Abstract
Background
The infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has rapidly spread all over the world, becoming pandemic. Several studies have shown that diabetes mellitus (DM) is an independent risk factor that increases mortality and other adverse outcomes of coronavirus disease-19 (COVID-19). Studies have suggested that SARS-CoV-2 may bind dipeptidyl peptidase-4 (DPP4) for entering cells of the respiratory tract. Besides, DPP4 takes part in immune system regulation. Thus, DPP-4 inhibitors (DPP4i) may play a role against COVID-19.
Methods
We focused on the impact of DPP4i treatment on COVID-19-related outcomes in people with DM. For this purpose, we conducted a systematic review and meta-analysis to summarize the existing evidence on this topic.
Results
Retrospective observational studies provide inconsistent results on the association between use of DPP4i and outcomes of COVID-19. While two studies reported significantly lower mortality rates among patients with DM who received DPP4i versus those who did not, a series of other studies showed no effect of DPP4i or even worse outcomes. A meta-analysis of 7 studies yielded a neutral estimate of the risk ratio of COVID-19-related mortality among users of DPP4i (0.81; 95% CI 0.57–1.15).
Conclusion
In the absence of randomized controlled trials, observational research available so far provides inconclusive results and insufficient evidence to recommend use of DPP4i against COVID-19.
Keywords: Diabetes, Incretins, Epidemiology, Coronavirus, Pandemic
Introduction
In December 2019, an infectious disease caused by a newly discovered coronavirus, the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has emerged in Wuhan (China) and rapidly spread all over the world. Coronavirus disease 2019 (COVID-19) has become a global pandemic [1]. At the time this review was written, it has been diagnosed in over 92 million people worldwide, causing 2 million deaths, thus, making COVID-19 the major contingent healthcare focus in most countries [2]. Both the earliest [3] and the most recent and comprehensive meta-analysis of observational studies have shown that pre-existing diabetes mellitus (DM) almost doubled the risk for severe/critical COVID-19 and almost tripled in-hospital mortality [4]. Moreover, new-onset DM and poor glucose control and during hospitalization has been associated with worse outcomes [5, 6].
Dipeptidyl peptidase-4 (DPP4), also named CD26, is a transmembrane glycoprotein almost ubiquitously expressed in the surface of many cells including epithelial and endothelial cells of many tissues, and immune cells [7]. DPP4 plays an essential role in immune system regulation by promoting T cell activation and proliferation, modulating the function of other immune cells, and stimulating the production of pro-inflammatory cytokines [7]. DPP4 exists also as soluble form in the circulation, where it maintains its enzymatic activity [8]. DPP4 physiologically degrades glucagon-like peptide-1 (GLP-1) playing a role in glucose metabolism. DPP4 has many other substrates including cytokines, chemokines, and growth factors. It also acts as a binding protein and a ligand of extracellular factors [8]. Evidence suggested that human DPP4 is a functional receptor for the spike glycoprotein of the Middle East Respiratory Syndrome coronavirus (MERS-CoV), which is phylogenetically correlated to SARS-CoV-2 [9]. Viral binding is important for cell adhesion, entry into host cells and developing virulence. Thus, DPP4 inhibition might block the entering of the virus in host cells. However, to date, no data firmly support that DPP4 is also a receptor for SARS-CoV-2 [10, 11]. DPP4i has been identified as a potential candidate by structural studies [12, 13] which so far await confirmation in human cells [10]. Furthermore, it has been hypothesized that DPP4 inhibition may antagonize SARS-CoV-2 virulence through a reduction of the cytokine storm [7] and lung inflammation [14].
DPP4 inhibitors (DPP4i) are widely used for the treatment of type 2 diabetes [15]. For example in Italy, it has become one of the most popular second-line glucose-lowering medications (GLMs) [16, 17], thanks to their optimal safety and tolerability profile. Since quite limited treatment options are available against COVID-19 and there is basically no pharmaceutical strategy to prevent SARS‐CoV‐2 infection, DPP4i have been hypothesized to represent a potential approach [18].
The aim of this review is to summarize the current state of the art on DPP4i treatment and COVID-19-related outcomes in patients with DM, especially in-hospital mortality, intensive care unit (ICU) admission and need of mechanical ventilation. In addition, we evaluated whether DPP4i exposure was associated with a lower risk of SARS-CoV-2 infection. As a disclaimer, we would like to note that evidence is accumulating at a fast pace during the various pandemic phases and the conclusions we reached by analysing existing literature might not be confirmed in the near future.
Methods
We screened the English literature for studies reporting SARS-CoV-2 infection and/or COVID-19-related outcomes in DPP4i users. We employed the following search string: (‘DPP-4’ or ‘DPP-IV’ or ‘DPP4’ or ‘dipeptidyl peptidase-4’ or ‘dipeptidyl peptidase-IV’) and (‘inhibitor’ or ‘inhibitors’) and (‘COVID’ or ‘SARS-CoV-2’). Full text of the retrieved articles was accessed. The search string was run in PubMed, Scopus and Cochrane Library, and further refined by the screening cross-references of the retrieved articles. We included in the analysis all studies reporting separately data for patients with diabetes treated with DPP4i or other GLMs. Observational (cohort or nested case–control) studies published up to December 2020 were included.
The following data were extracted from eligible studies: first author, year of publication, design, sample size, patient characteristics including mean age, haemoglobin A1c (HbA1c), prevalence of comorbidities and complications (e.g. hypertension, cardiovascular disease, chronic kidney disease and cancer), and outcomes.
The meta-analysis was performed including all observational studies reporting mortality in patients with DM and COVID-19. We compared crude mortality data between patients treated with DPP4i and any other GLMs. A sensitivity analysis was performed including the most adjusted estimate available from the studies. Odd ratios were transformed into risk ratios according to the equation proposed by Zhang et al. [18]. All estimates were thus reported as risk ratios and 95% confidence interval (CI). The random effect model was used to obtain pooled RR. Heterogeneity was assessed using the I2 test. Review Manager version 5.3 was used to perform the meta-analysis.
Summary of the evidence
Details and characteristic of observational studies evaluating the impact of DPP4i on COVID-19 outcomes are summarized in Table 1. At baseline, the pooled mean age was 71 years, HbA1c was 7.9%. Results on COVID-19-related outcomes are shown in Table 2.
Table 1.
Clinical characteristics of trials included in the review
| Author | Type of study | Location(s) | Subjects with diabetes | DPP4i users | Age | % Male | HbA1c | % Hypertension | % CVD | % CKD |
|---|---|---|---|---|---|---|---|---|---|---|
| Solerte | Multicenter, case control study | Northern Italy | 338 | 169 | 69 | 70 | 7.5 | 70 | 39 | 24 |
| Mirani | Single center, case series | Milan (Italy) | 90 | 11 | 71 | 72 | NA | 77 | 39 | 19 |
| Rhee | Healthcare insurance database | Korea | 832 | 263 | 62 | 54 | NA | 68 | 27 | 19 |
| Yan | Multicenter, case control study | China | 57 | 6 | NA | NA | NA | NA | NA | NA |
| Kim | Multicenter, observational study | South Korea | 235 | 85 | 68 | 45 | 7.7 | 63 | 22 | 8 |
| Chen | Single center, observational | Wuhan (China) | 100 | 20 | 66 | NA | 7.9 | 33 | 21 | 12 |
| Silverii | Multicenter, observational study | Sicily (Italy) | 159 | 13 | 73 | 54 | NA | NA | NA | NA |
| Fadini | Single center, case series | North-East Italy | 85 | 9 | 70 | 65 | 7.6 | 69 | 27 | 15 |
| Cariou | Multicenter, observational study | France | 1317 | 285 | 70 | 65 | 8.1 | 77 | 41 | NA |
| Pérez-Belmonte | Multicenter, observational study (with PSM) | Spain | 2666 (210 matched) | 105 | 75 | 62 | NA | 76 | 23 | 13 |
| Dalan | Single center observartional study | Singapore | 76 | 27 | NA | NA | NA | NA | NA | NA |
| Nafakhi | Single center observartional study | Iraq | 67 | 14 | 60 | 43 | NA | 66 | 15 | NA |
| Overall | – | – | 6022 | 1007 | 68 | 61 | 7.6 | 73 | 29 | 15 |
CVD cardiovascular disease; CKD chronic kidney disease, NA not available
Table 2.
Clinical outcomes of COVID-19-related to DPP4i treatment
| Author | Death | ICU admission | Mechanical Ventilation | ICU admission and/or death | ICU admission, mechanical ventilation, death | Mechanical Ventilation and/or death | Disease severity |
|---|---|---|---|---|---|---|---|
| Solerte | adj OR 0.23 (0.12–0.46) | HR 0.51 (0.27–0.95) | HR 0.27 (0.11–0.62) | ||||
| Mirani | adj HR 0.13 (0.02–0.92) | OR 0.54 (0.11–2.70) | |||||
| Rhee | adj OR 0.362 (0.14–0.97) | ||||||
| Yan | adj OR 0.32 (0.02–2.18) | ||||||
| Kim | adj OR 1.47 (0.45–4.78) | ||||||
| Chen | adj OR 1.48 (0.40, 5.53) | ||||||
| Silverii | RR 1.0 (0.51–2.14) | ||||||
| Fadini | RR 0.80 (0.12–5.54) | OR 1.74 (0.62–4.90) | |||||
| Cariou | OR 0.85 (0.55–1.32) | OR 1.01 (0.75, 1.34) | |||||
| Pérez-Belmonte | adj OR 1.05 (0.67–2.11) | OR 1.12 (0.65–1.95) | |||||
| Dalan | adj RR 4.07, (1.42–11.66) | adj RR 60.2 (3.17–1140.1) |
ICU intensive care unit, Adj adjusted, OR odds ratio, HR hazard ratio, RR relative risk
In our cohort study including 403 patients hospitalized for COVID‐19 from February to April 2020 at the University Hospital of Padova, 85 had DM, 9 of whom were on treatment with DPP4i. No significant difference in the rate of ICU admission or death was observed between DPP4i users and non-users [19]. Our analysis revealed that exposure to DPP4i in matched patients with DM was similar in patients with (10.6%) and without (8.8%) COVID-19, or in those attending the local outpatient clinic (15.4%) and in those hospitalized for other reasons (8.5%). The rate of DPP4 use was also similar in patients with DM hospitalized with COVID-19 pneumonia (11.3%) and with pneumonia of other aetiology (10.3%). Overall, these data do not support the hypothesis that DPP4i might be protective against COVID-19.
Sainsbury et al. performed a cohort study in a large UK-based primary care dataset aiming to investigate whether treatment with SGLT2 inhibitors (SGLT2i) was associated with SARS-CoV-2 infection. 7676 SGLT2i users were matched with a propensity score analysis with 7,676 DPP4i users and followed-up from 30th January to 27th July 2020. The primary outcome was confirmed or clinically suspected infection. The incidence rate of infection was similar among SGLT2i users and DPP4i users (19.7/1000 vs 24.7/1000 person-years). The adjusted HR was 0.92 (95% CI 0.66–1.29) [20].
The CORONADO study, a nationwide multicentre observational study conducted in France, included 1317 patients hospitalized for SARS-CoV-2 with a history of DM or newly diagnosed DM. The primary outcome was a composite of tracheal intubation for mechanical ventilation and/or death within 7 days of admission. 21.6% of the participants were on DPP4i. No association was reported between a severe course of COVID-19 and a treatment with DPP4i prior to admission (OR 1.01; 95% CI 0.75–1.34) [21].
Consistent with these findings, in a retrospective observational study of 120 patients with DM conducted by Chen et al. DPP4i users showed similar clinical outcomes and laboratory findings compared to nonuser. In-hospital death was non-significantly higher in DPP4i users (25%) than in nonusers (14%; p = 0.31) [6].
Similarly, in Pérez-Belmonte et al. on the univariate models, DPP4i treatment was associated with higher in-hospital deaths (OR 1.56, 95% CI 1.13–2.14, p = 0.006), though this association was not confirmed when models were adjusted using multivariate logistic regression analysis (OR 1.39, 95% CI 0.64–1.67, p = 0.876) [22]. After propensity score matching, the use of DPP4i was not associated with other adverse outcomes, such as need of ICU admission, mechanical ventilation, in-hospital complications, or long-time hospital stays [22]. A few other observational studies reported no association between DPP4i treatment and COVID-19-related mortality [23, 24] or COVID‐19 severity [25].
On the other side, there are notable observational studies suggesting beneficial effects of DPP4i on COVID-19-related outcomes.
In a case series involving 387 patients with diagnosis of COVID-19 admitted to a research hospital in Lombardy (Northern Italy) between February 20 and 9 April 2020, 90 patients had DM, 12.2% of whom were treated with DPP4i, a prevalence similar to that observed in another Italian study. The study was performed to evaluate factors that predicted adverse outcomes related to COVID-19 in patients with diabetes. In-hospital mortality rate in patients with DM was twice as higher that of patients without DM. However, contrary to what observed in many other studies, such association was lost after adjustment for confounders. Use of DPP4i was associated with a lower mortality rate independently from confounders (adjusted HR 0.13; 95% CI 0.02–0.92). Moreover, DPP4i users needed non-invasive mechanical ventilation less frequently suggesting a less severe pneumonia [26]. It should be noted that this result was based on 11 patients only, who were treated with DPP4i.
In a multicentre, case–control, retrospective, observational study, conducted in Northern Italy hospitals, 169 subjects treated with sitagliptin plus insulin were matched for age and sex with 169 subjects treated with standard care (insulin) at the time of hospitalization [27]. Primary outcomes were hospital discharge and death. Admission to the ICU, the need for mechanical ventilation, and extracorporeal membrane oxygenation were among secondary outcomes. The use of sitagliptin was associated with a reduced mortality (18% vs 37%, p < 0.001). The association between sitagliptin treatment and reduction in mortality was confirmed after adjustment for clinically relevant factors (age, sex, comorbidities, ongoing treatment), yielding an adjusted OR of 0.23 (95% CI 0.12–0.46). A greater number of patients treated with sitagliptin were discharged from the hospital at day 30 as compared with the number of discharged patients in the standard-of-care group (71% vs 59%, p < 0.001). The use of sitagliptin was associated with a lower risk for the need of mechanical ventilation and ICU as compared to the standard of care. Patients treated with sitagliptin showed a significant reduction in inflammatory parameters such as procalcitonin and C-reactive protein at follow-up. Lymphocyte counts of sitagliptin-treated patients were increased compared with baseline and compared with patients treated with standard of care. In the group treated with sitagliptin, mean blood glucose level measured during the hospitalization was lower than in standard-of-care group. As shown in other studies, authors found that better glucose control during hospitalization was associated with better outcomes. However, outcome analysis was not adjusted for glucose control associated with sitagliptin use. Therefore, the influence of better glycaemic control in the sitagliptin group cannot be excluded.
Similarly, among 67 patients with DM hospitalized for COVID-19 pneumonia in a single centre in Iraq, DPP4i use was associated with decreased length of ICU stay, also after the adjustment for confounder variables (OR − 0.3, 95% CI 0.2–3, p = 0.04). However, no data on in-hospital death were reported [28].
In line with these results, a retrospective study using Korean insurance claims data and including 832 subjects with DM and 263 on DPP4i, showed that DPP4i treatment was significantly associated with better clinical outcomes compared to patients with COVID-19 not treated with DPP4i after adjusting for age, gender, comorbidity, and medications (adjusted OR 0.362, 95% CI 0.135–0.971). While we write, this article is still in pre-print meaning that reported data and their interpretation have not yet passed to peer-review [29].
Opposite findings were observed in a retrospective analysis of 717 patients hospitalized for COVID-19 in a single health care centre in Singapore [30]. Among them, 76 (10.6%) had DM. Patients receiving a DPP4i (n = 27) experienced higher rates of ICU admission compared to 49 patients treated with other glucose-lowering medications, also after the adjustment for confounder variables (adjusted RR 5.14, 95% CI 1.49–17.70). In the diabetes cohort, patients on DPP4i were more likely to require mechanical ventilation, but no data were available for mortality rates.
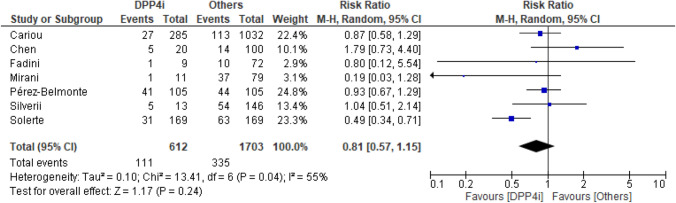
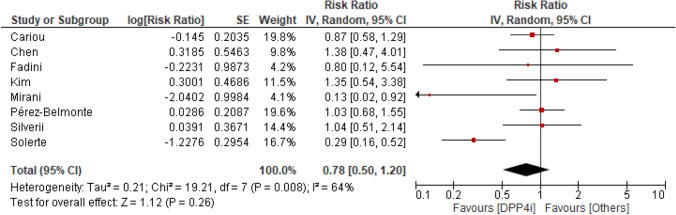
Seven studies reported crude data on mortality. Death occurred in 111 of 612 (18%) patients treated with DPP4i and in 335 of 1703 (19.7%) patients treated with other glucose-lowering medication (RR 0.81, 95% CI 0.57–1.15) (Fig. 1). The I2 statistics suggested possible heterogeneity. Adjusted RR was available for three of seven studies. When the analysis was updated using the most adjusted RR result remained similar (RR 0.74, 95% CI 0.47–1.16) (Fig. 2).
Fig. 1.
Risk for mortality for DPP4i users and other glucose-lowering medication users (crude data). Risk ratio with 95% confidence intervals
Fig. 2.
Risk for mortality for DPP4i users and other glucose-lowering medication users (adjusted risk ratio). Risk ratio with 95% confidence intervals
Interpretation
The evidence accrued so far on the association between DPP4i and outcomes of patients with DM and COVID-19 showed a certain degree of heterogeneity across the studies. Overall, DPP4i appear neutral in the setting of COVID-19 infection, but available studies are still insufficient to made definitive conclusions. It is noteworthy that all findings were derived from retrospective observational studies and that most studies were not designed to directly investigate the role of DPP-4i. For this reason and due to the intrinsic limitations of observational studies, even the positive results of a few studies should be considered hypothesis generating [31].
The differences in the methodology used, in the baseline characteristics, and the sample size could explain the differences observed for the association between DPP4i and COVID-19 outcomes.
Several potential biases should be considered to interpret the inconsistently reported positive outcomes among people with DM who received DPP4i during SARS-CoV-2 infection or hospitalization for COVID-19. These are summarised in Fig. 3. In general, comparative effectiveness research on GLMs is biased by the so-called confounding by indication [32], whereby the reasons why patients received DPP4i or not may have driven differential outcomes more than the drugs themselves. For example, it should be noted that, in Italy, where two positive studies were performed, DPP4i can be prescribed only by diabetes specialists. Therefore, it is possible that pre-hospital or in-hospital use of DPP4i was linked to the patient being seen by a diabetes specialist, which can be per se associated with better outcomes. Indeed, epidemiological studies clearly suggest that outpatients attending specialist diabetes clinics have much better global outcomes than those not attending diabetes clinics [33]. Methods have been designed to reduce the impact of so-called channelling bias, including propensity score matching. Yet, quality of the studies available so far was suboptimal in several cases, with no or incomplete adjustment.
Fig. 3.
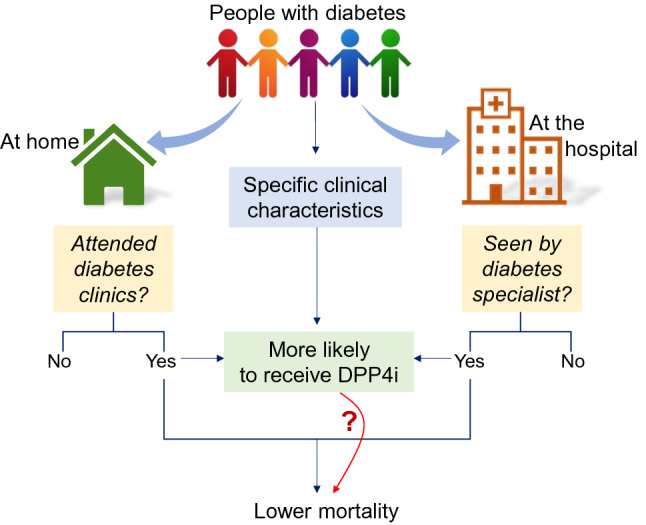
Interpretation of a possible spurious association between use of DPP4i and better outcomes of COVID-19. Specific clinical characteristics are typically associated with the probability of receiving a DPP4i. In addition, having attended diabetes clinics before COVID-19 or being seen by a diabetes specialist during hospitalization increases the likelihood of receiving a DPP4i. In both cases, the observed outcome can be confounded by different clinical characteristics or by indirect effect of diabetes specialist care. Thus, the direct link between use of DPP4i and lower mortality is highlighted with a question mark
It has been recognized that inflammation plays a key role in the development and severity of COVID-19. Severe COVID-19 is associated with a hyperinflammatory state and cytokine storm, which can lead to multi-organ damage [34, 35]. DPP4i has been proposed to reduce the overproduction of proinflammatory cytokines and mediators, such as IL-1, IL-6 and tumour necrosis factor-α (TNF-α), C-reactive protein (CRP), possibly mitigating the clinical course of COVID-19. In several human studies, a treatment with sitagliptin significantly decreased plasma concentrations of IL‐6 [36] and CRP [37]. In another study, a treatment with vildagliptin was associated with a significant reduction in IL-6 levels [38]. Saxagliptin attenuated the activation of the inflammasome and reduce the serum levels of CRP, TNF-α, IL‐1β, IL‐18, and IL‐6 [39]. However, other studies failed to demonstrate any effect of DPP4i on levels of inflammatory biomarkers [40–42]. It should also be noted that treatment with DPP4i in patients with DM exerted no protective effects against cardiovascular disease, which is based on a chronic low-grade inflammation. Furthermore, the effects of DPP4 inhibition on the concentrations of active intact cytokines and chemokines can be paradoxical. By protecting inflammatory factors from enzymatic degradation by DPP-4, DPP4i could even potentiate some inflammatory network [43]. This is the mechanism postulated to form the basis for the development of arthritis during treatment with DPP4i [44, 45], although this issue is still controversial [46]. Conflicting results were also detected in the observational studies included in this review. In Solerte et al., patients treated with sitagliptin showed a significant reduction in inflammatory parameters such as procalcitonin and CRP at follow-up [27]. On the contrary, in the study conducted by Chen and colleagues, DPP4i users showed similar levels of CRP, IL-6 and procalcitonin compared to nonuser [6]. In other studies, no data were available for laboratory analysis.
Acute respiratory distress syndrome (ARDS) is frequently encountered complication of COVID-19, which is the main cause of SARS‐CoV‐2‐induced death. Interestingly, in an experimental model of ARDS, DPP4 inhibition by sitagliptin alleviated histological findings of lung injury by inhibiting pro-inflammatory cytokines IL-1β, TNFα, and IL-6 [14]. However, there is no evidence of similar findings in the human lung.
Most of the studies and the meta-analyses suggest that DPP4i treatment is at least safe. Due to their favourable safety profile, DPP4i remain a valid therapeutic option for the management of patients with DM and COVID‐19.
Conclusion
In the absence of results coming from well-design, randomized control trials, data on the effectiveness or safety of DPP4i in the treatment of COVID-19 should be interpreted with caution and no definite conclusion can be made. Randomized phase III and phase IV trials are ongoing or have been approved to evaluate the effect of DPP4i linagliptin (NCT04341935 and NCT04371978) and sitagliptin (NCT04365517) in reducing the severity and all-cause mortality of COVID-19. The results of these studies might help reveal implications of the use of DPP4i in such patients or DPP4i might finally add to the list of failed drugs against COVID-19.
Funding
University of Padova, though a contribution from the CARIPARO Foundation (COVIDIMED project of the Department of Medicine).
Compliance with ethical standards
Conflict of interest
B.M.B. received lecture or advisory board fees from Astra Zeneca, Boehringer Ingelheim, Eli Lilly, Mundipharma, Novartis, Novo Nordisk, and Sanofi. A.A. received research grants, lecture fees, or advisory board fees from Merck Sharp & Dome, AstraZeneca, Novartis, Boehringer Ingelheim, Sanofi, Mediolanum, Janssen, Novo Nordisk, Eli Lilly, Servier, and Takeda. G.P.F. received grant support, lecture fees, or advisory board fees from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novartis, Novo Nordisk, Sanofi, Genzyme, Servier, and Merck Sharp & Dohme.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Helmy YA, Fawzy M, Elaswad A, Sobieh A, Kenney SP, Shehata AA. The COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control. J Clin Med. 2020;9(4):1225. doi: 10.3390/jcm9041225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Coronavirus disease (COVID-19) Dashboard [Internet]. Available from: https://covid19.who.int/. Cited 28 Nov 2020
- 3.Fadini GP, Morieri ML, Longato E, Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Investig. 2020;43:867–869. doi: 10.1007/s40618-020-01236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantovani A, Byrne CD, Zheng M-H, Targher G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: a meta-analysis of observational studies. Nutr Metab Cardiovasc Dis. 2020;30(8):1236–1248. doi: 10.1016/j.numecd.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu L, She Z-G, Cheng X, Qin J-J, Zhang X-J, Cai J, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(6):1068–1077.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Yang D, Cheng B, Chen J, Peng A, Yang C, et al. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. 2020;43(7):1399–1407. doi: 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- 7.Shao S, Xu Q, Yu X, Pan R, Chen Y. Dipeptidyl peptidase 4 inhibitors and their potential immune modulatory functions. Pharmacol Ther. 2020;209:107503. doi: 10.1016/j.pharmthera.2020.107503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambeir A-M, Durinx C, Scharpé S, De Meester I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003;40(3):209–294. doi: 10.1080/713609354. [DOI] [PubMed] [Google Scholar]
- 9.Lu G, Hu Y, Wang Q, Qi J, Gao F, Li Y, et al. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;500(7461):227–231. doi: 10.1038/nature12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xi CR, Di Fazio A, Nadvi NA, Patel K, Xiang MSW, Zhang HE, et al. A novel purification procedure for active recombinant human DPP4 and the inability of DPP4 to bind SARS-CoV-2. Molecules. 2020;25(22):5392. doi: 10.3390/molecules25225392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5(4):562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vankadari N, Wilce JA. Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microb Infect. 2020;9:601–604. doi: 10.1080/22221751.2020.1739565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526(1):135–140. doi: 10.1016/j.bbrc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawasaki T, Chen W, Htwe YM, Tatsumi K, Dudek SM. DPP4 inhibition by sitagliptin attenuates LPS-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 2018;315(5):L834–L845. doi: 10.1152/ajplung.00031.2018. [DOI] [PubMed] [Google Scholar]
- 15.Sesti G, Avogaro A, Belcastro S, Bonora BM, Croci M, Daniele G, et al. Ten years of experience with DPP-4 inhibitors for the treatment of type 2 diabetes mellitus. Acta Diabetol. 2019;56(6):605–617. doi: 10.1007/s00592-018-1271-3. [DOI] [PubMed] [Google Scholar]
- 16.Bonora E, Cataudella S, Marchesini G, Miccoli R, Vaccaro O, Fadini GP, et al. Clinical burden of diabetes in Italy in 2018: a look at a systemic disease from the ARNO Diabetes Observatory. BMJ Open Diabetes Res Care. 2020;8(1):e001191. doi: 10.1136/bmjdrc-2020-001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonora E, Cataudella S, Marchesini G, Miccoli R, Vaccaro O, Fadini GP, et al. A view on the quality of diabetes care in Italy and the role of diabetes clinics from the 2018 ARNO Diabetes Observatory. Nutr Metab Cardiovasc Dis. 2020;30(11):1945–1953. doi: 10.1016/j.numecd.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Filardi T, Morano S. COVID-19: is there a link between the course of infection and pharmacological agents in diabetes? J Endocrinol Invest. 2020;43(8):1053–1060. doi: 10.1007/s40618-020-01318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fadini GP, Morieri ML, Longato E, Bonora BM, Pinelli S, Selmin E, et al. Exposure to dipeptidyl-peptidase-4 inhibitors and COVID-19 among people with type 2 diabetes: a case-control study. Diabetes Obes Metab. 2020;22(10):1946–1950. doi: 10.1111/dom.14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sainsbury C, Wang J, Gokhale K, Acosta-Mena D, Dhalla S, Byne N, et al. Sodium-glucose co-transporter-2 inhibitors and susceptibility to COVID-19: a population-based retrospective cohort study. Diabetes Obes Metab. 2021;23(1):263–269. doi: 10.1111/dom.14203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cariou B, Hadjadj S, Wargny M, Pichelin M, Al-Salameh A, Allix I, et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pérez-Belmonte LM, Torres-Peña JD, López-Carmona MD, Ayala-Gutiérrez MM, Fuentes-Jiménez F, Huerta LJ, et al. Mortality and other adverse outcomes in patients with type 2 diabetes mellitus admitted for COVID-19 in association with glucose-lowering drugs: a nationwide cohort study. BMC Med. 2020;18(1):359. doi: 10.1186/s12916-020-01832-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silverii GA, Monami M, Cernigliaro A, Vigneri E, Guarnotta V, Scondotto S, et al. (2020) Are diabetes and its medications risk factors for the development of COVID-19? Data from a population-based study in Sicily. Nutr Metab Cardiovasc Dis [DOI] [PMC free article] [PubMed]
- 24.Kim MK, Jeon JH, Kim SW, Moon JS, Cho NH, Han E, et al. The clinical characteristics and outcomes of patients with moderate-to-severe Coronavirus disease 2019 infection and diabetes in Daegu South Korea. Diabetes Metab J. 2020;44(4):602–613. doi: 10.4093/dmj.2020.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan H, Valdes AM, Vijay A, Wang S, Liang L, Yang S, et al. Role of drugs used for chronic disease management on susceptibility and severity of COVID-19: a large case-control study. Clin Pharmacol Ther. 2020;108(6):1185–1194. doi: 10.1002/cpt.2047. [DOI] [PubMed] [Google Scholar]
- 26.Mirani M, Favacchio G, Carrone F, Betella N, Biamonte E, Morenghi E, et al. Impact of comorbidities and glycemia at admission and dipeptidyl peptidase 4 inhibitors in patients with type 2 diabetes with COVID-19: a case series from an academic hospital in Lombardy. Italy Diabetes Care. 2020;43(12):3042–3049. doi: 10.2337/dc20-1340. [DOI] [PubMed] [Google Scholar]
- 27.Solerte SB, D’Addio F, Trevisan R, Lovati E, Rossi A, Pastore I, et al. Sitagliptin treatment at the time of hospitalization was associated with reduced mortality in patients with type 2 diabetes and COVID-19: a multicenter, case-control, retrospective, observational study. Diabetes Care. 2020;43(13):2999–3006. doi: 10.2337/dc20-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nafakhi H, Alareedh M, Al-Buthabhak K, Shaghee F, Nafakhi A, Kasim S. Predictors of adverse in-hospital outcome and recovery in patients with diabetes mellitus and COVID-19 pneumonia in Iraq. Diabetes Metab Syndr. 2020;15(1):33–38. doi: 10.1016/j.dsx.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhee SY, Lee J, Nam H, Kyoung D-S, Kim DJ (2020) Effects of a DPP-4 inhibitor and RAS blockade on clinical outcomes of patients with diabetes and COVID-19. medRxiv. 2020.05.20.20108555 [DOI] [PMC free article] [PubMed]
- 30.Dalan R, Ang LW, Tan WYT, Fong S-W, Tay WC, Chan Y-H et al (2020) The association of hypertension and diabetes pharmacotherapy with COVID-19 severity and immune signatures: an observational study. Eur Hear J Cardiovasc Pharmacother [DOI] [PMC free article] [PubMed]
- 31.Nauck MA, Meier JJ. Reduced COVID-19 mortality with sitagliptin treatment? weighing the dissemination of potentially lifesaving findings against the assurance of high scientific standards. Diabetes Care. 2020;43(12):2906–2909. doi: 10.2337/dci20-0062. [DOI] [PubMed] [Google Scholar]
- 32.Gerstein HC, McMurray J, Holman RR. Real-world studies no substitute for RCTs in establishing efficacy. Lancet (Lond, Engl) 2019;393(10168):210–211. doi: 10.1016/S0140-6736(18)32840-X. [DOI] [PubMed] [Google Scholar]
- 33.Bonora E, Monami M, Bruno G, Zoppini G, Mannucci E. Attending Diabetes Clinics is associated with a lower all-cause mortality: a meta-analysis of observational studies performed in Italy. Nutr Metab Cardiovasc Dis. 2018;28(5):431–435. doi: 10.1016/j.numecd.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (Lond Engl). 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8:e46–e47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Mei T, Chen W, Ye S. Comparison of antidiabetic medications during the treatment of atherosclerosis in T2DM patients. Mediators Inflamm. 2017;2017:5032708. doi: 10.1155/2017/5032708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makdissi A, Ghanim H, Vora M, Green K, Abuaysheh S, Chaudhuri A, et al. Sitagliptin exerts an antinflammatory action. J Clin Endocrinol Metab. 2012;97(9):3333–3341. doi: 10.1210/jc.2012-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rizzo MR, Barbieri M, Marfella R, Paolisso G. Reduction of oxidative stress and inflammation by blunting daily acute glucose fluctuations in patients with type 2 diabetes: role of dipeptidyl peptidase-IV inhibition. Diabetes Care. 2012;35(10):2076–2082. doi: 10.2337/dc12-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birnbaum Y, Bajaj M, Qian J, Ye Y. Dipeptidyl peptidase-4 inhibition by Saxagliptin prevents inflammation and renal injury by targeting the Nlrp3/ASC inflammasome. BMJ open diabetes Res care. 2016;4(1):e000227. doi: 10.1136/bmjdrc-2016-000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nomoto H, Kimachi K, Miyoshi H, Kameda H, Cho KY, Nakamura A, et al. Effects of 50 mg vildagliptin twice daily vs 50 mg sitagliptin once daily on blood glucose fluctuations evaluated by long-term self-monitoring of blood glucose. Endocr J. 2017;64(4):417–424. doi: 10.1507/endocrj.EJ16-0546. [DOI] [PubMed] [Google Scholar]
- 41.Sromova L, Busek P, Posova H, Potockova J, Skrha P, Andel M, et al. The effect of dipeptidyl peptidase-IV inhibition on circulating T cell subpopulations in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2016;118:183–192. doi: 10.1016/j.diabres.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 42.Tsurutani Y, Omura M, Matsuzawa Y, Saito J, Higa M, Taniyama M, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin on atherosclerosis, β-cell function, and glycemic control in Japanese patients with type 2 diabetes mellitus who are treatment naïve or poorly responsive to antidiabetes agents: a mu. Curr Ther Res Clin Exp. 2017;84:26–31. doi: 10.1016/j.curtheres.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fadini GP, Albiero M, Avogaro A. Direct effects of DPP-4 inhibition on the vasculature: reconciling basic evidence with lack of clinical evidence. Vascul Pharmacol. 2015;73:1–3. doi: 10.1016/j.vph.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Padron S, Rogers E, Demory Beckler M, Kesselman M. Republished: DPP-4 inhibitor (sitagliptin)-induced seronegative rheumatoid arthritis. Drug Ther Bull. 2020;58(1):12–15. doi: 10.1136/dtb.2019.228981rep. [DOI] [PubMed] [Google Scholar]
- 45.Saito T, Ohnuma K, Suzuki H, Dang NH, Hatano R, Ninomiya H, et al. Polyarthropathy in type 2 diabetes patients treated with DPP4 inhibitors. Diabetes Res Clin Pract. 2013;102(1):e8–12. doi: 10.1016/j.diabres.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 46.Kathe N, Shah A, Said Q, Painter JT. DPP-4 inhibitor-induced rheumatoid arthritis among diabetics: a nested case-control study. Diabetes Ther Res Treat Educ Diabetes Relat Disord. 2018;9(1):141–151. doi: 10.1007/s13300-017-0353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]