Abstract
One year after the first human case of SARS-CoV-2, two nanomedicine-based mRNA vaccines have been fast-tracked, developed, and have received emergency use authorization throughout the globe with more vaccine approvals on the heels of these first two. Several SARS-CoV-2 vaccine compositions use nanotechnology-enabled formulations. A silver lining of the COVID-19 pandemic is that the fast-tracked vaccine development for SARS-CoV-2 has advanced the clinical translation pathway for nanomedicine drug delivery systems. The laboratory science of lipid-based nanoparticles was ready and rose to the clinical challenge of rapid vaccine development. The successful development and fast tracking of SARS-CoV-2 nanomedicine vaccines has exciting implications for the future of nanotechnology-enabled drug and gene delivery; it demonstrates that nanomedicine is necessary and critical to the successful delivery of advanced molecular therapeutics such as nucleic acids, it is establishing the precedent of safety and the population effect of phase four clinical trials, and it is laying the foundation for the clinical translation of more complex, non-lipid nanomedicines. The development, fast-tracking, and approval of SARS-CoV-2 nanotechnology-based vaccines has transformed the seemingly daunting challenges for clinically translating nanomedicines into measurable hurdles that can be overcome. Due to the tremendous scientific achievements that have occurred in response to the COVID-19 pandemic, years, perhaps even decades, have been streamlined for certain translational nanomedicines.
Keywords: Nanomedicine, Clinical translation, SARS-CoV-2 vaccines, Drug delivery systems, COVID-19, Nanotechnology
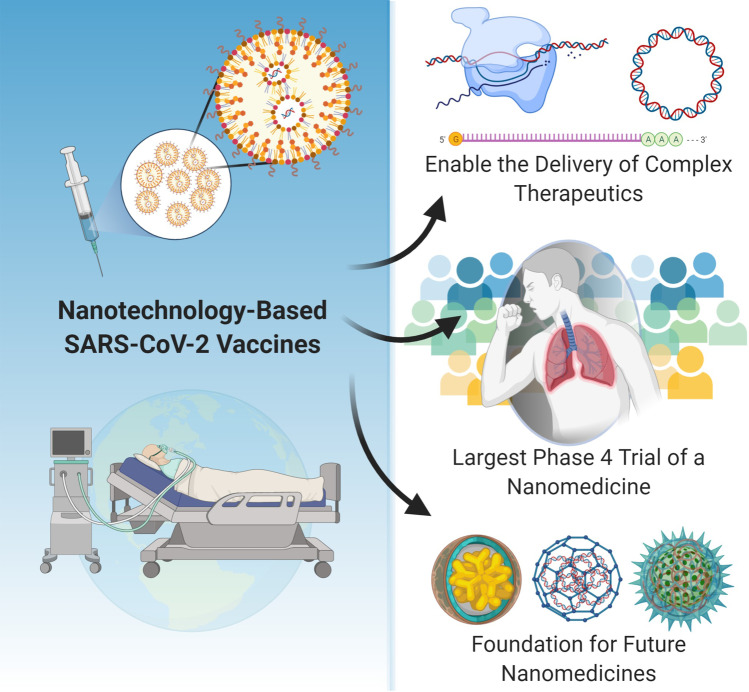
As the globe continues through the trenches of the SARS-CoV-2 pandemic, it is clear we are in “unchartered waters” for almost every aspect of human life; from politics to the economy, from education to communication, and of course, from scientific discovery to medical advancements. Before March 2020, the word “unprecedented” was rare and seldom used, but during the SARS-CoV-2 pandemic, every day seems to result in unprecedented statistics, changes, or advancements. There is an incredible race to develop vaccines and treatments for SARS-CoV-2/ COVID-19 with unprecedented open access, rapid dissemination, and collaboration within the global scientific community. The rampant global spread of SARS-CoV-2 and political initiatives such as the US government’s “Operation Warp Speed” and Russia’s Direct Investment Fund support are accelerating and fueling the fast-tracking, streamlined process of vaccine development. A shining-star advancement that has emerged at the forefront of SARS-CoV-2/COVID-19 science is the development, fast-tracking, and approval of SARS-CoV-2-based mRNA vaccines from Pfizer/BioNTech and Moderna that both employ lipid nanoparticle technology. The two approved SARS-CoV-2 vaccines are nanotechnology-based formulations and many more of the 77 additional vaccines in fast-tracked trials are nanomedicines [1]. This fast-tracking and approval has astounding and exciting implications for the future of nanotechnology-enabled drug and gene delivery systems and has undoubtedly streamlined many years of clinical challenges and obstacles in the translational pathway. As illustrated in Fig. 1, the fast-track developments and approvals of SARS-CoV-2 nanomedicine vaccines are demonstrating that nanomedicine is enabling and necessary, is establishing the precedent for safety and the population effect, and is solidifying the foundation for the clinical translation of future nanomedicines.
Fig. 1.
Implications of nanotechnology-based SARS-CoV-2 vaccines
Figure 1 illustrates the significant implications of the current nanotechnology-based SARS-CoV-2 vaccines on enabling nucleic acid delivery, initiating the largest phase 4 study of a nanomedicine, and establishing the foundation for the clinical translation of future nanomedicines
Nanotechnology-based vaccines are the leading alternative to viral vector vaccines in current clinical trials for SARS-CoV-2. The expedited approvals of mRNA1273 and BNT162b2 are truly remarkable accomplishments in the history of medicine. The closest precedent for comparison to the fast-tracked, clinical race for mRNA SARS-CoV-2 vaccines would be Onpattro® (patisiran) by Alnylam Pharmaceuticals, a siRNA-targeting transthyretin protein for treating a form of amyloidosis. Patisiran was granted fast-track review and orphan drug status which streamlined clinical trials to ~ 5 years with a $450 K treatment cost [2]. On August 3, 2018, the UK’s Medicines and Healthcare Products Regulatory Agency (MHRA) was the first agency to grant patient access to Patisiran through the Early Access to Medicines Scheme (EAMS), followed by approval by the US Federal Drug Administration (FDA) (Aug 10, 2018), marketing authorization by the European Medicines Agency (EMA) (Aug 30 2018), and approvals in Canada, Japan, and Germany (Sep-Oct 2018) [3].
Remarkably, the design, development, approval, and distribution of mRNA1273 and BNT162b2 were achieved in less than 1 year. Attesting to the great demand for SARS-CoV-2 vaccines, the rapid wave of global approvals and authorizations for mRNA1273 and BNT162b2 has been more extraordinary than the swift approvals of Patisiran. On December 2, 2020, UK’s MHRA granted BNT162b2 emergency supply authorization, marking the first approval of a SARS-CoV-2 vaccine [4]. This was followed by the US FDA emergency authorization on December 11 and European Union authorization on December 21, 2020 [4]. On December 18, 2020, the US FDA granted emergency authorization of mRNA1273 followed by approvals in Canada (12.23.20), Israel (1.4.21), the European Union (1.6.21), the UK (1.8.21), and Switzerland (1.12.21) [5]. Six weeks after the first SARS-CoV-2 vaccine authorization, mRNA1273 and BNT162b2 are both approved in over 35 countries, and 5 other (non-nanomedicine) vaccines have received approval [1]. Although there are many hurdles to overcome with mass production, cold storage, and acquiring more safety and efficacy data, this is a landmark in modern medicine, and the lipid nanoparticle delivery systems enabled this achievement.
As shown in Table 1 [6–13], additional nanotechnology-based vaccines will surely follow the path to expedited approval. Pfizer/BioNTech have one approved vaccine, but they also have three additional mRNA vaccines in development. A unique mRNA approach taken by ARCT-021 and one of BioNTech SE/Pfizer’s strategies are the application of self-replicating mRNA. Self-replicating mRNA constructs include the replicase enzyme to self-propagate, and comparative trials seem to require only one administration and/or a lower dose relative to the other mRNA strategies [14].
Table 1.
| Vaccine (Phase) | Encoding | Drug Delivery System | Company | Status |
|---|---|---|---|---|
| mRNA | ||||
| mRNA1273 | Full-length, prefusion stabilized spike protein |
Lipid nanoparticles (LNPs) composition: Proprietary Ionic lipid SM-102, polyethylene glycol (PEG) 2000, dimyristoyl glycerol (DMG), cholesterol, 1,2-distearoyl-sn-glycero-3-phosphocholine [DSPC]), tromethamin hydrochloride, acetic acid, sodium acetate, and sucrose |
Moderna *Operation Warp Speed *Supply agreement with USA for first 100 million doses |
Approved in multiple countries, emergency use in other countries 94.5% efficacy (separate phase 2a, 3, 4 studies) |
| BNT162 program (4 vaccines) |
BNT162b2: full spike mRNA Also in development: BNT162a1: uridine mRNA BNT162b1: RBD mRNA BNT162c2: self-amplifying RNA |
BNT162b2 LNPs composition: lipids ((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate), 2 [(polyethylene glycol)-2000]-N,N-ditetradecylacetamide, 1,2-distearoyl-sn-glycero-3- phosphocholine, and cholesterol), potassium chloride, monobasic potassium phosphate, sodium chloride, dibasic sodium phosphate dihydrate, and sucrose |
BioNTech SE and Pfizer *Supply agreements with Canada, Japan, UK, and USA *Operation Warp Speed |
Approved (BNT162b2) in multiple countries, Emergency use in other countries. 95% efficacy (integrated phase 1/2/3 studies, phase 4 of BNT162b2) |
| ARCoV | S1 receptor binding domain (RBD 319-541) | LNPs: undisclosed compositions, similar to mRNA1273 and BNT162b2 | Academy of Military Medical Sciences, Suzhou Abogen Biosciences and Walvax Biotechnology | Phase 1 |
| COVAC1 | Self-replicating mRNA |
Imperial College London and Morningside Ventures *Partnership as VacEquity Global Health to provide low-cost vaccine to low income regions |
Phase 1/2 | |
| LUNAR-COV19 (ARCT-021) | Self-replicating mRNA prefusion spike protein; Lipid-enabled and Unlocked Nucleomonomer Agent modified RNA | Arcturus Therapeutics and Duke-NUS Medical School | Phase 1/2 | |
| CVnCoV | Spike mRNA |
CureVac *Support from Germany and The Bill and Melinda Gates Foundation and the Coalition for Epidemic Preparedness Innovations (CEPI) |
Phase 2b/3 | |
| Proteins | ||||
| NVX-CoV2373 (Novavax) | Full-length recombinant S protein of SARS-CoV-2 combined with saponin-based Matrix-M™ adjuvant | Virus-like nanoparticle vaccine with saponin-based Matrix-M™ adjuvant | Novavax and the Coalition for Epidemic Preparedness Innovations (CEPI) | Phase 1, 2/3 |
| DNA | ||||
| INO-4800 (Inovio) | plasmid pGX9501 expressing a synthetic, optimized sequence of the SARS-CoV-2 full length spike glycoprotein | DNA coated-poly(D,L-lactide-co-glycolide) (PLGA) nanoparticles | Inovio Pharmaceuticals, International Vaccine Institute, Coalition for Epidemic Preparedness Innovations (CEPI) | Phase 2 on hold due to electroporation device clarifications |
| Covigenix | DNA plasmid with gene for SARS-CoV-2 nucleocapsid | P Fusogenix™ is formulated with neutral lipids and FAST proteins (fusion-associated small transmembrane) |
Entos Pharmaceuticals Inc., Canadian Institutes of Health Research (CIHR), Research Nova Scotia (RNS), and the Institute for Ageing (IA) |
Phase 1 |
Table 1 illustrates the range of nanoparticle-based vaccines in current clinical trials. Most of the mRNA vaccines in trial use liposomes or lipid nanoparticles. Some of the protein subunit and DNA vaccines also use nanotechnology platforms.
On the other hand, Novavax’s protein subunit vaccine relies on a virus-like nanoparticle delivery system [15]. Multiple vaccines in clinical trial are employing the biomimetic approach of using virus-like nanoparticles, similar to the HPV vaccines [7]. The novelty of Novavax’s approach is the use of Matrix-M1, a saponin-based adjuvant [15]. For Novavax’s system, saponin (plant-based glycoside) is mixed with phospholipids and cholesterol to form 40-nm nanoparticles used for drug delivery [16]. Covigenix is another example of a nanomedicine platform for DNA delivery; this proprietary drug delivery system uses Entos Pharmaceuticals Trademarked technology Fusogenix™ to promote nanoparticle fusion with cell membranes [17].
Considering the technologies shown in Table 1, it is clear that most of these vaccines are delivery platforms that can be easily tailored for future applications. Although this is certainly promising and exciting, each formulation has challenges to face and overcome. An important aspect of SARS-CoV-2 vaccination that has not been addressed by the nanotechnology-based vaccines in trial is the potential need for an upper respiratory tract secretory IgA1 response; such a response might benefit from a pulmonary or nasal delivery strategy [12].
Translational nanomedicine is enabling
The past year of SARS-CoV-2/COVID-19 science and the recent approval of two lipid nanoparticle-based vaccines have demonstrated that nanomedicine is necessary and enabling. Nanomedicine enables the delivery of drugs and molecules that would not otherwise be useful or viable therapeutics. Most of the nanomedicine-based vaccines are for the delivery of nucleic acids including the approved Moderna and Pfizer/BioNT vaccines for the delivery of mRNA. Although using mRNA is a highly effective vaccination strategy, delivery of mRNA alone would have poor efficacy as mRNAs have low permeability due to their extremely large size (300–5000 kDa), high negative charge, and are easily degraded by RNAases or cleared by immune cells [18, 19]. The application of mRNA (and other nucleic acids) requires a delivery system [19]. Prior to the urgency of vaccine development for SARS-CoV-2, nanomedicines have been approved for indications ranging from cancer to fungal infections. However, the timeline and process were markedly different. For example, the development and approval of Doxil® took 7 and a half years, but the foundation of Doxil® began 16 years before approval [20]. Prior to the COVID-19 pandemic, the most significant challenge facing nanomedicines in clinical translation was addressing the unknown question of “how will the nanoscale properties of the materials effect the safety and toxicity of the formulation?” It is well known that nanoscale materials have different properties than microscale materials. In 2004, the National Cancer Institute, the Federal Drug Administration, and the National Institute of Standards and Technology formed the Nanotechnology Characterization Laboratory to assist with pre-clinical characterization of nanomedicines, to standardize the process of nanomedicine safety and efficacy analysis, and to gain insight of the health risks and safety of nanoscale materials. The pre-COVID-19 focus was on how the less-characterized nanoproperties of a therapy could jeopardize safety or lead to an unforeseen health risk. The imperative need for SARS-CoV-2 vaccines has demanded that the best scientific solutions accelerate through clinical translation at an unprecedented pace. And it just so happened that nano-drug delivery systems are a part of these scientific solutions in current demand. The COVID-19 focus is on the solution, and nanomedicine is the part of the solution that enables delivery of mRNA and DNA. This paradigm shift from “what are the unknown risks of nanomedicines?” to “what clinical solutions can we solve with nanomedicine” has opened the door to translational nanomedicine applications beyond SARS-CoV-2 vaccines.
The first large-scale phase 4 clinical trial of a nanomedicine is underway
Although the FDA’s approval of Doxil® in 1995 marked one of the first nanomedicine approvals, all approvals to date are for treatments administered to a relatively small population of patients following clinical approval. According to the University of Oxford’s Global Change Data Lab, as of December 29, 2020, 4.68 million people have received their first dose of a lipid nanoparticle SARS-CoV-2 vaccine [21]. Of the 4.68 million people to receive a vaccine, 2.13 million people have been vaccinated in the USA; 1 million people have been vaccinated in China; 800,000 in the UK; 491,600 people in Israel; between 60 and 40 K each in Canada, Bahrain, Russia, and Germany; and less than 10 K each in 13 additional countries [21]. This translates to 0.64% of the US population, 5.68% of the Israeli population, and 1.18% of the UK’s population receiving a vaccination less than 1 month after approvals and emergency use authorizations began. By January 12, 2021, out of every 100 people in the country, 22.34 people in Israel have received at least one dose of a SARS-CoV-2 vaccination, followed by 12.9 in the United Arab Emirates, 5.75 in Bahrain, 4.52 in the UK, 2.82 in the USA, and 1.32 in Italy [21]. In a November press release, Pfizer Inc. projected manufacturing up to 1.3 billion doses of BNT162b2 in 2021 while Moderna is expected to supply 500 million doses of mRNA1273 in 2021 [22, 23]. The rapid development, approval, and administration of the SARS-CoV-2 vaccines are truly one of the most astonishing accomplishments in the history of medicine, and nanomedicine is a part of this history. As more vaccines progress through the pipeline, the number of people vaccinated with a nanotechnology-based vaccine will escalate. Although each of these nanomedicine vaccines uses a slightly different and propriety formulation, most of these vaccines are liposome or solid lipid nanoparticle formulations. The first large-scale study of the population effect of nanomedicines is underway, and this study is expected to be ongoing and sustained. The field of nanomedicine will have large-scale, validated, and sustained safety and efficacy data.
Valuable safety data is already emerging. Allergic reactions as severe as anaphylactic shock have already emerged to both the Pfizer/BioNT and the Moderna vaccines [24]. The allergic reactions may be caused from the polyethylene glycol (PEG) content of the solid lipid nanoparticles [24]. It is possible that the reaction is from a different component of the formulations such as the ionic condensing lipids that have not been previously tested in humans. In addition to PEG, four other ingredients are being investigated by the US FDA’s Center for Biologics Evaluation and Research [24, 25]. Once the component is identified, future formulations can be adjusted to avoid immunogenicity or screen for immune reactions. For example, if PEG is the immunogenic component, screening patients for anti-PEG antibodies before administration could identify patients likely to have an adverse allergic reaction. This highlights the need for paralleled research and development in patient screening and monitoring. Efficacy data collection is paramount as well; as the SARS-CoV-2 virus continues to mutate, it will be important to correlate viral strain exposure with vaccine efficacy.
Global data collection, transparency, and scientific insight from agencies such as the Nanotechnology Characterization Laboratory will be key to making the emerging safety data as useful as possible and applying it toward future translational nanomedicines. This large-scale phase 4 clinical trial is a period of discovery for lipid nanoparticles and will actually help to address the pre-COVID-19 focus of nanomedicine translation, addressing the unknown risks. As the process of discovery and data collection progresses, comparing adverse events between the different nano-drug delivery systems will be critical in characterizing the safety profile of different liposome and solid lipid nanoparticle components. As adverse events are analyzed and understood, this will help to maintain the paradigm shift away from the unknown risks and on future applications.
The foundation is solidified for the clinical translation of future nanomedicines
The benefit of the current SARS-CoV-2 vaccines (approved and in development) using liposomes or other types of nanoparticles is that there will be a large amount of data on an entire category of nano-drug delivery systems (lipid nanoparticles). Future translational nanomedicines will have a control nanoparticle formulation to use for comparison. This will not be applicable to all studies but will be useful for many initial safety screenings. The current composition of lipid nanoparticles in SARS-CoV-2 vaccines is similar to liposomes because they are suited to deliver the nucleic acid payload, and there are also precedents of liposome approvals for drug delivery. The approval of SARS-CoV-2 nanomedicine-based vaccines is establishing the foundation for developing more complex systems such as actively targeted systems and systems for combination drug delivery. Liposomes and lipid nanoparticles will no longer be a rare novelty. Although the translational pathway will not be expedited for applications outside of high-demand, unmet-need applications (such as a pandemic), the pathway for clinically translating polymeric nanomedicines, metallic nanomedicines, and hybrid nanomedicines is more established than ever before.
Liposomes and solid lipid nanoparticles are highly biocompatible and biodegradable, and to date, the reports of adverse immune reactions have been low. Although, generally, the biodegradation of polymeric and metallic nanoparticles is slower, they may also be beneficial depending on the disease being treated and the drug being delivered. The most remarkable property of nanomedicine is the high surface area to volume ratio; this enables highly efficient drug packaging. The encapsulated drug is protected from degradation and immune clearance, and a lower dose can be administered due to efficient drug packaging. Nanomedicine can dramatically improve (or enable) the safety and efficacy of a drug. Taking simple formulations one step further, besides PEGylating the surface of the nanoparticles to help avoid aggregation and immune clearance, the dramatic surface area of nanoparticles can be functionalized with targeting residues that bind to disease-specific receptors. A second drug can be added and even included in a different compartment or absorbed to the surface of the particle depending on the desired release kinetics. Imaging and detection modalities can be added. Although the pathway will still be challenging; the current SARS-CoV-2 nanomedicine vaccines are solidifying the foundation for the future translation of complex and non-lipid nanomedicines.
In brief, without the nanomedicine component, the mRNA- and DNA-based vaccines would have little efficacy. The current large phase 4 clinical trial has initiated the beginning of a paradigm shift away from the “unknown risks of nanomedicine” toward “how nanomedicine can enable clinical solutions.” The tremendous production, distribution, and administration of the first 4.68 million doses of the Pfizer and Moderna vaccines have initiated the largest population study of nanomedicine. This phase 4 clinical trial is helping to answer the historical question of “what are the unknown risk of lipid nanoparticles?” As safety and efficacy data is gathered and adverse events are analyzed, a smooth and clear pathway for the development of more complex and non-lipid nanomedicines will be established. As the globe continues through the unchartered waters of the SARS-CoV-2 pandemic, it is clear we are in the midst of unprecedented scientific excellence and collaboration and unprecedented successes for translational nanomedicine. As nanomedicine is a critical component of many SARS-CoV-2 vaccine solutions, translational nanomedicine has finally advanced beyond infancy, and the future developments and applications are assured to be astounding.
Biographies
Lara Milane
is pursuing mitochondrial nanomedicine research
and developing nanotechnology based solutions to inhibit tunneling nanotube
formation in cancer. Assistant Teaching Professor, Department of Pharmaceutical
Sciences, Northeastern University, Boston, MA, USA.

Mansoor Amiji
is a University Distinguished Professor,
Professor of Pharmaceutical Science, and Professor of Chemical Engineering at
Northeastern University in Boston, MA, USA. His primary areas of research interest
are in the development of targeted therapeutic solutions for chronic diseases
such as cancer, neurodegenerative diseases, and inflammatory diseases.

Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lara Milane, Email: l.milane@northeastern.edu.
Mansoor Amiji, Email: m.amiji@northeastern.edu.
References
- 1.McGill COVID19 Vaccine Tracker Team https://covid19.trackvaccines.org/vaccines/
- 2.Al Shaer D, Al Musaimi O, Albericio F, de la Torre BG. FDA Tides Harvest Pharmaceuticals (Basel) 2018;12:2019. doi: 10.3390/ph12020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindenboom CR. https://investors.alnylam.com/press-releases(2018).
- 4.Pfizer Inc, https://www.pfizer.com/news/press-release/press-releases-archive(2021).
- 5.Moderna, Inc. https://investors.modernatx.com/news-releases(2021).
- 6.Jain S, Batra H, Yadav P. & Chand S. COVID-19 vaccines currently under preclinical and clinical studies, and associated antiviral immune response. Vaccines8, 10.3390/vaccines8040649 (2020). [DOI] [PMC free article] [PubMed]
- 7.Huang L, et al. SARS-CoV-2 vaccine research and development: conventional vaccines and biomimetic nanotechnology strategies. Asian J Pharm Sci. 2020 doi: 10.1016/j.ajps.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin MD, et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat Nanotechnol. 2020;15:646–655. doi: 10.1038/s41565-020-0737-y. [DOI] [PubMed] [Google Scholar]
- 9.Campos EVR, et al. How can nanotechnology help to combat COVID-19? Opportunities and urgent need. J Nanobiotechnol. 2020;18:125. doi: 10.1186/s12951-020-00685-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lundstrom K. The current status of COVID-19 vaccines. Frontiers in Genome Editing2, 10.3389/fgeed.2020.579297 (2020). [DOI] [PMC free article] [PubMed]
- 11.Dong Y, et al. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduction and Targeted Therapy. 2020;5:237. doi: 10.1038/s41392-020-00352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 13.Zimmer C, Corum J, Wee. SL. Coronavirus Vaccine Tracker, https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html
- 14.Fuller DH, Berglund P. Amplifying RNA vaccine development. N Engl J Med. 2020;382:2469–2471. doi: 10.1056/NEJMcibr2009737. [DOI] [PubMed] [Google Scholar]
- 15.Keech C, et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.JM Reimer et al 2012 Matrix-M™ adjuvant induces local recruitment, activation and maturation of central immune cells in absence of antigen PLoS ONE 7 e41451 e41451 10.1371/journal.pone.0041451 [DOI] [PMC free article] [PubMed]
- 17.Pharmaceuticals E. Fusogenixhttps://www.entospharma.com/fusogenix(2020).
- 18.Houseley J, Tollervey D. The many pathways of RNA degradation. Cell. 2009;136:763–776. doi: 10.1016/j.cell.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 19.Kowalski PS, Rudra A, Miao L, Anderson DG. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol Ther. 2019;27:710–728. doi: 10.1016/j.ymthe.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barenholz Y. Doxil® — the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Lab GCD. https://ourworldindata.org/covid-vaccinations(2020).
- 22.Pfizer Inc, Pfizer and BioNTech announce vaccine candidate against covid-19 achieved success in first interim analysis from phase 3 study, https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-vaccine-candidate-against
- 23.Mrinalika Roy TR. Moderna CEO confident of producing 500 million COVID-19 vaccine doses in 2021, https://www.reuters.com/article/us-health-coronavirus-moderna/moderna-ceo-confident-of-producing-500-million-covid-19-vaccine-doses-in-2021-idUSKBN28E2QX(2020).
- 24.Vrieze Jd. Suspicions grow that nanoparticles in Pfizer’s COVID-19 vaccine trigger rare allergic reactions. Science Magazine, 10.1126/science.abg2359 (2020).
- 25.Diaz A. Boston health care worker who experienced reaction to Moderna coronavirus vaccine has history of allergies, 2020).