time and experience with the HemosIL AcuStar ADAMTS 13 Activity assay are needed to find a perfect strategy balancing its ease of use and diagnostic performances. Reference methods using both full‐length VWF and VWF73 substrates should therefore be kept for backup if clinical presentation, including the French and/or PLASMIC score, and ADAMTS13 assessed by the HemosIL AcuStar ADAMTS13 activity assay appear discrepant.
Other original features of our study are the extensive evaluation of the analytic performances of the HemosIL AcuStar ADAMTS13 activity assay and of the robustness of this assay in the low detectable ADAMTS13 activity range (0‐25 IU/dL). This bracket is crucial for both adequate diagnosis and follow‐up supporting either caplacizumab‐driven therapy or preemptive rituximab‐driven therapy but remains critically difficult to assess robustly and precisely with currently available assays. Another specific end point of this work was also to determine whether this assay could be performed on serum to better suit real‐life needs.
While clinical scores such as the FRENCH score 46 or the PLASMIC score 47 have significantly made TTP detection easier, differential diagnosis with other TMAs or coagulopathies can be challenging, and rapid results could accelerate initial diagnosis. The excellent analytical performances of this new assay are combined with a time of execution of 1 hour for a run including 25 samples, which could make ADAMTS13 activity available faster for clinical care and improve initial management. 48 Providing rapidly available results should nevertheless not be done at the expense of their reliability, and time should be taken for IQC, technical, and medical validation, ensuring that correct results are provided to physicians for clinical care. In terms of benefit for current medical practice, the comparison for plasma samples of HemosIL AcuStar ADAMTS13 activity assay and FRETS‐VWF73 showed satisfactory correlation overall and lack of interferences, in addition to its ease of use compared to FRETS‐VWF73, a laborious time‐consuming and expertise‐requiring technique. Moreover, calibration must be performed each time the reagent lot is changed and not every time testing is performed. Linearity and interassay precision are excellent, and no contamination was detected in this automated system. We chose to work with the manufacturer’s IQC for interassay precision and with our usual supplier for intra‐assay precision to check whether working with the manufacturer’s IQC would result in better quality controls, which was not the case.
Analytical robustness in the low detectable ADAMTS13 activity range is also noticeably better compared to FRETS‐VWF73, and the 2 out of 18 intra‐assay precisions that are much higher (22.2% for both) appear to be overestimated by a random error, as the other values are much lower. This could allow for more standardized and reliable monitoring of TTP patients in remission, particularly for patients with low detectable ADAMTS13 activity, and for a more rapid identification of relapsing patients calling for preemptive rituximab. This assay also appears adequate for caplacizumab‐driven therapy, given that a yet‐to‐be‐determined cutoff ADAMTS13 activity level (likely around 20‐25 IU/dL) could determine the continuation or discontinuation of caplacizumab. The determination of this cutoff ADAMTS13 activity level could be a determining parameter for the overall cost‐effectiveness of caplacizumab in TTP.
The HemosIL AcuStar ADAMTS13 activity assay has a much lower limit of detection than the gold standard FRETS‐VWF73. The ADAMTS13 activity threshold value for the diagnosis of acute‐phase TTP is < 10 IU/dL, but the choice of this cutoff is closely related to the limit of detection of previously available methods. While acute‐phase acquired TTP tends to have very low (<5 IU/dL) ADAMTS13 activity, it appears that relapsing patients or patients with USS display a somewhat higher ranger of ADAMTS13 activity (between 5 and 10 IU/dL) (data not shown). Within the < 10 IU/dL range, the comparison of the clinical context and the ADAMTS13 activity measured by the HemosIL ADAMTS13 activity remains to be performed. This new method offers a much lower limit of detection, which could in turn lower the threshold for TTP diagnosis and incidentally lower the threshold for initiating preemptive rituximab. The 10 IU/dL threshold for preemptive rituximab remains up for debate. This new assay could help identify a more adequate threshold and separate patients with < 10 IU/dL ADAMTS13 activity into a group with ADAMTS13 activity closer to 0.2 IU/dL who would benefit from preemptive treatment and into another group with ADAMTS13 activity closer to 10 IU/dL for whom the risk‐benefit balance might lean toward monitoring alone. In addition, it greatly improves the possibility of studying USS samples for genotype‐phenotype studies, which were so far hindered by the high limit of detection of reference methods. Our study, the first to include USS, reports notably an adult patient with USS with pregnancy‐onset TTP that was misdiagnosed with the HemosIL AcuStar ADAMTS13 activity assay at 13 IU/dL. Finally, regarding the use of serum in addition to citrated plasma, ADAMTS13 activity assay is performed in limited expert laboratories, sometimes covering a wide geographic area. Preanalytical handling and transport of aliquoted frozen citrated plasma can be difficult in such conditions, with the risk of faulty results if mishandled. Serum samples are easier to handle and already regularly used for that matter, leading our team to investigate the use of serum for performing FRETS‐VWF73. The AcuStar is indeed an automated system capable of performing many specialized hemostasis assays on plasma or on serum samples, for example, antiphospholipid antibodies. While reports that thrombin generated during the clotting process may degrade ADAMTS13 have been published, 49 our data show that testing on serum does not provide FP results. Moreover, our real‐life experience of measuring ADAMTS13 on serum rather than plasma show identical diagnostic performance. Serum testing should, however, be performed with caution until further validation has been performed. Finally, since EDTA completely eliminates the enzyme activity, EDTA plasma specimens are absolutely not to be used.
The HemosIL AcuStar ADAMTS13 activity assay appears very reliable and easy to perform. This method could thus replace current techniques as a first‐line assay for both TTP initial diagnosis and follow‐up, with an obvious benefit for therapy‐driven monitoring. However, confrontation with clinical data remains crucial, and if ADAMTS13 activity appears to conflict with clinical presentation, it must be controlled with a reference method in experienced laboratories.
AUTHOR CONTRIBUTIONS
NB performed the biological part of the study, collected clinical data, analyzed and interpreted the results, and wrote the manuscript. SB performed the technical/biological part of the study, analyzed the data, and critically reviewed the manuscript. BJ and AS supervised the biological study, interpreted the results, and critically reviewed the manuscript. SC and AD collected clinical data and critically reviewed the manuscript. PC supervised the patients’ enrollment, codesigned the study, and critically reviewed the manuscript. AV designed and supervised the global study, interpreted the results, and critically reviewed the manuscript. All authors approved the final version of the manuscript.
RELATIONSHIP DISCLOSURE STATEMENT
PC is member of the advisory board for Sanofi, Takeda, Roche, and Alexion. AV is a member of the French advisory boards for Sanofi, Takeda, and Roche‐Chugai. NB, SB, SC, AD, BJ, and AS declare no conflict of interest.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
The authors thank all the members of the Reference Center for Thrombotic Microangiopathies (CNR‐MAT): Augusto Jean‐François (Service de Néphrologie, dialyse et transplantation ; CHU Larrey, Angers); Azoulay Elie (Service de Réanimation Médicale, Hôpital Saint‐Louis, Paris); Barbay Virginie (Laboratoire d'Hématologie, CHU Charles Nicolle, Rouen); Benhamou Ygal (Service de Médecine Interne, CHU Charles Nicolle, Rouen); Bordessoule Dominique (Service d'Hématologie, Hôpital Dupuytren, Limoges); Charasse Christophe (Service de Néphrologie, Centre Hospitalier de Saint‐Brieuc); Charvet‐Rumpler Anne (Service d'Hématologie, CHU de Dijon) ; Chauveau Dominique (Service de Néphrologie et Immunologie Clinique, CHU Rangueil, Toulouse); Choukroun Gabriel (Service de Néphrologie, Hôpital Sud, Amiens); Coindre Jean‐Philippe (Service de Néphrologie, CH Le Mans); Coppo Paul (Service d'Hématologie, Hôpital Saint‐Antoine, Paris); Corre Elise (Service d'Hématologie, Hôpital Saint‐Antoine, Paris); Delmas Yahsou (Service de Néphrologie, CHU de Bordeaux, Bordeaux); Deschenes Georges (Service de Néphrologie Pédiatrique, Hôpital Robert Debré, Paris); Devidas Alain (Service d'Hématologie, Hôpital Sud‐Francilien, Corbeil‐Essonnes); Dossier Antoine (Service de Néphrologie, Hôpital Bichat, Paris); Fain Olivier (Service de Médecine Interne, Hôpital Saint‐Antoine, Paris); Fakhouri Fadi (Service de Néphrologie, CHU Hôtel‐Dieu, Nantes) ; Frémeaux‐Bacchi Véronique (Laboratoire d'Immunologie, Hôpital Européen Georges Pompidou, Paris); Galicier Lionel (Service d'Immunopathologie, Hôpital Saint‐Louis, Paris); Grangé Steven (Service de Réanimation Médicale, CHU Charles Nicolle, Rouen) ; Guidet Bertrand (Service de Réanimation Médicale, Hôpital Saint‐Antoine, Paris); Halimi Jean‐Michel (Service de Néphrologie Pédiatrique, Hôpital Bretonneau, Tours); Hamidou Mohamed (Service de Médecine Interne, Hôtel‐Dieu, Nantes); Herbrecht Raoul (service d'Oncologie et d'Hématologie, Hôpital de Hautepierre, Strasbourg); Hié Miguel (Service de Médecine Interne, Groupe Hospitalier Pitié‐Salpétrière, Paris) ; Jacobs Frédéric (Service de Réanimation Médicale, Hôpital Antoine Béclère, Clamart); Joly Bérangère (Service d'Hématologie Biologique, Hôpital Lariboisière, Paris) ; Kanouni Tarik (Unité d'Hémaphrèse, Service d'Hématologie, CHU de Montpellier) ; Kaplanski Gilles (Service de Médecine Interne, Hôpital la Conception, Marseille) ; Lautrette Alexandre (Hôpital Gabriel Montpied, Service de Réanimation médicale, Clermont‐Ferrand); Le Guern Véronique (Unité d'Hémaphérèse, Service de Médecine Interne, Hôpital Cochin, Paris) ; Loirat Chantal (Service de Néphrologie Pédiatrique, Hôpital Robert Debré, Paris); Moulin Bruno (Service de Néphrologie, Hôpital Civil, Strasbourg); Mousson Christiane (Service de Néphrologie, CHU de Dijon); Ojeda Uribe Mario (Service d'Hématologie, Hôpital Emile Muller, Mulhouse); Ouchenir Abdelkader (Service de Réanimation, Hôpital Louis Pasteur, Le Coudray); Parquet Nathalie (Unité de Clinique Transfusionnelle, Hôpital Cochin, Paris); Peltier Julie (Urgences Néphrologiques et Transplantation Rénale, Hôpital Tenon, Paris) ; Pène Frédéric (Service de Réanimation Médicale, Hôpital Cochin, Paris) ; Perez Pierre (Service de Réanimation polyvalente, CHU de Nancy) ; Poullin Pascale (Service d'hémaphérèse et d'autotransfusion, Hôpital la Conception, Marseille); Pouteil‐Noble Claire (Service de Néphrologie, CHU Lyon‐Sud, Lyon); Presne Claire (Service de Néphrologie, Hôpital Nord, Amiens); Provôt François (Service de Néphrologie, Hôpital Albert Calmette, Lille); Rondeau Eric (Urgences Néphrologiques et Transplantation Rénale, Hôpital Tenon, Paris); Saheb Samir (Unité d'Hémaphérèse, Hôpital la Pitié‐Salpétrière, Paris) ; Schlemmer Benoît (Service de Réanimation Médicale, Hôpital Saint‐Louis, Paris); Seguin Amélie (Service de Réanimation Médicale, centre hospitalier de Vendée) ; Servais Aude (Service de Néphrologie, CHU Necker‐Enfants Malades) ; Stépanian Alain (Laboratoire d'Hématologie, Hôpital Lariboisière, Paris); Vernant Jean‐Paul (Service d'Hématologie, Hôpital la Pitié‐Salpétrière, Paris); Veyradier Agnès (Service d'Hématologie Biologique, Hôpital Lariboisière, Paris); Vigneau Cécile (Service de Néphrologie, Hôpital Pontchaillou, Rennes); Wynckel Alain (Service de Néphrologie, Hôpital Maison Blanche, Reims); and Zunic Patricia (Service d'Hématologie, Groupe Hospitalier Sud‐Réunion, la Réunion).
Beranger N, Benghezal S, Joly BS, et al. Diagnosis and follow‐up of thrombotic thrombocytopenic purpura with an automated chemiluminescent ADAMTS13 activity immunoassay. Res Pract Thromb Haemost.2021;5:81–93. 10.1002/rth2.12461
Nicolas Beranger and Sandrine Benghezal contributed equally to this paper.
Handling Editor: Dr Neil Zakai
Funding information This work was partly funded by a grant from the Délégation Régionale à la Recherche Clinique, Assistance Publique‐Hôpitaux de Paris (PHRC AOM05012). This work was also partly supported by the French National Plan for Rare Diseases of the French Ministry of Health.
REFERENCES
- 1. Furlan M, Robles R, Galbusera M, Remuzzi G, Kyrle PA, Brenner B, et al. Von Willebrand factor–cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic‐uremic syndrome. N Engl J Med. 1998;339:1578–84. [DOI] [PubMed] [Google Scholar]
- 2. Sadler JE. Pathophysiology of thrombotic thrombocytopenic purpura. Blood. 2017;130:1181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mariotte E, Azoulay E, Galicier L, Rondeau E, Zouiti F, Boisseau P, et al. The French Reference Center for Thrombotic Microangiopathies. Epidemiology and pathophysiology of adulthood‐onset thrombotic microangiopathy with severe ADAMTS13 deficiency (thrombotic thrombocytopenic purpura): a cross‐sectional analysis of the French national registry for thrombotic microangiopathy. Lancet Haematol. 2016;3:237–45. [DOI] [PubMed] [Google Scholar]
- 4. Tsai H‐M, Lian EC. Antibodies to Von Willebrand factor–cleaving protease in acute TTP. N Engl J Med. 1998;339:1585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, Mcgee BM, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488–94. [DOI] [PubMed] [Google Scholar]
- 6. Coppo P, Bengoufa D, Veyradier A, Wolf M, Bussel A, Millot GA, et al. Severe ADAMTS13 deficiency in adult idiopathic thrombotic microangiopathies defines a subset of patients characterized by various autoimmune manifestations, lower platelet count, and mild renal involvement. Medicine (Baltimore). 2004;83:233–44. [DOI] [PubMed] [Google Scholar]
- 7. Morgand M, Buffet M, Busson M, Loiseau P, Malot S, Amokrane K, et al. High prevalence of infectious events in thrombotic thrombocytopenic purpura and genetic relationship with toll‐like receptor 9 polymorphisms: experience of the French Thrombotic Microangiopathies Reference Center. Transfusion. 2014;54:389–97. [DOI] [PubMed] [Google Scholar]
- 8. Scully M, Thomas M, Underwood M, Watson H, Langley K, Camilleri RS, et al. Thrombotic thrombocytopenic purpura and pregnancy : presentation, management, and subsequent pregnancy outcomes. Blood. 2014;124:211–20. [DOI] [PubMed] [Google Scholar]
- 9. Moatti‐Cohen M, Garrec C, Wolf M, Boisseau P, Galicier L, Azoulay E, et al. Unexpected frequency of Upshaw‐Schulman syndrome in pregnancy‐onset thrombotic thrombocytopenic purpura. Blood. 2012;119:5888–97. [DOI] [PubMed] [Google Scholar]
- 10. Page EE, Kremer Hovinga JA, Terrell DR, Vesely SK, George JN. Clinical importance of ADAMTS13 activity during remission in patients with acquired thrombotic thrombocytopenic purpura. Blood. 2016;128:2175–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129:2836–46. [DOI] [PubMed] [Google Scholar]
- 12. Scully M, Hunt BJ, Benjamin S, Liesner R, Rose P, Peyvandi F, et al. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol. 2012;158:323–35. [DOI] [PubMed] [Google Scholar]
- 13. Bell WR, Ness PM, Kickler TS, Braine HG. Improved survival in thrombotic thrombocytopenic purpura–hemolytic uremic syndrome: clinical experience in 108 patients. N Engl J Med. 1991;325:398–403. [DOI] [PubMed] [Google Scholar]
- 14. Balduini CL, Gugliotta L, Luppi M, Laurenti L, Klersy C, Pieresca C, et al. High versus standard dose methylprednisolone in the acute phase of idiopathic thrombotic thrombocytopenic purpura: a randomized study. Ann Hematol. 2010;89:591–6. [DOI] [PubMed] [Google Scholar]
- 15. Froissart A, Buffet M, Veyradier A, Poullin P, Provôt F, Malot S, et al. Efficacy and safety of first‐line rituximab in severe, acquired thrombotic thrombocytopenic purpura with a suboptimal response to plasma exchange. Experience of the French Thrombotic Microangiopathies Reference Center. Crit Care Med. 2012;40:104–11. [DOI] [PubMed] [Google Scholar]
- 16. Scully M, McDonald V, Cavenagh J, Hunt BJ, Longair I, Cohen H, et al. A phase 2 study of the safety and efficacy of rituximab with plasma exchange in acute acquired thrombotic thrombocytopenic purpura. Blood. 2011;118:1746–53. [DOI] [PubMed] [Google Scholar]
- 17. Peyvandi F, Duby C. Caplacizumab, Anti‐VWF nanobody potentially changing the treatment paradigm in thrombotic thrombocytopenic purpura: results of the TITAN trial. Blood. 2014;124:229.24850757 [Google Scholar]
- 18. Peyvandi F, Scully M, Kremer Hovinga JA, Cataland S, Knöbl P, Wu H, et al. Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2016;374:511–22. [DOI] [PubMed] [Google Scholar]
- 19. Scully M, Cataland SR, Peyvandi F, Coppo P, Knöl P, Kremer Hovinga JA, et al. Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2019;380:335–46. [DOI] [PubMed] [Google Scholar]
- 20. Veyradier A, Obert B, Houllier A, Meyer D, Girma JP. Specific von Willebrand factor–cleaving protease in thrombotic microangiopathies: a study of 111 cases. Blood. 2001;98:1765–72. [DOI] [PubMed] [Google Scholar]
- 21. Peyvandi F, Lavoretano S, Palla R, Feys HB, Vanhoorelbeke K, Battaglioli T, et al. ADAMTS13 and anti‐ADAMTS13 antibodies as markers for recurrence of acquired thrombotic thrombocytopenic purpura during remission. Haematologica. 2008;93:232–9. [DOI] [PubMed] [Google Scholar]
- 22. Kremer Hovinga JA, Vesely SK, Terrell DR, Lämmle B, George JN. Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2010;115:1500–11. [DOI] [PubMed] [Google Scholar]
- 23. Jin M, Casper TC, Cataland SR, Kennedy MS, Lin S, Li YJ, et al. Relationship between ADAMTS13 activity in clinical remission and the risk of TTP relapse. Br J Haematol. 2008;141:651–8. [DOI] [PubMed] [Google Scholar]
- 24. Callewaert F, Ulrichts H, Kremer Hovinga JA, De Swert K, Tersago D. The predictive value of ADAMTS13 activity for treatment monitoring of patients with acquired TTP: data from the phase II TITAN trial with caplacizumab. J Thromb haemostasis. 2015;13:24. [Google Scholar]
- 25. Studt JD, Böhaa M, Budde U, Girma JP, Varadi K, Lämmle B. Measurement of von Willebrand factor–cleaving protease (ADAMTS‐13) activity in plasma: a multicenter comparison of different assay methods. J Thromb Haemost. 2003;1:1882–7. [DOI] [PubMed] [Google Scholar]
- 26. Peyvandi F, Palla R, Lotta LA, Mackie I, Scully MA, Machin SJ. ADAMTS‐13 assays in thrombotic thrombocytopenic purpura. J Thromb Haemost. 2010;8:631–40. [DOI] [PubMed] [Google Scholar]
- 27. Tripodi A, Peyvandi F, Chantarangkul V, Palla R, Afrasiabi A, Canciani MT, et al. Second international collaborative study evaluating performance characteristics of methods measuring the von Willebrand factor cleaving protease (ADAMTS‐13). J Thromb Haemost. 2008;6:1534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Remuzzi G, Galbusera M, Noris M, Canciani MT, Daina E, Bresin E, et al. Von Willebrand factor cleaving protease (ADAMTS13) is deficient in recurrent and familial thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. Blood. 2002;100:778–85. [DOI] [PubMed] [Google Scholar]
- 29. Furlan M, Robles R, Solenthaler M, Lämmle B. Acquired deficiency of von Willebrand factor–cleaving protease in a patient with thrombotic thrombocytopenic purpura. Blood. 1998;91:2839–46. [PubMed] [Google Scholar]
- 30. Gerritsen HE, Turecek PL, Schwarz HP, Lämmle B, Furlan M. Assay of von Willebrand factor (vWF)‐cleaving protease based on decreased collagen binding affinity of degraded vWF. A tool for the diagnosis of thrombotic thrombocytopenic purpura (TTP). Thromb Haemost. 1999;82:1386–9. [PubMed] [Google Scholar]
- 31. Böhm M, Vigh T, Scharrer I. Evaluation and clinical application of a new method for measuring activity of von Willebrand factor–cleaving metalloprotease (ADAMTS13). Ann Hematol. 2002;81:430–5. [DOI] [PubMed] [Google Scholar]
- 32. Obert B, Tout H, Veyradier A, Fressinaud E, Meyer D, Girma JP. Estimation of the von Willebrand factor–cleaving protease in plasma using monoclonal antibodies to vWF. Thromb Haemost. 1999;82:1382–5. [PubMed] [Google Scholar]
- 33. Joly BS, Stepanian A, Hajage D, Thouzeau S, Capdenat S, Coppo P, et al. Evaluation of a chromogenic commercial assay using VWF‐73 peptide for ADAMTS13 activity measurement. Thromb Res. 2014;134:1074–80. [DOI] [PubMed] [Google Scholar]
- 34. Mackie I, Langley K, Chitolie A, Liesner R, Scully M, Machin S, et al. Discrepancies between ADAMTS13 activity assays in patients with thrombotic microangiopathies. Thromb Haemost. 2013;109:488–96. [DOI] [PubMed] [Google Scholar]
- 35. Thouzeau S, Capdenat S, Stepanian A, Coppo P, Veyradier A. Evaluation of a commercial assay for ADAMTS13 activity measurement. Thromb Haemost. 2013;110:852–3. [DOI] [PubMed] [Google Scholar]
- 36. Jin M, Cataland S, Bissell M, Wu HM. A rapid test for the diagnosis of thrombotic thrombocytopenic purpura using surface enhanced laser desorption/ionization time‐of‐flight (SELDI‐TOF)‐mass spectrometry. J Thromb Haemost. 2006;4:333–8. [DOI] [PubMed] [Google Scholar]
- 37. Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS‐VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005;129:93–100. [DOI] [PubMed] [Google Scholar]
- 38. Mirabet M, Blanch S, Puig J, Serra J. Highly sensitive fully automated chemiluminescent immunoassay for rapid quantification of ADAMTS13 activity. Res Pract Thromb Haemost. 2017;1:1297. [Google Scholar]
- 39. Kato S, Matsumoto M, Matsuyama T, Isonishi A, Hiura H, Fujimura Y. Novel monoclonal antibody‐based enzyme immunoassay for determining plasma levels of ADAMTS13 activity. Transfusion. 2006;46:1444–52. [DOI] [PubMed] [Google Scholar]
- 40. Valsecchi C, Mirabet M, Mancini I, Biganzoli M, Schiavone L, Faraudo S, et al. Evaluation of a new, rapid, fully automated assay for the measurement of ADAMTS13 activity. Thromb Haemost. 2019;119:1767–72. [DOI] [PubMed] [Google Scholar]
- 41. Stratmann J, Ward JN, Miesbach W. Evaluation of a rapid turn‐over, fully‐automated ADAMTS13 activity assay: a method comparison study. J Thromb Thrombolysis. 2020;50(3):628–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Favresse J, Lardinois B, Chatelain B, Jacqmin H, Mullier F. Evaluation of the fully automated HemosIL Acustar ADAMTS13 Activity assay. Thromb Haemost. 2018;118:942–4. [DOI] [PubMed] [Google Scholar]
- 43. Joly BS, Stepanian A, Leblanc T, Hajage D, Chambost H, Harambat J, et al. The French Reference Center for Thrombotic Microangiopathies. Child‐onset and adolescent‐onset acquired thrombotic thrombocytopenic purpura with severe ADAMTS13 deficiency : a cohort study of the French national registry for thrombotic microangiopathy. Lancet Haematol. 2016;3026:1–10. [DOI] [PubMed] [Google Scholar]
- 44. Hubbard AR, Heath AB, Kremer Hovinga JA. Establishment of the WHO 1st International Standard ADAMTS13, plasma (12/252): Communication from the SSC of the ISTH. J Thromb Haemost. 2015;13:1151–3. [DOI] [PubMed] [Google Scholar]
- 45. Lu RN, Yang S, Wu HM, Zheng XL. Unconjugated bilirubin inhibits proteolytic cleavage of von Willebrand factor by ADAMTS13 protease. J Thromb Haemost. 2015;13:1064–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Benhamou Y, Assie C, Boelle P‐Y, Buffet M, Grillberger R, Malot S, et al. Development and validation of a predictive model for death in acquired severe ADAMTS13 deficiency‐associated idiopathic thrombotic thrombocytopenic purpura: The French TMA Reference Center experience. Haematologica. 2012;97:1181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bendapudi PK, Li A, Hamdan A, Fry AM, Uhl L, Marques M, et al. Derivation and prospective validation of a predictive score for the rapid diagnosis of thrombotic thrombocytopenic purpura: the Plasmic score. Blood. 2014;124:231. [Google Scholar]
- 48. Connell NT, Cheves T, Sweeney JD. Effect of ADAMTS13 activity turnaround time on plasma utilization for suspected thrombotic thrombocytopenic purpura. Transfusion. 2016;56:354–9. [DOI] [PubMed] [Google Scholar]
- 49. Crawley JTB, Lam JK, Rance JB, Mollica LR, O’Donnell JS, Lane DA. Proteolytic inactivation of ADAMTS13 by thrombin and plasmin. Blood. 2005;105:1085–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


