Abstract
Background:
Post-operative atrial fibrillation (pAF) following coronary artery bypass grafting (CABG) is a common complication. Whether pAF is associated with an increased risk of cerebrovascular accident (CVA) remains uncertain. We investigated the association between pAF and long-term risk of CVA by performing a post-hoc analysis of 10-year outcomes of the Arterial Revascularization Trial (ART).
Methods:
For the present analysis, among patients enrolled in the ART (n=3102), we excluded those who did not undergo surgery (n=25), had a prior history of atrial fibrillation (n=45), or had no information regarding the incidence of pAF (n=9). The final population consisted of 3023 patients of whom 734 (24.3%) developed pAF with the remaining 2289 maintaining sinus rhythm (SR). Competing risk and Cox regression analysis were used to investigate the association between pAF and the risk of CVA.
Results:
At 10 years, the cumulative incidence of CVA was 6.3% (4.6–8.1) vs 3.7% (2.9–4.5) in patients with pAF and SR respectively. pAF was an independent predictor of CVA at 10 years (HR 1.53; 95%CI 1.06–2.23; P-value=0.025) even when CVAs that occurred during the index admission were excluded from the analysis (HR 1.47; 95% 1.02–2.11; P=0.04).
Conclusions:
Patients with pAF after CABG are at higher risk of CVA. These findings challenge the notion that pAF is a benign complication.
Keywords: post-operative atrial fibrillation, stroke, coronary artery bypass graft surgery, cerebrovascular accidents
Introduction
The incidence of postoperative atrial fibrillation (pAF) following coronary artery bypass grafting (CABG) surgery ranges between 20% and 40%. pAF typically develops within the first week post-surgery, at a median time of 2 days after the operation. It generally resolves, with or without medication, within 24–48 hours and most patients are discharged in sinus rhythm (SR) [1]. While pAF has been traditionally considered a transient and benign complication of CABG [2], more recent studies have reported an association between pAF and increased early mortality and morbidity including stroke, renal and respiratory failure and a prolonged intensive care unit duration [3]. Although pAF may not be directly responsible for these poor outcomes, it is likely contributory and is, at least, a surrogate for increased morbidity and mortality following cardiac surgery [4,5].
What remains unclear is whether patients who develop transient pAF are at higher risk of stroke after discharge as conflicting findings have been reported on the association between pAF and an increased risk of late stroke [6–8]. Consequently, current guidelines do not support long-term anticoagulation in patients with pAF following CABG [9].
The Arterial Revascularization Trial (ART) is one of the largest randomized trials of surgical coronary revascularization which was designed to compare 10-year outcomes after bilateral vs single internal thoracic artery grafts [10]. In the this post-hoc analysis, we investigated the association between pAF and risk of stroke during 10-year follow-up in patients undergoing CABG.
Methods
The authors declare that all data are available to other researchers on reasonable request.
A post-hoc analysis of the ART trial was conducted. For the present analysis, among patients enrolled in the ART (n=3102) from 2004 to 2007, we excluded those who did not undergo surgery (n=25), had a prior history of atrial fibrillation/flutter before surgery (n=45), and those with no information regarding the incidence of pAF (n=9), as shown in Figure I in the Supplement.
The remaining 3023 patients were classified based on the occurrence of pAF during their index admission. pAF was defined as the occurrence of any episode of atrial fibrillation or flutter (collectively termed pAF for this analysis) following the index procedure through the time of discharge that lasted at least 30 seconds and was captured on a standard 12-lead electrocardiogram or cardiac telemetry [11]. After discharge, all patients underwent 12-lead electrocardiogram within 6 weeks.
Trial design
The ART was approved by the institutional review board of all participating centers, and informed consent was obtained from each participant. The protocol of ART has been published [12]. Briefly, ART was a 2-arm, randomized multicenter trial conducted in 28 hospitals in 7 countries, with patients being randomized equally to single or bilateral internal thoracic artery grafts. Eligible patients were those with multivessel coronary artery disease undergoing CABG including urgent patients. Only emergency patients (refractory myocardial ischemia/cardiogenic shock) and those requiring single grafts, redo CABG and concomitant procedures were excluded.
Follow-up
Questionnaires were sent to study participants by post every year after surgery. No clinic visits were planned apart from the routine clinical 6-week post-operative visit. Participants were sent stamped addressed envelopes to improve the return rates of postal questionnaires. Study co-ordinators contacted participants by telephone to alert them to the questionnaire’s arrival and to ask them about medications, adverse events and health services resource use.
Study Endpoints
The primary endpoint was the incidence of cerebrovascular accident (CVA) which occurred after discharge during the 10-year follow-up. Secondary endpoints were 10-year cardiovascular (CV) and all-cause mortality.
Endpoints definition
CVA was defined as a new neurological deficit evidenced by clinical signs of paresis, plegia or new cognitive dysfunction including any mental status alteration lasting more than 24 hours and/or evidence on CT or MRI scan of recent brain infarct (less than 6 months). The modified Rankin Scale [13] was used to evaluate the degree of disability in patients who suffered a CVA. The scale runs from 0–6, running from perfect health without symptoms to death: 0 - No symptoms; 1 - No significant disability. Able to carry out all usual activities, despite some symptoms; 2 - Slight disability. Able to look after own affairs without assistance, but unable to carry out all previous activities; 3 - Moderate disability. Requires some help, but able to walk unassisted; 4 - Moderately severe disability. Unable to attend to own bodily needs without assistance, and unable to walk unassisted; 5 - Severe disability. Requires constant nursing care and attention, bedridden, incontinent; 6 - Dead.
Death was classified as cardiovascular and non-cardiovascular, using autopsy reports and death certificates. Cardiovascular deaths were defined as deaths due to cardiac causes (i.e. congestive heart failure, arrhythmias, myocardial infarction) and vascular causes (i.e. CVA, dissection and pulmonary embolism).
Major bleeding was defined according to the Bleeding Academic Research Consortium (BARC) definition as any haemorrhage requiring blood transfusion (type 3a), compromising patient hemodynamically (3b), requiring surgical reintervention (type 4) or resulting in patient death (type 5) [14].
Statistical analysis
A set of baseline characteristics were selected to adjust the association between pAF and the risk of CVA: age, female sex, New York Heart Association (NYHA) class, left ventricular ejection fraction (LVEF), diabetes, smoking status, chronic obstructive pulmonary disease (COPD), arterial hypertension (medically treated), prior myocardial infarction (MI), body mass index (BMI), creatinine, previous CVA, peripheral vascular disease, unstable angina, previous percutaneous coronary intervention (PCI), off-pump surgery and total number of grafts. The rate of missing data for these variables was low (Figure II in the Supplement) and missing data were handled with multiple imputation (i.e. 10 imputed datasets) with multivariate imputation by chained equations (MICE). Individual coefficients from each imputed dataset were combined based upon Rubin’s rules for pooling [15]. For the comparison of baseline characteristics between patients with stable sinus rhythm (SR group) and with pAF (pAF group), categorical variables were compared between the two groups with the chi-square test or Fisher’s exact test for categorial variables and Student’s t-test or the Wilcoxon rank-sum test for non-normally distributed continuous variables. The 10-year cumulative incidence of CVA and CV mortality in the two groups was calculated using competing risk analysis for CVA and CV mortality, accounting for the competing risk of death, as proposed by Fine and Gray [16]. The cumulative incidence for all-cause mortality was calculated using 1-Kaplan–Meier estimates. The association between pAF and primary and secondary endpoints was estimated as a sub-distribution hazard ratio (HR) and its 95% confidence interval derived from univariable and bi-directional stepwise multivariable Cox models. Variables included in the final multivariable model were selected based on Akaike information criterion (AIC). Proportional hazard assumption was assessed with Schoenfeld residuals and global p was reported. The SR group was used as the reference group in all analyses. Adjusted P for multiple comparisons (primary and secondary endpoints) was calculated using Bonferroni-Holm correction.
For sensitivity analysis, the association between pAF and the primary outcomes was recalculated restricting the analysis only to CVA that occurred after discharge or excluding patients with evidence of pAF within 6 weeks following discharge. Finally, we calculated the CHA2DS2VASc for each patient, which is a widely adopted tool to stratify patients based on their predicted risk of CVA [17]. To investigate if pAF was associated with an additional risk across CHA2DS2VASc score categories, the interaction between CHA2DS2VASc and pAF on the risk of CVA was explored by forcing their interaction term in a Cox regression model. Relative Hazard was calculated and plotted for each CHA2DS2VASc categories in the pAF and SR groups. We then defined high risk patients as those with CHA2DS2VASc ≥4 which corresponds to the 75th percentile of its distribution. The cumulative incidence of CVA is reported in 4 groups stratified by rhythm status (pAF vs SR) and baseline CHA2DS2VASc (<4 vs ≥4). The relative risk of CVA was calculated across the groups as Hazard ratio and 95%CI using univariable Cox regression and using patients with SR and CHA2DS2VASc score<4 for reference. As only a small number of patients in the pAF group received vitamin K antagonists (i.e. Warfarin), we reported the cumulative incidence of major bleeding and CVA stratified for anticoagulation therapy received for descriptive purpose only. P values <0.05 were considered significant. Statistical analyses were performed using R Statistical Software (version 3.2.3; R Foundation for Statistical Computing, Vienna, Austria) and the following packages: mice for multiple imputation, survival for survival analysis, finalfit for univariate and multivariate regression tables generation and ggplot2 for figures.
Results
Study population
The final population consisted of 3023 patients. Of whom, 734 (24.3%) and 2289 (75.7%) patients presented with pAF or stable SR respectively during the index hospitalization. Baseline characteristics in the two groups are presented in Table 1. The incidence of CVA during index admission was 14 (1.9%) and 23 (1.0%) in the pAF and SR groups, respectively. Hospital mortality was 13 (1.8%) and 16 (0.7%) in the pAF and SR groups, respectively. In 676 (92.1%) patients in the pAF group, stable SR was restored before discharge. In the SR group, 20 patients presented with new onset of AF within 6 weeks after discharge.
Table 1.
Baseline characteristics in patients with and without postoperative atrial fibrillation
| pAF | SR | p | |
|---|---|---|---|
| n | 734 | 2289 | |
| Age, year (mean (SD)) | 66.41 (8.16) | 62.58 (8.93) | <0.001 |
| Ethnicity, n (%) | <0.001 | ||
| Caucasian | 710 (96.7) | 2064 (90.2) | |
| East Asian | 1 (0.1) | 5 (0.2) | |
| South Asian | 19 (2.6) | 129 (5.6) | |
| Afro-Caribbean | 0 (0.0) | 2 (0.1) | |
| African | 0 (0.0) | 5 (0.2) | |
| Hispanic | 4 (0.5) | 84 (3.7) | |
| Female, n (%) | 99 (13.5) | 331 (14.5) | 0.55 |
| LVEF, n (%) | 0.035 | ||
| ≥ 50% | 527 (71.8) | 1743 (76.1) | |
| 30–50% | 186 (25.3) | 503 (22.0) | |
| < 30% | 21 (2.9) | 43 (1.9) | |
| Peripheral Vascular Disease, n (%) | 55 (7.5) | 154 (6.7) | 0.53 |
| Creatinine, mmol/l (mean (SD)) | 98.63 (23.72) | 95.90 (21.07) | 0.003 |
| Body Mass Index, (mean (SD)) | 28.29 (3.88) | 28.18 (4.08) | 0.51 |
| COPD, n (%) | 18 (2.5) | 55 (2.4) | 1.000 |
| Smoking, n (%) | 0.07 | ||
| Current | 90 (12.3) | 346 (15.1) | |
| Ex-smoker | 435 (59.3) | 1258 (55.0) | |
| Never smoked | 209 (28.5) | 685 (29.9) | |
| Previous cerebrovascular accident, n (%) | 48 (6.5) | 129 (5.6) | 0.41 |
| NYHA class 3 or 4, n (%) | 134 (18.3) | 509 (22.2) | 0.025 |
| Diabetes, n (%) | 0.17 | ||
| No | 546 (74.4) | 1771 (77.4) | |
| Insulin-dependent | 49 (6.7) | 119 (5.2) | |
| Non-insulin dependent | 139 (18.9) | 399 (17.4) | |
| Arterial hypertension, n (%) | 579 (78.9) | 1766 (77.2) | 0.35 |
| Unstable angina, n (%) | 53 (7.2) | 182 (8.0) | 0.57 |
| Prior MI, n (%) | 326 (44.4) | 938 (41.0) | 0.11 |
| Prior PCI, n (%) | 124 (16.9) | 354 (15.5) | 0.39 |
| Off-pump surgery, n (%) | 268 (36.5) | 969 (42.3) | 0.006 |
| Number of grafts, mean (SD) | 3.23 (0.81) | 3.17 (0.81) | 0.06 |
pAF: post-operative atrial fibrillation; SR: sinus rhythm; LVEF: left ventricular ejection fraction; COPD: chronic obstructive pulmonary disease; CVA: cerebrovascular accident: NYHA: New York Heart Association; MI: myocardial infarction; PCI: percutaneous coronary intervention
Medications at discharge in the two groups are presented in Table I in the Supplement. In the pAF group, Warfarin was prescribed in 61 (8.3%) patients (12 with persistent pAF and the remaining 49 with SR restoration), while in the SR group, Warfarin was prescribed in 18 (0.8%) patients. As expected, the proportion of patients discharged on amiodarone was higher in the pAF group (47.7% vs 1.7%).
Association between pAF and 10-year outcomes
During 10-year follow-up, a total of 46 (6.3%) CVA were recorded (23 ischemic, 4 haemorrhagic and 19 unknown aetiology) in the pAF group. Their median modified Rankin score was 3.0 [interquartile range 1–5]. A total of 83 (3.6%) CVA were recorded in the SR group (55 ischemic, 7 haemorrhagic and 21 unknown aetiology). Their median modified Rankin score was 2.5 [interquartile range 1–4] (Table II in the Supplement). The cumulative incidence of CVA at 10 years was 6.3% (4.6–8.1) vs 3.7% (2.9–4.5) in the pAF and SR groups respectively (Table III in the Supplement). With univariable and multivariable Cox regression (Table 2), pAF was found to be an independent predictor of CVA at 10 years (HR 1.53; 95%CI 1.06–2.23; Bonferroni-Holm corrected P-value=0.025; Global P =0.40; Figure 1 Panel A). Causes of death are reported in Table IV in the Supplement. CVA was reported as cause of death in 21 (9.7%) out of 216 total deaths and in 15 (3.8%) out of 394 total deaths in the pAF and SR groups, respectively. At 10 years the cumulative incidence of cardiovascular and all-cause mortality was 11.1% (8.8–13.4) vs 6.4% (5.3–7.4) and 30.2% (26.8–33.6) vs 18% (16.4–19.6) in the pAF and SR groups, respectively. pAF was independently associated with increased risk of cardiovascular mortality (HR 1.48; 95%CI 1.11–1.97; Bonferroni-Holm corrected P-value=0.01; Global P=0.60; Table 3, Figure 2 Panel A) and all-cause mortality (HR 1.34; 95%CI 1.13–1.59; Bonferroni-Holm corrected P-value=0.03; Global P=0.10; Table V in the Supplement; Figure 2 Panel B). Sensitivity analysis confirmed that an independent association between pAF and risk of CVA at 10 years also existed when CVA that occurred during the index admission was excluded (HR 1.47; 95% 1.02–2.11; P=0.04; Table VI in the Supplement; Figure 1 Panel B) and when patients with evidence of atrial fibrillation within 6 weeks from discharge were excluded (HR 1.49; 95%CI 1.04–2.16; P=0.032; Table VII in the Supplement). In the pAF group, the cumulative incidence of CVA and major bleeding at 10 years was 3.6%(0–8.4%) vs 5.3%(3.5–7.0) (Table VIII in the Supplement) and 3.4%(0.0–8.1) vs 4.1%(2.6–5.7) (Table IX in the Supplement) in patients discharged with and without Warfarin.
Table 2.
Association between clinical variables and cerebrovascular accidents at 10 years.
| Variables | HR 95% CI (univariable) |
HR 95% CI (multivariable) |
|
|---|---|---|---|
| pAF (SR as reference) | 1.79 (1.25–2.57, p=0.001) | 1.53 (1.06–2.23, p=0.025) | |
| Age | 1.07 (1.05–1.10, p<0.001) | 1.07 (1.05–1.10, p<0.001) | |
| Ethnicity | Caucasian | Reference | Reference |
| East-Asian | 0.00 (0.00-Inf, p=0.995) | 0.00 (0.00-Inf, p=0.996) | |
| South-Asian | 0.46 (0.15–1.45, p=0.186) | 0.89 (0.28–2.87, p=0.847) | |
| Afro-Caribbean | 0.00 (0.00-Inf, p=0.997) | 0.00 (0.00-Inf, p=0.998) | |
| African | 0.00 (0.00-Inf, p=0.996) | 0.00 (0.00-Inf, p=0.996) | |
| Hispanic | 2.88 (1.51–5.49, p=0.001) | 4.95 (2.48–9.90, p<0.001) | |
| Female gender | 1.99 (1.33–2.97, p=0.001) | 1.62 (1.07–2.46, p=0.024) | |
| LVEF | ≥ 50% | Reference | |
| 30–50 % | 1.38 (0.94–2.03, p=0.101) | - | |
| < 30% | 1.36 (0.43–4.29, p=0.603) | - | |
| Peripheral Vascular Disease | 1.05 (0.53–2.06, p=0.892) | - | |
| Creatinine | 1.01 (1.00–1.01, p=0.026) | - | |
| Body Mass Index | 0.99 (0.95–1.04, p=0.709) | - | |
| COPD | 1.74 (0.71–4.26, p=0.224) | - | |
| Smoking | Current | Reference | |
| Ex-smoker | 0.77 (0.47–1.27, p=0.311) | 0.52 (0.31–0.87, p=0.013) | |
| Never smoked | 1.05 (0.62–1.77, p=0.857) | 0.60 (0.35–1.05, p=0.072) | |
| Prior CVA | 3.26 (2.02–5.25, p<0.001) | 2.56 (1.58–4.15, p<0.001) | |
| NYHA class 3 or 4 | 0.73 (0.46–1.16, p=0.184) | 0.61 (0.37–0.98, p=0.043) | |
| Diabetes | No | Reference | |
| Insulin-dependent | 1.07 (0.50–2.30, p=0.869) | - | |
| Non-insulin dependent | 1.12 (0.72–1.74, p=0.602) | - | |
| Arterial hypertension | 1.59 (0.98–2.55, p=0.058) | - | |
| Unstable angina | 0.79 (0.39–1.61, p=0.514) | - | |
| Prior MI | 1.06 (0.75–1.50, p=0.740) | - | |
| Prior PCI | 1.57 (1.04–2.37, p=0.033) | 1.85 (1.22–2.80, p=0.004) | |
| Off-pump surgery | 1.27 (0.90–1.80, p=0.173) | - | |
| Number of grafts | 1.04 (0.84–1.28, p=0.737) | - |
pAF: post-operative atrial fibrillation; SR: sinus rhythm; LVEF: left ventricular ejection fraction; COPD: chronic obstructive pulmonary disease; CVA: cerebrovascular accident: NYHA: New York Heart Association; MI: myocardial infarction; PCI: percutaneous coronary intervention
Figure 1.
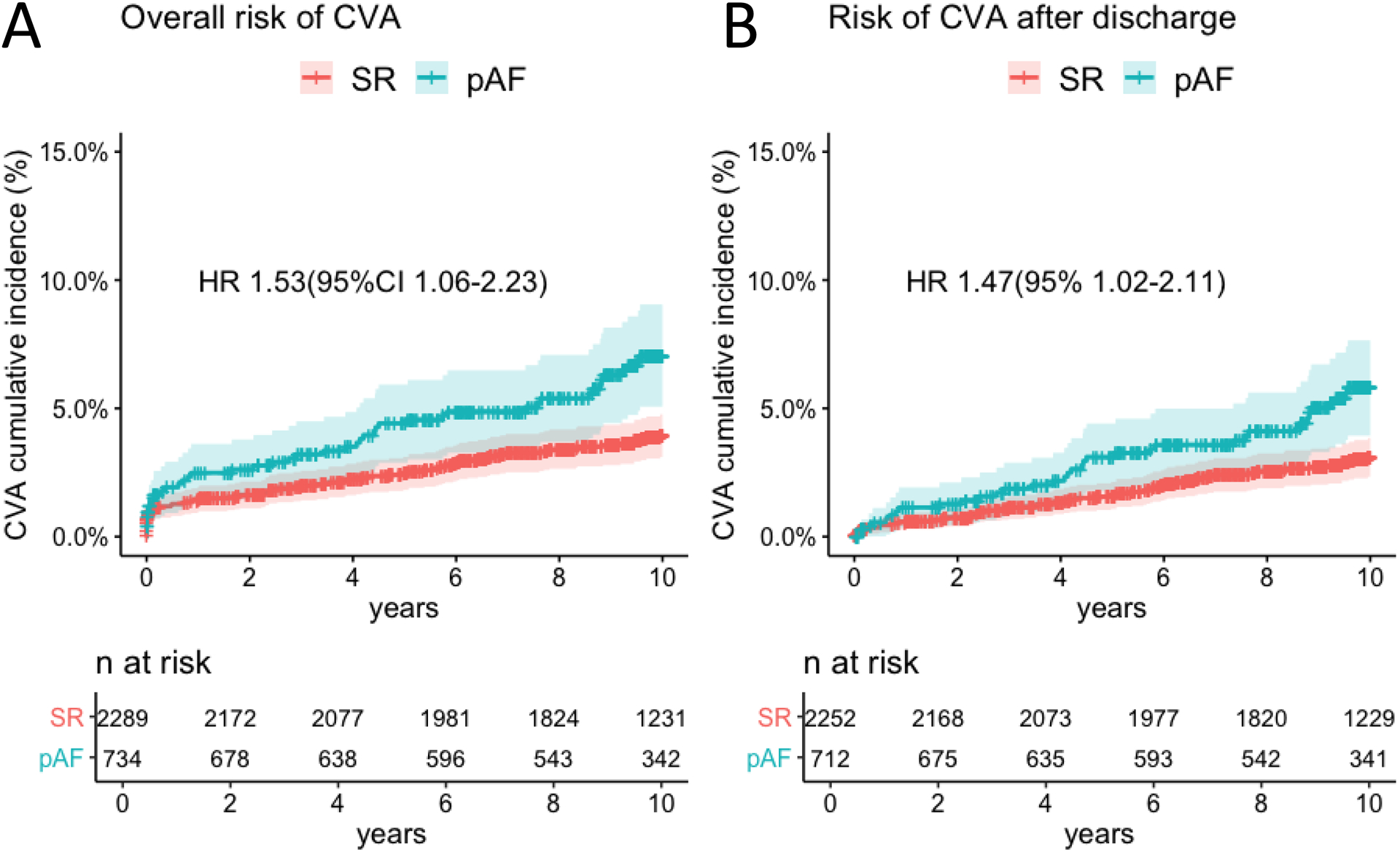
Cumulative incidence of cerebrovascular accident (CVA) in patients with postoperative atrial fibrillation (pAF) and stable sinus rhythm (SR) (Panel A) in the overall sample. Cumulative incidence of CVA in patients with pAF and stable SR after discharge (i.e. CVA that occurred during index hospitalization were excluded) (Panel B).
Table 3.
Association between clinical variables and cardiovascular mortality at 10 years.
| Variables | HR 95% CI (univariable) |
HR 95% CI (multivariable) | |
|---|---|---|---|
| pAF (SR as reference) | 1.86 (1.42–2.45, p<0.001) | 1.48 (1.11–1.97, p=0.007) | |
| Age | 1.08 (1.06–1.10, p<0.001) | 1.08 (1.06–1.10, p<0.001) | |
| Ethnicity | Caucasian | Reference | |
| East-Asian | 2.19 (0.31–15.65, p=0.433) | 7.77 (1.07–56.53, p=0.043) | |
| South-Asian | 0.53 (0.23–1.19, p=0.123) | 1.11 (0.49–2.56, p=0.798) | |
| Afro-Caribbean | 0.00 (0.00-Inf, p=0.991) | 0.00 (0.00-Inf, p=0.994) | |
| African | 3.49 (0.49–24.90, p=0.212) | 4.48 (0.59–34.16, p=0.148) | |
| Hispanic | 1.88 (1.05–3.37, p=0.033) | 2.99 (1.61–5.57, p=0.001) | |
| Female gender | 2.18 (1.61–2.95, p<0.001) | 2.13 (1.54–2.94, p<0.001) | |
| LVEF | ≥ 50% | Reference | |
| 30–50 % | 2.38 (1.80–3.15, p<0.001) | 2.18 (1.63–2.92, p<0.001) | |
| < 30% | 5.35 (3.13–9.15, p<0.001) | 3.96 (2.27–6.90, p<0.001) | |
| Peripheral Vascular Disease | 1.91 (1.27–2.87, p=0.002) | - | |
| Creatinine | 1.01 (1.01–1.02, p<0.001) | 1.01 (1.00–1.01, p<0.001) | |
| Body Mass Index | 1.01 (0.98–1.05, p=0.378) | - | |
| COPD | 1.88 (0.97–3.67, p=0.063) | - | |
| Smoking | Current | Reference | |
| Ex-smoker | 0.70 (0.49–1.00, p=0.051) | 0.43 (0.30–0.63, p<0.001) | |
| Never smoked | 0.65 (0.44–0.97, p=0.034) | 0.35 (0.23–0.53, p<0.001) | |
| Prior CVA | 2.35 (1.56–3.54, p<0.001) | 1.70 (1.12–2.59, p=0.012) | |
| NYHA class 3 or 4 | 1.48 (1.10–1.99, p=0.009) | - | |
| Diabetes | No | Reference | |
| Insulin-dependent | 2.70 (1.79–4.07, p<0.001) | 1.86 (1.22–2.85, p=0.004) | |
| Non-insulin dependent | 1.21 (0.86–1.71, p=0.265) | 1.11 (0.78–1.57, p=0.567) | |
| Arterial hypertension | 1.49 (1.04–2.13, p=0.028) | - | |
| Unstable angina | 1.39 (0.90–2.14, p=0.137) | - | |
| Prior MI | 1.76 (1.35–2.30, p<0.001) | 1.47 (1.11–1.94, p=0.007) | |
| Prior PCI | 1.05 (0.73–1.50, p=0.792) | - | |
| Off-pump surgery | 1.38 (1.06–1.79, p=0.017) | - | |
| Number of grafts | 0.95 (0.81–1.12, p=0.561) | - |
pAF: post-operative atrial fibrillation; SR: sinus rhythm; LVEF: left ventricular ejection fraction; COPD: chronic obstructive pulmonary disease; CVA: cerebrovascular accident: NYHA: New York Heart Association; MI: myocardial infarction; PCI: percutaneous coronary intervention
Figure 2.
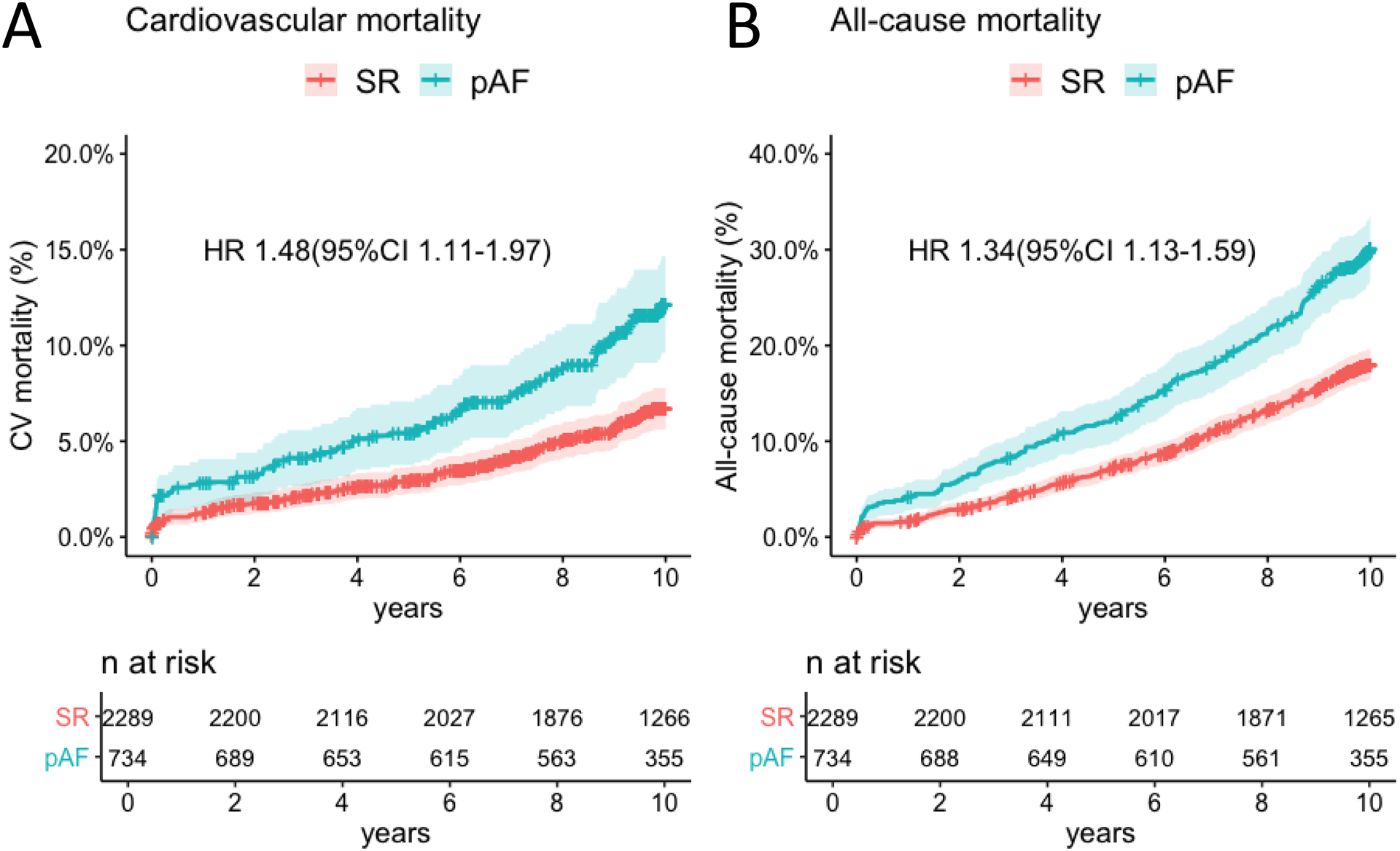
Cumulative incidence of cardiovascular (CV) deaths in patients with postoperative atrial fibrillation (pAF) and stable sinus rhythm (SR) (Panel A). Cumulative incidence of all-cause deaths in patients with pAF and stable SR (Panel B).
Prognostic implication of CHA2DS2-VASc score in patients with pAF
The distribution of baseline CHA2DS2-VASc score in the pAF and SR groups is reported in Table X in the Supplement. Mean CHA2DS2-VASc score was 3.46±1.31 vs 3.17±1.29 in the pAF and SR groups respectively (P<0.001). We found a significant interaction between CHA2DS2-VASc and pAF on the risk of CVA (p=0.01; Figure III in the Supplement). The cumulative incidence of CVA in the pAF and SR groups stratified by CHA2DS2-VASc score is reported in Table XI in the Supplement. The risk of CVA was comparable between pAF and SR groups for CHA2DS2-VASc score <4 but was significantly higher in patients with pAF for CHA2DS2-VASc score ≥4. Compared to patients with CHA2DS2-VASc score <4 in the SR group, patients with CHA2DS2-VASc score ≥4 and SR were associated with 2-fold relative risk increase of CVA (HR 2.13[95%CI 1.38–3.27]) whilst those with CHA2DS2-VASc score ≥4 and pAF were associated with 4-fold relative risk increase of CVA (HR 4.05[95%CI 2.54–6.46])(Figure 3).
Figure 3.
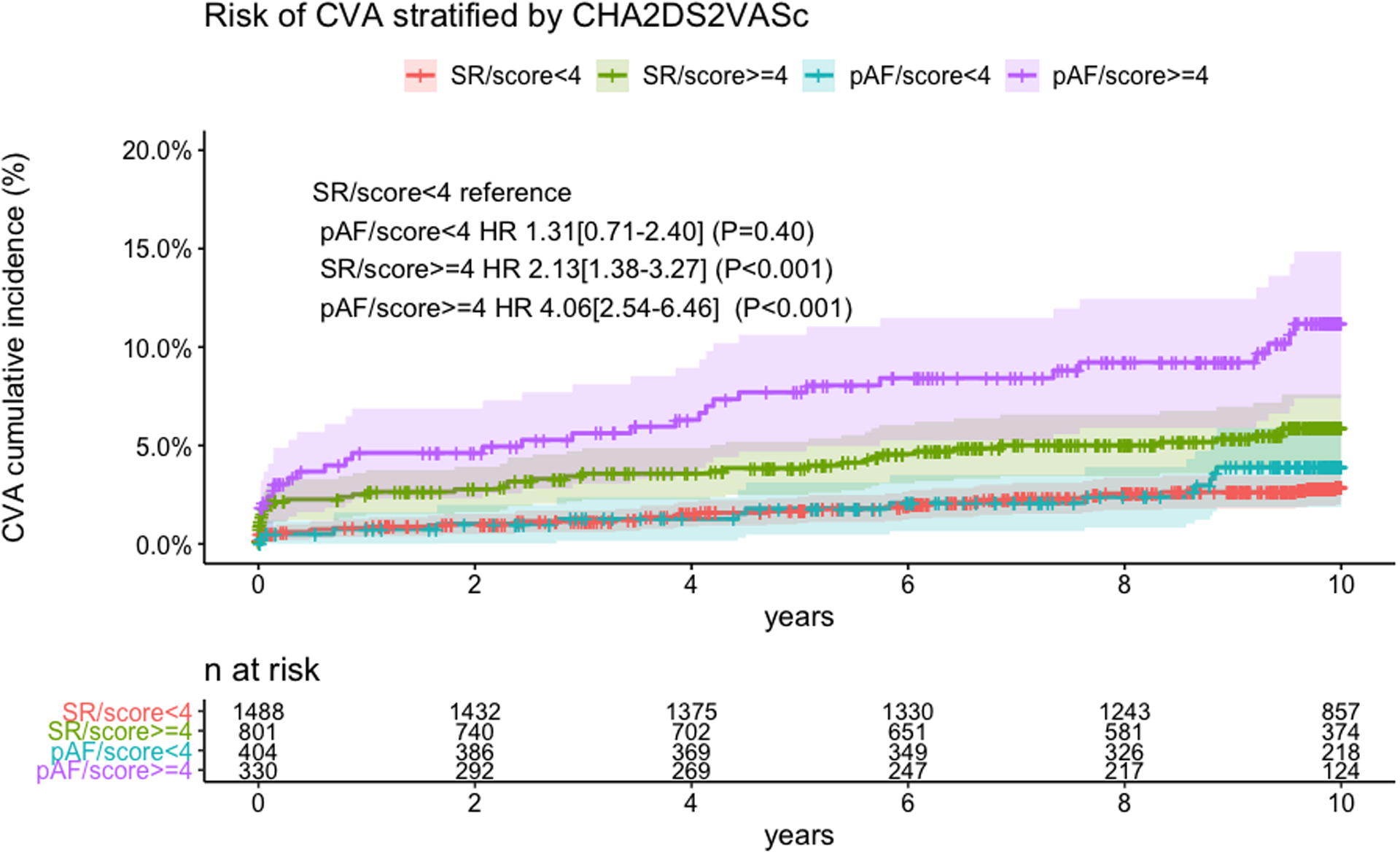
Cumulative incidence of cerebrovascular accident (CVA) with postoperative atrial fibrillation (pAF) and stable sinus rhythm (SR) stratified by CHA2DS2VASc < 4 or ≥4.
Results regarding the prognostic implication of CHADS2 score [18], an earlier and simplified form of CHA2DS2-VASc score are presented in Supplemental material (Tables XII and XIII in the Supplement; Figure IV in the Supplement).
Discussion
In the cohort of patients undergoing CABG surgery in the ART trial, we found a significant association between pAF and the risk of CVA at 10 years and this association continued after controlling for potential confounders. Half the patients developing CVA, presented with the least moderate disability (i.e. median Rankin = 3). In patients with pAF, CVA was more frequently reported as the cause of death compared to patients who remained in SR. Overall, pAF was associated with an increased risk of cardiovascular and all-cause mortality.
pAF has long been considered a benign, self-limiting condition which converts to SR before hospital discharge in most cases [2]. However, recent evidence suggested that the occurrence of pAF is associated with patients with larger burden of comorbidities, so that causality between pAF and long-term mortality remains unclear [4,5].
Limited and conflicting data are available on the association between pAF and CVA. To the best of our knowledge, no previous study has demonstrated the association between pAF and long-term (>10 years) risk of CVA. By using linked administrative data in the province of Ontario between 1996 and 2006, Whitlock et al.reported that pAF was associated with an increased incidence of stroke and death up to 2 years after CABG [6]. A post-hoc analysis of the Excel trial reported an increased risk of stroke at 3 years following CABG in patients with pAF.[7] In both studies, the association between pAF and the risk of stroke was mainly driven by events occurring before discharge, In contrast, a recent analysis from the Danish nationwide registries showed no significant increase in the risk of thromboembolism in patients with pAF after CABG. However, AF was associated with an increased risk of rehospitalization for atrial fibrillation [8].
In the present analysis we found an independent association between pAF and 10-year risk of CVA. Although a relevant number of CVA occurred within 30 days, the incidence of CVA continued to be higher in the pAF patients at mid- and long-term follow-up and the association between pAF and CVA persisted when CVA that occurred during index hospitalization was excluded. The association of pAF with CVA was evident even in patients with high CHA2DS2-VASc score (≥4). If this association is validated in other studies, this finding could be used to stratify patients with pAF and identify those who may benefit from stricter surveillance with continuous heart rhythm monitoring or anticoagulation therapy. In particular, little evidence exists on the efficacy and safety of anticoagulation therapy in patients with pAF and current recommendations are mainly driven by the therapy for non-surgical AF modified by the potential risk of bleeding in the postoperative period [19]. Current guidelines recommend anticoagulation for patients with a prolonged duration of pAF (>48 hours) [20] for at least 4 weeks. However, it remains unclear if the benefit of long-term anticoagulation for thromboembolism prevention outweighs the bleeding risk in this population. It must also be considered that post-CABG patients usually receive one or two antiplatelet agents to prevent graft failure, and the combination of antiplatelet and anticoagulation therapies might significantly increase the risk of bleeding. The present post-hoc analysis was largely underpowered to investigate whether the prescription of anticoagulation therapy was associated with the risk of CVA or bleeding. However, it is worth noting that in the present cohort the cumulative incidence of CVA in the pAF group was lower among patients discharged on Warfarin and that patients discharged on Warfarin presented a comparable risk of severe bleeding when compared to patients discharged without it. Further benefit may be achieved by the use of novel oral anticoagulants (NOAC), which can prevent new CVA in high risk patients with a reduced risk of bleeding compared to Warfarin. This is related to a safer pharmacodynamic profile and fewer interactions with other drugs and food, which prevent supratherapeutic INR, traditionally associated with Warfarin. In a retrospective analysis of 960 patients undergoing isolated CABG, 29 patients with pAF were discharged on NOAC compared to 77 discharged on Warfarin. Late post-operative outcomes showed three readmissions for major bleeding, all in patients discharged on Warfarin, while no readmissions for major bleeding were recorded in patients discharged on a novel anticoagulation regimen [21].
Moreover, attention to each episode of pAF, regardless of its duration, should be raised in order to tailor a stricter surveillance in patients developing this complication. In fact, pAF has been shown to be associated with the late recurrence of atrial fibrillation, which exposes the patients to the risk of CVA and late mortality [22–23]. Hence, this requires the clinician to undertake a more intense follow-up with the aim of identifying late recurrence of arrhythmias in a timely manner.
The present analysis has several limitations. We had no information on the duration of pAF in those cases with sinus rhythm restoration before discharge and therefore we were unable to correlate the duration of pAF with clinical outcomes. Moreover, the ART cohort enrolled a relatively low-risk subset of CABG patients and these results may not be generalizable to the real-world CABG population. The ART trial used questionnaires for follow-up, and it is possible that adverse events may have been underreported. However, for patients requiring hospital admission, copies of medical records were retrieved and information on adverse events were collected. As previously highlighted, this post-hoc analysis was largely underpowered to detect any difference in clinical outcomes in patients discharged on anticoagulation therapy and larger studies are needed to define risks and benefits of such therapy in patients with pAF. Finally, studies using continuous heart rhythm monitoring are needed to assess the risk of recurrence of atrial dysrhythmias in patients with pAF.
In conclusion, in the ART trial the occurrence of pAF following CABG was associated with an increased 10-year risk of CVA and mortality. This association highlights the need to revisit the notion that pAF is a transient, benign condition. In particular, special consideration should be given to patients at higher baseline risk of CVA (CHA2DS2-VASc≥4) who develop pAF.
Supplementary Material
Clinical perspective.
What is new?
Postoperative atrial fibrillation (pAF) after CABG is independently associated with a higher risk of cerebrovascular accidents at 10 years.
The association between pAF and risk of cerebrovascular accident persists when CVAs that occurred before discharge are excluded.
pAF is also independently associated with a higher risk of cardiovascular and all-cause mortality.
What are the clinical implications?
Our findings highlight the need to revisit the notion that pAF is a transient, benign condition.
Patients with pAF after CABG should be considered for stricter surveillance with continuous heart rhythm monitoring and anticoagulation therapy in those at very high risk (i.e. CHA2DS2-VASc≥4).
Founding:
The Arterial Revascularization Trial was supported by grants from the British Heart Foundation (SP/03/001), the U.K. Medical Research Council (G0200390), and the National Institute of Health Research Efficacy and Mechanism Evaluation Programme (09/800/29). Umberto Benedetto contribution was supported by the National Institute of Health Research Bristol Biomedical Research Centre. Alastair Gray is partly supported by the National Institute of Health Research Oxford Biomedical Research Centre.
Abbreviations:
- pAF
post-operative atrial fibrillation
- CABG
coronary artery bypass grafting
- CVA
cerebrovascular accident
- SR
sinus rhythm
Footnotes
A complete list of the investigators in the Arterial Revascularization Trial is provided in the Supplemental Material.
Disclosure: authors have no conflicts of interest to disclose.
Supplemental materials
List of the investigators in the Arterial Revascularization Trial
Expanded results
Supplemental Tables I – XIII
Supplemental Figures I – IV
References
- 1.Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, Barash PG, Hsu PH, Mangano DT Investigators of the Ischemia Research and Education Foundation; Multicenter Study of Perioperative Ischemia Research Group. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004; 291:1720–1729 [DOI] [PubMed] [Google Scholar]
- 2.Greenberg JW, Lancaster TS, Schuessler RB, Melby SJ. Postoperative atrial fibrillation following cardiac surgery: a persistent complication. Eur J Cardiothorac Surg. 2017; 52:665–672. [DOI] [PubMed] [Google Scholar]
- 3.Saxena A, Dinh DT, Smith JA, Shardey GC, Reid CM, Newcomb AE. Usefulness of postoperative atrial fibrillation as an independent predictor for worse early and late outcomes after isolated coronary artery bypass grafting (multicenter Australian study of 19,497 patients). Am J Cardiol. 2012;109:219–225. [DOI] [PubMed] [Google Scholar]
- 4.El-Chami MF, Kilgo P, Thourani V, Lattouf OM, Delurgio DB, Guyton RA, Leon AR, Puskas JD New-onset atrial fibrillation predicts long-term mortality after coronary artery bypass graft. J Am Coll Cardiol. 2010;55:1370–1376. [DOI] [PubMed] [Google Scholar]
- 5.Mariscalco G, Klersy C, Zanobini M, Banach M, Ferrarese S, Borsani P, Cantore C, Biglioli P, Sala A Atrial fibrillation after isolated coronary surgery affects late survival. Circulation. 2008;118:1612–1618 [DOI] [PubMed] [Google Scholar]
- 6.Whitlock R, Healey JS, Connolly SJ, Wang J, Danter MR, Tu JV, Novick R, Fremes S, Teoh K, Khera V, et al. Predictors of early and late stroke following cardiac surgery. CMAJ. 2014;186:905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kosmidou I, Chen S, Kappetein AP, Serruys PW, Gersh BJ, Puskas JD, Kandzari DE, Taggart DP, Morice MC, Buszman PE, et al. New-Onset Atrial Fibrillation After PCI or CABG for Left Main Disease: The EXCEL Trial. J Am Coll Cardiol. 2018;71:739–748 [DOI] [PubMed] [Google Scholar]
- 8.Butt JH, Xian Y, Peterson ED, Olsen PS, Rørth R, Gundlund A, Olesen JB, Gislason GH, Torp-Pedersen C, Køber L, et al. Long-term Thromboembolic Risk in Patients With Postoperative Atrial Fibrillation After Coronary Artery Bypass Graft Surgery and Patients With Nonvalvular Atrial Fibrillation. JAMA Cardiol. 2018;3:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:2246–2280. [DOI] [PubMed] [Google Scholar]
- 10.Taggart DP, Altman DG, Gray AM, Lees B, Gerry S, Benedetto U, Flather M. ART Investigators. Randomized Trial of Bilateral versus Single Internal-Thoracic-Artery Grafts. N Engl J Med. 2016;375:2540–2549. [DOI] [PubMed] [Google Scholar]
- 11.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, DiMarco J, et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012;14:528–606. [DOI] [PubMed] [Google Scholar]
- 12.Taggart DP, Lees B, Gray A, Altman DG, Flather M, Channon K; ART Investigators Protocol for the Arterial Revascularisation Trial (ART). A randomised trial to compare survival following bilateral versus single internal mammary grafting in coronary revascularisation [ISRCTN46552265]. Trials. 2006;7:7–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farrell B, Godwin J, Richards S, Warlow C. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry. 1991;54:1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. [DOI] [PubMed] [Google Scholar]
- 15.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley and Sons; 2004. [Google Scholar]
- 16.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. JASA. 1999;94:496–509. [Google Scholar]
- 17.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 18.Gage BF, van Walraven C, Pearce L, Hart RG, Koudstaal PJ, Boode BS, Petersen P. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004;110:2287–2292. [DOI] [PubMed] [Google Scholar]
- 19.Epstein AE, Alexander JC, Gutterman DD, Maisel W, Wharton JM. Anticoagulation: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest 2005; 128:24S–27S. [DOI] [PubMed] [Google Scholar]
- 20.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. [DOI] [PubMed] [Google Scholar]
- 21.Woldendorp K, Khadra S, Bannon P, Robinson B. Novel Oral Anticoagulants Compared to Warfarin for Postoperative Atrial Fibrillation After Isolated Coronary Artery Bypass Grafting. Hear Lung Circ. In press. DOI: 10.1016/j.hlc.2020.04.018. [DOI] [PubMed] [Google Scholar]
- 22.Ahlsson A, Fengsrud E, Bodin L, Englund A. Postoperative atrial fibrillation in patients undergoing aortocoronary bypass surgery carries an eightfold risk of future atrial fibrillation and a doubled cardiovascular mortality. Eur J Cardiothorac Surg. 2010;37:1353–1359. [DOI] [PubMed] [Google Scholar]
- 23.Antonelli D, Peres D, Freedberg NA, Feldman A And Rosenfeld T Incidence of Postdischarge Symptomatic Paroxysmal Atrial Fibrillation in Patients Who Underwent Coronary Artery Bypass Graft:. Pacing and Clinical Electrophysiology. 2004; 27: 365–367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


