Abstract
BACKGROUND
Dipeptidyl peptidase 4 (DPP-4; also known as CD26), a transmembrane receptor expressed on T cells, has a costimulatory function in activating T cells. In a mouse model, down-regulation of CD26 prevented graft-versus-host disease (GVHD) but preserved graft-versus-tumor effects. Whether inhibition of DPP-4 with sitagliptin may prevent acute GVHD after allogeneic stem-cell transplantation is not known.
METHODS
We conducted a two-stage, phase 2 clinical trial to test whether sitagliptin plus tacrolimus and sirolimus would reduce the incidence of grade II to IV acute GVHD from 30% to no more than 15% by day 100. Patients received myeloablative conditioning followed by mobilized peripheral-blood stem-cell transplants. Sitagliptin was given orally at a dose of 600 mg every 12 hours starting the day before transplantation until day 14 after transplantation.
RESULTS
A total of 36 patients who could be evaluated, with a median age of 46 years (range, 20 to 59), received transplants from matched related or unrelated donors. Acute GVHD occurred in 2 of 36 patients by day 100; the incidence of grade II to IV GVHD was 5% (95% confidence interval [CI], 1 to 16), and the incidence of grade III or IV GVHD was 3% (95% CI, 0 to 12). Nonrelapse mortality was zero at 1 year. The 1-year cumulative incidences of relapse and chronic GVHD were 26% (95% CI, 13 to 41) and 37% (95% CI, 22 to 53), respectively. GVHD-free, relapse-free survival was 46% (95% CI, 29 to 62) at 1 year. Toxic effects were similar to those seen in patients undergoing allogeneic stem-cell transplantation.
CONCLUSIONS
In this nonrandomized trial, sitagliptin in combination with tacrolimus and sirolimus resulted in a low incidence of grade II to IV acute GVHD by day 100 after myeloablative allogeneic hematopoietic stem-cell transplantation. (Funded by the National Heart, Lung, and Blood Institute; ClinicalTrials.gov number, NCT02683525.)
ACUTE GRAFT-VERSUS-HOST DISEASE (GVHD) remains a major complication and cause of death after allogeneic hematopoietic stem-cell transplantation (HSCT).1 With the use of standard prophylaxis regimens that include a calcineurin inhibitor with either methotrexate or sirolimus, grade II to IV acute GVHD occurs in 34 to 51% of patients by 100 days after HLA-matched allogeneic transplantation.2,3
Dipeptidyl peptidase 4 (DPP-4) is a homodimeric type II transmembrane receptor identical to the leukocyte surface antigen CD26 and is also present in a soluble, enzymatically active form in plasma.4 DPP-4 is involved in a broad range of biologic processes, including insulin release,5 stromal-cell–derived factor 1–induced homing of stem cells,4 hematopoietic cytokine activity,4 and T-cell immune function.6 On T cells, CD26 or DPP-4 has a costimulatory function in enhancing T-cell activation.6 The interaction of CD26 or DPP-4 on T cells with its ligand caveolin 1 on antigen-presenting cells (APCs) enhances T-cell activation and up-regulates CD86 on APCs, which results in costimulation.7 In a mouse model, down-regulation of CD26 expression by a monoclonal antibody prevented acute GVHD and preserved graft-versus-tumor effects.8
Sitagliptin is a selective inhibitor of DPP-4 that is approved for the treatment of type 2 diabetes mellitus. In a trial investigating whether sitagliptin enhances engraftment of cord-blood transplants, sitagliptin had an acceptable side-effect profile and unexpectedly resulted in a decreased incidence of acute GVHD.9 On the basis of this observation and preclinical studies showing that DDP-4 inhibitors lessened T-cell activation,10 we hypothesized that DPP-4 inhibition may reduce the incidence of acute GVHD after allogeneic HSCT. In a phase 2 trial, we investigated the efficacy of sitagliptin in combination with tacrolimus and sirolimus for the prevention of acute GVHD after myeloablative allogeneic peripheral-blood stem-cell transplantation.
Methods
Trial Design and Oversight
This was an investigator-initiated, single-group, phase 2 trial evaluating the safety and efficacy of sitagliptin combined with tacrolimus and sirolimus in the prophylaxis of grade II to IV acute GVHD after HSCT from matched related or unrelated donors. The protocol, available with the full text of this article at NEJM.org, was approved by the institutional review board at Indiana University. The trial was conducted in accordance with the principles of the Declaration of Helsinki and the International Council for Harmonisation guidelines for Good Clinical Practice. All the patients gave written informed consent. An independent data and safety monitoring committee oversaw the conduct of the trial. The trial was designed by all nine authors. Data were collected and trial procedures were overseen by the trial investigators and the supporting research staff of the Clinical Trials Office of the Indiana University Comprehensive Cancer Center. The first author was responsible for data analysis. All the authors had access to the data, vouch for the completeness and accuracy of the data and for the fidelity of the trial to the protocol, solely contributed to the writing and review of the manuscript, had final responsibility for the manuscript content, and made the decision to submit the manuscript for publication. No commercial support was obtained.
Patients
Eligible patients were 18 to 60 years of age and had acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL), a myelodysplastic disorder with a score on the Revised International Prognostic Scoring System of more than 3 (on a scale from 0 to 10, with higher scores indicating a worse prognosis), or chronic myeloid leukemia refractory to more than two tyrosine kinase inhibitors or that was beyond the first chronic phase. Patients were required to have a Karnofsky performance-status score of at least 70% (on a percentage scale in which lower values indicate greater disability), a left ventricular ejection fraction of at least 45%, a diffusing capacity of the lung for carbon monoxide of at least 50%, a creatinine clearance of at least 60 ml per minute, and serum levels of alanine aminotransferase and aspartate aminotransferase that were no more than 2 times the upper limit of the normal range. Patients were candidates for myeloablative conditioning and had suitable 6/6 HLA-matched related donors or 10/10 HLA-matched (at HLA-A, -B, -C, -DRB1, and -DQB1 by high-resolution typing) unrelated donors. Patients were excluded if they had received a previous transplant or were taking insulin or insulin secretagogues for diabetes mellitus. To minimize bias, consecutive patients referred to our program from January 2016 through November 2018 who were deemed to be suitable candidates for myeloablative conditioning and had matched related or unrelated donors were approached about participating in this trial and screened if they gave written consent.
Treatment
Patients received myeloablative conditioning that included either fractionated total body irradiation (total dose, 13.5 Gy) and cyclophosphamide (120 mg per kilogram of body weight) or thiotepa (15 mg per kilogram) and cyclophosphamide (120 mg per kilogram), followed by infusion of filgrastim-mobilized peripheral-blood stem cells on day 0 (the day of transplantation). Prophylaxis of acute GVHD with tacrolimus at a dose of 0.02 mg per kilogram per day was started on day −3 as a continuous intravenous infusion and was later switched to an oral formulation when the patient could swallow comfortably. Sirolimus was started on day −3 at a dose of 4 mg orally daily. Doses of tacrolimus and sirolimus were modified to target serum trough levels of 5 to 10 ng per milliliter. In the absence of GVHD, tacrolimus and sirolimus were tapered at day 100 and discontinued by approximately day 180. In the event of renal toxic effects, mycophenolate mofetil (1000 mg twice daily) was substituted for tacrolimus at the physicians’ discretion. Sitagliptin (600 mg orally every 12 hours) was started on day −1 and taken until day 14. The dose and schedule of sitagliptin were based on previous pharmacodynamic studies involving recipients of cord-blood transplants.11,12 Supportive care was provided according to institutional guidelines.
End-Point Measurements
The primary end point was grade II to IV acute GVHD by day 100. Acute GVHD was scored according to modified Glucksberg criteria (with I indicating minimal disease and IV severe disease)13 (Table S1 in the Supplementary Appendix, available at NEJM.org). For the purpose of evaluating the primary end point, additional patients could be enrolled if patients died from causes other than GVHD or were lost to follow-up before day 100. Secondary end points were neutrophil and platelet engraftment (assessed in time-to-event analyses), nonrelapse mortality, relapse, overall survival, and chronic GVHD. Nonhematologic adverse events were described and graded according to the revised National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0, and their potential relationship to sitagliptin was assessed by the treating physician and the team of investigators.
Correlative Studies
Plasma samples were collected at baseline and at defined times after the first dose of sitagliptin and then before each subsequent morning dose to evaluate plasma DPP-4 activity. Plasma DPP-4 activity was measured with the use of the DPPIV-Glo Protease Assay (Promega), as reported previously.9 Plasma concentrations of interleukin-1β, −2, −6, −8, and −10, interferon-γ, tumor necrosis factor (TNF) α (TNF-α), soluble interleukin-2 receptor α, soluble TNF receptor 1 (TNFR1), suppressor of tumorigenicity 2 (ST2), chemokine (C-X-C motif) ligand 9 (Mig), and regenerating islet-derived protein 3α (Reg3α) were measured on day −1 and at later-defined times with the use of Luminex multiplex assay kits (Invitrogen), according to the manufacturer’s instructions.
Statistical Analysis
This trial used a Simon minimax two-stage design with the primary objective to reduce the incidence of grade II to IV acute GVHD by day 100 from 30%, on the basis of previous reports,2 to no more than 15% with a one-sided type I error to 0.1 and a type II error to 0.2. A total of 23 patients who could be evaluated were to be enrolled in the first stage, and if fewer than 6 patients had grade II to IV acute GVHD by day 100, additional patients were to be enrolled for a total accrual of 36 patients who could be evaluated. In the final analysis, if fewer than 8 of the 36 patients who could be evaluated had grade II to IV acute GVHD by day 100, it could be concluded that sitagliptin was efficacious at reducing the incidence of grade II to IV acute GVHD. The cumulative incidences of relapse, nonrelapse mortality, acute GVHD, and chronic GVHD were estimated with the use of Gray’s method.14,15 Relapse and nonrelapse mortality were considered to be competing risks for each other, and relapse and death due to non-GVHD causes were competing risks for acute and chronic GVHD. Overall survival was estimated with the use of the Kaplan–Meier method.16 Although not a prespecified end point in the protocol, the composite end point of GVHD-free, relapse-free survival (defined as the absence of grade III or IV acute GVHD, chronic GVHD for which systemic glucocorticoid therapy was warranted, relapse, or death in the first year after transplantation) was estimated as reported.17 For all analyses, a two-sided alpha level of 0.05 was considered to indicate statistical significance. Statistical analyses were performed with the use of SPSS software, version 22 (IBM), and R software, version 3.6.1 (Comprehensive R Archive Network, www.cran.r-project.org).
Results
Patients
From January 2016 through November 2018, a total of 37 patients were enrolled, and the last follow-up was on October 1, 2019. In stage 1 of the trial, 1 patient who had engraftment and in whom GVHD did not develop declined further follow-up and was withdrawn from the trial on day 38. Another patient was added to the trial to allow for 23 patients who could be evaluated for the primary end point. The trial did not meet its stopping rule after stage 1, and an additional 13 patients were enrolled in stage 2 for a total of 36 patients who could be evaluated. Baseline characteristics of the 36 patients are summarized in Table 1. Because of programmatic changes unrelated to the trial, the first patient received total body irradiation and high-dose cyclophosphamide, whereas subsequent patients received thiotepa and high-dose cyclophosphamide.
Table 1.
Clinical and Transplantation Characteristics at Baseline.*
| Characteristic | All Patients (N = 36) |
|---|---|
| Median age (range) – yr | 46 (20–59) |
| Sex – no. (%) | |
| Male | 17 (47) |
| Female | 19 (53) |
| Karnofsky performance-status score – no. (%)† | |
| ≥90% | 34 (94) |
| <90% | 2 (6) |
| HCT-specific comorbidity index score – no. (%)‡ | |
| 0 | 11 (31) |
| 1–2 | 9 (25) |
| 3–4 | 13 (36) |
| ≥5 | 3 (8) |
| Disease – no. (%) | |
| Acute myeloid leukemia§ | 19 (53) |
| Acute lymphoblastic leukemia§ | 9 (25) |
| Myelodysplastic syndrome | 4 (11) |
| Chronic myeloid leukemia | 4 (11) |
| Disease Risk Index group – no. (%)¶ | |
| Low risk | 2 (6) |
| Intermediate risk | 24 (67) |
| High and very high risk | 10 (28) |
| Median time from diagnosis to transplantation (range) – days | 138 (41–2227) |
| Donor type – no. (%) | |
| Matched related | 13 (36) |
| Matched volunteer unrelated | 23 (64) |
| Cytomegalovirus status – no. (%) | |
| Recipient negative, donor negative | 9 (25) |
| Recipient positive, donor negative | 12 (33) |
| Recipient negative, donor positive | 4 (11) |
| Recipient positive, donor positive | 11 (31) |
Percentages may not total 100 because of rounding.
Scores are on a percentage scale in which lower values indicate greater disability.
Scores on the hematopoietic-cell transplantation (HCT)–specific comorbidity index range from 0 to 29, with higher scores indicating a greater burden of illness.18
Of the 19 patients with acute myeloid leukemia, 17 were in first complete remission and 2 were in second complete remission before transplantation. Of the 9 patients with acute lymphoblastic leukemia, 8 were in first complete remission and 1 was in untreated early first relapse. Complete remission was defined as the presence of fewer than 5% blasts in the bone marrow aspirate with no morphologic or cytogenetic evidence of leukemia. No measurement for minimal residual disease by sensitive flow cytometric or molecular assays was performed in any of the patients with acute leukemia, and minimal residual disease was not a criterion for decision making.
Risk was defined according to the refined Disease Risk Index of the Center for International Blood and Marrow Transplant Research.19
Engraftment
All the patients had engraftment. Median times to neutrophil and platelet engraftment were 13 days (range, 10 to 20) and 15 days (range, 13 to 114), respectively. All the patients had at least 95% donor chimerism in whole blood or bone marrow by day 30. One patient who initially had 98% donor chimerism at day 30 had a decrease to 5% on day 98 in the absence of leukemia relapse. He reverted to 100% donor chimerism on abrupt discontinuation of immune suppression on day 110 and remained in remission at last follow-up.
Acute GVHD
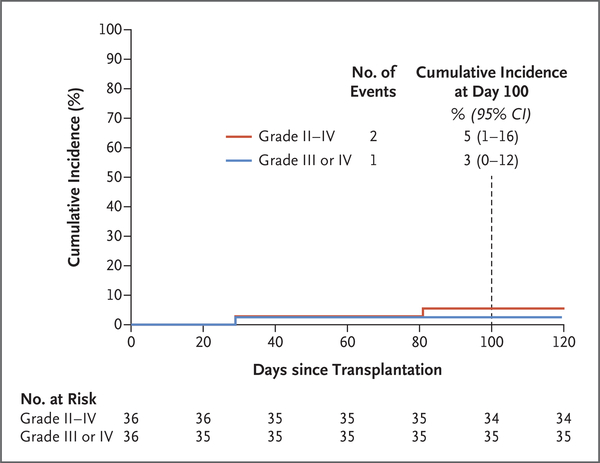
Acute GVHD occurred in 2 of the 36 patients by day 100. Both received peripheral-blood stem cells from unrelated donors. One patient had grade II acute GVHD involving the gut (stage 1), liver (stage 1), and skin (stage 3) on day 81, and another had grade IV acute GVHD involving the skin (stage 3), gut (stage 4), and liver (stage 4) on day 29, for a cumulative incidence of grade II to IV acute GVHD of 5% (95% confidence interval [CI], 1 to 16) and of grade III or IV acute GVHD of 3% (95% CI, 0 to 12) (Fig. 1). An additional patient who received peripheral-blood stem cells from a related donor had late-onset grade II acute GVHD on day 140 after abrupt stopping of immunosuppression on day 110 for low donor chimerism (see above). No other patients had acute GVHD after day 100.
Figure 1.
Cumulative Incidence of Acute Graft-versus-Host Disease (GVHD).
A total of 28 patients received 80% or more of the planned 32 doses of sitagliptin, with 8 patients receiving fewer owing to difficulty swallowing because of mucositis, a declining of treatment because of nausea or vomiting, and, in the case of the patient who had grade IV acute GVHD by day 100, the development of acute kidney failure. The 2 patients who had grades II and IV acute GVHD by day 100 received 65% and 70% of the planned total dose, respectively.
Toxicity, Infectious Complications, and Nonrelapse Mortality
No toxic effects were attributed to sitagliptin by the investigators. Specifically, and related to the mechanism of action of the drug, no episodes of hypoglycemia were noted. The frequencies of grade 3 or 4 nonhematologic toxic effects occurring in the first 30 days after transplantation are shown in Table 2. In addition, acute hemolysis due to passenger lymphocyte syndrome (in two patients, with subsequent acute renal failure in one patient) and thrombotic microangiopathy that resolved with discontinuation of tacrolimus (in one patient) were also observed. Infections included cytomegalovirus viremia (in six patients), BK virus cystitis (in five patients), and bacteremia (in three patients, with infection due to Granulicatella adiacens, enterococcus, and coagulase-negative staphylococcus in one patient each). The patient with grade IV acute GVHD had concurrent human herpes virus 6 reactivation and Epstein–Barr virus reactivation that resolved with rituximab. All toxic effects were reversible, and the 1-year nonrelapse mortality was zero.
Table 2.
Grade 3 or 4 Adverse Events within 30 Days after Transplantation.*
| Adverse Event | Grade 3 | Grade 4 |
|---|---|---|
| no. of patients | ||
| Gastrointestinal event | ||
| Anorexia, nausea, or vomiting | 10 | 1 |
| Oropharyngeal mucositis | 19 | 2 |
| Diarrhea | 3 | 0 |
| Ileus | 1 | 0 |
| Hemorrhage | 1 | 0 |
| Cardiovascular event | ||
| Hypotension | 2 | 0 |
| Hypertension | 3 | 0 |
| Fluid overload or edema | 1 | 1 |
| Chest pain | 1 | 0 |
| Arrhythmia (supraventricular tachycardia) | 1 | 0 |
| Renal or urologic event | ||
| Acute kidney injury | 6 | 3 |
| Urinary tract infection | 4 | 0 |
| Neurologic event | ||
| Headache | 1 | 0 |
| Syncope | 2 | 0 |
| Neutropenic fever | 4 | 0 |
| Sepsis | 0 | 3 |
Nonhematologic adverse events were described and graded according the revised National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Transient biochemical abnormalities that resolved with electrolyte replacement and that resolved within 30 days after transplantation are not included.
Relapse, Chronic GVHD, and Survival
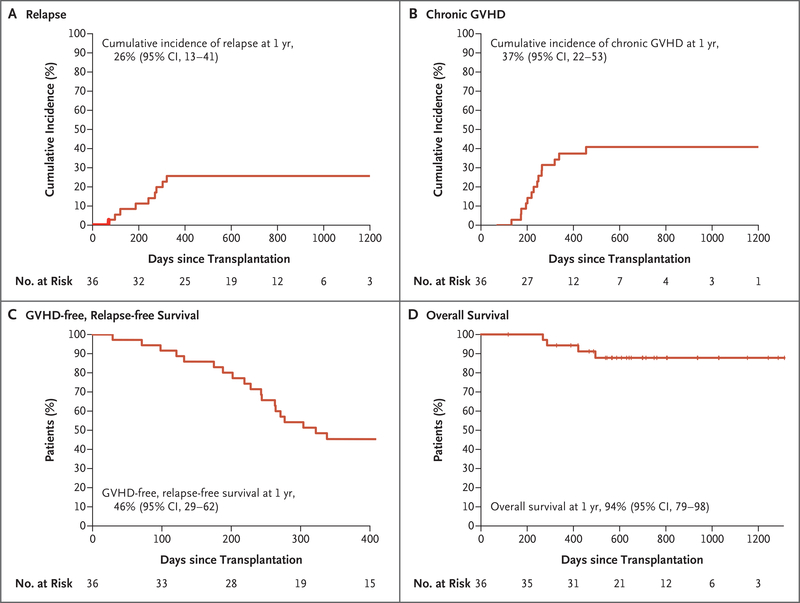
With the exclusion of a patient lost to follow-up at day 118, the median follow-up of surviving patients was 700 days (range, 327 to 1313). Overall, 9 patients had a relapse at a median of 243 days (range, 71 to 322) after transplantation with a cumulative incidence of relapse at 1 year of 26% (95% CI, 13 to 41) (Fig. 2A). The incidence of relapse at 1 year was similar in patients who received peripheral-blood stem cells from related donors (4 of 13 patients) and those who received them from unrelated donors (5 of 23 patients) and was also similar among those with ALL (2 of 9 patients) and those with myeloid cancers (7 of 27 patients). Chronic GVHD developed in 15 of 34 patients who survived without relapse beyond day 100, for an overall 1-year cumulative incidence of 37% (95% CI, 22 to 53) (Fig. 2B). Among these 15 patients, 5 had mild, 7 moderate, and 3 severe chronic GVHD according to National Institutes of Health global severity scoring20 (Table S2). A total of 9 patients with chronic GVHD received systemic glucocorticoids, resulting in a 1-year GVHD-free, relapse-free survival of 46% (95% CI, 29 to 62) (Fig. 2C). Overall survival at 1 year was 94% (95% CI, 79 to 98) (Fig. 2D). GVHD-free, relapse-free survival and overall survival curves for patients receiving peripheral-blood stem cells from related or unrelated donors, and also for patients with ALL as compared with those with myeloid cancers, are presented in Figure S1A through S1D.
Figure 2. Time-to-Event Curves for 36 Patients Who Could Be Evaluated.
Cumulative-incidence curves and estimates at 1 year are shown for relapse (Panel A) and chronic GVHD (Panel B). Kaplan–Meier curves are shown for the composite end point of GVHD-free, relapse-free survival (Panel C) and overall survival (Panel D).
DPP-4 Inhibition, Plasma Cytokines, and Immune Reconstitution
Maximal plasma DPP-4 inhibition was observed at 2 hours and 4 hours after dosing with mean (±SD) nadir plasma DPP-4 activities of 22±10% and 22±5%, respectively. Plasma DPP-4 inhibition was sustained with a mean trough residual activity of 29±8% (Fig. S2A and S2B). No obvious difference in DPP-4 inhibition was apparent in the patients who had acute GVHD by day 100 as compared with others. Plasma cytokines and acute GVHD biomarkers were measured on day −1 and days 7, 14, and 30 (Fig. S3). Levels were low in most patients, with the two patients who had acute GVHD by day 100 having higher median levels of ST2, Reg3α, Mig, soluble TNFR1, interferon-γ, interleukin-10, and interleukin-2 receptor α, although small numbers of events precluded formal comparison. Reconstitution of different immune subsets, including B cells, natural killer cells, regulatory T cells, CD4 conventional T cells, and CD8 T cells, is shown in Figure S4.
Discussion
This phase 2 trial showed the feasibility and safety of sitagliptin in combination with sirolimus plus tacrolimus. The combination was associated with a low incidence of acute GVHD among patients undergoing matched allogeneic peripheral-blood stem-cell transplantation after myeloablative conditioning. The findings are consistent with our previous results regarding sitagliptin in patients undergoing cord-blood transplantation, in whom sitagliptin also had an acceptable side-effect profile and resulted in a substantially lower than expected incidence of acute GVHD.9,11 The current findings also support preclinical studies of an anti-CD26 antibody, which suggested that DPP-4 is a viable target for the prevention of acute GVHD.8
The incidence of acute GVHD in our trial appears to be lower than in other trials testing other agents. Although a control group was not included, the risks of grade II to IV and grade III or IV acute GVHD were substantially lower than previously observed with sirolimus plus tacrolimus alone, for which the incidence has varied from 26 to 47% and from 7 to 19%, respectively,2,3,21–25 even when other agents such methotrexate, mycophenolate, or antithymocyte globulin were added to the backbone regimen.19–21 Our results also compare favorably with other approaches, including post-transplantation cyclophosphamide in combination with tacrolimus and mycophenolate, for which an incidence of grade II to IV acute GVHD of 27% was reported after reduced-intensity, matched allogeneic HSCT.26 A number of new agents, including abatacept,27 atorvastatin (for treatment of donor and recipient),28 vorinostat,29 and vedolizumab,30 have also shown promising results in single-group trials, although none appears to achieve a lower level of acute GVHD than sitagliptin. Furthermore, its ready availability, ease of administration, safety, and relatively inexpensive cost as compared with the new agents make sitagliptin a clinically attractive candidate. Randomized trials are needed to confirm the efficacy of sitagliptin.
The evaluation of new preventative approaches to acute GVHD remains complex, because such interventions may have adverse effects on relapse and nonrelapse mortality, and has motivated the use of composite end points such as GVHD-free, relapse-free survival.17 It is notable that the 1-year cumulative incidence of relapse of 26% (95% CI, 13 to 41) that was observed with sitagliptin is within the range of 20 to 40% reported in studies of established GVHD prophylaxis regimens,26,31 which suggests that sitagliptin does not compromise graft-versus-leukemia effects. Despite the use of peripheral-blood stem cells, which are known to result in a higher incidence of chronic GVHD than the use of bone marrow,32 we observed a 1-year incidence of chronic GVHD of 37%, which compares favorably with estimates of 39 to 53% reported in other studies.32–34 Together with a 1-year non-relapse mortality of zero, we observed a 1-year GVHD-free, relapse-free survival of 46% (95% CI, 29 to 62), which compares favorably with that observed with regimens based on tacrolimus and methotrexate26 or with post-transplantation cyclophosphamide, which is associated with a low incidence of chronic GVHD.26
High-dose sitagliptin was associated with very mild adverse effects and resulted in degrees of plasma DPP-4 inhibition that were similar to those that we reported previously in a trial of cord-blood transplantation.11 Sitagliptin did not appear to increase the frequency or severity of infections that would be otherwise observed in a similar population. However, we observed two cases of passenger lymphocyte syndrome. In both cases, minor ABO incompatibility between recipient and donor was present (recipients, A+; donors, O+). Although this complication has been reported more frequently when methotrexate is not used in GVHD prophylaxis,35 further study is needed to determine whether sitagliptin increases this risk.
The appropriate duration of sitagliptin treatment for prevention of acute GVHD is not known. In the trial investigating whether sitagliptin can enhance engraftment of cord-blood transplants, the drug was administered for only 4 days from days −1 to 2, the time during which homing of stem cells is thought to occur, and yet a low incidence of acute GVHD was observed.9 In the current trial, treatment was continued through day 14 so as to extend beyond the period when recipient APCs might persist to activate donor-alloreactive T cells.36 Therefore, it is not known whether a shorter duration of treatment could have been equally efficacious. However, both patients who had acute GVHD before day 100 in this trial received less than 80% of the planned total dose of sitagliptin. Alternatively, we cannot rule out the possibility that a longer duration of treatment might further reduce the risks of GVHD and improve overall outcomes.
We analyzed the plasma levels of a number of inflammatory cytokines and biomarkers predictive of acute GVHD, including ST2 and Reg3α. Although there was no control group for comparison, cytokine and biomarker levels remained low in the majority of patients, with possible trends toward higher levels in the patients who had acute GVHD before day 100, although formal comparison is not possible. Future trials that include a control group and have a larger sample size with more events could better assess the relationship of markers to acute GVHD. In addition, we did not observe any obvious deleterious effect of sitagliptin on immune-cell recovery, which appeared similar to that previously reported after peripheral-blood stem-cell transplantation.37 Studies of immune-cell recovery, including functional T-cell activity, that directly compare patients receiving sitagliptin with controls in randomized trials are required to better address this issue.
Our trial confirmed the safety of high-dose sitagliptin and showed a low incidence of grade II to IV acute GVHD by day 100 when used in combination with tacrolimus plus sirolimus after myeloablative allogeneic peripheral-blood stem-cell transplantation. Inhibition of DPP-4 should be further investigated in randomized trials that compare sitagliptin with current standard GVHD prophylaxis regimens.
Supplementary Material
Acknowledgments
Supported by grants (R01 HL112669, to Drs. Farag and Broxmeyer, and R35 HL139599, to Dr. Broxmeyer) from the National Heart, Blood, and Lung Institute of the National Institutes of Health. Data management and regulatory support were provided by the Clinical Trials Office of the Indiana University Simon Comprehensive Cancer Center.
Dr. Farag reports receiving grant support from Bristol-Myers Squibb and Incyte; and Dr. Broxmeyer, receiving consulting fees from Elixell Therapeutics. No other potential conflict of interest relevant to this article was reported.
Footnotes
Contributor Information
Sherif S. Farag, Indiana University School of Medicine, Indianapolis Indiana University Health, Indianapolis; Indiana University Simon Comprehensive Cancer Center, Indianapolis.
Mohammad Abu Zaid, Indiana University School of Medicine, Indianapolis Indiana University Health, Indianapolis.
Jennifer E. Schwartz, Indiana University School of Medicine, Indianapolis Indiana University Health, Indianapolis.
Teresa C. Thakrar, Indiana University Health, Indianapolis
Ann J. Blakley, Indiana University Simon Comprehensive Cancer Center, Indianapolis
Rafat Abonour, Indiana University School of Medicine, Indianapolis Indiana University Health, Indianapolis.
Michael J. Robertson, Indiana University School of Medicine, Indianapolis Indiana University Health, Indianapolis.
Hal E. Broxmeyer, Indiana University School of Medicine, Indianapolis Indiana University Simon Comprehensive Cancer Center, Indianapolis.
Shuhong Zhang, Indiana University School of Medicine, Indianapolis
References
- 1.Tanaka Y, Kurosawa S, Tajima K, et al. Analysis of non-relapse mortality and causes of death over 15 years following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2016; 51:553–9. [DOI] [PubMed] [Google Scholar]
- 2.Cutler C, Logan B, Nakamura R, et al. Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood 2014; 124:1372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Törlén J, Ringdén O, Garming-Legert K, et al. A prospective randomized trial comparing cyclosporine/methotrexate and tacrolimus/sirolimus as graft-versus-host disease prophylaxis after allogeneic hematopoietic stem cell transplantation. Haematologica 2016;101:1417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broxmeyer HE, Hoggatt J, O’Leary HA, et al. Dipeptidylpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoiesis. Nat Med 2012; 18:1786–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerich J. Pathogenesis and management of postprandial hyperglycemia: role of incretin-based therapies. Int J Gen Med 2013;6:?877–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatano R, Ohnuma K, Yamamoto J, Dang NH, Morimoto C. CD26-mediated co-stimulation in human CD8(+) T cells provokes effector function via pro-inflammatory cytokine production. Immunology 2013;138:165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohnuma K, Yamochi T, Uchiyama M, et al. CD26 up-regulates expression of CD86 on antigen-presenting cells by means of caveolin-1. Proc Natl Acad Sci U S A 2004;101:14186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatano R, Ohnuma K, Yamamoto J, Dang NH, Yamada T, Morimoto C. Prevention of acute graft-versus-host disease by humanized anti-CD26 monoclonal antibody. Br J Haematol 2013;162:263–77. [DOI] [PubMed] [Google Scholar]
- 9.Farag SS, Srivastava S, Messina-Graham S, et al. In vivo DPP-4 inhibition to enhance engraftment of single-unit cord blood transplants in adults with hematological malignancies. Stem Cells Dev 2013; 22:1007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohnuma K, Takahashi N, Yamochi T, Hosono O, Dang NH, Morimoto C. Role of CD26/dipeptidyl peptidase IV in human T cell activation and function. Front Biosci 2008;13:2299–310. [DOI] [PubMed] [Google Scholar]
- 11.Farag SS, Nelson R, Cairo MS, et al. High-dose sitagliptin for systemic inhibition of dipeptidylpeptidase-4 to enhance engraftment of single cord umbilical cord blood transplantation. Oncotarget 2017;8: 110350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vélez de Mendizábal N, Strother RM, Farag SS, et al. Modelling the sitagliptin effect on dipeptidyl peptidase-4 activity in adults with haematological malignancies after umbilical cord blood haematopoietic cell transplantation. Clin Pharmacokinet 2014;53:247–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995;15:825–8. [PubMed] [Google Scholar]
- 14.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94: 496–509. [Google Scholar]
- 15.Scrucca L, Santucci A, Aversa F. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant 2010;45:1388–95. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81. [Google Scholar]
- 17.Holtan SG, DeFor TE, Lazaryan A, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood 2015;125:1333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005;106:2912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood 2014;123:3664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SJ. Classification systems for chronic graft-versus-host disease. Blood 2017;129:30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho VT, Aldridge J, Kim HT, et al. Comparison of tacrolimus and sirolimus (Tac/Sir) versus tacrolimus, sirolimus, and mini-methotrexate (Tac/Sir/MTX) as acute graft-versus-host disease prophylaxis after reduced-intensity conditioning allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 2009; 15:844–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khaled SK, Palmer J, Stiller T, et al. A phase II study of sirolimus, tacrolimus and rabbit anti-thymocyte globulin as GVHD prophylaxis after unrelated-donor PBSC transplant. Bone Marrow Transplant 2013;48:278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kornblit B, Maloney DG, Storer BE, et al. A randomized phase II trial of tacrolimus, mycophenolate mofetil and sirolimus after non-myeloablative unrelated donor transplantation. Haematologica 2014;99:1624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pulsipher MA, Langholz B, Wall DA, et al. The addition of sirolimus to tacrolimus/methotrexate GVHD prophylaxis in children with ALL: a phase 3 Children’s Oncology Group/Pediatric Blood and Marrow Transplant Consortium trial. Blood 2014;123:2017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez R, Nakamura R, Palmer JM, et al. A phase II pilot study of tacrolimus/sirolimus GVHD prophylaxis for sibling donor hematopoietic stem cell transplantation using 3 conditioning regimens. Blood 2010;115:1098–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolaños-Meade J, Reshef R, Fraser R, et al. Three prophylaxis regimens (tacrolimus, mycophenolate mofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: a randomised phase 2 trial with a non-randomised contemporaneous control group (BMT CTN 1203). Lancet Haematol 2019;6(3):e132–e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koura DT, Horan JT, Langston AA, et al. In vivo T cell costimulation blockade with abatacept for acute graft-versus-host disease prevention: a first-in-disease trial. Biol Blood Marrow Transplant 2013;19: 1638–49. [DOI] [PubMed] [Google Scholar]
- 28.Hamadani M, Gibson LF, Remick SC, et al. Sibling donor and recipient immune modulation with atorvastatin for the prophylaxis of acute graft-versus-host disease. J Clin Oncol 2013;31:4416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi SW, Braun T, Henig I, et al. Vorinostat plus tacrolimus/methotrexate to prevent GVHD after myeloablative conditioning, unrelated donor HCT. Blood 2017; 130:1760–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y-B, Shah NN, Renteria AS, et al. Vedolizumab for prevention of graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Blood Adv 2019;3:4136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boudreau JE, Giglio F, Gooley TA, et al. KIR3DL1/HLA-B subtypes govern acute myelogenous leukemia relapse after hematopoietic cell transplantation. J Clin Oncol 2017;35:2268–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med 2012;367:1487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saber W, Cutler CS, Nakamura R, et al. Impact of donor source on hematopoietic cell transplantation outcomes for patients with myelodysplastic syndromes (MDS). Blood 2013;122:1974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saber W, Opie S, Rizzo JD, Zhang M-J, Horowitz MM, Schriber J. Outcomes after matched unrelated donor versus identical sibling hematopoietic cell transplantation in adults with acute myelogenous leukemia. Blood 2012;119:3908–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gajewski JL, Petz LD, Calhoun L, et al. Hemolysis of transfused group O red blood cells in minor ABO-incompatible unrelated-donor bone marrow transplants in patients receiving cyclosporine without posttransplant methotrexate. Blood 1992; 79:3076–85. [PubMed] [Google Scholar]
- 36.Sadeghi B, Al-Hashmi S, Hassan Z, et al. Expansion and activation kinetics of immune cells during early phase of GVHD in mouse model based on chemotherapy conditioning. Clin Dev Immunol 2010;2010:142943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ando T, Tachibana T, Tanaka M, et al. Impact of graft sources on immune reconstitution and survival outcomes following allogeneic stem cell transplantation. Blood Adv 2020;4:408–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.