Abstract
Aims
The effect of the gut microbiota (GM) and its metabolite on bone health is termed the gut-bone axis. Multiple studies have elucidated the mechanisms but findings vary greatly. A systematic review was performed to analyze current animal models and explore the effect of GM on bone.
Methods
Literature search was performed on PubMed and Embase databases. Information on the types and strains of animals, induction of osteoporosis, intervention strategies, determination of GM, assessment on bone mineral density (BMD) and bone quality, and key findings were extracted.
Results
A total of 30 studies were included, of which six studies used rats and 24 studies used mice. Osteoporosis or bone loss was induced in 14 studies. Interventions included ten with probiotics, three with prebiotics, nine with antibiotics, two with short-chain fatty acid (SCFA), six with vitamins and proteins, two with traditional Chinese medicine (TCM), and one with neuropeptide Y1R antagonist. In general, probiotics, prebiotics, nutritional interventions, and TCM were found to reverse the GM dysbiosis and rescue bone loss.
Conclusion
Despite the positive therapeutic effect of probiotics, prebiotics, and nutritional or pharmaceutical interventions on osteoporosis, there is still a critical knowledge gap regarding the role of GM in rescuing bone loss and its related pathways.
Cite this article: Bone Joint Res 2021;10(1):51–59.
Keywords: Gut microbiota, Bone, Osteoporosis, Gut-bone axis, Fragility fracture, Probiotic
Article focus
In this review, we summarized current animal models, interventions, and outcome measurements on gut-bone axis studies.
To identify potential therapeutic targets to provide directions for future study and experimental design.
Key messages
The use of the mouse model is recommended for gut microbiota (GM) studies, as there is currently a well-established gut metagenome, as well as knowledge on gastroenterology, genetics, and immunology in mice.
Future studies should not only focus on the changes of GM composition at higher taxonomic levels, but also the functionalities carried by each specific bacterial flora.
Metagenomic techniques serve as a useful tool in identifying the key molecules and pathways involved in the gut-bone axis.
Strengths and limitations
Our findings provide valuable information for further studies designed to explore the gut-bone axis based on animal models, and serve as a platform to translate these preclinical findings to clinical application.
Due to the heterogeneity of the studies, only a qualitative analysis was performed.
Introduction
The collection of microbes that inhabit the human gastrointestinal tract is termed the gut microbiota (GM), and it has been estimated that the total amount of microbes can reach up to 1014.1 GM has been shown to play an indispensable role in host physiology including nutrition absorption, modulation of the immune system, and homeostasis.2 However, the disturbance of the dynamic ecosystem of the GM including ageing, prolonged use of antibiotics, and alteration of diet can lead to multiple host disorders. GM dysbiosis is found to be closely related to increased risk of bone loss,3 inflammatory bowel disease,4 diabetes, and obesity.5
Osteoporosis is a multifactorial skeletal disease that is characterized by loss of bone density and quality. The condition increases the risk of fragility fractures, which commonly occurs in the proximal femur, spine, and distal radius, leading to pain, disability, and even mortality.6,7 The mainstay treatment of osteoporosis includes bisphosphonates,8,9 but compliance rates are low and often lead to common side effects including gastrointestinal upset and even atypical fractures. Therefore, the development of novel diagnostics and treatments for bone loss has become a priority.10 With this, many studies have investigated the roles of various parameters including Casein kinase 2-interacting protein-1 (CKIP-1) in strengthening bone formation,11 angelica polysaccharide in promoting mesenchymal stem cell (MSC) proliferation and osteoblast differentiation,12 and aminoguanidine and pyridoxamine13 in mitigating bone quality deterioration. Novel predictors of osteoporosis, including cortical bone thickness and the distal femoral cortex index,14 and rehabilitation methods, have also been explored.15 Recently, there has been increasing evidence suggesting that the GM plays an important role in bone homeostasis, and GM modulation shows promising effects in reversing bone loss.3 The emergence of next-generation sequencing (NGS) technology has substantially facilitated the research on GM. Multiple studies have identified that GM can regulate bone metabolism with its own metabolites or modulate nutritional absorption and the immune system.16,17 The relationship between GM and bone metabolism has been collectively defined as the gut-bone axis.18
As of now, few clinical studies on the beneficial effect of probiotics have been performed in an attempt to reverse bone loss, and the exact mechanisms are still unknown.19,20 With the current knowledge gap in the pathophysiology, animal experiments remain the most powerful means to explore the gut-bone interaction comprehensively. Furthermore, selecting an appropriate animal model that mimics common clinical scenarios, including postmenopausal osteoporosis (PMO) and senile osteoporosis, would allow the study of novel interventions and potentially treat our ageing population. The purpose of this study was to identify and analyze existing animal models, intervention strategies, outcome measures, and potential therapeutic targets to provide recommendations for future study and experimental design.
Methods
Search strategy
PubMed and Embase (date last accessed 7 June 2020) were searched. Keywords used for search criteria were “(microbiota* OR microbiome*) AND bone”.
Search criteria
The inclusion criteria were: 1) preclinical studies; 2) use of animal model; and 3) study on the gut-bone axis. The exclusion criteria were: 1) lack of analysis on bone mineral density (BMD); 2) review articles; and 3) abstract or conference paper.
Selection of studies
Two independent reviewers (JL and CL) performed the selection process on two databases. Each reviewer screened the titles and abstracts of all published studies. Articles were selected based on inclusion and exclusion criteria. Each article was reviewed, and any disagreement was solved by consensus and discussion.
Data extraction
For eligible studies, the two reviewers extracted information on: 1) types and strains of animal used; 2) osteoporosis or bone loss induction approaches; 3) intervention protocols; 4) methods of GM determination; 5) assessment methodology; and 6) key findings.
Statistical analysis
Due to large heterogeneity in animal models and methodology, a qualitative review was performed.
Results
Characteristics of the papers
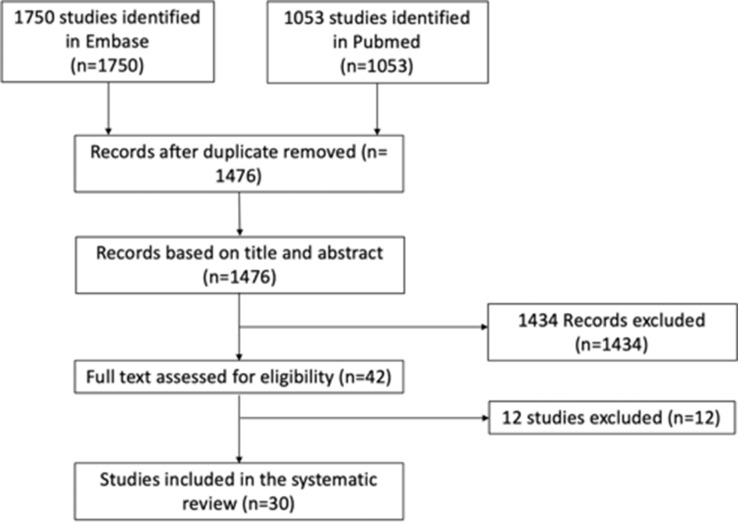
A total of 1,750 and 1,053 studies were identified from Embase and PubMed, respectively. All duplicate entries were removed, leaving 1,476 records. Each title and abstract was reviewed, and 1,434 records were excluded. Upon detailed review of full text, an additional 12 studies were excluded: three lacked the analysis on BMD;21-23 five were performed to study the role of GM on rheumatoid arthritis and osteoarthritis;24-28 three were unrelated to gut-bone axis;29-31 and one concerned bone marrow transplantation.32 A total of 30 studies were included for our systematic review, which were published from 2012 to 2020 (Supplementary Table i and Figure 1).
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart of study selection.
Animals
Mice were used in 23 studies including C57BL6 in 15 studies,33-47 CB6F1 in one study,48 BALB/c in three studies,39,48,49 Swiss Webster in one study,17 ddY in one study,50 senescence-accelerated mouse prone 6 (SAMP6) and senescence-accelerated mouse resistant 1 (SAMR1) in two studies,51,52 and Institute of Cancer Research (ICR) mice in two studies.53,54 Germ-free mice were used in six studies33,34,36,38,45,48 and rats were used in six studies, including four Sprague-Dawley55-58 and two Wistar.59,60 Genetically modified animals were used in five studies.35,36,39,42,61
Induction of osteoporosis or bone loss
Ovariectomy was performed on mice in three studies,44,49,50 and rats in five studies.55-58,62 Other methods included high-fat diet (HFD),60 tenofovir disoproxil fumarate (TDF),40 and glucocorticoid (GC) in two studies.46,53 One study performed sex steroid depletion by leuprolide.34 Six studies induced bone loss by GM dysbiosis following broad-spectrum antibiotic treatment.35,41,42,47,48,63 Two studies used SAMP6 mice as the age-related bone loss model.51,52 One study used D-galactose (D-gal) and sodium nitrite (NaNO2) to induce age-related bone loss.54
Interventions
The most common intervention was probiotics including Lactobacillus rhamnosus GG (LGG),34,39,40,46 Lactobacillus reuteri (LR),42,46,49,63 Lactobacillus paracasei,44,60 Lactobacillus plantarum,44 and Lactobacillus bulgaricus and Lactococcus lactis.51 Prebiotics included xylooligosaccharide,60 fructooligosaccharide (FOS), glucomannan,52 and acid-hydrolyzed high amylose corn starch (AH-HAS).50 One study had Escherichia coli as the intervention.63 Five studies performed colonization of germ-free (GF) mice with faeces from conventionally raised (CONV-R) animals,33,34,38,48 humans,38 and a single strain of bacteria.45 Nine studies had antibiotics treatment with different protocols.35,41-43,46-48,61,63 Other supplements include short-chain fatty acid (SCFA),39,48 sialoglycoprotein (SGP),59 nitrate,56 vitamin D,37 vitamin E,54 Duck Egg White-Derived Peptide VSEE (Val-Ser-Glu-Glu),57 and tuna bone powder (TBP).53 Two studies used traditional Chinese medicine (TCM) herb including Eclipta prostrata 51 and Fructus Ligustri Lucidi (FLL).54 One study had neuropeptide Y1 receptor (Y1R) antagonist intervention.58 Two studies had a high-weight polymer (MDY) mucus supplement to repair the intestinal barrier function.46,63
Measurement and analysis
Gut microbiota was determined in 24 studies, and 19 studies performed 16S ribosomal RNA (rRNA) sequencing.35,38-40,42,43,45,46,49-51,53,54,56-59,61,63 One study performed metagenomic analysis.41 Two studies used culture-dependent analysis,52,55 and six studies performed quantitative polymerase chain reaction (qPCR) analysis to quantify bacteria load.35,37,47,48,59,61
For bone assessment, a total of 25 studies performed micro-CT to measure BMD and microstructural parameters.33-40,42,44-51,53-58,61,63 Two studies performed peripheral quantitative CT (pQCT).33,44 Six performed dual-energy X-ray absorptiometry (DXA).37,43,50,56,59,61 One study performed X-rays,52 one used Raman spectroscopy,41 and another study performed Fourier-transform infrared spectroscopy (FTIR).54 In total, 14 studies performed static and dynamic bone histomorphometry analysis.33,34,38-40,46-49,55,56,60,61,63 Eight studies performed mechanical testing.35,37,42,46,54,55,57,63 Nine studies had bone histology including haematoxylin and eosin (H&E), tartrate-resistant acid phosphatase (TRAP), and toluidine blue and safranin O/fast staining.33,40,45-48,54,55,58
Regarding screening for inflammation, seven studies performed serum inflammatory cytokines assay including tumour necrosis factor (TNF)-α, CRP, and interleukin (IL)-17.34,40,45,47,52,54,61 In total, 17 studies performed polymerase chain reaction (PCR) test to quantify gene expression of inflammatory cytokines including IL-1, IL-6, IL-10, IL-17, TNF-α, and bone-related markers including alkaline phosphatase (ALP), osterix, collagen alpha-1 type I (Col-α1), and osteocalcin (OC). The studies also quantified the expression of the nuclear factor kappa-B (NF-κB) pathway and Wnt/β-catenin pathway in the bone tissue,33,34,36,39,44-50,53,55,56,61,63 colon,33,34,38,50,53,61 liver,48 and spleen.53
A total of 20 studies tested serum markers including calcium, vitamin D, parathyroid hormone (PTH), serotonin, type 1 collagen fragments, OC, TRAP5b, N-terminal propeptide of type I procollagen (P1NP), insulin-like growth factor 1 (IGF-1), glucose, triglyceride, and cholesterol levels.33,34,37,39,42-45,47-49,52,54-58,60,61,63 Three studies performed urine analysis,44,50,52 two had calcium concentrations analysis of faeces and femoral bone,52,53 three had western blot to test the osteogenic protein expression in bone marrow,39,51,54 three had intestinal permeability assay,34,40,63 and one had serum endotoxin measurement for barrier function assessment.46 Nine studies performed flow cytometry to quantify CD4+, CD8+, CD11b+, and Foxp3+ cells in bone marrow,33,38,39,44-47,49,55 spleen, and small intestine.34,35,55 Four studies had faecal SCFA measurement.39,43,48,53
Key findings
Three studies reported increased BMD in GF mice compared with CONV-R mice,33,34,36 and two studies showed decreased BMD after colonization of GM from CONV-R donor mice.33,45 Yan et al48 reported increased C-terminal telopeptide-1 (CTX-1) and procollagen I N-terminal propeptide (PINP), and reduced trabecular bone volume at one month after colonization with specific-pathogen-free (SPF) microbiota. However, at eight months following intervention, colonized mice had increased periosteal and endosteal area, and similar bone volume compared with GF siblings.48 However, Quach et al38 performed gavage on GF mice to introduce microbiota originated from human or CONV-R mice and found no change of GM and bone mass despite successful colonization. One study reported gavage with control or glucocorticoid-treated (GC-Tx) mice faecal slurry (3 × 105 colony-forming units (CFUs)) for eight weeks, and recipient mice showed a significant decrease of bone volume fraction (BV/TV) compared to the control group.46 Li et al34 compared the effect of leuprolide on bone in GF mice, CONV-R mice, and recolonized GF mice, and found that GF mice are protected against trabecular bone loss induced by sex steroid depletion.
Treatment of probiotics including LGG, LR, L. paracasei, Bifidobacterium longum, or prebiotic were found to prevent osteoporosis induced by ovariectomy (OVX),44,49,55 GC,46 HFD,60 and TDF.40 One study found that four-week supplementation with LGG or Bifidobacterium and Lactobacillus preparation could completely protect against the loss of BV/TV induced by OVX.34 Ohlsson et al44 started six-week supplementation of L. paracasei from two weeks before OVX and found that probiotics reduced the expression of TNF-α and IL-1β, and prevented OVX-induced cortical bone loss.44 Briton et al49 found that LR decreased the osteoclastic bone resorption markers TRAP5 and receptor activator of nuclear factor kappa-Β ligand (RANKL), suppressed OVX-induced increase of CD4+ T lymphocytes, and protected OVX mice from bone loss. Schepper et al46 found that LR treatment reversed the GM alteration featured by decreased levels of Verrucomicrobiales and Bacteriodales and increased Clostridiales, enhanced the intestinal barrier function, and ameliorated the decrease in both bone volume and trabecular bone microstructure induced by GCs. Eaimworawuthikul et al60 found that 12-week supplementation with L. paracasei or the prebiotic xylooligosaccharide improved the obese-insulin resistance and systemic inflammation, as well as improving trabecular thickness induced by HFD. Another study found that LGG treatment could prevent BMD decrease and microarchitecture deterioration induced by TDF through increasing intestinal barrier integrity and immunomodulating effect.40
Dietary supplementation, including SGP, VSEE, AH-HAS, Y1R antagonist, or TBP were found to reverse the GM dysbiosis and rescue osteoporosis induced by OVX50,57-59 or GC.53 Wang et al59 found that SGP treatment significantly reversed the increase of E. coli and Bacteroides fragilis, and the decrease of Clostridium leptum, Faecalibacterium prausnitzii, and Lactobacillus, and offset the decreased BMD by 16.9% induced by OVX. Guo et al57 reported that VSEE improved BMD and inhibited dyslipidaemia in an OVX model through the regulation of intestinal microbiota and the Wnt/β-catenin pathway.57 Tousen et al50 found that AH-HAS treatment increased the abundance of Bifidobacterium spp., downregulated the expression of osteoclastogenic cytokine RANKL and interleukin-7 receptor (IL-7R) genes in the bone marrow, and attenuated bone loss in OVX mice.50 Xie et al58 reported that Y1R antagonist lowered Firmicutes versus Bacteroidetes ratio and increased the abundance of Lactobacillus in the OVX model, and these changes were found to be significantly associated with the bone microstructure and serum Ca2+ levels.58 Li et al53 found that TBP increased the abundance of Bacteroidetes and Proteobacteria and decreased Firmicutes in a glucocorticoid-induced osteoporosis (GIOP) model, and low dosage of TBP alleviated BMD and bone microarchitecture through reversing the GM alteration, repairing the intestinal barrier, and increasing Wnt/β-catenin pathway in the bone.53 Li et al54 reported that FLL could reverse the abnormal changes of Bifidobacterium and the ratio of Firmicutes/Bacteroidetes, which resulted in increased oxidative stress in the serum and ageing-related osteoporosis triggered by D-gal and NaNO2. Tanabe et al52 reported that FOS and glucomannan treatment increased levels of Lactobacillus and Bacteroides, decreased the levels of Clostridium, reduced serum TNF-α and CRP levels, and restored the bone area and the calcium content in the femur in SAMP6 mice.52 Zhao et al51 treated SAMP6 mice with 12 weeks of E. prostrata, which promoted Lactobacillus growth that enhanced the femur bone volume and trabecular bone structure.51
Yan et al48 found that SCFA supplementation increased IGF-1 levels, and reduced bone mass in antibiotic-treated mice to normal levels.48 Tyagi et al39 found that LGG treatment increased the production of SCFA, which could promote bone formation via regulatory T cell (Treg cell)-mediated regulation of CD8+ T cell Wnt 10b production.39 One study found that TBP treatment increased the SCFA-producing bacteria and calcium concentration, and promoted bone formation.53 Interestingly, Villa et al37 found that dietary vitamin D affects Bacteroides in male offspring only, which was positively correlated with BMD.37
Six studies reported that antibiotics-induced GM dysbiosis led to bone loss.35,41,42,47,48,63 Guss et al35 found that 12 weeks of antibiotic treatment depleted Bacteroidetes and enriched Proteobacteria, and decreased cortical BMD and femur bending strength.35 Yan et al48 found that one-month antibiotic intervention decreased SCFA production, decreased serum IGF-1 levels, and inhibited bone formation.48 Rios-Arce et al42 reported successful depletion of GM by two weeks of ampicillin/neomycin treatment.42 Four weeks after intervention, GM repopulation with reversed Firmicutes versus Bacteroidetes ratio and reduced trabecular BMD was observed. Furthermore, Schepper et al63 found that LR treatment could reverse the above GM alteration, increase the intestinal barrier integrity, and rescue postantibiotic bone loss. Hathaway-Schrader et al47 found that antibiotic treatment from six to 12 weeks caused an increase in γ-proteobacteria in males and Firmicutes in females, respectively, altering trabecular bone mass in both sexes.47 Conversely, Cho et al43 examined the effects of seven weeks of antibiotics from weaning, and found that antibiotic treatment elevated Firmicutes to bacteria ratio, and increased BMD at three weeks after intervention.43 Tavakoli and Xiao61 found that seven weeks of antibiotic treatment depleted GM and rescued the decreased BMD in SCD mice.61
Discussion
In our review, 24 out of 30 studies used mice, while six studies used rats. The preference of mice as an animal model for GM studies is related to a well-established gut metagenome, as well as robust knowledge on gastroenterology, genetics, and immunology in mice.64 Notably, a recently published study had constructed a mouse gut microbial biobank that contains 126 species, represented by 244 strains, which provides valuable information for future study of microbe-host interactions.65 Among the 24 studies on mice, 15 chose the C57BL6 strain.33-46 The C57BL6 mouse is currently the most widely used inbred strain in biomedical research. This strain is easy to breed and is characterized by low BMD.66 The mice are also a commonly used background strain for genetic modification. Using genetically modified C57BL6 mice, four studies have identified the pathways involved in the gut-bone axis, including nucleotide-binding oligomerization domain-containing protein 1 (NOD1) and NOD2 signalling,36 and immune cell (lymphocyte, Treg cell) mediated regulation.35,39,42 Although a previous study comparing the gut metagenomes between mice and humans found that GM showed high functional similarity, there is still a substantial difference at the gene level.67 Hence, the translation of findings into clinical practice still needs further research. The germ-free mice raised in an aseptic isolating environment also serve as an ideal animal model for negative control or recipient of GM transplantation. Despite the altered immune systems and physiological abnormalities of GF mice compared with CONV-R mice, GM colonization is a useful approach as it mimics the natural process of gaining microbial communities at birth as in humans.
To explore the conventional roles of GM in bone homeostasis, germ-free33,34,36,38,45,48 and antibiotic-induced35,41,42,47,48,63 GM dysbiosis models were widely utilized. Bone loss occurred in many conditions, and various animal models emerged.68 In humans, the most common primary osteoporosis types were postmenopausal and age-related osteoporosis. OVX,44,49,50,55-58,62 leuprolide-induced sex steroid depletion animal models,34 SAMP6,51,52 and ageing models induced by D-gal and NaNO2 54 corresponded to the above clinical definitions, respectively. Hypercortisolism was one of the secondary osteoporosis causes,69 and high daily dose of GC use significantly increased the risks of fracture.70 This review included two studies based on GC-induced models.46,53 Previous studies also suggested that HFD disrupted bone remodelling,71 so the diet-fed model was involved.60 TDF is an effective antiviral medicine, however it also accelerates bone loss.72 One study executed TDF animal model to investigate the prevention methods.40 Due to the diverse pathogenic mechanisms of bone loss, it is essential to study the effects and potential pathways of GM and interventions in the relevant animal models separately. Translation and treatment to clinical patients would be the future target.
Eight studies performed ovariectomy to generate PMO models.44,49,50,55-58,62 OVX animal models have been shown to have altered immune status and increased bone inflammation and resorption.73 These features match the intervention with probiotics due to their immunomodulating effects. Nevertheless, the majority of studies applied interventions before OVX,44,49,58 or immediately or four weeks after OVX,50,59 which shows a preventive effect on PMO. Future studies should focus on the effect of probiotics treatment on established osteoporosis. Interestingly, a shift of GM profile characterized by the increase of Firmicutes, E. coli, and B. fragilis, and the decrease of Bacteroidaceae, Alcaligenaceae, C. leptum, F. prausnitzii, and Lactobacillus were observed after OVX in two studies,56,59 which was reversed by sialoglycoprotein of Carassius auratus (Ca-SGP) treatment.59 However, it remains unanswered how these GM profile alterations in OVX model rats induce bone inflammation and result in bone loss. In the study by Li et al,34 osteoporosis was induced by steroid depletion with gonadotropin-releasing hormone (GnRH) agonist leuprolide administration, which was reversed by LGG supplementation.34
Additionally, SAMP6 mice were used in two studies to investigate the effect of probiotics or TCM on senile osteoporosis.51,52 One study utilized SAMR1 as a control group and found that the GM profile in ageing-accelerated P6 mice differed from the R1 strain in the abundance of decreased Firmicutes and Actinobacteria, and increased Bacteroidetes.51 Notably, the authors found that the effects of E. prostrata on osteoporosis were mediated by GM, which was evidenced by increased Lactobacillus and Lactococcus and reversed GM profile alteration in P6 mice after treatment. Decreased Firmicutes versus Bacteroidetes ratio in their study was also identified in the GIOP mice model.53 This ratio was reportedly related to ageing and weight loss, but its implication in osteoporosis has not been specified.74,75 Additionally, Li et al54 used D-gal and NaNO2 injection to induce age-related osteoporosis.54 They observed an increased abundance of pro-ageing microbiota including Clostridium, Clostridiaceae SMB53, and Oscillospira in ageing mice, which led to increased circulating trimethylamine and TMAO levels, and subsequent bone loss.
The role of GM in secondary osteoporosis has also been investigated, including studies on GIOP,46,53 bone loss induced by HFD,60 and TDF.40 It has been shown that GIOP is the most common form of secondary osteoporosis.76 Schepper et al46 were first to report GM alteration with increased Clostridiales and decreased Bacteroidales, Verrucomicrobiales, and intestinal barrier dysfunction in a GIOP mouse model.46 This was comparable with the changes observed in patients with GIOP.77 Furthermore, probiotics LR or MDY treatment were found to enhance barrier function, reduce serum endotoxin levels, and rescue bone loss, which indicates the intestinal barrier function as a potential novel therapeutic target in treating GIOP. Notably, transplantation of GC-treated mouse faecal material into untreated wild-type (WT) mice caused bone loss.45 Li et al53 also found that TBP treatment could rescue GIOP, modulate GM, and slightly increase SCFA production in the gut.53 Another study used HFD to induce bone loss with insulin resistance and systemic inflammation, and found that probiotics and prebiotics treatment improved bone structure and ameliorated bone resorption.60
Bacteria culture and CFU counting is the traditional method for GM quantification.52,55 The major pitfall of this method is the difficulty in culturing obligate anaerobes species, as well as the limited capacity to cover the huge amount of intestinal flora. Quantitative PCR was performed to count bacterial load in six studies.35,37,47,48,59,61 This approach allows quantification of the total commensal bacteria,37,48 or target microbe flora.37 In recent years, NGS including 16S rRNA and metagenomic gene sequencing techniques have become the mainstream approach in determining the GM. The most commonly used approach for GM analysis is 16S rRNA sequencing, which has been applied in 19 studies.35,38-40,42,43,45,46,49-51,53,54,56-59,61,63 The main advantage of 16S rRNA sequencing is that the entire microbiota within one sample could be determined based on quantitative PCR amplification. More recently, metagenomic approach has been used, which can provide additional information on the potential links between the GM and bone due to its versatile functional analysis and increased sequencing depth.41 The authors recommend the 16S rRNA and metagenomic sequencing techniques for future research, which would allow functional analysis.
Micro-CT is the fundamental approach for assessing BMD and microarchitecture in both trabecular and cortical bone,33-40,42,44-51,53-58,61,63 whereas DXA was performed in six studies.37,43,50,56,59,61 DXA enables body composition analysis but can only provide 2D images with relatively low resolution, and without 3D bone structure information. Bone histomorphometry analysis was also common.33,34,38-40,47,48,55,56,60,61,63 This is important in gut-bone analysis, as the change of the GM composition and function is a dynamic process. Meanwhile, mechanical test was applied in eight studies,35,37,42,46,54,55,57,63 which is a well-established standard for bone quality.
The most commonly used treatment was probiotics supplementation, including LGG,34,39,40,46 LR, 42,46,49,63 L. paracasei, 44,60 Bifidobacteria,55 and other strains of Lactobacillus.44,51 These probiotics have been collectively shown to modify the GM profile and functional activities, regulate the immune responses in the host, augment epithelial barrier function, and positively impact BMD and structure, thereby exerting a beneficial effect on bone health.34,35,39,40,42,44,46,49,50,53,55,60,63,78 The review by Villa et al78 showed that the bacterial genus with the greatest potential for future clinical trials was Lactobacillus. However, there is still controversy regarding the effective dose, timing, and duration of treatment.78 Prebiotics including FOS, GM, and AH-HAS were applied in three studies.50,52,60 These prebiotics could yield a similar beneficial effect on bone including GM regulation, altering the immune status, and relieving systemic inflammation. Additionally, other nutritional interventions including various protein extracts and vitamin D were also applied.37,39,53,57,59 These supplementations were found to reverse the GM alteration induced by OVX59 and increase SCFA-producing bacteria in the GIOP model,53 thereby rescuing bone loss. Interestingly, Villa et al37 observed increased Bacteroides in male adult offspring following maternal vitamin D supplementation, which was negatively correlated with systemic inflammation and positively correlated with bone strength and structure.37 Despite the current evidence, there is still a lack of translation to clinical trials.
SCFA produced by intestinal flora is the best-studied group of microbial metabolites, which has been reported to regulate bone homeostasis through direct inhibition of bone resorption, stimulation of calcium absorption, and the immunomodulatory effect.18 In our review, Yan et al48 found that long-term antibiotic treatment reduced SCFA production and increased bone mass, whereas SCFA supplementation reduced bone mass to normal levels.48 Tyagi et al39 revealed the effect of SCFA-induced bone formation through Treg cell-mediated regulation of CD8+ T cell Wnt10b production.39 Similarly, Lucas et al79 indicated the crucial role of SCFAs as potent regulators of osteoclast metabolism and bone homeostasis.79 Ovariectomized mice treated with SFCA significantly increased bone mass and prevented postmenopausal and inflammation-induced bone loss due to the downregulation of essential osteoclast genes including tumour necrosis factor receptor (TNFR)-associated factor 6 (TRAF6) and nuclear factor of activated T cells 1 (NFATc1).79 Positive results were also seen in steady-state conditions. This reinforces the theory that supplements of SFCA or diets increasing the production of SFCA could be a powerful therapeutic. One study also reported that SCFA producers increased following TBP supplementation.53 Meanwhile, the SCFA production in the gut is reportedly affected by antibiotic treatment or colonization.43,48 These findings further confirm the critical role of SCFA in the gut-bone axis, which also serves as a potential therapeutic target for GM manipulation. The central role of SCFA on the gut-bone axis has been extensively reviewed in the study of Zaiss et al.18 The mechanisms including creating a tolerogenic immune environment in the gut epithelium and directly downregulating the osteoclastic genes of osteoclast precursors have been summarized.18 Notably, the advantage of SCFA is that its production could be modified by prebiotics and probiotics, which may serve as an effective, safe, and cost-effective therapeutic option for treating osteoporosis.
There are some limitations in this review. Due to the heterogeneity of the animal strains, interventions, and outcome measurements, the meta-analysis was not performed. Also, indirect mechanisms including the effects of GM on lipid metabolism and adiposity were not discussed, which could also contribute to the systemic inflammation and subsequent bone loss.
In this review, we have summarized the current animal models, intervention protocols, and outcome measurements on gut-bone axis studies. Our findings provide valuable information for further studies designed to explore the gut-bone axis based on animal models, and hopefully shed light on translating these preclinical findings to clinical application. Mice are the preferred animal model for GM studies due to their well-established gut metagenome, and complement the robust knowledge of gastroenterology, genetics, and immunology. The germ-free mice are the ideal animal model for negative control. Treatment of probiotics including LGG, LR, L. paracasei, B. longum, or prebiotic were found to prevent osteoporosis induced by OVX. With the ageing population, future studies should focus on the effect of treatment on established osteoporosis as these patients are at high risk of fragility fractures and subsequent mortality.
In conclusion, future studies should not only focus on the changes of GM composition at higher taxonomic levels but also the functionalities carried by each specific bacterial flora. Metagenomic techniques could serve as a useful tool in identifying the key molecules and pathways involved in the gut-bone axis. In addition to the immunomodulating effects of probiotics, intervention targets including intestinal barrier function and SCFA production enable future studies to explore the gut-bone interaction from new perspectives. Regarding the effect of antibiotics on bone, the GM depletion by chronic treatment should be distinguished from the GM dysbiosis induced by short-term antibiotic treatment. GM transplantation is of important clinical interest; however, its effects on bone homeostasis are still disputable, which entails further validation to obtain more generalized results. Close attention should also be paid to avoid the potential bias caused by disparities in experimental settings, animal strains, and sex, as well as the timepoint and duration of intervention.
Author contributions
J. Li: Acquired and interpreted the data, Wrote and revised the manuscript.
W. T. P. Ho: Drafted the manuscript.
C. Liu: Conceptualized and designed the study, Revised the manuscript.
S. K-H. Chow: Conceptualized and designed the study, Revised the manuscript.
M. Ip: Conceptualized and designed the study.
J. Yu: Conceptualized and designed the study.
H. S. Wong: Conceptualized and designed the study.
W-H. Cheung: Conceptualized and designed the study, Revised the manuscript.
J. J. Y. Sung: Conceptualized and designed the study.
R. M. Y. Wong: Conceptualized and designed the study, Wrote the manuscript.
W-H. Cheung and R. M. Y. Wong are joint senior authors.
W-H. Cheung and R. M. Y. Wong contributed equally to this work.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Supplementary material
Table showing a summary of the characteristics shown in the 30 studies included in this systematic review.
© 2021 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- 1.Sommer F, Bäckhed F. The gut microbiota--masters of host development and physiology. Nat Rev Microbiol. 2013;11(4):227–238. [DOI] [PubMed] [Google Scholar]
- 2.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohlsson C, Sjögren K. Effects of the gut microbiota on bone mass. Trends Endocrinol Metab. 2015;26(2):69–74. [DOI] [PubMed] [Google Scholar]
- 4.Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9(10):599–608. [DOI] [PubMed] [Google Scholar]
- 5.Marik PE. Colonic flora, probiotics, obesity and diabetes. Front Endocrinol. 2012;3:87. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Cheung WH, Miclau T, Chow SK-H, Yang FF, Alt V. Fracture healing in osteoporotic bone. Injury. 2016;47 Suppl 2:S21–S26. [DOI] [PubMed] [Google Scholar]
- 7.Wong RMY, Choy MHV, Li MCM, et al. . A systematic review of current osteoporotic metaphyseal fracture animal models. Bone Joint Res. 2018;7(1):6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong RMY, Law SW, Lee KB, Chow SKH, Cheung WH. Secondary prevention of fragility fractures: instrumental role of a fracture liaison service to tackle the risk of imminent fracture. Hong Kong Med J. 2019;25(3):338–242. [DOI] [PubMed] [Google Scholar]
- 9.Cehic M, Lerner RG, Achten J, Griffin XL, Prieto-Alhambra D, Costa ML. Prescribing and adherence to bone protection medications following hip fracture in the United Kingdom: results from the world hip trauma evaluation (white) cohort study. Bone Joint J. 2019;101-B(11):1402–1407. [DOI] [PubMed] [Google Scholar]
- 10.Khosla S, Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017;5(11):898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng X, Wu X, Zhang J, Zhang G, Li G, Pan X. The role of CKIP-1 in osteoporosis development and treatment. Bone Joint Res. 2018;7(2):173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie X, Liu M, Meng Q. Angelica polysaccharide promotes proliferation and osteoblast differentiation of mesenchymal stem cells by regulation of long non-coding RNA H19: an animal study. Bone Joint Res. 2019;8(7):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abar O, Dharmar S, Tang SY. The effect of aminoguanidine (Ag) and pyridoxamine (PM) on ageing human cortical bone. Bone Joint Res. 2018;7(1):105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Q-F, Sun H, Shu L-Y, et al. . Radiographic predictors for bone mineral loss: cortical thickness and index of the distal femur. Bone Joint Res. 2018;7(7):468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu W-B, Hsu W-H, Hung J-S, Shen W-J, Hsu RW-W. Transcriptome analysis of osteoblasts in an ovariectomized mouse model in response to physical exercise. Bone Joint Res. 2018;7(11):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez CJ, Guss JD, Luna M, Goldring SR. Links between the microbiome and bone. J Bone Miner Res. 2016;31(9):1638–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quach D, Britton RA. Gut Microbiota and Bone Health. Adv Exp Med Biol. 2017;1033:47–58. [DOI] [PubMed] [Google Scholar]
- 18.Zaiss MM, Jones RM, Schett G, Pacifici R. The gut-bone axis: how bacterial metabolites bridge the distance. J Clin Invest. 2019;129(83018–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nilsson AG, Sundh D, Bäckhed F, Lorentzon M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J Intern Med. 2018;284(3):307–317. [DOI] [PubMed] [Google Scholar]
- 20.Lambert MNT, Thybo CB, Lykkeboe S, et al. . Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: a randomized controlled trial. Am J Clin Nutr. 2017;106(3):909–920. [DOI] [PubMed] [Google Scholar]
- 21.Latorre JD, Hernandez-Velasco X, Bielke LR, et al. . Evaluation of a Bacillus direct-fed microbial candidate on digesta viscosity, bacterial translocation, microbiota composition and bone mineralisation in broiler chickens fed on a rye-based diet. Br Poult Sci. 2015;56(6):723–732. [DOI] [PubMed] [Google Scholar]
- 22.Latorre JD, Hernandez-Velasco X, Vicente JL, Wolfenden R, Hargis BM, Tellez G. Effects of the inclusion of a Bacillus direct-fed microbial on performance parameters, bone quality, recovered gut microflora, and intestinal morphology in broilers consuming a grower diet containing corn distillers dried grains with solubles. Poult Sci. 2017;96(8):2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tellez G, Latorre JD, Kuttappan VA, et al. . Utilization of rye as energy source affects bacterial translocation, intestinal viscosity, microbiota composition, and bone mineralization in broiler chickens. Front Genet. 2014;5:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doonan J, Tarafdar A, Pineda MA, et al. . The parasitic worm product ES-62 normalises the gut microbiota bone marrow axis in inflammatory arthritis. Nat Commun. 2019;10(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guss JD, Ziemian SN, Luna M, et al. . The effects of metabolic syndrome, obesity, and the gut microbiome on load-induced osteoarthritis. Osteoarthritis Cartilage. 2019;27(1):129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rios JL, Bomhof MR, Reimer RA, Hart DA, Collins KH, Herzog W. Protective effect of prebiotic and exercise intervention on knee health in a rat model of diet-induced obesity. Sci Rep. 2019;9(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan H, Guo R, Ju Y, et al. . A single bacterium restores the microbiome dysbiosis to protect bones from destruction in a rat model of rheumatoid arthritis. Microbiome. 2019;7(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henrotin Y, Patrier S, Pralus A, Roche M, Nivoliez A. Protective actions of oral administration of Bifidobacterium longum CBi0703 in spontaneous osteoarthritis in Dunkin Hartley guinea pig model. Cartilage. 2019:194760351984167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duan Y, Prasad R, Feng D, et al. . Bone marrow-derived cells restore functional integrity of the gut epithelial and vascular barriers in a model of diabetes and ACE2 deficiency. Circ Res. 2019;125(11):969–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia X, Jia L, Mo L, et al. . Berberine ameliorates periodontal bone loss by regulating gut microbiota. J Dent Res. 2019;98(1):107–116. [DOI] [PubMed] [Google Scholar]
- 31.Yang T, Ahmari N, Schmidt JT, et al. . Shifts in the gut microbiota composition due to depleted bone marrow beta adrenergic signaling are associated with suppressed inflammatory transcriptional networks in the mouse colon. Front Physiol. 2017;8:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staffas A, Burgos da Silva M, Slingerland AE, et al. . Nutritional support from the intestinal microbiota improves hematopoietic reconstitution after bone marrow transplantation in mice. Cell Host Microbe. 2018;23(4):447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sjögren K, Engdahl C, Henning P, et al. . The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27(6):1357–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J-Y, Chassaing B, Tyagi AM, et al. . Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016;126(6):2049–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guss JD, Horsfield MW, Fontenele FF, et al. . Alterations to the gut microbiome impair bone strength and tissue material properties. J Bone Miner Res. 2017;32(6):1343–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohlsson C, Nigro G, Boneca IG, Bäckhed F, Sansonetti P, Sjögren K. Regulation of bone mass by the gut microbiota is dependent on Nod1 and NOD2 signaling. Cell Immunol. 2017;317:55–58. [DOI] [PubMed] [Google Scholar]
- 37.Villa CR, Taibi A, Chen J, Ward WE, Comelli EM. Colonic Bacteroides are positively associated with trabecular bone structure and programmed by maternal vitamin D in male but not female offspring in an obesogenic environment. Int J Obes. 2018;42(4):696–703. [DOI] [PubMed] [Google Scholar]
- 38.Quach D, Collins F, Parameswaran N, McCabe L, Britton RA. Microbiota reconstitution does not cause bone loss in germ-free mice. mSphere. 2018;3(1):e00545–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tyagi AM, Yu M, Darby TM, et al. . The microbial metabolite butyrate stimulates bone formation via T regulatory cell-mediated regulation of Wnt10b expression. Immunity. 2018;49(6):1116–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu H, Gu R, Li W, et al. . Lactobacillus rhamnosus GG attenuates tenofovir disoproxil fumarate-induced bone loss in male mice via gut-microbiota-dependent anti-inflammation. Ther Adv Chronic Dis. 2019;10:204062231986065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guss JD, Taylor E, Rouse Z, et al. . The microbial metagenome and bone tissue composition in mice with microbiome-induced reductions in bone strength. Bone. 2019;127:146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rios-Arce ND, Schepper JD, Dagenais A, et al. . Post-antibiotic gut dysbiosis-induced trabecular bone loss is dependent on lymphocytes. Bone. 2020;134:115269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho I, Yamanishi S, Cox L, et al. . Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488(7413):621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohlsson C, Engdahl C, Fåk F, et al. . Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One. 2014;9(3):e92368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hathaway-Schrader JD, Poulides NA, Carson MD, et al. . Specific commensal bacterium critically regulates gut microbiota Osteoimmunomodulatory actions during normal postpubertal skeletal growth and maturation. JBMR Plus. 2020;4(3):e10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schepper JD, Collins F, Rios‐Arce ND, et al. . Involvement of the gut microbiota and barrier function in Glucocorticoid‐Induced osteoporosis. J Bone Miner Res. 2020;35(4):801–820. [DOI] [PubMed] [Google Scholar]
- 47.Hathaway-Schrader JD, Steinkamp HM, Chavez MB, et al. . Antibiotic perturbation of gut microbiota dysregulates osteoimmune cross talk in postpubertal skeletal development. Am J Pathol. 2019;189(2):370–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan J, Herzog JW, Tsang K, et al. . Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci U S A. 2016;113(47):E7554–E7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Britton RA, Irwin R, Quach D, et al. . Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol. 2014;229(11):1822–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tousen Y, Matsumoto Y, Nagahata Y, Kobayashi I, Inoue M, Ishimi Y. Resistant starch attenuates bone loss in ovariectomised mice by regulating the intestinal microbiota and bone-marrow inflammation. Nutrients. 2019;11(2):297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao X, Ai J, Mao H, Gao X. Effects of Eclipta prostrata on gut microbiota of SAMP6 mice with osteoporosis. J Med Microbiol. 2019;68(3):402–416. [DOI] [PubMed] [Google Scholar]
- 52.Tanabe K, Nakamura S, Moriyama-Hashiguchi M, et al. . Dietary Fructooligosaccharide and Glucomannan Alter Gut Microbiota and Improve Bone Metabolism in Senescence-Accelerated Mouse. J Agric Food Chem. 2019;67(3):867–874. [DOI] [PubMed] [Google Scholar]
- 53.Li J, Yang M, Lu C, et al. . Tuna Bone Powder Alleviates Glucocorticoid-Induced Osteoporosis via Coregulation of the NF-κB and Wnt/β-Catenin Signaling Pathways and Modulation of Gut Microbiota Composition and Metabolism. Mol Nutr Food Res. 2020;64(5):1900861. [DOI] [PubMed] [Google Scholar]
- 54.Li L, Chen B, Zhu R, et al. . Fructus Ligustri Lucidi preserves bone quality through the regulation of gut microbiota diversity, oxidative stress, TMAO and SIRT6 levels in aging mice. Aging. 2019;11(21):9348–9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parvaneh K, Ebrahimi M, Sabran MR, et al. . Probiotics (Bifidobacterium longum) increase bone mass density and upregulate SPARC and BMP-2 genes in rats with bone loss resulting from ovariectomy. Biomed Res Int. 2015;2015:897639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Conley MN, Roberts C, Sharpton TJ, Iwaniec UT, Hord NG. Increasing dietary nitrate has no effect on cancellous bone loss or fecal microbiome in ovariectomized rats. Mol Nutr Food Res. 2017;61(5):1600372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo D, Liu W, Zhang X, et al. . Duck Egg White-Derived Peptide VSEE (Val-Ser-Glu-Glu) Regulates Bone and Lipid Metabolisms by Wnt/β-Catenin Signaling Pathway and Intestinal Microbiota. Mol Nutr Food Res. 2019;63(24):1900525. [DOI] [PubMed] [Google Scholar]
- 58.Xie W, Han Y, Li F, et al. . Neuropeptide Y1 receptor antagonist alters gut microbiota and alleviates the ovariectomy-induced osteoporosis in rats. Calcif Tissue Int. 2020;106(4):444–454. [DOI] [PubMed] [Google Scholar]
- 59.Wang F, Yu P, Gui X, Wang Y, Xue C, Wang J. Sialoglycoprotein isolated from the eggs of Carassius auratus prevents bone loss: an effect associated with the regulation of gut microbiota in ovariectomized rats. Food Funct. 2016;7(12):4764–4771. [DOI] [PubMed] [Google Scholar]
- 60.Eaimworawuthikul S, Tunapong W, Chunchai T, et al. . Altered gut microbiota ameliorates bone pathology in the mandible of obese-insulin-resistant rats. Eur J Nutr. 2020;59(4):1453–1462. [DOI] [PubMed] [Google Scholar]
- 61.Tavakoli S, Xiao L. Depletion of intestinal microbiome partially rescues bone loss in sickle cell disease male mice. Sci Rep. 2019;9(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang J, Wang Y, Gao W, et al. . Diversity analysis of gut microbiota in osteoporosis and osteopenia patients. PeerJ. 2017;5:e3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schepper JD, Collins FL, Rios-Arce ND, et al. . Probiotic Lactobacillus reuteri Prevents Postantibiotic Bone Loss by Reducing Intestinal Dysbiosis and Preventing Barrier Disruption. J Bone Miner Res. 2019;34(4):681–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nguyen TLA, Vieira-Silva S, Liston A, Raes J. How informative is the mouse for human gut microbiota research? Dis Model Mech. 2015;8(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu C, Zhou N, Du M-X, et al. . The mouse gut microbial Biobank expands the coverage of cultured bacteria. Nat Commun. 2020;11(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone. 1996;18(5):397–403. [DOI] [PubMed] [Google Scholar]
- 67.Xiao L, Feng Q, Liang S, et al. . A catalog of the mouse gut metagenome. Nat Biotechnol. 2015;33(10):1103–1108. [DOI] [PubMed] [Google Scholar]
- 68.Komori T. Animal models for osteoporosis. Eur J Pharmacol. 2015;759:287–294. [DOI] [PubMed] [Google Scholar]
- 69.Hudec SMD, Camacho PM. Secondary causes of osteoporosis. Endocr Pract. 2013;19(1):120–128. [DOI] [PubMed] [Google Scholar]
- 70.Buckley L, Humphrey MB. Glucocorticoid-Induced osteoporosis. N Engl J Med. 2018;379(26):2547–2556. [DOI] [PubMed] [Google Scholar]
- 71.Montalvany-Antonucci CC, Zicker MC, Ferreira AVM, et al. . High-fat diet disrupts bone remodeling by inducing local and systemic alterations. J Nutr Biochem. 2018;59:93–103. [DOI] [PubMed] [Google Scholar]
- 72.Seto W-K, Asahina Y, Brown TT, et al. . Improved bone safety of tenofovir Alafenamide compared to tenofovir disoproxil fumarate over 2 years in patients with chronic HBV infection. Clin Gastroenterol Hepatol. 2018;S1542-3565(18):30633–30635. [DOI] [PubMed] [Google Scholar]
- 73.Clowes JA, Riggs BL, Khosla S. The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev. 2005;208:207–227. [DOI] [PubMed] [Google Scholar]
- 74.Mariat D, Firmesse O, Levenez F, et al. . The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Indiani CMDSP, Rizzardi KF, Castelo PM, Ferraz LFC, Darrieux M, Parisotto TM. Childhood obesity and Firmicutes/Bacteroidetes ratio in the gut microbiota: a systematic review. Child Obes. 2018;14(8):501–509. [DOI] [PubMed] [Google Scholar]
- 76.Buckley L, Guyatt G, Fink HA, et al. . American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis Rheumatol. 2017;69(8):1521–1537. [DOI] [PubMed] [Google Scholar]
- 77.Qiu D, Xia Z, Deng J, Jiao X, Liu L, Li J. Glucorticoid-induced obesity individuals have distinct signatures of the gut microbiome. Biofactors. 2019;45(6):892–901. [DOI] [PubMed] [Google Scholar]
- 78.Villa CR, Ward WE, Comelli EM. Gut microbiota-bone axis. Crit Rev Food Sci Nutr. 2017;57(8):1664–1672. [DOI] [PubMed] [Google Scholar]
- 79.Lucas S, Omata Y, Hofmann J, et al. . Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat Commun. 2018;9(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]