Abstract
Aims
Unicompartmental knee arthroplasty (UKA) and bicompartmental knee arthroplasty (BCA) have been associated with improved functional outcomes compared to total knee arthroplasty (TKA) in suitable patients, although the reason is poorly understood. The aim of this study was to measure how the different arthroplasties affect knee extensor function.
Methods
Extensor function was measured for 16 cadaveric knees and then retested following the different arthroplasties. Eight knees underwent medial UKA then BCA, then posterior-cruciate retaining TKA, and eight underwent the lateral equivalents then TKA. Extensor efficiency was calculated for ranges of knee flexion associated with common activities of daily living. Data were analyzed with repeated measures analysis of variance (α = 0.05).
Results
Compared to native, there were no reductions in either extension moment or efficiency following UKA. Conversion to BCA resulted in a small decrease in extension moment between 70° and 90° flexion (p < 0.05), but when examined in the context of daily activity ranges of flexion, extensor efficiency was largely unaffected. Following TKA, large decreases in extension moment were measured at low knee flexion angles (p < 0.05), resulting in 12% to 43% reductions in extensor efficiency for the daily activity ranges.
Conclusion
This cadaveric study found that TKA resulted in inferior extensor function compared to UKA and BCA. This may, in part, help explain the reported differences in function and satisfaction differences between partial and total knee arthroplasty.
Cite this article: Bone Joint Res 2021;10(1):1–9.
Keywords: Arthroplasty, Knee extension, Unicompartmental, Bicompartmental, Extensor mechanism
Article focus
What is the effect of unicompartmental (UKA), bicompartmental (BCA), and total knee arthroplasty (TKA) on knee extensor function?
Key messages
Medial unicompartmental, medial bicompartmental, and lateral unicompartmental arthroplasty preserved extensor efficiency.
Lateral bicompartmental arthroplasty preserved extensor efficiency for four of five daily-activity ranges of flexion.
TKA reduced extensor efficiency for all daily-activity ranges of flexion investigated.
Strengths and limitations
Comprehensive investigation of partial, combined partial, and total knee arthroplasty with direct comparison to the native knee.
Repeated measures study design with minimal soft-tissue disruption and quadriceps loading applied in a physiological direction.
Constant loading, time-zero data with no healing/adaptation.
Introduction
More than 1.5 million total knee arthroplasty (TKA) procedures are performed annually worldwide1 to treat end-stage osteoarthritis (OA) in one, two, or three knee compartments.2-5 While many patients benefit from TKA, for some, poor postoperative function can lead to disability, especially on stairs or when walking on slopes,6,7 and patient dissatisfaction rates of up to 25% have been reported.8-11 Consequently, there has been much recent debate in the orthopaedic community regarding how to improve knee function and patient satisfaction following arthroplasty.9,12-16
For patients with functional cruciate ligaments, and disease isolated to one tibiofemoral knee compartment, the use of unicompartmental knee arthroplasty (UKA) instead of TKA can lead to improved patient satisfaction and knee function, with reported benefits including faster gait, greater range of motion, and earlier return to desired activity.7,17-21 There is also renewed interest in combined partial knee arthroplasty (CPKA)22,23 where combinations of two or more partial knee arthroplasty (PKA) implants are used in the same knee.24 Recent data have suggested that two-thirds of TKA patients could be candidates for PKA or CPKA.25 When UKA and patellofemoral arthroplasty (PFA) are used in combination, this is referred to as bicompartmental knee arthroplasty (BCA), and may be medial (BCA-M) or lateral (BCA-L) depending on which tibiofemoral compartment is resurfaced.22 BCA is an option for patients with bicompartmental gonarthrosis and a spared tibiofemoral compartment, or as an alternative to TKA for patients who underwent UKA or PFA, and subsequently developed disease in a second knee compartment.24 Recent data have suggested that CPKA patients have improved function and satisfaction compared to matched TKA patients.26 While differences have been observed clinically, there remains limited understanding of the fundamental biomechanical mechanisms which lead to this improved function following UKA and BCA. This understanding is important for selecting the right procedure for the right patient, and for identifying targets for implant/procedure innovation to address patient dissatisfaction and poor function.
Gait analysis has revealed differences between UKA, BCA, and TKA patients during weight acceptance, heel-strike, and mid-stance, but not at toe-off.21,27 Given that the quadriceps are highly active from weight acceptance through to mid-stance, but less so at toe-off,28 we hypothesized that the differences in function between PKA and TKA may in part be explained through differences in extensor function. Therefore, the aim of this study was to quantify the efficiency of knee extension in vitro following UKA, BCA, and TKA to reveal how the different arthroplasties affect knee extensor function. Our null hypothesis was that there would be no differences in extension moment or efficiency between the different forms of arthroplasty compared to the native knee, or relative to each other.
Methods
Study design and specimen preparation
A total of 16 fresh-frozen cadaveric knees (mid-femur to mid-tibia) from ten Caucasian donors were sourced (Science Care, Phoenix, Arizona, USA). Ethical approval was granted by our hospital tissue bank (Imperial College Healthcare Tissue Bank Project R15022 and HTA licence 12275). Donors had no history or visible signs of gonarthrosis, trauma, deformity, restricted range of motion, or history of diseases which typically affect musculoskeletal health.
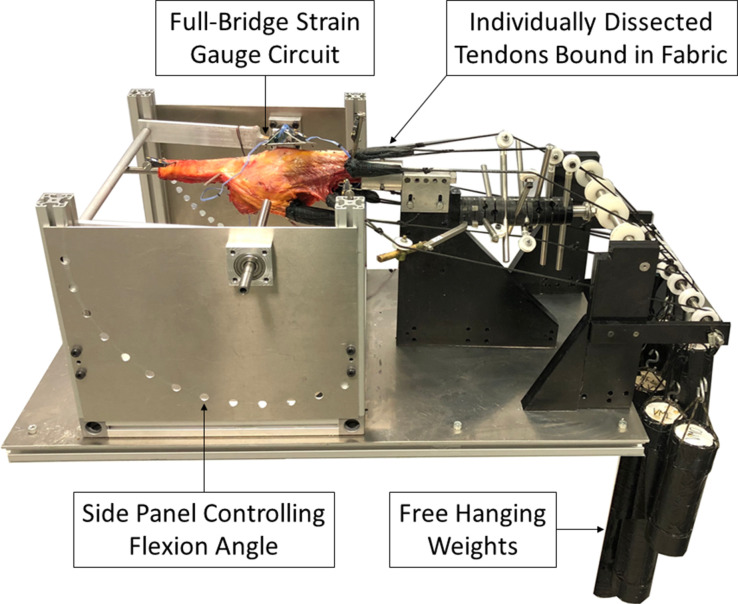
Defrosted specimens were dissected to remove the skin and subcutaneous fat, carefully preserving the fascia. To preserve lateral collateral ligament function and anatomical position, the head of the fibula was screwed to the tibia.29 The femur was mounted on a knee extension rig (Figure 1) with the transepicondylar axis of the femur aligned to the extension axis of the rig, enabling measurement of the knee sagittal plane extension moment, as previously described.29 The quadriceps tendons, iliotibial band (ITB), and hamstrings were individually dissected, bound with fabric, and attached to hanging weights via cables. These were arranged so the resulting tension in each tendon was directed anatomically,30 simulating physiological knee extension.29 The applied load totalled 225 N, distributed according to the reported cross-sectional area of each tendon (Table I). An intramedullary rod, inserted into the tibial canal, was restrained by a bar and connected to a calibrated full-bridge strain gauge circuit. This previously validated method of restrained extension under muscle loading conditions enabled measurement of the extension moment, using a repeated measures design.29-33 Measurements were taken from 110° to 0° knee extension, at 10° increments, and repeated three times for each implant state.
Fig. 1.
Knee extension rig mounted with knee specimen demonstrating individually dissected and loaded quadriceps, iliotibial band, and hamstring tendons.
Table I.
Loading weight and direction of pull relative to the femoral shaft axis for individually dissected quadriceps, iliotibial band, and hamstring tendons.
| Tendon(s) | Load direction relative to femoral shaft axis | Load, N | |
|---|---|---|---|
| Anteroposterior | Mediolateral | ||
| Rectus femoris and vastus intermedius | 0° Anterior | 0° Lateral | 61.25 |
| Vastus lateralis longus | 0° Anterior | 14° Lateral | 57.75 |
| Vastus lateralis obliquus | 33° Posterior | 35° Lateral | 15.75 |
| Vastus medialis longus | 0° Anterior | 15° Medial | 24.5 |
| Vastus medialis obliquus | 44° Posterior | 47° Medial | 15.75 |
| Iliotibial band | 6° Posterior | 0° Lateral | 30 |
| Medial hamstrings (semimembranosus and semitendinosus) | 0° Posterior | 0° Medial | 10 |
| Lateral hamstrings (long and short head of biceps femoris) | 0° Posterior | 0° Lateral | 10 |
Testing order and arthroplasty procedures
Prior to testing, all specimens were scanned with CT and subject to 3D surgical planning for sizing (Embody Orthopaedic, London, UK). A transpatellar approach was used to access the joint, eliminating disruption to the soft-tissues and the variability of suture tension between tests associated with a parapatellar approach.34 The patellar osteotomy was performed 10 mm from its lateral border, leaving sufficient room in the medial fragment for subsequent cemented fixation of the patella button without compromise to the optimal button position. The osteotomy was reduced with two partially threaded cannulated screws to ensure adequate compression. The reduced osteotomy was inspected before and after each test to ensure no visible distraction, displacement, or loss of reduction had occurred. The longitudinal splits in the quadriceps and patellar tendons were opposed with absorbable, synthetic, and braided 2.0 interrupted sutures, tied by the same surgeon (AG) for tension consistency.
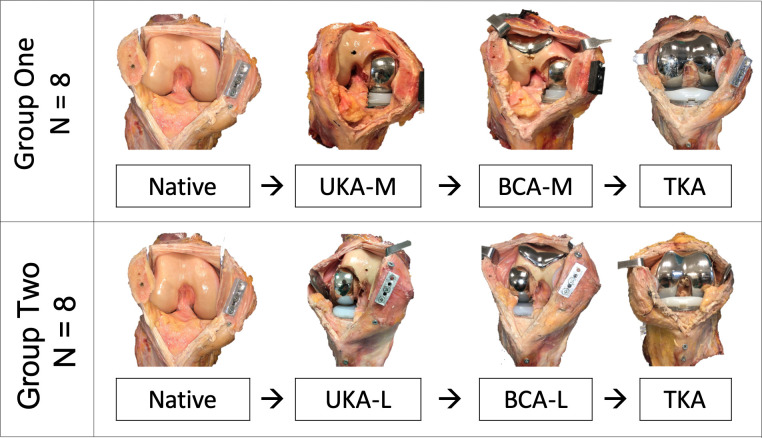
The specimens were divided into two groups of eight and tested in their native state, and then again following each arthroplasty (Figure 2). Group 1 was implanted with a cemented mobile-bearing medial UKA (UKA-M, Oxford Partial Knee Microplasty System; Zimmer Biomet, Warsaw, Indiana, USA) and then a Gender Solutions patellofemoral arthroplasty (PFA; Zimmer Biomet), converting it to a BCA-M. Finally, the metal PKA components were removed, carefully preserving bone stock. The patellar button was retained and the knee was implanted with a posterior-cruciate retaining TKA (NexGen CR-Flex; Zimmer Biomet). Group 2 was subject to the same sequence of testing (native → UKA → BCA → TKA), except that lateral UKA was used (UKA-L, Fixed Lateral Oxford; Zimmer Biomet), before being converted to a BCA-L through the addition of a PFA, then posterior-cruciate retaining TKA. All procedures were carried out by the same surgeon (AG) using standard instrumentation as per the manufacturer’s instructions, with techniques approved by the senior surgeon (JPC) and a Zimmer Biomet representative.
Fig. 2.
Arthroplasty sequences for Groups 1 and 2: medial unicompartmental knee arthroplasty (UKA-M), medial bicompartmental arthroplasty (BCA-M), lateral unicompartmental knee arthroplasty (UKA-L), lateral bicompartmental knee arthroplasty (BCA-L), and posterior-cruciate retaining total knee arthroplasty (TKA).
The largest UKA bearing size required was 5 mm. During conversion to TKA, the UKA and PFA components were removed using a fine saw blade at the cement-bone interface, to preserve bone stock. A measured resection TKA technique was employed with the femoral reference pins placed prior to removal of the UKA femoral component, on the assumption that the UKA technique had restored femoral length. Similarly, the TKA tibial resection was referenced prior to removal of the UKA tibial tray. Since the minimum resection for the NexGen CR-Flex is 10 mm, the tibial resection removed all the bone impacted by the prior UKA implantation except for where the UKA keel was located. The TKA keel slot then removed any residual evidence of the UKA keel slot such that the TKA bone-cement interface was as it would be for a primary joint. One specimen required a 14 mm TKA bearing, while for all others a 10 mm or 12 mm bearing was sufficient to restore the native joint line.
The specimens in Groups 1 and 2 were initially paired; however, two specimens in Group 1 were unsuitable: the CT scan revealed a tibial fracture on one specimen, while another was unable to reach full extension on native testing. Arthroplasties were sourced. The mean age at death was 66 years (SD 5.8) (Group 1) and 67 years (SD 5.3) (Group 2), and mean body mass index was 24.9 kg/m2 (SD 4.0) (Group 1) and 23.2 kg/m2 (SD 4.1) (Group 2). Three of the eight specimens in Group 1 and four of Group 2 were female.
To ensure TKA was not disadvantaged by being the last state in the sequence, a pilot knee was tested native, then following TKA, without the intervening implant states (no UKA and no PFA). Testing was repeated over a 16-hour period including overnight storage in a refrigerator set to 4°C, with no significant degradation of data between the first and last TKA measurements.
Statistical analysis
Extensor efficiency was defined as the ratio of energy output by the extending knee through a range of flexion following arthroplasty, compared to the native knee. This work output was calculated as the integral of the extension moment over a chosen flexion range. Five flexion ranges were examined. Four corresponded to daily activities that require the quadriceps activity to generate knee extension moments: the stance phase of gait at fast walking speeds (30° to 0°);28 stair ascent (40° to 10°);35 uphill walking (80° to 10°);36 and sit-to-stand (100° to 0°).37 The full experimental range was also examined.
For both extension moment and efficiency data, intra-state repeats were averaged in MatLab then subject to statistical analysis in SPSS v.26 (IBM, Armonk, New York, USA). Normality was tested using a Shapiro-Wilk test, with data then analyzed with repeated measures analysis of variance (RMANOVA) (α = 0.05) along the following criteria: 1) one-way RMANOVA of native knee extension moment data with independent variable of flexion angle for all 16 knees; 2) two-way RMANOVA of extension moment data, with the independent variables of flexion angle (0° to 110° at 10° increments) and implant state (native, UKA, BCA, and TKA), with Groups 1 and 2 analyzed separately; and 3) one-way RMANOVA of work output data for each of the five flexion ranges, with independent variable of implant state (native, UKA, BCA, and TKA), with Groups 1 and 2 analyzed separately.
Post-hoc paired t-tests with Bonferroni correction were undertaken when differences across tests were found. The reported p-values were adjusted and multiplied in SPSS by the appropriate Bonferroni correction. Statistical significance was set at p < 0.05. All means were reported with standard deviation.
Results
Native knee
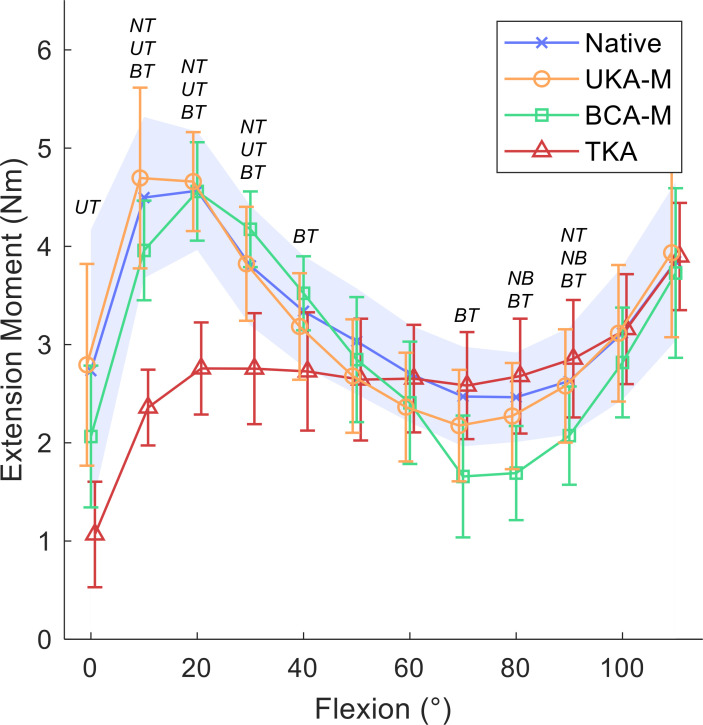
Between 10° and 20° of flexion, the mean extension moment of the native knee was at its highest, at 4.7 Nm (SD 1.2) (Figures 3 and 4). There was a large reduction in mean extension moment to 3 Nm (SD 1.7) in terminal extension (paired t-tests p < 0.015), and to a minimum of 2.5 Nm (SD 0.5) between 70° and 80° flexion (paired t-tests p < 0.001).
Fig. 3.
Static flexion angles against mean extension moment (Nm) for native knees, medial unicompartmental knee arthroplasty (UKA-M), medial bicompartmental arthroplasty (BCA-M), and total knee arthroplasty (TKA); 95% confidence intervals with a shaded blue area for the native knee, and bars for implanted knees. Italicized letters indicate pairwise statistical differences (p < 0.05): NU, native versus UKA-M; NB, native versus BCA-M; NT, native versus TKA; UB, UKA-M versus BCA-M; UT, UKA-M versus TKA; BT, BCA-M versus TKA.
Fig. 4.
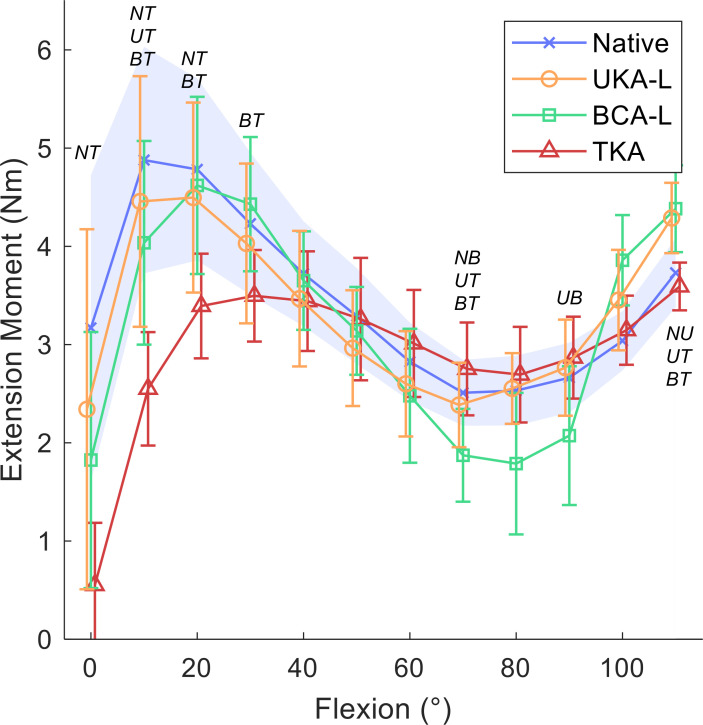
Static flexion angles against mean extension moment (Nm) for native knees, lateral unicompartmental knee arthroplasty (UKA-L), lateral bicompartmental knee arthroplasty (BCA-L), and total knee arthroplasty (TKA); 95% confidence intervals with a shaded blue area for the native knee, and bars for implanted knees. Italicized letters indicate pairwise statistical differences (p < 0.05): NU, native versus UKA-L; NB, native versus BCA-L; NT, native versus TKA; UB, UKA-L versus BCA-L; UT, UKA-L versus TKA; BT, BCA-L versus TKA.
Medial compartment procedures
The addition of a UKA-M or a BCA-M caused no reduction in mean peak extension moment at 20° flexion (UKA-M 4.7 Nm (SD 0.6), BCA-M 4.6 Nm (SD 0.6)) while the insertion of a TKA resulted in a mean extension moment of 2.8 Nm (0.6), only 60% of the normal value (paired t-test p < 0.001; Figure 3). The size of the difference depended on the angle of knee flexion (RMANOVA p < 0.001; Figure 3). Large reductions in knee extension moment were measured following TKA at low angles of flexion (10° to 30°) compared to the native knee and the PKA procedures (mean decreases of 1 Nm (SD 0.6) to 2.3 Nm (SD 0.9), or 28% to 61%; paired t-tests p < 0.01). In mid-flexion (40° to 60°), no difference was detected between any implant state, except for at 40° flexion, where the mean extension moment was 0.8 Nm (SD 0.6) higher for BCA-M than TKA (paired t-test p = 0.04). From 70° to 90°, BCA-M generated lower extension moment compared to TKA (mean differences < 1.0 Nm (SD 0.7); paired t-tests p < 0.04) and the native knee (mean difference < 0.9 Nm (SD 0.6); paired t-tests p = 0.05 at 70° and 90°; paired t-test p < 0.01 at 80°). A very small increase in moment was also measured following TKA compared to the native knee at 90° (mean 0.2 Nm (SD 0.1); paired t-test p = 0.02). All states were similar at flexion angles of 100° or higher.
Lateral compartment procedures
For the lateral group, a similar pattern was seen (Figure 4). The normal knee delivered its maximal mean extension moment of 4.9 Nm (SD 1.4) at 10°, while the lateral UKA’s maximum mean extension moment of 4.5 Nm (SD 1.2) was at 20°, as was the BCA-L, which delivered a mean 4.6 Nm (SD 1.1). Conversely, the low-flexion peak for TKA was observed at 30° and was 29% lower than normal at a mean 3.5 Nm (SD 0.6). TKA generated reduced extension moment compared to: the native knee in full extension; all other states at 10°; native and BCA-L at 20°; and BCA-L at 30° (mean decreases of 0.9 Nm (SD 0.5) to 2.6 Nm (SD 1.8), or 22% to 83%; paired t-tests p < 0.03). All states were similar from 40° to 60°. At 70°, BCA-L produced a smaller extension moment compared to native and TKA states (mean decrease < 0.9 Nm (SD 0.3); paired t-tests p < 0.01), and TKA had a higher mean extension moment than UKA-L (0.4 Nm (SD 0.3); paired t-test p = 0.04). At 90°, BCA-L produced a lower moment than UKA-L (mean difference 0.7 Nm (SD 0.4); paired t-test p = 0.02). At 110°, the extension moment after UKA-L was higher than native and TKA, while BCA-L was higher than TKA (mean increases < 0.8 Nm (SD 0.4); paired t-tests p < 0.01).
Extensor efficiency
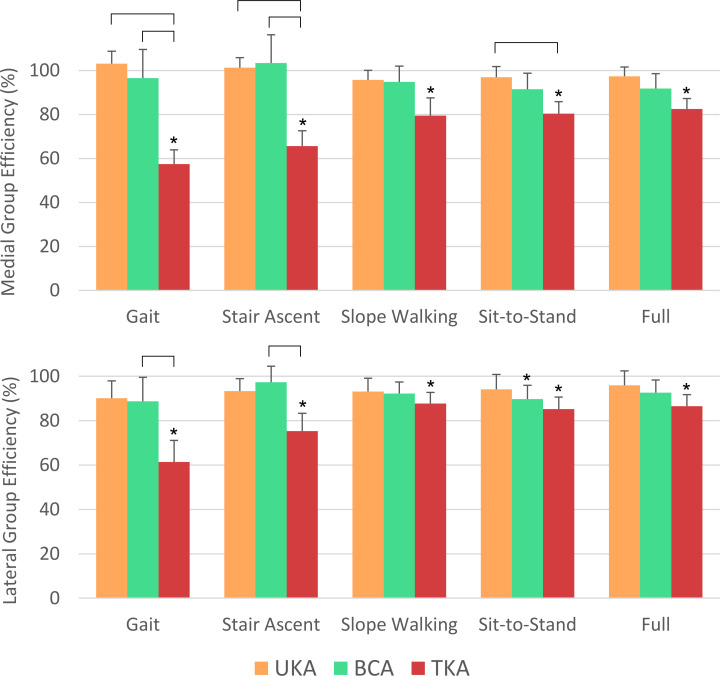
Compared to the native knee, arthroplasty affected the work output of the extensor mechanism (Figure 5). For the full range of motion (0° to 110°), TKA demonstrated a mean > 14% (SD 9%) reduction in extensor efficiency (paired t-tests p < 0.02). Conversely, differences were not found for both medial and lateral UKA and BCA. Similarly, for the quadriceps active range of uphill walking (80° to 10°), stair ascent (40° to 10°), and gait (30 to 0°), no differences were detected between medial and lateral UKA and BCA and native knee work output. For the sit-to-stand range (100° to 0°), UKA-M, UKA-L, and BCA-M were similar to native, but BCA-L was a mean 10% (SD 8%) less efficient (paired t-test p = 0.04). Efficiency after TKA was greatly reduced for all examined daily activity ranges compared to the native state (paired t-tests p ≤ 0.02), with the least reduction seen for uphill walking (mean 12% (SD 7%) reduction; paired t-test p = 0.01) and the greatest seen for the stance phase of gait (mean 43% (SD 13%) reduction; paired t-test p < 0.001).
Fig. 5.
Mean with 95% confidence interval work output by the extending knee over different ranges of motion for the different arthroplasty procedures as a percentage of the native knee work output. Top row: medial unicompartmental knee arthroplasty (UKA-M), medial bicompartmental arthroplasty (BCA-M), and total knee arthroplasty (TKA). Bottom row: lateral unicompartmental knee arthroplasty (UKA-L), lateral bicompartmental knee arthroplasty (BCA-L), and TKA. Data for gait (30 to 0°), stair ascent (40° to 10°), uphill slope walking (80° to 10°), sit-to-stand (100° to 0°), and full (110° to 0°) ranges of knee flexion are shown. The asterisks (*) indicate data statistically different from the native knee, and brackets indicate differences between the arthroplasties (paired t-tests p < 0.05).
Compared to the medial compartment procedures, TKA was less efficient than UKA-M and BCA-M in stair ascent and gait (mean decreases 35% (SD 10%) to 45% (SD 11%); all paired t-tests p < 0.001), and UKA-M for sit-to-stand (mean reduction 17% (SD 10%); paired t-test p = 0.01). Comparing to the lateral compartment procedures, TKA was less efficient than BCA-L for the stair ascent (mean 23% (SD 10%) reduction; paired t-test p < 0.01) and gait ranges (mean 32% (SD 19%) reduction; paired t-test p = 0.01).
Discussion
The most important finding of this study is that UKA and BCA make little impact on the efficiency of the extensor mechanism, while the negative impact of TKA on extensor function is substantial, with a reduction in extension moment of over 40% towards terminal knee extension. Following UKA, no differences were detected in either extension moment or efficiency. The addition of a PFA resulted in a small drop in extension moment between 70° and 90° flexion, but when examined in the context of the full range associated with the quadriceps active range of activities of daily living, extensor efficiency was largely unaffected. Conversely, following TKA, large decreases in extension moment at low angles of knee flexion resulted in 12% to 43% drops in extensor efficiency over ranges of knee flexion associated with common activities of daily living. This suggests that in order to achieve the same function during these activities, TKA patients expend significantly more energy, particularly during activities associated with increased levels of quadriceps activity and high cycle numbers, such as fast walking.28,38,39
The vast majority of patients benefit from TKA, experiencing relief of pain and low revision rates compared to PKA.2 Patients with increased extensor strength report better outcomes,40 however TKA is known to reduce extensor strength relative to native knees,41,42 impacting gait and other functional activities. PKA has been associated with restoration of kinematics and gait toward that of the native knee,17 improved range of motion, and faster return to desired physical function.5 The improved function of PKA is, in part, a consequence of retention of the anterior cruciate ligament (ACL), which is otherwise sacrificed for nearly all TKA designs. Posterior-stabilized TKA designs also require resection of the posterior cruciate ligament (PCL). In one study of consecutive patients, functional cruciate ligaments were resected in 95% of cases,43 regardless of the functional integrity of either ligament. Loss of the cruciate ligaments, which is classically associated with knee instability, has been recently shown to affect intraoperative gap balancing,44 and now the data from both this and a prior study29 suggest it may also affect knee extensor efficiency. The mechanism for this may be the increased anterior translation and the associated reduction in patellar tendon angle resulting from the loss of ACL function.45,46 The majority of patients with primary bicompartmental disease receive a TKA.2 Similarly, those who go on to develop native compartment arthrosis in a knee that has previously undergone PKA are typically revised to a TKA, which may involve removal of a well-functioning PKA, with mixed reports of the impact of such a procedure on patient outcomes.47-51 Our study suggests that conversion of a PKA to a BCA in the event of subsequent degeneration may preserve extensor efficiency and potentially improve functional outcomes for the patient.
An in vivo kinematic study found abnormal sagittal kinematics following TKA, but not UKA-M, for the terminal 20° of open-chain knee extension.46 Similarly, previous in vitro studies have demonstrated that single UKA-M implants retain near-native kinematics of the knee,52 which are significantly altered following TKA.53 Isolated PFA has been reported to replicate native extension moments, meanwhile significant reductions from 0° to 50° flexion are seen with cruciate-retaining and posterior-stabilized TKA designs.29 Our study supports the findings of these studies, but furthers those findings to address isolated arthrosis of the lateral compartment, and bicompartmental arthrosis.
By undertaking a repeated measures design, with the only variable being implant state, this cadaveric model eliminated confounding factors, including muscle strength and variation in anatomy. However, since testing was under static conditions with constant loading, the model is unable to replicate true in vivo kinematics or physiological muscle loading conditions, or the soft-tissue response of the healed postoperative knee. Binding tendons to muscle loads dictated a maximum load that could be applied before tearing occurred. The applied loads were an order of magnitude lower than normal physiological conditions. Here, we prioritized loading in a physiological direction, over magnitude of force applied, with the consequence being that our efficiency data required normalization. Repeated access to the knee was required, necessitating reopening and closure of the transpatellar approach. While the same surgeon (AG) tensioned the sutures and tightened the screws to minimize possible variation, some disparity may have occurred. Previous work suggested that the impact of this was minimal34 and hence it was unlikely to change the outcome of this work. TKA had to be the final implant state due to its invasiveness. The TKA-only pilot data suggested that there was no deleterious effect on the TKA of prior implantation of UKA or PFA, or the length of time between specimen dissection and TKA implantation. Our surgical technique referenced the TKA from the implanted UKA; some authors consider UKA→TKA to be effectively a primary TKA with no differences in patient outcomes, while others report that prior UKA can negatively influence TKA outcomes.47-51 Clinically, data suggest that walking on a slope with a TKA in situ is functionally more difficult than post-UKA.7 However, in this study, no differences were detected over this range of flexion. Differences in slope walking may be a consequence of other factors, such as loss of proprioception following resection of the cruciate ligaments, rather than differences in extensor function.
In conclusion, this study found that PKA preserves near-normal extensor function, while TKA had substantially inferior extensor function compared to UKA and BCA. This may help explain the functional and satisfaction differences observed between partial and total knee arthroplasty. BCA offers an alternative to TKA with some biomechanical advantages for those whose arthrosis extends into a second compartment, and warrants further clinical investigation.
Author contributions
A. Garner: Conceptualized the work, Designed and carried out the study, Acquired, analyzed, and interpreted the data, Wrote, edited, and revised the manuscript.
O. Dandridge: Acquired, analyzed, and interpreted the data, Edited and reviewed the manuscript.
A. A. Amis: Designed the study, Reviewed, edited, and revised the manuscript.
J. P. Cobb: Conceptualized the study, Reviewed the data, Reviewed, edited, and revised the manuscript.
R. J. van Arkel: Conceptualized and designed the study, Analyzed the data, Wrote, reviewed, edited, and revised the manuscript.
Funding statement
This work was funded by the Sir Michael Uren Foundation, The Royal College of Surgeons of England, the Dunhill Medical Trust Clinical Research Fellowship, and The Sackler Trust. Infrastructure support was provided by the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre (BRC). Surgical instruments and implants for the study were supplied by Zimmer Biomet (Warsaw, Indiana, USA).The author or one or more of the authors have received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this article. In addition, benefits have been or will be directed to a research fund, foundation, educational institution, or other non- profit organization with which one or more of the authors are associated.
ICMJE COI statement
J. P. Cobb reports personal consultancy fees from Zimmer Biomet and personal and insitutional patents (paid to Imperial College London) from Embody Orthopaedic, all unrelated to this study.
Acknowledgements
The authors are grateful to Zimmer Biomet, in particular Christopher Friend, Michael Malon, and Timothy Lawes for supporting our in vitro investigation.
Ethical review statement
Ethical approval was granted by our hospital tissue bank (Imperial College Healthcare Tissue Bank Project R15022 and HTA license 12275).
© 2021 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- 1.Pabinger C, Lothaller H, Geissler A. Utilization rates of knee-arthroplasty in OECD countries. Osteoarthritis Cartilage. 2015;23(10):1664–1673. [DOI] [PubMed] [Google Scholar]
- 2.No authors listed 16th Annual Report 2019. National Joint Registry for England, Wales, Northern Ireland and Isle of Man. 2019. https://reports.njrcentre.org.uk/portals/0/pdfdownloads/njr%2016th%20annual%20report%202019.pdf (date last accessed 20 October 2020).
- 3.No authors listed Hip, Knee & Shoulder Arthroplasty: Annual Report 2019. Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). 2019. https://aoanjrr.sahmri.com/documents/10180/668596/Hip%2C+Knee+%26+Shoulder+Arthroplasty/c287d2a3-22df-a3bb-37a2-91e6c00bfcf0 (date last accessed 20 October 2020).
- 4.No authors listed Swedish Knee Arthroplasty Register; 2018. https://www.myknee.se/pdf/SVK_2018_Eng_1.0.pdf. Annual Report 2018 (date last accessed 20 October 2020). [Google Scholar]
- 5.Kleeblad LJ, van der List JP, Zuiderbaan HA, Pearle AD. Larger range of motion and increased return to activity, but higher revision rates following unicompartmental versus total knee arthroplasty in patients under 65: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2018;26(6):1811–1822. [DOI] [PubMed] [Google Scholar]
- 6.Hamai S, Okazaki K, Shimoto T, et al. Continuous sagittal radiological evaluation of stair-climbing in cruciate-retaining and posterior-stabilized total knee arthroplasties using image-matching techniques. J Arthroplasty. 2015;30(5):864–869. [DOI] [PubMed] [Google Scholar]
- 7.Wiik AV, Nathwani D, Akhtar A, et al. The unicompartmental knee is the preferred side in individuals with both a unicompartmental and total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2020;28(10):3193–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shan L, Shan B, Suzuki A, Nouh F, Saxena A. Intermediate and long-term quality of life after total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Am. 2015;97-A(2):156–168. [DOI] [PubMed] [Google Scholar]
- 9.Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KDJ. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunaratne R, Pratt DN, Banda J, et al. Patient Dissatisfaction Following Total Knee Arthroplasty: A Systematic Review of the Literature. J Arthroplasty. 2017;32(12):3854–3860. [DOI] [PubMed] [Google Scholar]
- 11.Nam D, Nunley RM, Barrack RL. Patient dissatisfaction following total knee replacement: a growing concern? Bone Joint J. 2014;96-B(11 Supple A):96–100. [DOI] [PubMed] [Google Scholar]
- 12.Yapp LZ, Clement ND, Macdonald DJ, Howie CR, Scott CEH. Changes in expectation fulfillment following total knee arthroplasty: a 10-year follow-up study. J Arthroplasty. 2020;35(7):1826–1832. [DOI] [PubMed] [Google Scholar]
- 13.Kaye AD, Urman RD, Cornett EM, et al. Enhanced recovery pathways in orthopedic surgery. J Anaesthesiol Clin Pharmacol. 2019;35(Suppl 1):S35–S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bączkowicz D, Skiba G, Czerner M, Majorczyk E. Gait and functional status analysis before and after total knee arthroplasty. Knee. 2018;25(5):888–896. [DOI] [PubMed] [Google Scholar]
- 15.Kayani B, Haddad FS. Robotic total knee arthroplasty. Bone Joint Res. 2019;8(10):438–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roussot MA, Haddad FS. The evolution and role of patellofemoral joint arthroplasty. Bone Joint Res. 2018;7(12):636–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal A, Miller S, Hadden W, et al. Comparison of gait kinematics in total and unicondylar knee replacement surgery. Ann R Coll Surg Engl. 2019;101(6):391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Argenson J-NA, Komistek RD, Aubaniac J-M, et al. In vivo determination of knee kinematics for subjects implanted with a unicompartmental arthroplasty. J Arthroplasty. 2002;17(8):1049–1054. [DOI] [PubMed] [Google Scholar]
- 19.Miller S, Agarwal A, Haddon WB, et al. Comparison of gait kinetics in total and unicondylar knee replacement surgery. Ann R Coll Surg Engl. 2018;100(4):267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiik AV, Manning V, Strachan RK, Amis AA, Cobb JP. Unicompartmental knee arthroplasty enables near normal gait at higher speeds, unlike total knee arthroplasty. J Arthroplasty. 2013;28(9 Suppl):176–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones GG, Kotti M, Wiik AV, et al. Gait comparison of unicompartmental and total knee arthroplasties with healthy controls. Bone Joint J. 2016;98-B(10 Supple B):16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garner A, van Arkel RJ, Cobb J. Classification of combined partial knee arthroplasty. Bone Joint J. 2019;101-B(8):922–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haddad FS, Masri BA. Compartmental arthroplasty: time for a clear nomenclature. Bone Joint J. 2019;101-B(8):889–890. [DOI] [PubMed] [Google Scholar]
- 24.Garner A, Cobb JP, Rivière C, Vendittoli C. Combined Partial Knee Arthroplasty : Rivière C, Vendittoli C, eds. Personalized hip and knee joint replacement. First ed Cham: Springer, 2020:243–254. [Google Scholar]
- 25.Scott CEH, Holland G, Krahelski O, et al. Patterns of cartilage loss and anterior cruciate ligament status in end-stage osteoarthritis of the knee: assessing suitability for partial knee arthroplasty. Bone Joint J. 2020;102-B(6):716–726. [DOI] [PubMed] [Google Scholar]
- 26.Garner A, Dandridge O, van Arkel R, Amis A, Cobb J. Bi-Unicondylar arthroplasty: improved biomechanical efficiency, superior gait characteristics and higher patient satisfaction compared to total knee arthroplasty. Orthopaedic research Society (ORS) 2020 annual meeting. 2020. https://www.ors.org/wp-content/uploads/2020/02/ORS_2020_Program_Book_FINAL.web20920.pdf (date last accessed 21 October 2020).
- 27.Garner A, Lambkin R, Cobb J. 2019. Medial Bicompartmental Arthroplasty enables near normal gait, similar to Unicompartmental Arthroplasty and 15% faster than age and sex matched Total Knee Arthroplasty [abstract]. British Association of Surgery of the Knee Conference 2019. [Google Scholar]
- 28.Winter DA. The Biomechanics and Motor Control of Human Gait. Waterloo: University of Waterloo Press, 1987. [Google Scholar]
- 29.Joseph MN, Carmont MR, Tailor H, Stephen JM, Amis AA. Total knee arthroplasty reduces knee extension torque in-vitro and patellofemoral arthroplasty does not. J Biomech. 2020;104:109739. [DOI] [PubMed] [Google Scholar]
- 30.Farahmand F, Senavongse W, Amis AA. Quantitative study of the quadriceps muscles and trochlear groove geometry related to instability of the patellofemoral joint. J Orthop Res. 1998;16(1):136–143. [DOI] [PubMed] [Google Scholar]
- 31.Christoforakis J, Bull AMJ, Strachan RK, et al. Effects of lateral retinacular release on the lateral stability of the patella. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):273–277. [DOI] [PubMed] [Google Scholar]
- 32.Farahmand F, Tahmasbi MN, Amis AA. Lateral force-displacement behaviour of the human patella and its variation with knee flexion--a biomechanical study in vitro. J Biomech. 1998;31(12):1147–1152. [DOI] [PubMed] [Google Scholar]
- 33.Ghosh KM, Blain AP, Longstaff L, et al. Can we define envelope of laxity during navigated knee arthroplasty? Knee Surg Sports Traumatol Arthrosc. 2014;22(8):1736–1743. [DOI] [PubMed] [Google Scholar]
- 34.Merican AM, Ghosh KM, Deehan DJ, Amis AA. The transpatellar approach for the knee in the laboratory. J Orthop Res. 2009;27(3):330–334. [DOI] [PubMed] [Google Scholar]
- 35.Adiputra LS, Parasuraman S, khan MKAA, Elamvazuthi I. Bio mechanics of Desending and ascending walk. Procedia Comput Sci. 2015;76:264–269. [Google Scholar]
- 36.Montgomery JR, Grabowski AM. The contributions of ankle, knee and hip joint work to individual leg work change during uphill and downhill walking over a range of speeds. R Soc Open Sci. 2018;5(8):180550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellis MI, Seedhom BB, Wright V. Forces in the knee joint whilst rising from a seated position. J Biomed Eng. 1984;6(2):113–120. [DOI] [PubMed] [Google Scholar]
- 38.Zeni JA, Flowers P, Bade M, et al. Stiff knee gait may increase risk of second total knee arthroplasty. J Orthop Res. 2019;37(2):397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orishimo KF, Kremenic IJ, Deshmukh AJ, Nicholas SJ, Rodriguez JA. Does total knee arthroplasty change frontal plane knee biomechanics during gait? Clin Orthop Relat Res. 2012;470(4):1171–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furu M, Ito H, Nishikawa T, et al. Quadriceps strength affects patient satisfaction after total knee arthroplasty. J Orthop Sci. 2016;21(1):38–43. [DOI] [PubMed] [Google Scholar]
- 41.Fokin A, Heekin D. Return of Quadriceps Strength After Primary Total Knee Arthroplasty With Single-Radius Knee System: Five-Year Follow-up. J Surg Orthop Adv. 2017;26(4):211–215. [PubMed] [Google Scholar]
- 42.Andriacchi TP, Hurwitz DE. Gait biomechanics and total knee arthroplasty. Am J Knee Surg. 1997;10(4):255–260. [PubMed] [Google Scholar]
- 43.Heekin RD, Fokin AA. Incidence of bicompartmental osteoarthritis in patients undergoing total and unicompartmental knee arthroplasty: is the time ripe for a less radical treatment? J Knee Surg. 2014;27(1):77–82. [DOI] [PubMed] [Google Scholar]
- 44.Kayani B, Konan S, Ahmed SS, Chang JS, Ayuob A, Haddad FS. The effect of anterior cruciate ligament resection on knee biomechanics: changes in flexion-extension gaps, mediolateral laxity, and maximum knee extension. Bone Joint J. 2020;102-B(4):442–448. [DOI] [PubMed] [Google Scholar]
- 45.Kang K-T, Koh Y-G, Park K-M, et al. The anterolateral ligament is a secondary stabilizer in the knee joint. Bone Joint Res. 2019;8(11):509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Price AJ, Rees JL, Beard DJ, et al. Sagittal plane kinematics of a mobile-bearing unicompartmental knee arthroplasty at 10 years: a comparative in vivo fluoroscopic analysis. J Arthroplasty. 2004;19(5):590–597. [DOI] [PubMed] [Google Scholar]
- 47.Lim JBT, Pang HN, Tay KJD, et al. Clinical outcomes and patient satisfaction following revision of failed unicompartmental knee arthroplasty to total knee arthroplasty are as good as a primary total knee arthroplasty. Knee. 2019;26(4):847–852. [DOI] [PubMed] [Google Scholar]
- 48.Zuo W, Ma J, Guo W, et al. Comparison of the clinical outcomes of revision of failed UKAs to TKAs with primary TKAs: a systematic review and meta-analysis. Medicine. 2018;97(50):e13408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun X, Su Z. A meta-analysis of unicompartmental knee arthroplasty revised to total knee arthroplasty versus primary total knee arthroplasty. J Orthop Surg Res. 2018;13(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lombardi AV, Kolich MT, Berend KR, Morris MJ, Crawford DA, Adams JB. Revision of Unicompartmental Knee Arthroplasty to Total Knee Arthroplasty: Is It as Good as a Primary Result? J Arthroplasty. 2018;33(7S):S105–S108. [DOI] [PubMed] [Google Scholar]
- 51.Thienpont E. Conversion of a unicompartmental knee arthroplasty to a total knee arthroplasty: can we achieve a primary result? Bone Joint J. 2017;99-B(1 Supple A):65–69. [DOI] [PubMed] [Google Scholar]
- 52.Peersman G, Slane J, Vuylsteke P, et al. Kinematics of mobile-bearing unicompartmental knee arthroplasty compared to native: results from an in vitro study. Arch Orthop Trauma Surg. 2017;137(11):1557–1563. [DOI] [PubMed] [Google Scholar]
- 53.Patil S, Colwell CW, Ezzet KA, D'Lima DD. Can normal knee kinematics be restored with unicompartmental knee replacement? J Bone Joint Surg Am. 2005;87-A(2):332–338. [DOI] [PubMed] [Google Scholar]