Abstract
At the time of cancer diagnosis, body mass index (BMI) is inversely correlated with lung cancer risk, which may reflect reverse causality and confounding due to smoking behavior. We used two-sample univariable and multivariable Mendelian randomization (MR) to estimate causal relationships of BMI and smoking behaviors on lung cancer and histological subtypes based on an aggregated genome-wide association studies (GWASs) analysis of lung cancer in 29 266 cases and 56 450 controls. We observed a positive causal effect for high BMI on occurrence of small-cell lung cancer (odds ratio (OR) = 1.60, 95% confidence interval (CI) = 1.24–2.06, P = 2.70 × 10−4). After adjustment of smoking behaviors using multivariable Mendelian randomization (MVMR), a direct causal effect on small cell lung cancer (ORMVMR = 1.28, 95% CI = 1.06–1.55, PMVMR = .011), and an inverse effect on lung adenocarcinoma (ORMVMR = 0.86, 95% CI = 0.77–0.96, PMVMR = .008) were observed. A weak increased risk of lung squamous cell carcinoma was observed for higher BMI in univariable Mendelian randomization (UVMR) analysis (ORUVMR = 1.19, 95% CI = 1.01–1.40, PUVMR = .036), but this effect disappeared after adjustment of smoking (ORMVMR = 1.02, 95% CI = 0.90–1.16, PMVMR = .746). These results highlight the histology-specific impact of BMI on lung carcinogenesis and imply mediator role of smoking behaviors in the association between BMI and lung cancer.
Keywords: body mass index, causal relationship, lung cancer, Mendelian randomization, smoking phenotypes
1 |. INTRODUCTION
Although body mass index (BMI) is known to be associated with the risk of several cancers,1 many epidemiological studies1–6 have found that BMI is inversely correlated with lung cancer risk. At the time of or 1 to 2 year leading up to cancer diagnosis, lung cancer cases tend to have lower BMI compared to age, population and sex-matched controls.7 This observation may reflect reverse causality related to the latent effects of lung cancer on BMI due to cachexia and the disease process8 and confounding due to smoking behavior,4,9–11 which suppresses appetite.
Mendelian randomization (MR) complements traditional epidemiological methods as it uses genetic variants as instrumental variables (IVs) to estimate causal effects and is free from the effects of confounders and reverse causality12 but could be influenced by pleiotropy or linkage disequilibrium (LD) among the markers.13 The conventional, univariable MR analysis evaluates the effect of a single predictor on an outcome. There are three assumptions for a valid IV—it must be: (a) associated with the exposure (the “relevance” assumption); (b) independent of the outcome given the exposure (the “exclusion restriction”); and (c) independent of all (both observed and unobserved) confounders (the “exchangeability” assumption).14,15 If an IV is associated with a confounder of exposure and outcome, then there is a conflict with these assumptions, which may lead to potential biases and erroneous conclusions. If associations between the IV and both the exposure and the confounder are measured, then their effects on the outcome can be estimated jointly using multivariable MR.16
Multivariable MR is an extension of univariable MR that takes pleiotropy among multiple traits into account. The assumptions of multivariable MR are broader than that of univariable MR: genetic variants could influence multiple measured exposures, and the exclusion restriction and exchangeability assumption extend accordingly.16 Under the scenario that there is a secondary exposure acting as a mediator of the relationship between primary exposure and outcome, multivariable MR can provide a consistent estimator of the direct effect of the exposure on the outcome that does not work via the mediator.17
In this study, we performed Mendelian randomization analyses to investigate causal effects of BMI and smoking phenotypes on lung cancer using univariable MR and multivariable MR methods. Sensitivity analyses were conducted to evaluate the impact that assumptions had on the findings and to ensure the robustness of the results.
2 |. MATERIALS AND METHODS
2.1 |. Genetic instruments for BMI and smoking phenotypes
Genetic instruments for BMI were identified using results from the largest available meta-analysis of genome-wide association studies (GWASs) for BMI, which combined summary statistics from the Genetic Investigation of ANthropometric Traits (GIANT) consortium18 with GWAS for BMI performed in UK Biobank participants of European ancestry, reaching about 700 000 individuals.19 The former association testing was performed with the inverse normally transformed BMI residuals using linear regression assuming an additive genetic model. The associations identified in the latter study were estimated using linear mixed model assuming an infinitesimal model. METAL20 was used to perform fixed-effect inverse variance-weighted meta-analysis.
Genetic instruments for smoking phenotypes were obtained from the most recent meta-analysis on tobacco and alcohol consumption based on over 30 GWASs, which identified 566 genetic variants associated with four smoking phenotypes in over 1.2 million individuals of European ancestry.21 Detailed information about the smoking phenotypes has been described elsewhere.21 Briefly, they included smoking status (Ever/Never), age of smoking initiation, smoking cessation (Current/Former) and number of cigarettes smoked per day (either as a current smoker or former smoker, quantitative measures were binned into five bins or collected with predefined bins as follows: 1 = 1–5, 2 = 6–15, 3 = 16–25, 4 = 26–35, and 5 = 36+ cigarettes per day). The association statistics were generated using a linear mixed model for all phenotypes in family studies and quasi-continuous phenotypes (age of smoking initiation and cigarettes per day) in unrelated individuals. A logistic model was utilized to estimate additive genetic effects for binary phenotypes (smoking status and smoking cessation) in studies of unrelated individuals. Meta-analysis was performed using rareGWAMA.22
The IVs for MR analyses were selected based on the following criteria: (a) r2 measure of LD among instruments <0.01 at a 10-Mb window; (b) P value less than the genome-wide significant level identified in the corresponding study (1 × 10−8 for BMI, 5 × 10−8 for smoking phenotypes); (c) minor allele frequency (MAF) > 0.01; (d) nonpalindromic single-nucleotide polymorphisms (SNPs) (A/T and G/C polymorphisms were considered for removal but there was no ambiguity in effects). We further split the genetic instruments for BMI into those that only affect BMI (“BMI-Only SNP”) and those that affect both smoking and BMI (“BMI&Smoking SNP”) by using the P values in the tobacco use GWAS meta-analysis: If the P value of any of smoking phenotypes was less than .05, it was classified as the “BMI&Smoking SNP”.
2.2 |. Genetic associations of SNPs with lung cancer risk
Summary statistics on lung cancer risk, including odds ratio (OR) estimates and standard errors for instrumental SNPs, were available from the Transdisciplinary Research in Cancer of the Lung (TRICL) and International Lung Cancer Consortium (ILCCO) based on an aggregated GWAS analysis of lung cancer in 29 266 cases and 56 450 controls.23 The associations between instrumental SNPs and histological subtypes of lung cancer were also provided in the original study. These analyses were restricted to European-descent participants, defined as having 80% or more derived European ancestry.
2.3 |. Mendelian randomization
The relationship between BMI, smoking and lung cancer is illustrated in Figure 1. The direct effect of BMI on lung cancer is the effect that formed not via smoking, which is equal to βXZ. The total effect of BMI on lung cancer is the effect of BMI on lung cancer directly plus the effect of BMI on lung cancer via smoking, which is equal to βXZ + αXYβYZ. We performed univariable MR analyses for BMI and smoking on lung cancer risk separately to estimate the total causal effects of BMI on lung cancer and smoking behaviors on lung cancer. To evaluate whether BMI was causal for smoking phenotypes or smoking phenotypes have causal effects on BMI, we conducted bidirectional MR for BMI and smoking phenotypes. Specifically, MR analyses were first performed from BMI to smoking phenotypes, and then the direction was reversed.
FIGURE 1.
Illustration of the total effect and direct effect of BMI on lung cancer. The direct effect of BMI on lung cancer is the effect BMI has on lung cancer not via any other exposure variables, which equal to βXZ. The total effect of BMI on lung cancer is the effect of BMI on lung cancer directly plus the effect of BMI on lung cancer via smoking, which is equal to βXZ + αXYβYZ. BMI, body mass index
To investigate the direct effects of BMI and smoking behaviors on lung cancer, we performed multivariable MR16 analysis, which is an extension of univariable MR that allows detecting causal effects of multiple risk factors jointly.17 Multivariable MR takes into account the relationship between BMI and smoking phenotypes and the fact that the SNPs used in the MR analyses are often associated with both BMI and smoking (ie, SNPs in our analyses were associated with BMI and at least one smoking phenotype with P < .05, the “BMI&Smoking SNP”). The SNPs used to conduct multivariable MR were combinations of IVs of each exposure. We restricted the analysis to SNPs that were clumped on r2 < 0.01 within 10-Mb. MR analyses and clumping were conducted using the packages “TwoSampleMR”24 and “MendelianRandomization” (https://cran.r-project.org/package=MendelianRandomization) in R.
2.4 |. Sensitivity analysis
We applied several MR methods to perform MR analyses. For univariable MR, we used inverse-variance weighted (IVW),25,26 weighted median,27 weighted mode28 and MR-Egger13 approaches. For multivariable MR, we used regression-based IVW16 and Egger.29 We calculated R2 to estimate the proportion of phenotypic variance explained and F-statistics to evaluate the strength of instruments.30 The two-sample conditional F-statistics (FTS)17,31 were also estimated to evaluate the strength of SNP-exposure conditional on other exposures. The intercept test of MR-Egger was used to assess horizontal pleiotropic effects, IGX2 statistic to check the violation of “NOME (no measurement error) assumption,” which states the potential relative bias due to measurement error. The heterogeneity estimated by Cochran’s Q test was to appraise whether any single IV was driving the results and to check for consistency of the analyses with MR assumptions. The outliers in IVW and MR-Egger regression models were identified using RadialMR.32 In addition, we calculated the power of our study using the mRnd power calculator for conventional MR.33
3 |. RESULTS
3.1 |. Genetic instruments
In total, 842 independent SNPs were included as IVs for BMI and 110, 7, 10 and 29 SNPs for smoking phenotypes of smoking status, age of smoking initiation, smoking cessation and cigarettes per day, respectively (Supporting Information Table S1). The F-statistic values (Supporting Information Table S2) for individual SNPs ranged from 30 to 1360, with means of 64, 41, 39, 53 and 92 for BMI, smoking status, age of smoking initiation, smoking cessation and cigarettes per day, respectively. However, the two-sample conditional F-statistics were all less than 10 for BMI and all smoking phenotypes, indicating that the strength of genetic instruments was significantly lower after conditioning on other exposures, which may result in greatly reduced power for multivariable MR to estimate causal effects. Despite a reduction in strength, the BMI instrument had an FTS over 8 reflecting moderate strength.31 The variability explained by these genetic instruments were 7.33% for BMI, 0.71% for smoking status, 0.1% for age of smoking initiation, 0.17% for smoking cessation and 1.03% for cigarettes per day (Supporting Information Table S2). Power calculations for the univariable IVW MR analyses (Supporting Information Figure S1) indicated greater than 80% statistical power to detect an OR bigger than1.077 for per 1 unit increase of BMI, 1.26 for ever smoking, 1.77 for one age younger of smoking initiation, 1.57 for current smoking and 1.213 for per 10 cigarettes increase of cigarettes per day. There were 451 IVs for BMI identified as “BMI&Smoking SNP” and 390 identified as “BMI-Only SNP” (Supporting Information Table S1).
3.2 |. Univariable MR analyses
From the univariable MR analyses (Table 1, Supporting Information Table S3 and Figure S2) we observed that increased BMI has a positive effect on occurrence of overall lung cancer when using all BMI associated SNPs (OR = 1.19, 95% confidence interval (CI) = 1.10–1.28, P = 7.24 × 10−6). This effect was also significant in histological subtypes of lung squamous cell carcinoma (OR = 1.36, 95% CI = 1.22–1.52, P = 3.59 × 10−8) and small cell lung cancer (OR = 1.73, 95% CI = 1.47–2.04, P = 5.50 × 10−11) but not in lung adenocarcinoma (OR = 1.00, 95% CI = 0.91–1.10, P = 0.935) (the first four rows of Table 1). The causal relationships estimated by BMI&Smoking SNP were consistent with the all SNPs set, with a slightly stronger effect (the 5–8 rows of Table 1). The BMI-Only SNP IVs were significant for small cell lung cancer (OR = 1.60, 95% CI = 1.24–2.06, P = 2.70 × 10−4); the associations between BMI and other lung cancer subtypes were weak or nonexistent (the last four rows of Table 1). As smoking behaviors are confounders of BMI and lung cancer, the BMI-Only SNPs, which have excluded genetic variants that have significant association with smoking phenotypes, should be instruments that are more valid for BMI in univariable MR analysis. The univariable MR using BMI-Only SNP estimates the total effect of BMI on lung cancer.17
TABLE 1.
Causal relationships of BMI on lung cancer and histological subtypes estimated by univariable MR
| Outcome | N.SNPs | OR (95% CI) | P |
|---|---|---|---|
| Using BMI-All SNPs | |||
| Overall | 791 | 1.19 (1.10–1.28) | 7.24E–06 |
| Adeno | 800 | 1.00 (0.91–1.10) | .935 |
| Squam | 793 | 1.36 (1.22–1.52) | 3.59E–08 |
| Small | 795 | 1.73 (1.47–2.04) | 5.50E-11 |
| Using BMI&Smoking SNPs | |||
| Overall | 422 | 1.28 (1.15–1.42) | 2.86E–06 |
| Adeno | 430 | 1.09 (0.97–1.22) | .160 |
| Squam | 427 | 1.48 (1.28–1.72) | 1.91E–07 |
| Small | 423 | 1.84 (1.48–2.29) | 3.76E–08 |
| Using BMI-Only SNPs | |||
| Overall | 368 | 1.06 (0.95–1.18) | .287 |
| Adeno | 369 | 0.89 (0.76–1.03) | .121 |
| Squam | 365 | 1.19(1.01–1.40) | .036 |
| Small | 371 | 1.60 (1.24–2.06) | 2.70E–04 |
Abbreviations: Adeno: lung adenocarcinoma; BMI-All SNPs: All genetic instruments associated with BMI; BMI&Smoking SNPs: the genetic instruments associated with both BMI and any one of the four smoking phenotypes; BMI-Only SNPs: the genetic instruments only associated with BMI; N.SNPs: number of SNPs used in MR; Overall: overall lung cancer; Small: small cell lung cancer; Squam: lung squamous cell carcinoma.
Among the four smoking phenotypes (Supporting Information Table S4 and Figure S3), smoking status was positively associated with lung cancer and histological subtypes, which is consistent with known associations of smoking with lung cancer risk. Age of smoking initiation was inversely associated with lung squamous cell carcinoma and small cell lung cancer, which indicated that the earlier an individual started regular smoking, the greater his/her risk of lung squamous cell carcinoma and small cell lung cancer. There was an expected strong evidence of causal effects of cigarettes per day (per 10 cigarettes increase) with lung cancer and histological subtypes (overall: OR = 2.89, 95% CI = 2.33–3.58, P = 3.59 × 10−22; adenocarcinoma: OR = 2.57, 95% CI = 2.06–3.20, P = 6.20 × 10−17; squamous cell: OR = 3.27, 95% CI = 2.60–4.10, P = 1.66 × 10−24; small cell: OR = 3.32, 95% CI = 2.52–4.37, P = 1.80 × 10−17). No significant causal effects of the genetic instrument modeling smoking cessation on lung cancer was observed. The sensitivity analyses using median-weighted IVs was similar for all exposures (ORs were comparable to those estimated by the IVW method), except for smoking cessation. Results for MR Egger regression were also qualitatively similar to results estimated by the IVW method, except smoking cessation for which protective effects were found (Supporting Information Table S4 and Figure S3). These results suggest heterogeneity in instrumental effects for the smoking cessation variable but not for other variables.
The MR analyses for evaluating the effect of BMI on smoking phenotypes yielded consistent results across three SNP sets (Supporting Information Table S5 and Figure S4). Increased BMI has positive effects on smoking status (OR = 1.24, 95% CI = 1.19–1.28, P = 6.60 × 10−36), smoking cessation (OR = 1.14, 95% CI = 1.11–1.18, P = 7.20 × 10−15) and cigarettes per day (OR = 1.39, 95% CI = 1.34–1.44, P = 7.54 × 10−64), while inverse effect on age of smoking initiation (OR = 0.91, 95% CI = 0.90–0.93, P = 3.68 × 10−26). There was limited evidence (Supporting Information Table S5 and Figure S5) for effects of smoking behaviors on BMI. In addition, no significant horizontal pleiotropic effects were detected in MR Egger (for the intercept of MR Egger, all P value more than 0.1). The low IGX2 statistics (less than 80%) for all smoking phenotypes suggest bias due to measurement errors in MR Egger (the last four rows of Supporting Information Table S5).
3.3 |. Multivariable MR analyses
We estimated mutually the effects of smoking and BMI on lung cancer using multivariable MR and observed a directly inverse effect of BMI on lung adenocarcinoma (OR = 0.86, 95% CI = 0.77–0.96, P = .008) and a weak risk effect on small-cell lung cancer (OR = 1.28, 95% CI = 1.06–1.55, P = .011) (Table 2 and Figure 2). After adjustment of BMI and other smoking phenotypes, smoking status still had a direct effect on overall lung cancer, lung adenocarcinoma and lung squamous cell carcinoma, but not on small cell lung cancer. There were positive direct effects of smoking cessation on overall lung cancer, squamous and small-cell lung cancer, but no direct effect on lung adenocarcinoma. Cigarettes per day has direct effects on overall lung cancer, squamous and small cell lung cancer. No significant direct effect was detected for age of smoking initiation on lung cancer risk, while jointly modeling BMI and other smoking phenotypes.
TABLE 2.
Causal relationships of BMI and smoking phenotypes on lung cancer and histological subtypes estimated by multivariable MR
| Exposure | N.SNPs | MVMR-IVW OR (95% CI) | P | MVMR-Egger OR (95% CI) | P | Pinter* |
|---|---|---|---|---|---|---|
| Overall lung cancer | .275 | |||||
| BMI | 776 | 0.95 (0.87–1.03) | .224 | 0.95 (0.88–1.04) | .270 | |
| SmkInit | 55 | 1.48 (1.30–1.70) | 6.87E–09 | 1.59 (1.33–1.90) | 4.62E–07 | |
| AgeSmk | 5 | 0.90 (0.68–1.20) | .477 | 0.91 (0.68–1.21) | .505 | |
| SmkCes | 3 | 1.33 (1.16–1.53) | 4.81E–05 | 1.34 (1.17–1.54) | 3.88E–05 | |
| CigDay | 7 | 1.29 (1.14–1.46) | 5.99E–05 | 1.29 (1.14–1.46) | 5.86E–05 | |
| Lung adenocarcinoma | .597 | |||||
| BMI | 784 | 0.86 (0.77–0.96) | .008 | 0.86 (0.77–0.96) | .008 | |
| SmkInit | 55 | 1.44 (1.21–1.71) | 3.71E–05 | 1.38 (1.09–1.74) | .007 | |
| AgeSmk | 5 | 0.88 (0.61–1.27) | .489 | 0.87 (0.60–1.26) | .473 | |
| SmkCes | 3 | 1.18 (0.99–1.41) | .070 | 1.18 (0.99–1.41) | .073 | |
| CigDay | 8 | 1.08 (0.92–1.26) | .346 | 1.08 (0.92–1.26) | .348 | |
| Lung squamous cell carcinoma | .177 | |||||
| BMI | 780 | 1.02 (0.90–1.16) | .746 | 1.03 (0.91–1.17) | .652 | |
| SmkInit | 58 | 1.54 (1.26–1.88) | 2.82E–05 | 1.75 (1.33–2.29) | 6.12E–05 | |
| AgeSmk | 5 | 0.90 (0.57–1.41) | .636 | 0.91 (0.58–1.42) | .679 | |
| SmkCes | 3 | 1.48 (1.20–1.83) | 2.39E–04 | 1.49 (1.21–1.84) | 1.95E–04 | |
| CigDay | 8 | 1.44 (1.20–1.73) | 1.11E–04 | 1.44(1.20–1.73) | 1.08E–04 | |
| Small cell lung cancer | .806 | |||||
| BMI | 782 | 1.28 (1.06–1.55) | .011 | 1.28 (1.05–1.54) | .012 | |
| SmkInit | 59 | 1.14 (0.84–1.53) | .403 | 1.10 (0.73–1.64) | .649 | |
| AgeSmk | 5 | 0.79 (0.41–1.53) | .485 | 0.79 (0.41–1.53) | .479 | |
| SmkCes | 3 | 1.78 (1.30–2.43) | 3.27E–04 | 1.78 (1.30–2.43) | 3.38E–04 | |
| CigDay | 8 | 1.71 (1.30–2.26) | 1.42E–04 | 1.71 (1.30–2.26) | 1.45E–04 |
Abbreviations: AgeSmk: age of smoking initiation; BMI: body mass index; CigDay: cigarettes per day; MVMR-Egger: multivariable Mendelian randomization using Egger regression; MVMR-IVW: multivariable Mendelian randomization using inverse variance-weighted approach; SmkCes: smoking cessation, Current/Former; SmkInit: smoking status, Ever/Never; N.SNPs: number of SNPs used in MR.
P value for intercept test of multivariable MR Egger.
FIGURE 2.
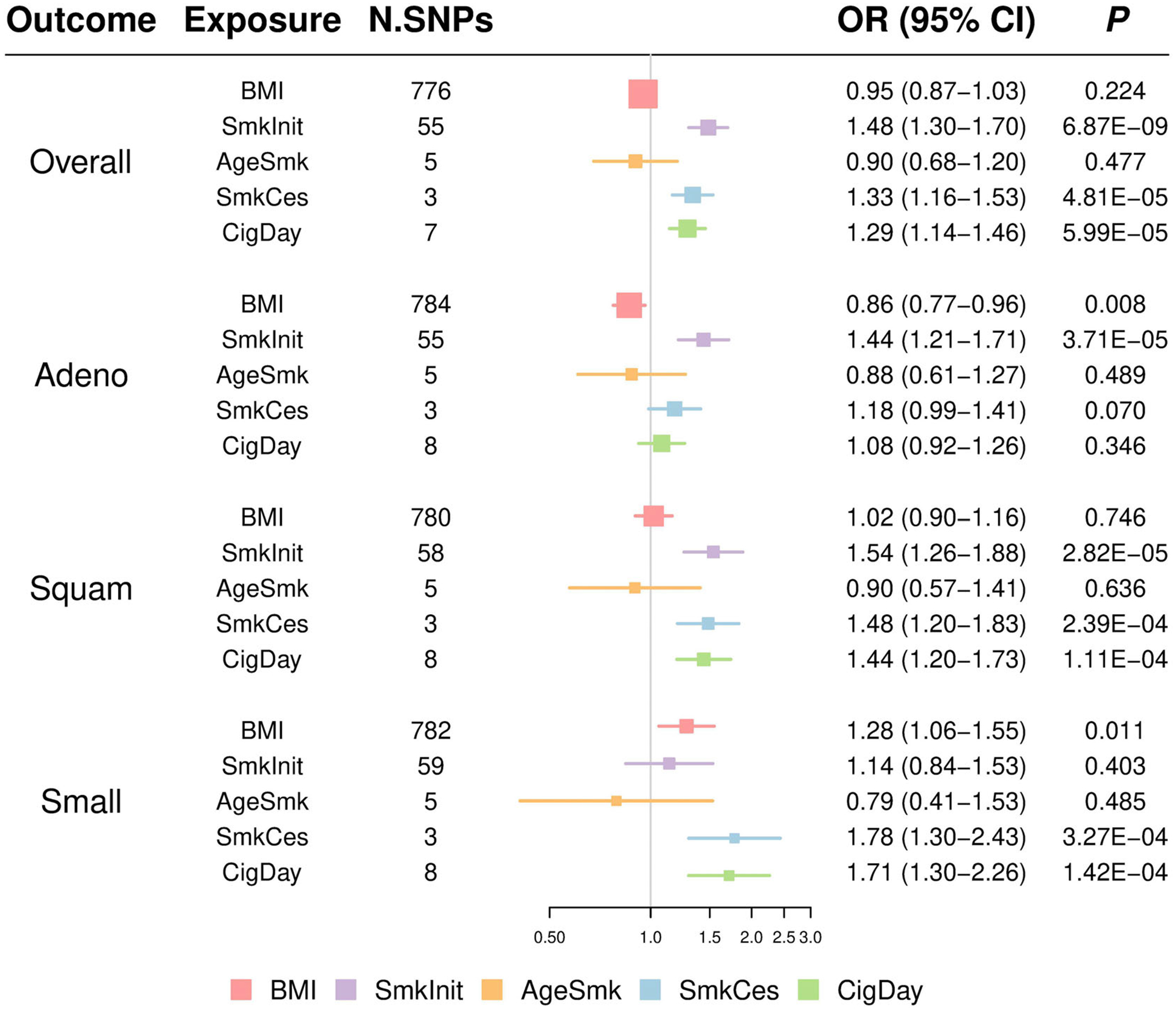
Odds ratios (ORs) and 95% confidence intervals (CIs) for the effect of BMI and smoking phenotypes on lung cancer and histological subtypes estimated using the multivariable Mendelian randomization (MR) inverse-variance weighted approach. Adeno: lung adenocarcinoma; Overall: overall lung cancer; Small: small cell lung cancer; Squam: lung squamous cell carcinoma. Colors indicate five exposures. BMI, body mass index; SmkInit, smoking status; AgeSmk, age of smoking initiation; SmkCes, smoking cessation; CigDay, cigarettes per day. For example, there were 776 BMI-associated SNPs used in multivariable MR jointly with other smoking-associated SNPs to investigate their causal effects on overall lung cancer. No significant effect of BMI on overall lung cancer was observed (OR = 0.95, 95% CI = 0.87–1.03, P = .224)
We also investigated the direct effects between BMI and smoking behaviors using multivariable MR (Supporting Information Table S6 and Table S7). Strong evidence for direct effects of BMI on smoking status and cigarettes per day was observed, as well as inverse effect on age of smoking initiation. There was also some evidence for direct effects of smoking behaviors on BMI, such as smoking status, smoking cessation and cigarettes per day.
3.4 |. Sensitivity analysis
Since genome-wide significant level for each exposure was determined according to the corresponding study of its genetic instruments,19,21 the thresholds used for BMI (1 × 10−8) and smoking phenotypes (5 × 10−8) were different. To provide a sensitivity analysis, we selected IVs based on instruments satisfying either 5 × 10−8 or 1 × 10−8 and performed additional MR analyses. As shown in Supporting Information Table S8–S10, the causal effects of BMI and smoking behaviors on lung cancer were robust using different thresholds.
In univariable MR analyses, the IGX2statistics of MR Egger for BMI and smoking phenotypes on lung cancer (Supporting Information Table S3 and Table S4) were less than 90%, except that of cigarettes per day on lung cancer and its subtypes (ranging from 96.2%−96.5%). The lower values of IGX2 indicate violation of the “NOME” assumption in the causal estimates due to variations in the genetic associations. In addition, the Cochran’s Q test in the IVW model and MR Egger model (Supporting Information Table S11) suggested that there was strong evidence of heterogeneity in most of the IVs. However, we did not observe significant directional pleiotropy using MR Egger, as the P value for the intercept tests was not significant (Supporting Information Table S3 and Table S4), which suggests balanced pleiotropy13 in the genetic instruments of univariable MR analyses. Balanced pleiotropy means that the pleiotropic effects of genetic instruments are balanced around the overall effect. The symmetry in the funnel plots also supported balanced pleiotropy (Supporting Information Figure S6–S7). However, outliers in the scatter plot suggested the presence of heterogeneity (Supporting Information Figure S8–S9). The outliers in the analysis of univariable MR for smoking cessation on lung cancer risk were rs518425 (intronic of CHRNA5) and rs56113850 (intronic of CYP2A6). The effects of these two large-effect SNPs appear to be opposite for BMI phenotypes, which explains the symmetry. After removing these two SNPs, no heterogeneity for smoking cessation remained and the causal relationship between smoking cessation and lung cancer was significant (Supporting Information Table S12 and Figure S10). In addition, the estimates of MR IVW, MR-weighted median, MR-weighted mode and MR Egger show nearly consistent effects except for smoking cessation, which has strong evidence of heterogeneity (Supporting Information Figure S2–S5).
The estimates were robust between multivariable MR IVW and multivariable MR Egger analyses, which suggested reliable evaluation of causal effects for BMI and smoking phenotypes on lung cancer risk (Table 2, Figure 2 and Supporting Information Figure S11). In addition, there was no significant evidence for a nonzero intercept of multivariable MR Egger regression, which also supports the reliability of the results for multivariable MR analyses.
3.5 |. MR analyses for lung cancer among ever −/never-smokers
To investigate the role of smoking phenotypes in the causal relationship between BMI and lung cancer, we further performed MR analyses for BMI and smoking phenotypes on ever- vs never-smokers for lung cancer. A positive effect of high BMI on occurrence of lung cancer among ever-smokers was observed using BMI-All SNPs or BMI&Smoking SNPs. An inverse effect of BMI on risk for lung cancer among never-smokers was identified using BMI-Only SNPs (Supporting Information Table S13). After adjustment of smoking phenotypes using multivariable MR, the estimated casual effects of BMI on ever-or never-smokers lung cancer were further attenuated (Supporting Information Table S14). A weakly but not significant protective effect of BMI on lung cancer risk among never smokers was observed, but further much larger studies are needed to evaluate these associations.
4 |. DISCUSSION
In this study, we observed that adjusted for smoking, BMI decreased the risk of lung adenocarcinoma and increased the risk of small-cell lung cancer. BMI increased the risk of lung squamous cell carcinoma but this effect was mediated by smoking. This study also highlights the effectiveness and necessity of multivariable MR in MR analyses, especially when large amounts of genetic variants are used as IVs.
Some previous observational studies5,6,34–37 have demonstrated that BMI was associated with decreasing risk of lung cancer, even after adjustment for smoking and other confounders,5 but separating out residual confounding with smoking is difficult in observational analyses. The inverse association for lung adenocarcinoma and the positive association for small-cell carcinoma were also observed in a prospective cohort study.6 There was no heterogeneity between males and females, but the association was stronger in ever-smokers than in never-smokers.6,34,35 In our findings, there was evidence of a causal effect for overall lung cancer among ever-smokers if using the SNPs associated with both BMI and smoking behavior, while reverse effect for overall lung cancer among never-smokers if using the SNPs only associated with BMI, suggests there might be some interactions between smoking phenotypes and BMI in the process of impacting lung carcinogenesis.
Carreras-Torres et al found that increased BMI, as defined by instruments for obesity from Mendelian randomization, was associated with increased risks for lung squamous cell and small cell lung cancers but not for lung adenocarcinomas.38 These were consistent with our univariable MR analyses using SNPs only associated with BMI, which indicated a total effect for BMI on lung cancer.17 Moreover, our multivariable MR analyses also identified direct effects for BMI with the risk of lung adenocarcinomas and small-cell lung cancers. Other Mendelian randomization studies for BMI and lung cancer also identified evidence of an increased risk for overall lung cancer,39 lung squamous cell carcinoma,39,40 and small cell lung cancer,40 as well as evidence of a decreased risk for lung adenocarcinoma.41 However, these analyses have not jointly considered modeling smoking with BMI, and we have seen that there are complex relationships among these factors, such that both need to be jointly modeled.
Smoking behavior is a confounder when evaluating the association between BMI and lung cancer. Previous MR study confirmed the role of obesity in smoking behaviors.9 By using multivariable MR, we removed bias caused by smoking phenotypes compared to using univariable MR. In the bidirectional analysis, we found that there was a much stronger path from BMI to smoking phenotypes rather than from smoking phenotypes to BMI, which indicates the role of smoking as a potential mediator between BMI and lung cancer risk. On the other hand, the effects of smoking (such as smoking cessation and cigarettes per day) on BMI were also observed. These results implied complicated effects of BMI and smoking behaviors on lung cancer risk. Further analyses such as network Mendelian randomization, which uses genetic instruments to investigate mediation in causal pathways, may provide insights into the causal relationships.42
The possible mechanism between obesity and lung cancer has been investigated in previous studies.43 Adipokines, which are secreted by adipose tissue, have properties affecting carcinogenesis, immunomodulation, appetite and energy homeostasis regulation, and variations in levels have been associated with tumor susceptibility and pathogenesis.44,45 In particular, leptin seems to mediate and maintain chronic inflammation after exposure to inhaled antigens (eg, smoke particles) and induce lung carcinogenesis.46 In addition, after adding recombinant human leptin, cell proliferation was observed in SQ-5 human clonal squamous lung cancer-derived cell lines.47 Adiponectin, unlike leptin, correlates inversely with body weight and is considered a protective hormone that exerts anti-inflammatory effects. Petridou et al48 demonstrated the expression of adiponectin receptors exclusively in lung cancer tissues, which supports the hypothesis that adiponectin mediates lung cancer development. Nevertheless, the role of obesity in lung carcinogenesis remains unclear and inconclusive. As obesity may influence different histological subtypes of lung cancer, further research is warranted to elucidate the role of obesity in lung cancer.
Although two sample Mendelian randomization is a powerful approach to make causal inference between exposures and outcome using summary statistics, we should be prudent with our findings because of several limitations. First, sample overlap can result in inflation of test results,49 but we expect little effect since there are no known overlaps in samples. In addition, the sample size for histological subtypes of lung cancer was limited, which will could lead to false-negative errors (lung adenocarcinoma: 11273 cases and 55 483 controls; lung squamous cell carcinoma: 7426 cases and 55 627 controls; small-cell lung cancer: 2664 cases and 21 444 controls; ever-smokers lung cancer: 23223 cases and 16 964 controls; never-smokers lung cancer: 2355 cases and 7504 controls23). Moreover, according to previous studies,50,51 if the situation of both exposure and outcome is binary, Wald-type estimators could introduce bias for causal OR. The causal estimations between binary smoking phenotypes (such as smoking status and smoking cessation) and lung cancer should be evaluated carefully. Nevertheless, we believe that the impact is small since the estimation will be close to the true value if the sample size is large,15 and we also had a large number of IVs. Furthermore, the BMI changes with age and the MR analysis was designed to estimate the lifetime effects of exposure,52 but the genetic instruments used here were associated with BMI at a specific age interval, so it may impact interpretability and validity of the results.
Taken together, we demonstrate that high BMI has direct inverse association with risk of lung adenocarcinoma and positive causal direct effects with small cell lung cancer. The total effects of BMI on lung squamous cell carcinoma and small cell lung cancer may be mediated by smoking phenotypes. These findings reveal heterogeneity of causal effects of BMI on histological subtypes of lung cancer. We expect that further investigations are needed to demystify the complex causal relationships between BMI, smoking and lung cancer. Our findings also suggest that future studies could explore the relevance of change BMI for evaluation risk of lung cancer.
Supplementary Material
What’s new?
Lung cancer risk appears to be inversely correlated with BMI, which could be due to a variety of factors. Here, the authors used Mendelian randomization (MR) to look for a causal effect of BMI on lung cancer. MR uses genetic variants as instrumental variables, and avoids the effects of confounding factors. However, linkage disequilibrium with causal variants may interfere with the results. After adjustment for smoking, the authors found a direct causal effect of BMI on small cell lung cancer, and an inverse effect on lung adenocarcinoma. These results highlight that the effect of BMI varies significantly depending on histology.
ACKNOWLEDGEMENTS
Dr. Amos is a research scholar of the Cancer Prevention Research Institute of Texas (CPRIT). His research is partially funded by CPRIT grant RR170048 and by NIH/NCI grant U19CA203654. Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization. The Boston Lung Cancer Study was funded by NIH (NCI) U01CA209414 (PI: Christiani). The EAGLE study was supported by the Intramural Research Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, DHHS. The Multiethnic Cohort Study is supported by the National Institutes of Health (CA164973). The CARET study was supported by the National Institutes of Health/National Cancer Institute: UM1 CA167462 (PI: Goodman), U01CA6367307 (PIs: Omen, Goodman), R01 CA111703 (PI: Chen) and U01 CA167462 (PI: Chen). Philip C Haycock is supported by Cancer Research UK (C18281/A19169). Wen Zhou was supported by China Scholarship Council and Nanjing Medical University.
Abbreviations:
- BMI
body mass index
- CI
confidence interval
- FTS
two-sample conditional F-statistic
- GIANT
Genetic Investigation of ANthropometric Traits
- GWAS
Genome wide association study
- GSCAN
GWAS and Sequencing Consortium of Alcohol and Nicotine use
- ILCCO
International Lung Cancer Consortium
- IV
instrumental variable
- IVW
inverse-variance weighted
- LD
linkage disequilibrium
- MAF
minor allele frequency
- MR
Mendelian randomization
- MVMR
multivariable Mendelian randomization
- NOME
no measurement error
- OR
odds ratio
- SNP
single-nucleotide polymorphism
- TRICL
Transdisciplinary Research in Cancer of the Lung
- UVMR
univariable Mendelian randomization
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
Each study has been approved by the Ethical Committees of the original studies.
DATA AVAILABILITY STATEMENT
GWAS summary statistics for BMI can be downloaded from the GIANT consortium website (https://portals.broadinstitute.org/collaboration/giant/index.php/GIANT_consortium_data_files) and from the Program in Complex Trait Genomics website (https://cnsgenomics.com/content/data). GWAS summary statistics for tobacco use can be downloaded from the GWAS and Sequencing Consortium of Alcohol and Nicotine use (GSCAN) consortium website (https://genome.psych.umn.edu/index.php/GSCAN). Other data that support the findings of our study are available on request from the corresponding author.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5[C1]24 million UK adults. Lancet. 2014;384:755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y, Dong J, Sun K, et al. Obesity and incidence of lung cancer: a meta-analysis. Int J Cancer. 2013;132:1162–1169. [DOI] [PubMed] [Google Scholar]
- 3.Song X, Pukkala E, Dyba T, et al. Body mass index and cancer incidence: the FINRISK study. Eur J Epidemiol. 2014;29:477–487. [DOI] [PubMed] [Google Scholar]
- 4.Smith L, Brinton LA, Spitz MR, et al. Body mass index and risk of lung cancer among never, former, and current smokers. J Natl Cancer Inst. 2012;104:778–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dewi NU, Boshuizen HC, Johansson M, et al. Anthropometry and the risk of lung cancer in EPIC. Am J Epidemiol. 2016;184:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu D, Zheng W, Johansson M, et al. Overall and central obesity and risk of lung cancer: a pooled analysis. J Natl Cancer Inst. 2018;110: 831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Zein M, Parent ME, Nicolau B, Koushik A, Siemiatycki J, Rousseau MC. Body mass index, lifetime smoking intensity and lung cancer risk. Int J Cancer. 2013;133:1721–1731. [DOI] [PubMed] [Google Scholar]
- 8.Schmitt R Quality of life issues in lung cancer. Chest. 1993;103: 51S–55S. [PubMed] [Google Scholar]
- 9.Carreras-Torres R, Johansson M, Haycock PC, et al. Role of obesity in smoking behaviour: Mendelian randomisation study in UK Biobank. BMJ. 2018;361:k1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winslow UC, Rode L, Nordestgaard BG. High tobacco consumption lowers body weight: a Mendelian randomization study of the Copenhagen general population study. Int J Epidemiol. 2015;44: 540–550. [DOI] [PubMed] [Google Scholar]
- 11.Malhotra J, Malvezzi M, Negri E, La Vecchia C, Boffetta P. Risk factors for lung cancer worldwide. Eur Respir J. 2016;48:889–902. [DOI] [PubMed] [Google Scholar]
- 12.Smith GD, Ebrahim S. “Mendelian randomization”: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1–22. [DOI] [PubMed] [Google Scholar]
- 13.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. 2015;44:512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Didelez V, Sheehan N. Mendelian randomization as an instrumental variable approach to causal inference. Stat Methods Med Res. 2007; 16:309–330. [DOI] [PubMed] [Google Scholar]
- 15.Didelez V, Meng S, Sheehan NA. Assumptions of IV methods for observational epidemiology. Statist Sci. 2010;25:22–40. [Google Scholar]
- 16.Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. 2015;181:251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanderson E, Davey Smith G, Windmeijer F, Bowden J. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol. 2019;48: 713–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yengo L, Sidorenko J, Kemper KE, et al. Meta-analysis of genome-wide association studies for height and body mass index in approximately 700000 individuals of European ancestry. Hum Mol Genet. 2018;27:3641–3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu M, Jiang Y, Wedow R, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019;51:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu DJ, Peloso GM, Zhan X, et al. Meta-analysis of gene-level tests for rare variant association. Nat Genet. 2014;46:200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKay JD, Hung RJ, Han Y, et al. Large-scale association analysis identifies new lung cancer susceptibility loci and heterogeneity in genetic susceptibility across histological subtypes. Nat Genet. 2017; 49:1126–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemani G, Zheng J, Elsworth B, et al. The MR-base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37:658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG, Consortium E-I. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. 2015;30:543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40: 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46:1985–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rees JMB, Wood AM, Burgess S. Extending the MR-egger method for multivariable Mendelian randomization to correct for both measured and unmeasured pleiotropy. Stat Med. 2017;36:4705–4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer TM, Lawlor DA, Harbord RM, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res. 2012;21:223–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanderson E, Spiller W, Bowden J. Testing and correcting for weak and pleiotropic instruments in two-sample multivariable mendelian randomisation. bioRxiv. 2020. 10.1101/2020.04.02.021980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bowden J, Spiller W, Del Greco MF, et al. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the radial plot and radial regression. Int J Epidemiol. 2018;47:1264–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. 2013;42:1497–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Everatt R, Virviciute D, Kuzmickiene I, Tamosiunas A. Body mass index, cholesterol level and risk of lung cancer in Lithuanian men. Lung Cancer. 2014;85:361–365. [DOI] [PubMed] [Google Scholar]
- 35.Duan P, Hu C, Quan C, et al. Body mass index and risk of lung cancer: systematic review and dose-response meta-analysis. Sci Rep. 2015;5: 16938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benn M, Tybjaerg-Hansen A, Smith GD, Nordestgaard BG. High body mass index and cancer risk-a Mendelian randomisation study. Eur J Epidemiol. 2016;31:879–892. [DOI] [PubMed] [Google Scholar]
- 37.Sanikini H, Yuan JM, Butler LM, et al. Body mass index and lung cancer risk: a pooled analysis based on nested case-control studies from four cohort studies. BMC Cancer. 2018;18:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carreras-Torres R, Haycock PC, Relton CL, et al. The causal relevance of body mass index in different histological types of lung cancer: a Mendelian randomization study. Sci Rep. 2016;6:31121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao C, Patel CJ, Michailidou K, et al. Mendelian randomization study of adiposity-related traits and risk of breast, ovarian, prostate, lung and colorectal cancer. Int J Epidemiol. 2016;45:896–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carreras-Torres R, Johansson M, Haycock PC, et al. Obesity, metabolic factors and risk of different histological types of lung cancer: a Mendelian randomization study. PLoS One. 2017;12:e0177875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brennan P, McKay J, Moore L, et al. Obesity and cancer: Mendelian randomization approach utilizing the FTO genotype. Int J Epidemiol. 2009;38:971–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burgess S, Daniel RM, Butterworth AS, Thompson SG, Consortium EP-I. Network Mendelian randomization: using genetic variants as instrumental variables to investigate mediation in causal pathways. Int J Epidemiol. 2015;44:484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ntikoudi E, Kiagia M, Boura P, Syrigos KN. Hormones of adipose tissue and their biologic role in lung cancer. Cancer Treat Rev. 2014;40:22–30. [DOI] [PubMed] [Google Scholar]
- 44.Chen J Multiple signal pathways in obesity-associated cancer. Obes Rev. 2011;12:1063–1070. [DOI] [PubMed] [Google Scholar]
- 45.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11:886–895. [DOI] [PubMed] [Google Scholar]
- 46.Ribeiro R, Araújo A, Lopes C, Medeiros R. Immunoinflammatory mechanisms in lung cancer development: is leptin a mediator? J Thorac Oncol. 2007;2:105–108. [DOI] [PubMed] [Google Scholar]
- 47.Tsuchiya T, Shimizu H, Horie T, Mori M. Expression of leptin receptor in lung: leptin as a growth factor. Eur J Pharmacol. 1999;365: 273–279. [DOI] [PubMed] [Google Scholar]
- 48.Petridou ET, Mitsiades N, Gialamas S, et al. Circulating adiponectin levels and expression of adiponectin receptors in relation to lung cancer: two case-control studies. Oncology. 2007;73:261–269. [DOI] [PubMed] [Google Scholar]
- 49.Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol. 2016;40: 597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palmer TM, Sterne JA, Harbord RM, et al. Instrumental variable estimation of causal risk ratios and causal odds ratios in Mendelian randomization analyses. Am J Epidemiol. 2011;173:1392–1403. [DOI] [PubMed] [Google Scholar]
- 51.Disney-Hogg L, Cornish AJ, Sud A, et al. Impact of atopy on risk of glioma: a Mendelian randomisation study. BMC Med. 2018;16:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Labrecque JA, Swanson SA. Interpretation and potential biases of Mendelian randomization estimates with time-varying exposures. Am J Epidemiol. 2019;188:231–238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


