Abstract
Objectives
To characterize real-world prescribing patterns and their clinical and healthcare resource utilization (HRU) implications in patients with metastatic renal cell carcinoma (mRCC) treated in Germany.
Methods
Eligible individuals were enrolled in the “Bundesverband der Betriebskrankenkassen” claims database and received targeted mRCC therapy between 1 January 2008 and 31 December 2016. Prescribing patterns and HRU were characterized by treatment line and summarized by descriptive statistics. Proxy progression-free survival (PFS) and overall survival (OS) were estimated using Kaplan-Meier curves.
Results
536 patients receiving mRCC treatment were included. The median treatment duration was 4.2 months (interquartile range [IQR]: 1.7–9.3) for first-line therapy and 3.8 months (IQR: 1.7–9.1) for second-line therapy. Median PFS and OS estimates were similar for the first- and second-line treatments: PFS, 7.4 versus 7.2 months; OS, 14.9 versus 13.6 months. Mean HRU costs were higher for patients receiving first-line therapy (€7,253.2) compared with those receiving second-line therapy (€6,242.9). Exploratory stratification of outcomes by centre expertise suggested a possible trend towards improved OS in the 10 most experienced centres versus all −others: first-line, 18.4 versus 13.2 months; second-line, 16.4 versus 12.4 months.
Conclusions
In routine care, German clinicians make rational prescribing decisions; possible variations in outcomes between centres warrant further investigation.
Keywords: Renal cell carcinoma, Sickness fund database, Treatment patterns and healthcare resources, Real-world practice, Retrospective observational study
Introduction
Renal cell carcinoma (RCC) is the world's twelfth most common cancer and accounts for >140,000 deaths per year [1, 2]. The incidence reported in European countries ranges from 13.5 to 31.4/100,000 person-years in men; the incidence in women is about half of that in men [3]. In 2008, the incidence of RCC was reported to be 22/100,000 for men and 10/100,000 for women [4]. In Germany, the estimated number of newly diagnosed RCC cases was 15,500 in 2014 [5, 6]. RCC affects men more frequently than women (63 vs. 37%), representing the eighth and tenth most frequent cancers for each sex in Germany, respectively [5, 6]. In Germany, the mean age at diagnosis is 68 years for men and 71 years for women [5, 6].
Risk factors for the development of RCC include smoking, obesity, hypertension, end-stage renal disease, acquired renal cystic disease, dialysis, receipt of a transplanted kidney, and tuberous sclerosis [7]. In addition, autosomal dominant syndromes, such as von Hippel-Lindau disease, account for 2-3% of all RCCs [8].
In the past decade, some of the identified targeted therapies approved for the treatment of metastatic RCC (mRCC) have included the use of tyrosine kinase inhibitors (TKIs; e.g., sunitinib, sorafenib, pazopanib, axitinib, and tivozanib). In addition to these TKIs, treatment options for mRCC include a monoclonal antibody (bevacizumab in combination with interferon alpha), mTOR inhibitors (temsirolimus and everolimus), and high-dose interleukin-2, which can be used as first-, second-, or later-line therapies [2, 9]. Since 2016, a new generation of drugs has been approved in the treatment of mRCC, including the immune checkpoint inhibitor nivolumab and the TKI cabozantinib.
The aim of the present study was to analyze treatment patterns in real-world practice in Germany and to estimate the outcomes and healthcare resource utilization (HRU) costs of patients treated with targeted therapies for mRCC, using information from the “Bundesverband der Betriebskrankenkassen” (BKK) sickness fund database for patients who were insured from 2008 to 2016.
Materials and Methods
Study Design
A retrospective, observational cohort study was conducted using routinely collected administrative claims data for patients with RCC and mRCC in Germany. Data were obtained from the BBK, a sickness fund claims database comprising records for up to 5.0 million insured individuals who were covered by statutory health insurance from 2008 to 2016. Patient data were fully anonymized (in compliance with the German data protection regulations). Analyses were performed by the Team Gesundheit Gesellschaft für Gesundheitsmanagement GmbH, Essen, Germany. All required study approvals were secured.
Data were collected for patients diagnosed with mRCC, according to the International Classification of Diseases, Tenth Revision, German Modification (ICD-10-GM) C64 (representing malignant neoplasms of the kidney and excluding neoplasms of the renal pelvis). To account for the length of the pre- and post-index periods, patients were enrolled between January 1, 2010, and December 31, 2015.
Inclusion and Exclusion Criteria
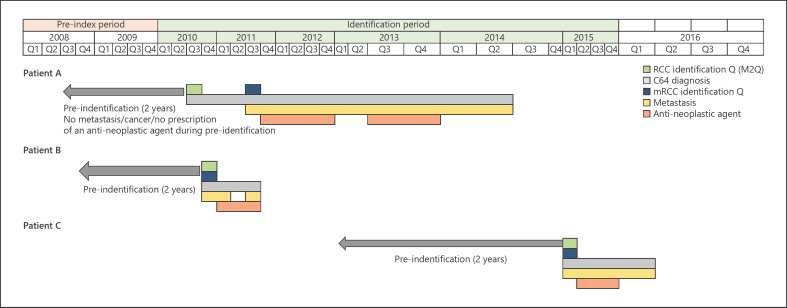
For inclusion in the analyses, patients had to be 18 years of age or older in the year of diagnosis with RCC, which could be either a hospital diagnosis or a diagnosis resulting from 2 separate outpatient visits. Patients had to be continuously enrolled in the BKK database for at least 24 months before identification and to have had at least one occurrence of metastasis (coded as secondary diagnosis in ICD-10-GM) or prescription of any anti-neoplastic agent used to treat mRCC; at least one ICD-10-GM code of C64 had to be present within the same quarter (Fig. 1). Patients were excluded if they had a diagnosis of other cancers or metastases during the pre-index period, except for non-melanoma skin cancers and a prescription of an anti-neoplastic agent that was administered to treat mRCC during the pre-index period.
Fig. 1.
VWe note that the following footnote which was submitted with this figure is missing: ‘Patient A: received two lines of therapy and survived beyond the end of their 949-day continuous enrolment; Patient B: died 192 days after initiating 1L therapy; Patient C: received two lines of therapy and died 313 days after initiating 1L (and 101 days after initiating 2L) therapy.’isualizations of the dates of RCC and mRCC onset (based on diagnostic codes) and anti-neoplastic agent treatment period(s) for 3 example patients included in the study. RCC, renal cell carcinoma; mRCC, metastatic renal cell carcinoma. Patient A received two lines of therapy and survived beyond the end of their 949-day continuous enrolment. Patient B died 192 days after initiating 1L therapy. Patient C received two lines of therapy and died 313 days after initiating 1L (and 101 days after initiating 2L) therapy.
Treatment Outcomes
The routine BKK billing data were used to describe HRU for patients with RCC. Prescribed therapy (drug prescription data), outpatient and ambulatory treatment, inpatient treatment/hospitalization, sickness absence, and other costs incurred were evaluated quarterly, reflecting the frequency of BKK database updates.
Initial treatment with an mRCC-specific anti-neoplastic agent was defined as first-line therapy. If treatment was discontinued (because of lack of efficacy or adverse events), a further agent was initiated and defined as second-line therapy (Fig. 2a). For first-line therapy, the active treatment period for a new prescription was the time from the start of therapy (index date) until the end of therapy (+28 days), to adjust for the intake time of medication from the last prescription. The surveillance treatment period was the time after the end of first-line therapy (>120-day gap in therapy) until second-line therapy (Fig. 2b). Patient follow-up was divided into a pre-index period (365 days to index date), an active treatment period (when the effective treatment was taken), a post-index period (time between end of treatment), death (if the patient died before new therapy initiation) or start of new treatment, and an end of the study period (December 31, 2016; Fig. 1).
Fig. 2.
Visualization of the line of therapy (a), duration of treatment (b), OS (c), and PFS (d) for 3 example patients included in the study. OS, overall survival; PFS, progression-free survival; 1L, first-line; 2L, second-line. Patient A received two lines of therapy and survived beyond the end of their 949-day continuous enrolment. Patient B died 192 days after initiating 1L therapy. Patient C received two lines of therapy and died 313 days after initiating 1L (and 101 days after initiating 2L) therapy.
Clinical outcomes included the following: duration of treatment, proxy progression-free survival (PFS), and proxy overall survival (OS). OS was calculated as the time between index date (either date of mRCC diagnosis or therapy initiation) and the end of follow-up which was at the end of the study period (if a patient survived at least until December 31, 2016), death, or end of continuous enrolment (Fig. 2c). PFS was estimated as the time from therapy initiation until the earliest of first disease recurrence/start of new line of therapy, end of continuous enrolment (presumably death), or end of the study period (censored) (Fig. 2d).
Bivariate analyses were used to examine all study measures, stratified by treatment line and prescribed therapy. PFS and OS were analyzed using Kaplan-Meier curves, hazard ratios (HR), and 95% confidence intervals (95% CI). Continuous variables were summarized by descriptive statistics (mean, standard deviation [SD], median, and interquartile range [IQR]). For all statistical tests, statistical significance was assumed at p < 0.05.
Healthcare Resource Utilization
The HRU and costs (€) were normalized and expressed as expenditures per month. HRU was measured in terms of costs in the pre-, active, and surveillance treatment periods, in order to assess the impact of treatment initiation on HRU. An exploratory analysis stratified the results by level of centre expertise: 10, 20, and 30 most experienced centres compared with all others. The 10 most experienced centres were identified by expert opinion. A further 20 centres were identified using the bed number as a proxy for experience.
Results
Patient Demographics
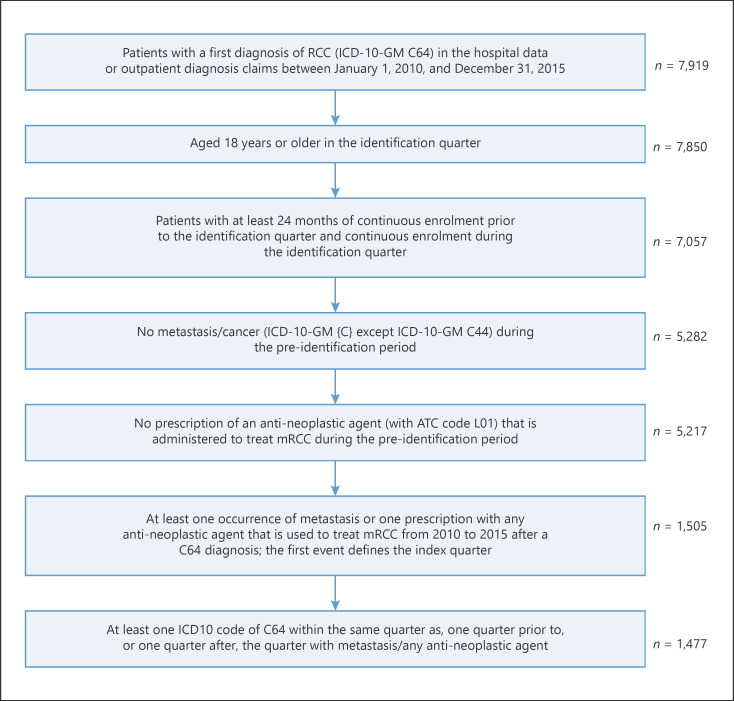
Between January 1, 2010, and December 31, 2015, a total of 7,919 patients received a diagnosis of RCC (Fig. 3), of which 536 patients received a specific anti-neoplastic agent and were eligible for inclusion (Table 1). The mean patient age at diagnosis was 66.7 years; most patients were men (73.7%).
Fig. 3.
Selection of mRCC study population. RCC, renal cell carcinoma; mRCC, metastatic renal cell carcinoma.
Table 1.
Study population stratified by therapeutic exposure
| Treated | Final study population | N | % | N |
|---|---|---|---|---|
| Yes | No specific anti-neoplastic agent | 236 | 16.0 | 772 |
| Yes | Specific anti-neoplastic agent | 536 | 36.3 | |
| No | No treatment at all | 705 | 47.7 | 705 |
| Total | 1,477 | 100.0 | 1,477 | |
Of the 536 patients who received first-line mRCC therapy, 286 (53.4%) also received second-line therapy. Overall, 83.6% of patients were prescribed sunitinib, pazopanib, or temsirolimus as first-line therapy and 79.7% of patients were prescribed second-line everolimus, sunitinib, axitinib, pazopanib, or sorafenib (Table 2). The mean (SD) age of patients receiving first- and second-line treatments was 67.1 (11.1) years and 65.5 (10.8) years, respectively. The ratio of men to women was approximately 3:1 irrespective of treatment line.
Table 2.
Most commonly prescribed therapies, by treatment line
| Treatment | N (%) |
|---|---|
| Patients receiving an agent specific to first-line treatment (N1 = 536)a | |
| Sunitinib (L01XE04) | 264 (49.3) |
| Pazopanib (L01XE11) | 136 (25.4) |
| Temsirolimus (L01XE09) | 48 (9.0) |
| Others | 88 (16.4) |
| Patients receiving an agent specific to second-line treatment (N2= 286)b | |
| Everolimus (L01XE10) | 62 (21.7) |
| Sunitinib (L01XE04) | 49 (17.1) |
| Axitinib (L01XE17) | 48 (16.8) |
| Pazopanib (L01XE11) | 38 (13.3) |
| Sorafenib (L01XE05) | 31 (10.8) |
| Others | 58 (20.3) |
The 3 most commonly prescribed first-line therapies account for n = 448 counts.
The 5 most commonly prescribed second-line therapies account for n = 228 counts.
Treatment Outcomes
The median (IQR) treatment duration was 4.2 (1.7–9.3) months for first-line therapy and 3.8 (1.7–9.1) months for second-line therapy. The median treatment duration for first-line agents was 5.8 months for pazopanib (n = 136), 4.2 months for sunitinib (n = 264), and 2.0 months for temsirolimus (n = 48). The median treatment duration for second-line agents was 4.6 months for axitinib (n = 48), 3.3 months for everolimus (n = 62), 4.5 months for pazopanib (n = 38), 3.0 months for sorafenib (n = 31), and 3.8 months for sunitinib (n = 50).
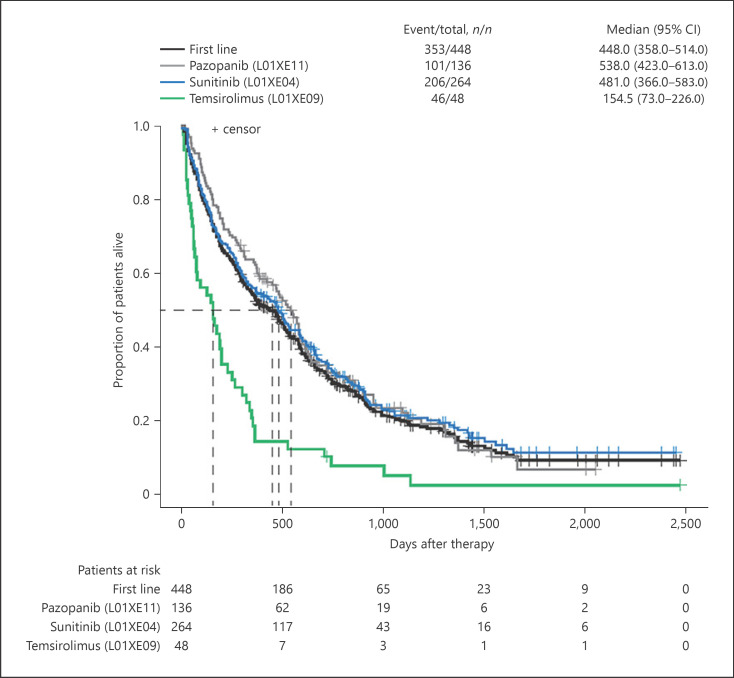
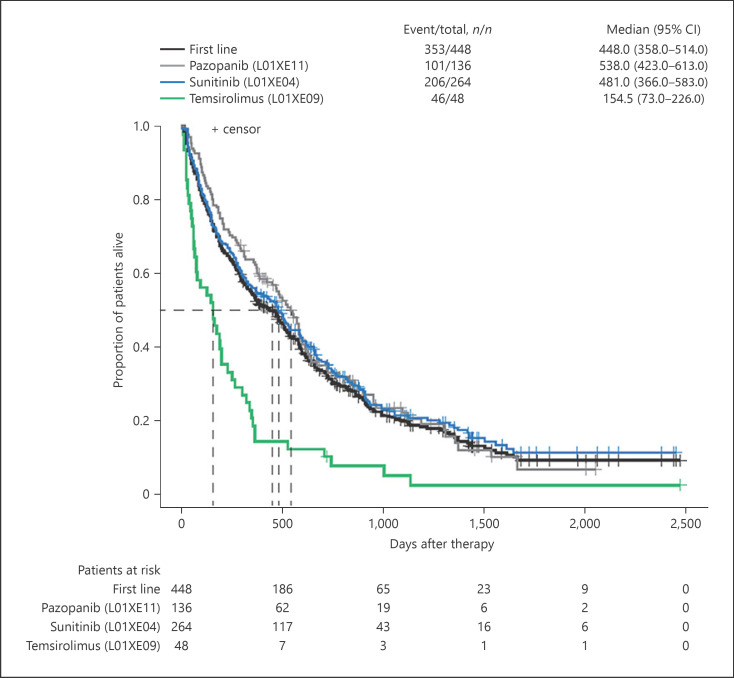
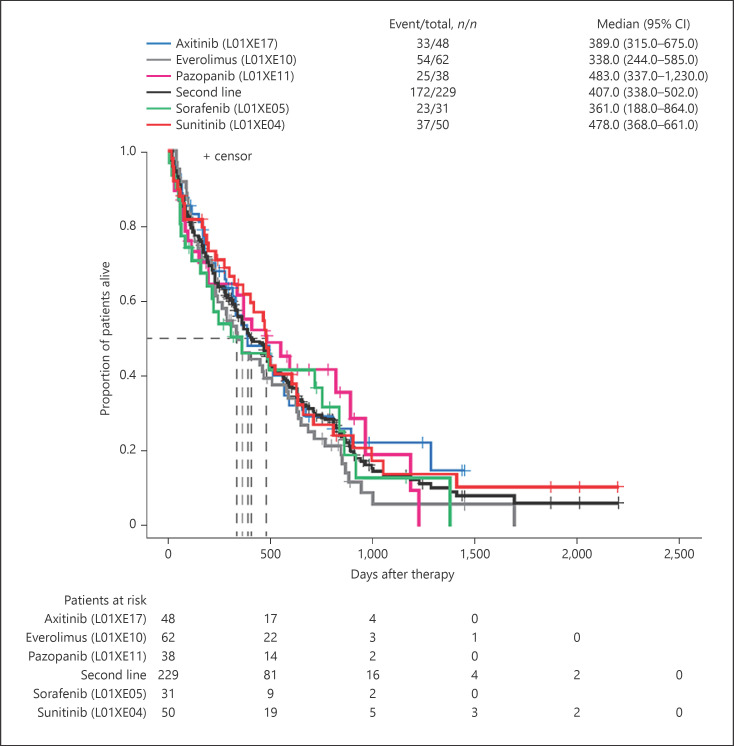
The median (95% CI) OS was 14.9 (11.9–17.1) months for first-line therapy and 13.6 (11.3–16.7) months for second-line therapy. Using median (95% CI) OS for temsirolimus as the reference for first-line therapies (5.1 [2.4–7.5] months), patients treated with pazopanib recorded the longest median OS of 17.9 months (HR [95% CI]: 0.40 [0.28–0.56]; p < 0.0001) and patients treated with sunitinib recorded a median OS of 16.0 months (HR [95% CI]: 0.41 [0.30–0.56]; p < 0.0001) (Fig. 4). For second-line therapies, median (95% CI) OS for sunitinib was used as the reference (15.9 [12.3–22.0] months). Median OS was longest among patients re-treated with pazopanib (16.1 months, HR [95% CI]: 1.02 [0.61–1.70]) and shortest for those receiving everolimus (11.3 months, HR [95% CI]: 1.31 [0.86–2.0]). The median OS for sorafenib was 12.0 months (HR [95% CI]: 1.22 [0.73–2.07]) and that for axitinib was 13.0 months (HR [95% CI]: 1.0 [0.63–1.61]) (Fig. 5).
Fig. 4.
The OS for patients in 1L therapy overall and per specified agent. OS, overall survival; 1L, first-line.
Fig. 5.
The OS for patients in 2L therapy overall and per specified agent. OS, overall survival; 2L, second-line.
Median PFS among patients treated with first- and second-line therapies was similar, at 7.4 (95% CI: 6.3–8.7) months and 7.2 (95% CI: 6.3–9.1) months, respectively. Temsirolimus was used as a reference for PFS comparisons of first-line therapies. There was a statistically significant difference between median (95% CI) PFS for patients treated with temsirolimus (3.5 [2.1–5.8] months) compared with those treated with pazopanib (10.5 months, HR [95% CI]: 0.35 [0.25–0.50]; p < 0.0001) and sunitinib (8.0 months, HR [95% CI]: 0.41 [0.30–0.57]; p < 0.0001). Median (95% CI) PFS for patients treated with sunitinib (7.5 [6.3–14.4] months) was used as a reference for second-line therapy comparisons. Patients re-treated with pazopanib had the longest median PFS (9.1 months, HR [95% CI]: 0.84 [0.50–1.40]; p = 0.15). Median PFS was similar for patients treated with axitinib (7.1 months, HR [95% CI]: 1.04 [0.65–1.67]; p = 0.15) and everolimus (7.1 months, HR [95% CI]: 1.45 [0.95–2.22]; p = 0.15). Among second-line agents, patients receiving sorafenib had the shortest median PFS (3.9 months, HR [95% CI]: 1.28 [0.76–2.15]; p = 0.15).
Healthcare Resource Utilization
The mean (SD) total healthcare cost was generally higher during the post-index period than the pre-index period (€3,033.6 [€2,132.6] vs. €1,384.8 [€1,150.7], respectively). The main cost drivers were outpatient pharmacy (€1,965.8 [€1,717.9]), followed by inpatient costs (€730.3 [€1,204.6]) for the prescribed treatments. Median outpatient pharmacy costs increased from €32.8 in the pre-index to €1,459.0 in the post-index period. Mean (SD) total monthly healthcare costs incurred during the post-index period were higher for patients receiving first-line therapy compared with second-line therapy (€3,761.2 [€7,675.0] vs. €234.3 [€705.2]). The mean (SD) total healthcare cost incurred during the active treatment period was also higher among patients receiving first-line therapy compared with second-line therapy (€7,253.2 [€7,860.4] vs. €6,242.9 [€3,802.7], respectively). For first-line patients, mean (SD) total healthcare costs were approximately twice as high during the index period compared with the post-index period (€7,253.2 [€7,860.4] vs. €3,761.2 [€7,675.0]). The most expensive treatment period was the first-line active treatment period (€7,253.2), during which the major cost drivers were outpatient pharmacy expenditures (€4,885.8), inpatient costs (€1,866.5), and practitioner expenditures (€237.3). Sunitinib was the most expensive in both first- and second-line treatments (€8,014.2/month and €7,632.1/month, respectively).
For first-line therapy, the mean number of outpatient prescriptions in days was higher during the active treatment period than during the post-index treatment period (32.3 vs. 21.5 days). The mean duration of work disability was also longer during the active treatment period than during the post-index treatment period (3.6 vs. 1.5 days). The length of stay was longer during the post-index treatment period (13.2 days) than during the active treatment period (11.6 days).
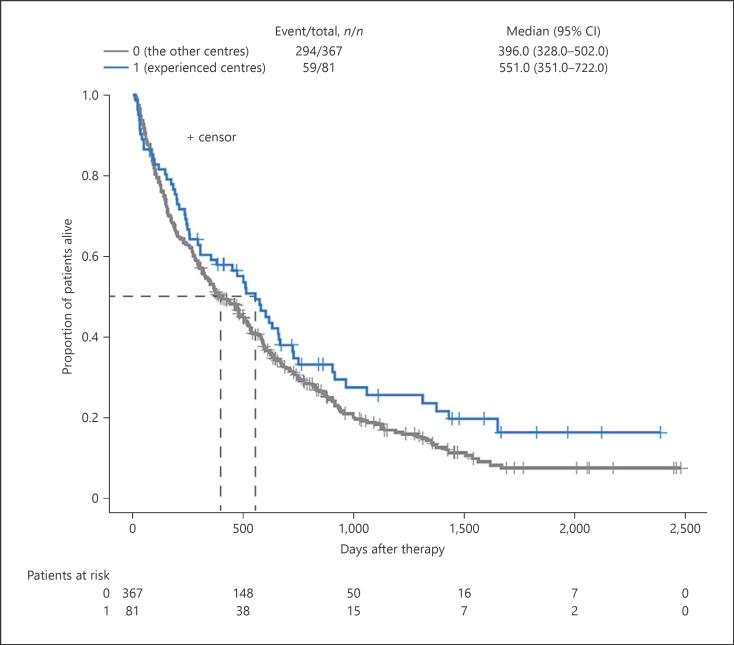
Exploratory stratification of the study results by level of centre expertise found median OS estimates for first-line therapies were longer for the 10, 20, and 30 most experienced German centres compared with all other centres, but the difference was not statistically significant (Table 3; Fig. 6). At the treatment level, however, median OS for first-line sunitinib was significantly higher for the 10 most experienced centres (21.9 vs. 15.3 months, respectively; HR [95% CI]: 1.46 [1.01–2.11]; p = 0.04) and for the 30 most experienced centres (21.9 vs. 12.4 months; HR [95% CI]: 1.36 [1.01–1.84]; p = 0.04; Fig. 7) compared with others.
Table 3.
OS in top 10, 20, and 30 most experienced centres compared with less experienced centres
| Centre experience comparison | First-line treatment |
Second-line treatment |
||||||
|---|---|---|---|---|---|---|---|---|
| n | median OS, months | HR | p value | n | median OS, months | HR | p value | |
| 10 most experienced centres vs. all other centres | 81 vs. 367 | 18.4 vs. 13.2 | 1.27 | 0.09 | 51 vs. 177 | 16.4 vs. 12.4 | 1.35 | 0.11 |
| 20 most experienced centres vs. all other centres | 106 vs. 342 | 17.1 vs. 12.9 | 1.14 | 0.29 | 64 vs. 164 | 15.9 vs. 12.3 | 1.22 | 0.25 |
| 30 most experienced centres vs. all other centres | 136 vs. 312 | 17.1 vs. 12.3 | 1.17 | 0.18 | 81 vs. 147 | 13.6 vs. 14.0 | 1.16 | 0.36 |
HR, hazard ratio; OS, overall survival.
Fig. 6.
The OS for all patients in 1L therapy at the top 10 experienced centres versus the other centres. OS, overall survival; 1L, first-line.
Fig. 7.
The OS for patients in 1L sunitinib therapy at the top 10 experienced centres versus the other centres. OS, overall survival; 1L, first-line.
For second-line treatments, there were trends for prolonged OS for the 10 and 20 most experienced centres, compared with all other centres, but the differences were not statistically significant. Median OS estimates were similar for the 30 most experienced centres and all other centres (Table 3). No significant differences were observed for specific second-line treatments.
Discussion
This retrospective analysis of the German BKK sickness fund claims database, between 2008 and 2016, was conducted to increase the understanding of treatment patterns, direct healthcare utilization, medical costs, OS, duration of treatment, and PFS in patients with mRCC. The most commonly prescribed first-line therapies were sunitinib, pazopanib, and temsirolimus. The most frequently prescribed second-line therapies were everolimus, sunitinib, axitinib, pazopanib, and sorafenib. These real-life treatment patterns are generally in accordance with German national guidelines, which (during the study period) recommended sunitinib, pazopanib, or bevacizumab in combination with interferon alpha as first-line therapies for intermediate-risk patients and temsirolimus for high-risk patients [10, 11].
Previous real-world studies have reported similar overall median PFS and OS for first-line treatments, but median PFS and OS for second-line treatments reported in the literature are more variable. In the current study, median PFS and OS for patients treated with first-line therapy were 7.4 and 14.9 months, respectively. The median PFS in the German clinical RCC registry was 7.9 months, whereas the median OS of trial-ineligible patients was 12.6 months [12]. Another study using the German RCC registry reported a median OS of 14.6 months [13]. The overall median PFS and OS for patients treated with second-line therapy were 7.2 and 13.6 months, respectively. Furthermore, this German clinical RCC registry data analysis reported overall median treatment durations (5.1 and 3.3 months for first- and second-line treatments, respectively) [13] similar to those in the present study (4.2 and 3.8 months for first- and second-line treatments, respectively). A much shorter median PFS of 3.4 months was reported in a German single-centre real-world experience study [14]. When examining clinical trial data, a systematic review of outcomes revealed substantial variability in PFS and OS across treatments and reported median PFS vs. OS in patients treated with sorafenib (4.7–8.6 vs. 17.8 months), pazopanib (9.2 vs. 22.9 months), axitinib (6.7–8.3 vs. 20.1 months [15]), temsirolimus (3.8 vs. 10.9 months), everolimus (4.9 vs. 14.8 months), and sunitinib (11 vs. 26.4 months) [16].
The most expensive treatment period in the present study was the active period during first-line treatment, driven by high outpatient pharmacy expenditures. These costs are considerably higher than data from France reporting overall healthcare costs of €5,546.0/month during first-line treatment, compared with €7,253.2/month observed in the current study [17]. Similar to the current findings, the driver of the costs in the French study was outpatient pharmacy expenditures, accounting for 53% of the overall monthly costs in France [17]. Moreover, in our study, sunitinib was the most expensive treatment among both first- and second-line therapies.
The results of the exploratory analysis by centre expertise suggest that German centres with greater perceived experience may achieve better clinical outcomes, most notably in terms of prolonged OS. This effect was particularly pronounced in patients treated with first-line sunitinib at the 10 and 30 most experienced centres, compared with all others. No such trend was seen, however, for the 20 most experienced centres. Across centres of all levels of experience, median OS was similar to that reported from randomized controlled trials of first-line sunitinib for mRCC [18]. Although exploratory, the trend towards prolonged OS in the 10 centres recognized by clinical experts as having the greatest expertise in RCC may suggest that physician experience can play a role in the effective real-world use of sunitinib, potentially because of the need for careful routine care management of treatment-related adverse events to ensure continuation of therapy and optimize treatment outcomes. No statistically significant differences were seen for the other treatment-specific comparisons evaluated. This might suggest that clinician experience is less relevant for other mRCC treatments, or it may reflect shortcomings in the proxy definition of centre expertise used in the study and/or the small sample sizes involved.
Although valuable in helping to characterize routine care practices and their clinical and cost implications, retrospective analyses of claims data are subject to inherent limitations that must be taken into consideration when interpreting their findings. Health insurance data are primarily generated for financial reimbursement transactions and not for clinical research purposes. They, therefore, contain only a limited number of clinical and demographic variables and insufficient detail to interpret the results in the context of patients' risk profiles, tumour burden and spread, or with respect to other clinically relevant considerations, such as the occurrence (and impact) of side effects, dose modifications, and treatment adherence. As a result, assumptions and proxies, such as those used to infer a diagnosis of mRCC and to generate PFS and OS estimates, must be used. Similarly, the stratification of the results by centre experience must be treated as exploratory given the opinion-based and proxy measures used to define centre experience. Nevertheless, the analysis does suggest between-centre variations in practices (and outcomes) that may warrant further investigation. It is also noteworthy that individuals enrolled in a statutory health insurance scheme may not be broadly representative of the German population as a whole. Furthermore, there may be a systematic bias in the data introduced by the quarterly documentation of diagnoses made in the outpatient setting. Finally, only the drugs approved for the treatment of mRCC in Germany until 2016 were included. New treatments available after 2016, such as cabozantinib or nivolumab, were not included here and will be investigated in subsequent analyses.
Conclusion
This observational study provides important insight into the real-life treatment patterns for patients with mRCC in Germany. It shows that the prescribing of systemic mRCC therapies is generally rational and aligned with the German national treatment guidelines. Analysis of the results by level of centre expertise suggests a possible trend towards improved OS in more experienced centres. This finding warrants further investigation and may point towards potential benefits of implementing a national, centralized approach to systemic RCC management, particularly for therapies that require greater monitoring and more complex care.
Statement of Ethics
This is a retrospective, observational cohort study using routinely collected administrative claims data of patients who are covered by statutory health insurance of the BBK. The sickness fund database consists of claims data of up to 5.0 million people insured from 2008 to 2016. Patient data were fully anonymized according to accepted standard procedures before analyses were performed by Team Gesundheit Gesellschaft für Gesundheitsmanagement GmbH, Essen, Germany. The BKK were informed about the project, and all the required approvals were obtained.
Conflict of Interest Statement
Martin Bögemann is an employee of the University of Münster and has received funds from Ipsen Pharma for providing expert consultations for this study. Aleksandra Zagorska is an employee of Ipsen Pharma. Divine Akumo is an employee of ZEG Berlin, a Kantar Health-owned company, and conducted work on behalf of Kantar Health. Kantar Health received funds from Ipsen Pharma for conducting the study and analysis and reporting of findings. Laila El Hadad is an employee of Team Gesundheit Gesellschaft für Gesundheitsmanagement GmbH, Essen, Germany, which received funding for this study from Kantar Health. Marc Pignot is an employee of Kantar Health.
Funding Sources
This study was funded by Ipsen Pharma.
Author Contributions
All authors contributed equally to the conception, design, and interpretation of the data, as well as revision of this publication.
Acknowledgements
The authors would like to thank Aranzazu Alba, an employee of Team Gesundheit Gesellschaft für Gesundheitsmanagement GmbH, Essen, Germany, for her contribution to the statistical analyses undertaken for this study. The authors thank Dr. Tim van Hartevelt and Dr. Tamzin Gristwood of Oxford PharmaGenesis for providing editorial support, which was sponsored by Ipsen Pharma in accordance with the Good Publication Practice guidelines.
References
- 1.Bersanelli M, Leonardi F, Buti S. Spotlight on cabozantinib for previously untreated advanced renal cell carcinoma: evidence to date. Cancer Manag Res. 2018;10:3773–80. doi: 10.2147/CMAR.S160485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zarrabi K, Fang C, Wu S. New treatment options for metastatic renal cell carcinoma with prior anti-angiogenesis therapy. J Hematol Oncol. 2017;10((1)):38. doi: 10.1186/s13045-016-0374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li P, Znaor A, Holcatova I, Fabianova E, Mates D, Wozniak MB, et al. Regional geographic variations in kidney cancer incidence rates in European countries. Eur Urol. 2015;67((6)):1134–41. doi: 10.1016/j.eururo.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Ljungberg B, Campbell SC, Choi HY, Cho HY, Jacqmin D, Lee JE, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60((4)):615–21. doi: 10.1016/j.eururo.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 5.Doehn C, Grünwald V, Steiner T, Follmann M, Rexer H, Krege S. The diagnosis, treatment, and follow-up of renal cell carcinoma. Dtsch Arztebl Int. 2016;113((35–36)):590–6. doi: 10.3238/arztebl.2016.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaatsch P, Spix C, Hentschel S, Katalinic A, Luttmann S, Luttmann S, et al. Krebs in Deutschland 2009/2010. Berlin, Germany: Robert-Koch-Institut; 2013. [Google Scholar]
- 7.Niederhuber JE, Armitage JO, Doroshow JH, Kastan MB, Tepper JE. Abeloff's clinical oncology e-book. Amsterdam, Netherlands: Elsevier Health Sciences; 2019. [Google Scholar]
- 8.Choyke PL, Glenn GM, Walther MM, Zbar B, Linehan WM. Hereditary renal cancers. Radiology. 2003;226((1)):33–46. doi: 10.1148/radiol.2261011296. [DOI] [PubMed] [Google Scholar]
- 9.Beisland C, Johannesen TB, Klepp O, Axcrona U, Torgersen KM, Kowalski J, et al. Overall survival in renal cell carcinoma after introduction of targeted therapies: a Norwegian population-based study. Onco Targets Ther. 2017;10:371–85. doi: 10.2147/OTT.S123061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF) Diagnostik. Therapie und Nachsorge des Nierenzellkarzinoms, Kurzversion 1.1. 2017. AWMF Registernummer: 043/017OL: Kurzversion S3-Leitlinie Nierenzellkarzinom [accessed 2019 Apr 9]. Available from: http://leitlinienprogramm-onkologie.de/Nierenzellkarzinom.85.0.html.
- 11.Alsmeier G, Bedke J, Doehn C, Eberhardt B, Flörcken A, Krege S, et al. Leitlinienprogramm Onkologie_S3_Nierenkrebs_metastasiert_2016–08 [accessed 2019 March 20]. Available from: https://www.awmf.org/uploads/tx_szleitlinien/043-017OLp_S3_Nierenkrebs_metastasiert_2018-08.pdf.
- 12.Marschner N, Staehler M, Müller L, Nusch A, Harde J, Koska M, et al. Survival of patients with advanced or metastatic renal cell carcinoma in routine practice differs from that in clinical trials-analyses from the German clinical RCC registry. Clin Genitourin Cancer. 2017;15((2)):e209–15. doi: 10.1016/j.clgc.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 13.Goebell PJ, Staehler M, Müller L, Nusch A, Scheffler M, Sauer A, et al. Changes in treatment reality and survival of patients with advanced clear cell renal cell carcinoma: analyses from the German clinical RCC-registry. Clin Genitourin Cancer. 2018;16((6)):e1101–15. doi: 10.1016/j.clgc.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Schwab M, Hofmann R, Heers H, Hegele A. mRCC outcome in the treatment of metastatic renal cell carcinoma: a German single-center real-world experience. In Vivo. 2018;32((6)):1617–22. doi: 10.21873/invivo.11422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motzer RJ, Escudier B, Tomczak P, Hutson TE, Michaelson MD, Negrier S, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013;14((6)):552–62. doi: 10.1016/S1470-2045(13)70093-7. [DOI] [PubMed] [Google Scholar]
- 16.Coppin C, Kollmannsberger C, Le L, Porzsolt F, Wilt TJ. Targeted therapy for advanced renal cell cancer (RCC): a Cochrane systematic review of published randomised trials. BJU Int. 2011;108((10)):1556–63. doi: 10.1111/j.1464-410X.2011.10629.x. [DOI] [PubMed] [Google Scholar]
- 17.Maroun R, Fleury L, Nachbaur G, Maunoury F, Vanhille JL, Durand-Zaleski I. Real-world costs and outcomes in metastatic renal cell carcinoma patients treated with targeted therapies: a cohort study from the French health insurance database. Curr Med Res Opin. 2017;33((10)):1755–62. doi: 10.1080/03007995.2017.1360850. [DOI] [PubMed] [Google Scholar]
- 18.Choueiri TK, Hessel C, Halabi S, Sanford B, Michaelson MD, Hahn O, et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): progression-free survival by independent review and overall survival update. Eur J Cancer. 2018;94:115–25. doi: 10.1016/j.ejca.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]