Abstract
Following an official request to EFSA from the European Commission, EFSA assessed the chronic dietary exposure to inorganic arsenic (iAs) in the European population. A total of 13,608 analytical results on iAs were considered in the current assessment (7,623 corresponding to drinking water and 5,985 to different types of food). Samples were collected across Europe between 2013 and 2018. The highest mean dietary exposure estimates at the lower bound (LB) were in toddlers (0.30 μg/kg body weight (bw) per day), and in both infants and toddlers (0.61 μg/kg bw per day) at the upper bound (UB). At the 95th percentile, the highest exposure estimates (LB–UB) were 0.58 and 1.20 μg/kg bw per day in toddlers and infants, respectively. In general, UB estimates were two to three times higher than LB estimates. The mean dietary exposure estimates (LB) were overall below the range of benchmark dose lower confidence limit (BMDL 01) values of 0.3–8 μg/kg bw per day established by the EFSA Panel on Contaminants in the Food Chain in 2009. However, for the 95th percentile dietary exposure (LB), the maximum estimates for infants, toddlers and other children were within this range of BMDL 01 values. Across the different age classes, the main contributors to the dietary exposure to iAs (LB) were ‘Rice’, ‘Rice‐based products’, ‘Grains and grain‐based products (no rice)’ and ‘Drinking water’. Different ad hoc exposure scenarios (e.g. consumption of rice‐based formulae) showed dietary exposure estimates in average and for high consumers close to or within the range of BMDL 01 values. The main uncertainties associated with the dietary exposure estimations refer to the impact of using the substitution method to treat the left‐censored data (LB–UB differences), to the lack of information (consumption and occurrence) on some iAs‐containing ingredients in specific food groups, and to the effect of food preparation on the iAs levels. Recommendations were addressed to improve future dietary exposure assessments to iAs.
Keywords: inorganic arsenic, dietary exposure assessment, rice, rice‐based commodities, drinking water
Summary
Following an official request to EFSA from the European Commission in December 2019, EFSA prepared a scientific report assessing chronic dietary exposure to inorganic arsenic (iAs) in the European population. In addition, EFSA was asked to provide an overview of the available occurrence data on total and iAs in food.
Human exposure to arsenic can occur via different routes; although dermal and inhalation exposure is possible, food and drinking water are the principal routes of exposure to arsenic (FAO/WHO, 2011; IARC, 2012). The International Agency for Research on Cancer (IARC) classified arsenic and iAs compounds as ‘carcinogenic to humans’ (Group 1) based on sufficient evidence of carcinogenicity in humans (IARC, 1973, 1980). More recently, the IARC also classified methylarsonic acid (MA) and dimethylarsinic acid (DMA) as ‘possibly carcinogenic to humans’ (Group 2B), and arsenobetaine (not metabolised in humans) and other organic arsenic compounds as ‘not classifiable as to their carcinogenicity to humans’ (Group 3) (IARC, 2012). In 2009, the EFSA Panel on Contaminants in the Food Chain (CONTAM Panel) established a reference point between 0.3 and 8 μg/kg bw per day as benchmark dose lower confidence limit (BMDL01) for a 1% increased risk of cancer of the lung, skin and bladder, as well as skin lesions (EFSA CONTAM Panel, 2009). The most recent EFSA dietary exposure assessment to arsenic refers back to the 2014 Scientific Report on dietary exposure to iAs in the European population (EFSA, 2014). In that report, very limited data on iAs were available and different factors were used to derive iAs values from analytical data on total arsenic (tAs). This approach was identified as the main source of uncertainty associated with the exposure estimates together with the overall high number of left‐censored data that impacted the lower and upper bound estimations.
In the current report, a total of 13,608 analytical results on iAs were initially considered for the dietary exposure assessment, among them 7,623 corresponding to drinking water and 5,985 to different types of food. Overall, the most represented food category was ‘Grain and grain‐based products’, in particular rice and rice‐based products. Samples were collected across Europe between 2013 and 2018. Consumption data from 23 different European countries and a total of 44 different dietary surveys (87,945 subjects) were used to estimate the chronic dietary exposure to iAs.
The highest dietary exposure was estimated in the young population (infants, toddlers and other children). The highest mean dietary exposure estimates at the lower bound (LB) were in toddlers (0.30 μg/kg bw per day), and in both infants and toddlers (0.61 μg/kg bw per day) at the upper bound (UB). At the 95th percentile, the highest exposure estimates (LB–UB) were 0.58 and 1.20 μg/kg bw per day in toddlers and infants, respectively. In general, UB estimates were two to three times higher than LB estimates. The mean dietary exposure estimates at the LB were overall below the range of benchmark dose lower confidence limit (BMDL01) values of 0.3–8 μg/kg bw per day established by the EFSA CONTAM Panel in 2009. However, for the 95th percentile dietary exposure (LB), the maximum estimates for infants, toddlers and other children were within this range of BMDL01 values. The main contributors to these LB–UB differences were some food categories (e.g. ‘Milk and dairy products’, ‘Grains and grain‐based products (no rice)’) and ‘Fruit and vegetable juices’, among others) with low LB values, a relatively high number of left‐censored data and relatively high consumption in different age classes. Across the different age classes, the main contributors to the dietary exposure to iAs (LB) were ‘Rice’, ‘Rice‐based products’, ‘Grains and grain‐based products (no rice)’ and ‘Drinking water’. Particular foodstuffs indicated for the young population (e.g. ‘Cereal‐based food for infants and young children’ and ‘Biscuits, rusks and cookies for children’) made a relevant contribution in the dietary exposure to iAs in this population. In the adult population, food groups such as ‘Vegetables and vegetable products’ and ‘Fish and other seafood’ were also apparent sources of iAs in certain countries.
As compared to the 2014 EFSA scientific report, the dietary exposure estimates to iAs were noticeably lower, with maximum mean and 95th percentile estimates being around 1.5–3 times lower across the different age classes. This difference is probably due to the sum of different factors related to the occurrence and consumption data used. Among these factors, the use of measured iAs allowed a more accurate and realistic dietary exposure assessment as compared to assessments that make use of assumptions and modelling to derive iAs values from tAs data which are affected by high inherent uncertainty. Likewise, the improvement of the linkage between consumption and occurrence thanks to the availability of additional information (e.g. facets for ingredients/processing in FoodEx2, FoodEx2 classification) led to more refined exposure estimations. Dietary exposure estimates in the current report are in good agreement with recently published scientific literature that also made use of measured iAs to estimate dietary exposure to iAs.
Different ad hoc dietary exposure scenarios were conducted to complement the general exposure scenario. These scenarios showed in some cases dietary exposure estimates in average and high consumers close to or within the range of BMDL01 values (0.3–8 μg/kg bw per day) established by the CONTAM Panel in 2009. As an example, the consumption of rice‐based formulae could lead to dietary exposure estimates of 0.30 and 0.39 μg/kg bw per day in mean and for high consumers, respectively.
The main uncertainties associated with the dietary exposure estimations refer to the impact of using the substitution method to treat the left‐censored data (LB–UB differences), to the lack of information (consumption and occurrence) on some iAs‐containing ingredients in specific food groups, and to the effect of food preparation on the iAs levels. Recommendations are provided to improve future dietary exposure assessments to iAs. These recommendations are mostly focused on asking data providers to submit analytical data following the requirements as specified in the Chemical monitoring reporting guidance which is annually updated (EFSA, 2020), to use validated analytical methods with adequate sensitivity and appropriate extraction methods for different arsenicals, and to further investigate the effect of processing/food preparation on the different arsenic species present in food. Likewise, it is important to collect consumption data, in particular on specific populations (e.g. people with coeliac disease and/or gluten intolerance) that might have a higher consumption of rice and/or rice‐based products and, therefore, higher dietary exposure to iAs.
1. Introduction
Arsenic is a ubiquitous metalloid that occurs in the environment as the result of both natural and anthropogenic activity. In the environment, arsenic generally occurs as pentavalent arsenic (As(V) or arsenate) and trivalent arsenic (As(III) or arsenite), both in inorganic and organic forms. In natural ground water, arsenic habitually appears in inorganic forms (As(III), As(V) or a combination of both); organic forms are rare in water as they are the result of biological activity. Organic arsenic species such as arsenobetaine and different arsenosugars are the most common water soluble forms in marine food, although lipid soluble arsenic species named arsenolipids can be found as a major form of organic arsenic in fish, fish products and some seaweed (Al Amin et al., 2020). In food of terrestrial origin, the predominant arsenic forms are inorganic arsenic (both As(V) and As(III)) and methylated arsenic species (methylarsonic acid (MA) and dimethylarsinic acid (DMA)).
Human exposure to arsenic can occur via different routes. Although dermal and inhalation exposure is possible, food and drinking water are the principal routes of exposure to arsenic (FAO/WHO, 2011; IARC, 2012). Although both forms of inorganic arsenic (iAs) are potentially harmful to human health, As(III) is considered more harmful than As(V) (Hughes et al., 2011). The International Agency for Research on Cancer (IARC) classified arsenic and iAs compounds as ‘carcinogenic to humans’ (Group 1) based on sufficient evidence of carcinogenicity in humans (IARC, 1973, 1980). More recently, the IARC also classified MA and DMA as ‘possibly carcinogenic to humans’ (Group 2B), and arsenobetaine (not metabolised in humans) and other organic arsenic compounds as ‘not classifiable as to their carcinogenicity to humans’ (Group 3) (IARC, 2012).
In its Scientific Opinion on arsenic in food, the EFSA Panel on Contaminants in the Food Chain (CONTAM Panel) established a reference point between 0.3 and 8 μg/kg bw per day as benchmark dose lower confidence limit (BMDL01) for a 1% increased risk of cancer of the lung, skin and bladder, as well as skin lesions (EFSA CONTAM Panel, 2009). Later, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) identified a BMDL05 of 3.0 μg/kg bw per day for an increased risk of lung cancer (range 2–7 μg/kg bw per day) based on epidemiological studies (JECFA, 2011).
There are different international standards or regulatory limits for the presence of arsenic in food and drinking water. For water intended for human consumption, a parametric value of 10 μg/L is established without distinguishing among different arsenic forms (Council Directive 98/83/EC)1 , while Commission Directive 2003/40/EC2 establishes a maximum level (ML) of 10 μg/L for total arsenic (tAs) in natural mineral waters. For food, Commission Regulation 2015/10063 provides MLs of iAs for rice and different types of rice‐containing food (applying from 1 January 2016). These MLs apply to non‐parboiled milled rice4 (polished or white rice) (200 μg/kg), to parboiled rice5 and husked rice6 (250 μg/kg), to rice waffles, rice wafers, rice crackers and rice cakes (300 μg/kg) and to rice destined for the production of food for infants and young children (100 μg/kg). Additionally, Codex Alimentarius has adopted through the years different MLs for tAs (10 μg/L for natural mineral water; 100 μg/kg for edible fats, oils (including fish oils), fat spreads and blended spreads; 500 μg/kg for food grade salt), and, more recently, for iAs (200 μg/kg for polished rice, and 350 μg/kg for husked rice) (FAO/WHO, 2018).
Previous assessments of dietary exposure to arsenic carried out by EFSA refer back to the 2009 Scientific Opinion on arsenic in food (EFSA CONTAM Panel, 2009) and, more recently, to the 2014 Scientific Report on dietary exposure to iAs in the European population (EFSA, 2014). In the 2014 output, the highest dietary exposure to iAs was estimated in the young population (infants, toddlers and other children), with the highest mean and 95th percentile estimates (at the upper bound scenario) being 1.37 μg/kg bw per day (infants) and 2.09 μg/kg bw per day (toddlers), respectively. In previous EFSA outputs, very limited data on iAs were available and different assumptions and modelling were used to derive iAs values from analytical data on tAs. This approach was identified as the main source of uncertainty associated with the exposure estimates, together with the overall high number of left‐censored data that impacted the lower and upper bound estimations.
In the current report, chronic dietary exposure to iAs is estimated across different European countries, using only measured iAs in combination with food consumption data from national dietary surveys. In addition, updated information is provided on the levels of arsenic (tAs and iAs) in a range of food sampled in the European market between 2013 and 2018.
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
In 2009, the EFSA Panel on Contaminants in the Food Chain (CONTAM) adopted a Scientific Opinion on the presence of arsenic in food (EFSA CONTAM Panel, 2009). At that time, the EFSA CONTAM Panel identified a range of BMDL01 values between 0.3 and 8 μg/kg bw day for a 1% increased risk of cancer of the lung, skin and bladder, as well as skin lesions, highlighting a possible risk to consumers on the basis of the estimated exposure.
In its 2014 scientific report on dietary exposure to inorganic arsenic in the European population (EFSA, 2014), EFSA identified grain‐based products as main contributors to the exposure together with rice and milk and dairy products. The heterogeneity of the food consumption data, the use of factors to derive inorganic arsenic from the reported total arsenic data, and the treatment of left censored data were important sources of uncertainties in the dietary exposure estimates.
Commission Recommendation (EU) 2015/13817 recommended to Member States to monitor during 2016, 2017 and 2018 the presence of arsenic, preferably by determining the content of inorganic and total arsenic and, if possible, other relevant arsenic species, in a wide variety of food, and to provide these data to EFSA on a regular basis at the latest by October 2018.
1.1.2. Terms of Reference
In accordance with Art. 31 of Regulation (EC) No 178/20028 the Commission asks EFSA:
For an updated exposure assessment for inorganic arsenic, covered by Recommendation 2015/1381/EU, taking into account the occurrence data in food, submitted after the publication of the 2014 EFSA scientific report and the updated comprehensive food consumption database.
An overview of the available occurrence data on total and inorganic arsenic in food.
2. Data and methodology
2.1. Occurrence data
2.1.1. Data collection and validation
Occurrence data on arsenic were collected as part of an annual call for collection of chemical contaminants occurrence data in food and feed,9 in the framework of Articles 23 and 33 of Regulation (EC) No 178/2002.8 The data submission followed the requirements of the EFSA Guidance on Standard Sample Description for Food and Feed (EFSA, 2010). Data collection was boosted by Commission Recommendation 2015/1381 to monitor the presence of arsenic in different food, preferably by determining the content of iAs, tAs and, if possible, other relevant arsenic species.7 An additional contribution to the data collection came from Commission Recommendation 2018/464 that asks to monitor different compounds, including arsenic, in seaweed, halophytes and products based on seaweed.10
Analytical data on arsenic were reported to EFSA as arsenic, arsenic and derivatives, total arsenic (tAs), inorganic arsenic (iAs), As(V), As(III), organic arsenic, methylarsonic acid (MA), dimethylarsinic acid (DMA) and arsenobetaine (AB). Data reported as ‘Arsenic and derivatives’ and ‘Arsenic’ were considered as tAs if no additional information was provided. After the exclusion of the analytical results already used in the 2014 EFSA scientific report (see terms of reference), a total of 67,401 analytical data were included from different food commodities sampled between 2013 and 2018 (Table 1). At the time of the extraction of the occurrence data (30 April 2020), only few analytical results for samples collected in 2019 were available (n = 194); based on this, it was decided to exclude them from the assessment as they were not considered representative of this year.
Table 1.
Analytical results on arsenic initially extracted from the EFSA Data Warehouse (for samples collected between 2013 and 2018)
| N | % | |
|---|---|---|
| Total Arsenic (tAs) | 58,013 | 86.1 |
| Inorganic Arsenic (iAs) | 6,021 | 9.0 |
| Arsenite ‐ As(III) | 730 | 1.1 |
| Arsenate ‐ As(V) | 737 | 1.1 |
| Organic Arsenic | 21 | 0.03 |
| Methylarsonic acid (MA) | 779 | 1.2 |
| Dimethylarsinic acid (DMA) | 783 | 1.2 |
| Arsenobetaine (AB) | 317 | 0.5 |
| TOTAL | 67,401 | 100 |
The initial data set of 67,401 analytical data is shown in Annex A.
2.1.2. Data cleaning and analysis
To ensure the appropriate quality of the occurrence data used for the dietary exposure estimations, different data cleaning and data validation steps were followed according to EFSA SOPs.11 Together with identifying duplicate samples, attention was paid to the information provided on analytical methods and their sensitivity, FoodEx classification, expression of the results, etc. Data providers were contacted when needed to confirm the information provided (e.g. reported arsenic levels initially identified as potential outliers).
Preliminary evaluation of the data set (67,401 analytical results) led to the exclusion of 1,316 analytical results (690 samples) mainly identified as duplicate entries (i.e. same sample submitted to EFSA at least twice). Other reasons to exclude samples were cases where submitted values could not be confirmed by the data providers, reported analytical method considered as not adequate for the analysis of arsenic, no information provided on the type of food analysed, etc. Some samples (less than 1%) were reported as ‘Suspect samples’. This sampling strategy typically focuses on samples to confirm or reject a suspicion of non‐conformity/high contamination and might not be representative. A careful analysis of the arsenic levels reported for these samples did not identify values that might be considered as outliers as compared to those reported within the corresponding food categories; therefore, these samples were retained in the data set. Data providers were also contacted as regards samples codified as ‘Grains as crops’, grain samples for which the final destination is unknown (food or feed chain). Those samples that could not be confirmed as entering the food chain were excluded (21 samples, 60 analytical results).
Following these first steps, the remaining 66,025 analytical results were grouped based on the arsenic speciation analysis (tAs, iAs, As(V), As(III), organic arsenic, MA, DMA, AB). Initially, a total of 57,299 samples with data on tAs were included together with 5,879 samples with analytical data on iAs; among these samples, few reported analytical data on both tAs and iAs (see Section 3.2).12 If analytical data on iAs were not reported, the iAs content was estimated as the sum of reported As(III) and As(V), using the left‐censoring limits when needed; for a total of 229 samples, the iAs content was derived as the sum of both species. Although a few studies have reported the presence of MA and DMA in water (Banerjee et al., 1999; Thirunavukkarasu et al., 2002), it is well established that almost all arsenic in drinking water is inorganic, either As(III) or As(V) (US EPA, 2001; FAO/WHO, 2011). Analytical results reported as tAs for drinking water (7,824 out of 7,887; the remaining 63 directly reported as iAs) were, therefore, considered as iAs.
As regards the samples with analytical data on tAs, a limit of quantification (LOQ) cut‐off of 200 μg/kg was applied on the food samples in line with the approach previously followed in the 2009 EFSA scientific opinion and in the 2014 EFSA scientific report (EFSA CONTAM Panel, 2009; EFSA, 2014). A total of 793 samples were excluded following this approach. For the samples with analytical data on iAs (except for drinking water, see below), an LOQ cut‐off of 100 μg/kg was applied (EFSA, 2018); out of the 67 samples identified with LOQs above this cut‐off, seven were quantified samples. Since they refer to food that usually contains relatively high levels of iAs (e.g. seaweed), the analytical methods were considered fit for purpose and, therefore, the seven samples were kept in the data set. The left‐censored data were treated by the substitution method using the lower bound (LB) and upper bound (UB) approach (WHO/IPCS, 2009; EFSA, 2010). At the LB, results below the limit of detection (LOD)/LOQ were replaced by zero; at the UB, the results below the LOD were replaced by the value reported as the LOD, and the results below the LOQ and above the LOD were replaced by the value reported as the LOQ. Further details on the analytical results excluded can be found in Annex A.
Drinking water
As mentioned in the introduction, for drinking water a value of 10 μg/L has been established both as parametric value and ML.1,2 This value was taken into account during the data cleaning to prepare the data set to estimate dietary exposure to iAs. Accordingly, samples of drinking water reporting analytical methods with LOQs higher than 10 μg/L were excluded (n = 264), as it was considered that these methods were not fit for purpose.
Rice and rice‐based food
The MLs of iAs for rice and rice‐based food as provided in Commission Regulation 2015/10063 were also considered during the preparation of the final data set. All analytical methods used for the analysis of rice and rice‐based food samples were fit‐for‐purpose, providing enough sensitivity to assess regulatory compliance.
A total of 51 out of 2,621 samples (< 2%) within the frame of this regulation were identified with levels above those permitted by legislation. Most of the samples belong to ‘Rice, brown’ (n = 15, < 4%) and ‘Rice, popped’ (n = 18, < 3%). ‘Rice popped’ refers to a food category under which are mainly codified rice waffles, rice wafers, rice crackers and rice cakes (see below Section 2.3). The levels of iAs reported for these samples as well as the sampling years are shown in Table 2; it can be seen that most of the samples were collected between 2013 and 2016. When looking at these samples from a non‐compliance perspective, it should be kept in mind that Commission Regulation 2015/1006 applied from 1 January 2016 and does not include samples lawfully placed on the market prior to this date. If the samples collected in 2016 were on the market before 1 January 2016, only 11 samples could be defined as non‐complaint with the legislation in place at the time of the sampling.13 Still, all 51 samples were kept in the data set used for the dietary exposure estimations. This decision was based on the fact that they were collected across different European countries indicating that these products are not limited to specific areas, and also to the fact that the population might have consumed them as they were on the market.
Table 2.
Samples of rice and rice‐based commodities with iAs levels above the MLs as provided in Commission Regulation 2015/1006a
| N | iAs levels (μg/kg) | Sampling year | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Maximum | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | ||
| Rice, unspecifiedb | 9 | 240 | 298 | 1 | – | – | 6 | 2 | – |
| Rice, brown | 15 | 327 | 570 | 4 | 5 | – | – | 4 | 2 |
| Rice, long‐grain | 4 | 255 | 370 | 1 | 1 | 1 | 1 | – | – |
| Rice, white | 5 | 244 | 320 | 1 | 1 | – | 3 | – | – |
| Rice, popped | 18 | 344 | 409 | 5 | 1 | – | 9 | 2 | 1 |
MLs for iAs as provided in Commission Regulation 2015/1006: non‐parboiled milled rice (polished or white rice) (200 μg/kg), parboiled rice and husked rice (250 μg/kg), rice waffles, rice wafers, rice crackers and rice cakes (300 μg/kg).
For samples of ‘Rice, unspecified’ the ML of 200 μg/kg was applied.
No samples of rice specifically identified as ‘destined for the production of food for infants and young children’ were submitted to EFSA. However, it is worth mentioning that for 50 samples codified as ‘Cereal‐based food for infants and young children’ (~ 22% of the total), the iAs values were above 100 μg/kg. Many of these samples specified the presence of rice and, therefore, it is likely that the rice used as an ingredient contained iAs levels above the ML of 100 μg/kg specified in the legislation. The samples were mainly collected in 2016 from different countries, with 15 collected in 2017 and 2018; all these samples were kept in the data set used for dietary exposure estimations.
A small number of samples were reported as pooled samples being part of Total Diet Studies (TDS). After verifying that the level of aggregation for the pooled samples matched the FoodEx classification of the individual samples, the pooled samples were retained in the data set. The number of samples pooled in each pooled sample was used to weight the reported analytical results before calculating the mean concentrations per food category. Analytical results were also corrected for recovery when this information was available.
2.2. Food consumption data
Food consumption data were retrieved from the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) in April 2020. The Comprehensive Database provides a compilation of national information on food consumption at individual level. Details on how the Comprehensive Database is used are published in the Guidance of EFSA (EFSA, 2011a).
The latest version of the Comprehensive Database updated in 2020 contains results from a total of 69 different dietary surveys carried out in 25 different European countries covering 134,929 individuals. Detailed information on the different dietary surveys available in the Comprehensive Database can be found on the dedicated page of the EFSA website.14 The age classes considered are the following:
Infants: < 12 months old;
Toddlers: ≥ 12 months to < 36 months old;
Other children: ≥ 36 months to < 10 years old;
Adolescents: ≥ 10 years to < 18 years old;
Adults: ≥ 18 years to < 65 years old;
Elderly: ≥ 65 years to < 75 years old;
Very elderly: ≥ 75 years old.
Seven additional surveys included in the Comprehensive Database provide information on specific population groups: ‘Pregnant women’ (15–45 years old for Latvia; 17–46 years for Portugal; 21–46 years for Spain; 17–43 years for Cyprus; 19–47 years for Austria) and ‘Lactating women’ (28–39 years old for Greece; 18–45 years for Estonia).
When for one country and age class two different dietary surveys were available, only the most recent survey was used. Only dietary surveys with more than one day per subject were used to estimate chronic dietary exposure to iAs following the recommendations issued by the EFSA Working Group on Food Consumption and Exposure (EFSA, 2011a). Similarly, subjects who participated only one day in the dietary studies, when the protocol prescribed more reporting days per individual, were also excluded from the chronic exposure assessment. This resulted in a total of 44 different dietary surveys (87,945 subjects) carried out in 23 different European countries used for the chronic dietary exposure assessment (Annex B.1). Owing to the differences in the methods used for data collection, direct country‐to‐country comparisons can be misleading.
2.3. Food classification
Consumption and occurrence data were both codified according to the FoodEx classification system. FoodEx is a food classification system developed by EFSA in 2009 with the objective of simplifying the linkage between occurrence and food consumption data when assessing the exposure to hazardous substances (EFSA, 2011b). It contains 20 main food categories (first level), which are further divided into subgroups having 140 items at the second level, 1,261 items at the third level and reaching about 1,800 endpoints (food names or generic food names) at the fourth level. Ad hoc use of the new version of FoodEx, named FoodEx2, allowed a more accurate linkage between occurrence and food consumption data thanks to the availability of more detailed food levels and the use of facets and facet descriptors (EFSA, 2011b, 2015).
Special attention was dedicated to the codification of rice waffles, rice wafers, rice crackers and rice cakes to guarantee an accurate and precise linkage with the consumption data of these key commodities in the exposure assessment to iAs. The consumption data of these foodstuffs are codified as ‘Rice, popped’ in FoodEx; the additional use of FoodEx2 codes allowed separating these consumption data among ‘Puffed rice textured bread’ and ‘Cakes’, these two codes covering rice waffles/rice wafers/rice crackers/rice cakes, and ‘Rice, popped’, referring to samples of loose popped rice typically used as breakfast cereal. Accordingly, and using the additional information provided with the analysed samples, the occurrence data were assigned with the corresponding FoodEx2 codes before the linkage with the consumption data. The samples initially reported in FoodEx as ‘Rice, popped’ were divided into two ad hoc groups: ‘Breakfast rice, popped (loose)’ and ‘Rice cakes/Rice waffles/Rice crackers’ (see below in Table 5 in Section 3.1.2). Additionally, few eating occasions codified in FoodEx2 as ‘Crackers and breadsticks’ and with rice identified as an ingredient were linked to the occurrence data derived for the group ‘Rice cakes/Rice waffles/Rice crackers’. The use FoodEx2 facets also permitted the identification of key ingredients in eating occasions of different food commodities, leading to a more accurate linkage with the corresponding occurrence data. This was the case, for instance, of the presence of rice in eating occasions of ‘Cereal‐based food for infants and young children’ or the presence of fish/rice in eating occasions of ready‐to‐eat meals for children.
Table 5.
Summary statistics (μg/kg) of the levels of iAs in different rice‐based food commoditiesa
| N | 25th percentile | 50th percentile | Mean | 75th percentile | 95th percentile | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | ||
| Rice flour | 19 | 65 | 66 | 74 | 75 | 104 | 109 | 110 | 110 | – | – |
| Rice bread | 10 | – | – | 59 | 59 | 75 | 80 | – | – | – | – |
| Noodle, rice | 17 | 47 | 47 | 59 | 68 | 66 | 76 | 79 | 80 | – | – |
| Rice flakes | 113 | 80 | 80 | 110 | 110 | 123 | 126 | 167 | 167 | 267 | 267 |
| ‘Rice cakes/rice waffles/rice crackers’ | 579 | 90 | 90 | 146 | 146 | 146 | 148 | 196 | 196 | 270 | 270 |
| Breakfast rice, popped (loose) | 62 | 36 | 75 | 95 | 100 | 100 | 114 | 150 | 150 | 220 | 220 |
| Rice porridge | 7 | – | – | 12 | 12 | 12 | 12 | – | – | – | – |
| Rice drink | 43 | 0 | 11 | 9 | 20 | 8 | 17 | 13 | 20 | – | – |
Percentiles are only provided when the number of samples is sufficient to provide statistically robust values (EFSA, 2011a).
2.4. Methodologies
2.4.1. Chronic dietary exposure
Dietary chronic exposure to iAs was assessed at individual level by multiplying the average daily consumption for each food with the corresponding mean occurrence estimated for iAs (LB and UB), summing up the respective intakes throughout the diet and finally dividing the results by the individual's body weight. For each dietary survey, the mean and 95th percentile dietary exposure to iAs were estimated from the distribution of the individual exposure results. In accordance with the specifications of the EFSA Guidance on the use of the EFSA Comprehensive Food Consumption Database, 95th percentile estimates for dietary surveys/age classes with less than 60 observations were not calculated since they may not be statistically robust (EFSA, 2011a).
The whole diet was taken into account, except for food not covered by occurrence data and for which an assumption on their contamination level was not possible. The different food commodities were grouped under different food categories to better explain their contribution to the total dietary exposure to iAs in each age class.
Different dilution factors were used to convert the occurrence data reported for solid food groups (e.g. coffee beans) to their respective liquid consumption amounts reported in the consumption database. The dilution factors used were 75 for ‘Tea and herbs for infusions (Solid)’, 60 for ‘Cocoa powder’, 10 for ‘Cocoa beverage‐preparation, powder’ and ‘Dried milk’, 18 for all types of coffees (except 7 for espresso and 63 for instant coffee), five for ‘Porridge’ and eight for infant and follow‐on formulae. Likewise, the same factors were applied on the consumption data when needed.
Additionally to the general chronic dietary exposure scenario, a few specific exposure scenarios were also conducted. They mainly cover consumers of different food commodities for which limited amount of consumption data/occurrence data were available (e.g. breast milk, infant and follow‐on formula), food commodities for which an accurate linkage consumption–occurrence data are not guarantee (e.g. cereal‐based food for infants), or consumers of food commodities with recognised relevance in the exposure to iAs such as rice and rice cakes/rice waffles/rice crackers. In these scenarios, the consumption and occurrence data were retrieved from the EFSA databases and, when not possible, scientific literature sources were used.
All analyses were run using the SAS Statistical Software (SAS enterprise guide 7.15).
3. Assessment
3.1. Occurrence data on inorganic arsenic
Dietary exposure assessment was carried out using only the occurrence data reported as iAs.15 Additionally, detailed concentration of tAs in different food categories is provided in Section 3.2 and Annex B.4.
Following the data cleaning and analysis steps described in Section 2.1.2, the data set used for dietary exposure estimations contained a total of 13,608 analytical results on iAs (Annex B.2). Among them, a total of 7,623 corresponded to drinking water15 and 5,985 to different types of food. As explained in Section 2.1.1, only samples collected between 2013 and 2018 were considered in the assessment. Figure 1 shows the distribution of the different samples across years. Samples analysed for iAs were collected in 23 different European countries (Figure 2). The main countries submitting occurrence data on iAs were Germany (n = 4,137), Hungary (n = 2,879) and Spain (n = 1,133).
Figure 1.
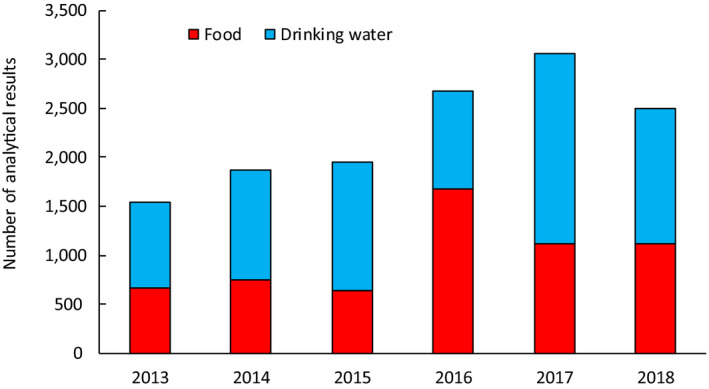
Distribution of analytical results on iAs in food and drinking water by sampling year
Figure 2.
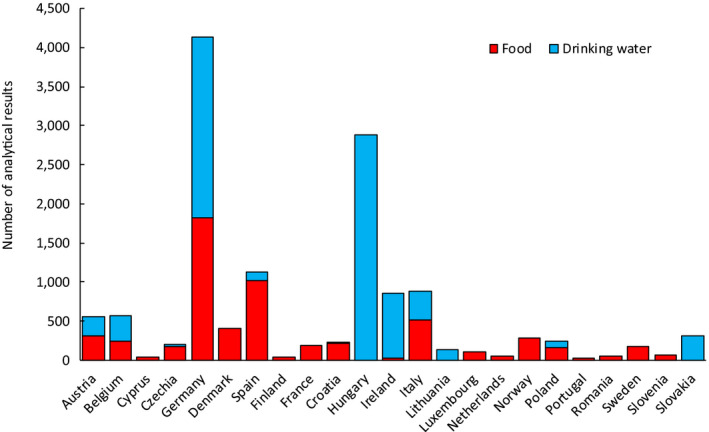
Distribution of analytical results on iAs in food and drinking water by sampling country
3.1.1. Analytical methods
Analysis of food commodities (n = 5,985)
Data providers reported information on the separation technique and/or the detection method used in the analysis of the different food samples. For around 25% of the samples, no information was provided, although in many cases data providers refer to the use of official methods without further details.
As concerns the separation technique, high‐performance liquid chromatography (HPLC) was the only technique reported (~ 40% of the samples). In this report, the analytical data reported as iAs were considered even if no information on arsenic speciation (e.g. separation techniques) was provided. The reported detection methods can be grouped into two categories: spectroscopic methods and mass spectrometry methods. Most of the samples (n = 3,521) were detected using inductively coupled plasma mass spectrometry (ICP‐MS). Among the spectroscopic methods, three different ones were indicated: Atomic Absorption Spectrometry (AAS), Atomic Emission Spectrometry (AES) and Atomic Fluorescence Spectrometry (AFS). The use of hydride generation (HG), a derivatisation step to improve selectivity and sensitivity in elemental analysis, was reported together with the three spectroscopic methods mentioned above. Figure 3 shows an overview on the different detection methods used.
Figure 3.
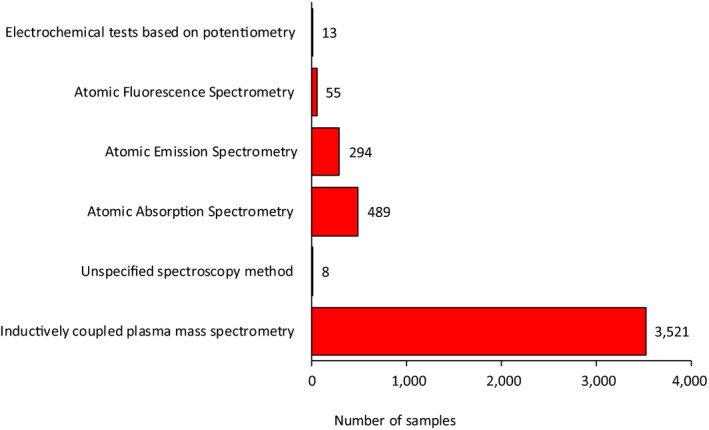
Detection methods reported in the analysis of iAs in food samples
The highest sensitivity was reported for a HPLC‐ICP‐MS method used for the analysis of different water molluscs (LOQ = 0.002 μg/kg). For the spectroscopic methods, the lowest LOQs varied between 0.008 μg/kg reported for HG‐AAS and 0.05 μg/kg reported for ICP‐HGAES, in both cases for the analysis of rice, with HPLC‐HGAFS reporting an LOQ of 4 μg/kg when used to analyse different types of rice.
Analysis of drinking water (n = 7,623)
Out of the 7,623 samples of drinking water included, only 63 were analysed for the presence of iAs and the rest for tAs. As mentioned in Section 2.1.2., all data reported as tAs for drinking water were treated as iAs.
Detection methods reported for drinking water were MS, AAS and AES (Figure 4). Similarly to the food analysis, for around 25% of the samples information on the analytical method was not reported. The use of separation techniques coupled to the detection methods was not provided for drinking water, with the exception of one sample analysed by gas chromatography with mass spectrometry detection (GC‐MS). Overall, higher sensitivity was reported for the analytical methods used to analyse drinking water as compared to the food analysis, being water a less complex matrix than food; the lowest LOQ was indicated for an ICP‐MS method (LOQ = 0.0002 μg/L). Among the spectroscopic methods, the highest sensitivities were 0.05 and 0.7 μg/L reported for AAS and AES, respectively.
Figure 4.
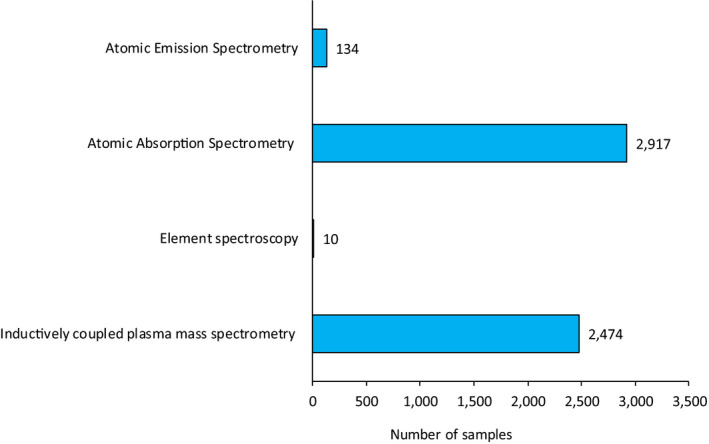
Detection methods reported in the analysis of inorganic arsenic in drinking water
3.1.2. Occurrence data on inorganic arsenic by food category
This section presents the 13,608 analytical results that were initially considered to estimate dietary exposure to iAs after the validation, cleaning and analysis of the data as explained in Section 2.1.2. Detailed FoodEx classification of each food group and summary statistics of the reported iAs levels (LB–UB) are shown in Annex B.2.
Table 3 shows an overview of the distribution of the samples across different food categories at FoodEx level 1. As can be seen, the most represented categories were ‘Drinking water’ (n = 7,623) and ‘Grains and grain‐based products’ (n = 2,928). For the food categories ‘Eggs and egg products’, ‘Sugar and confectionary’, ‘Animal and vegetable fats and oils’, ‘Legumes, nuts and oilseeds’, ‘Meat and meat products’ and ‘Alcoholic beverages’, almost all samples were reported as left‐censored data.
Table 3.
Samples analysed for iAs across FoodEx categories
| Number of samples | Left‐censored data (LC) | Limit of quantification (LOQ, μg/kg) | |||
|---|---|---|---|---|---|
| N | % LC | Min | Max | ||
| Grains and grain‐based products | 2,928 | 485 | 17 | 0.005 | 100 |
| Vegetables and vegetable products (including fungi) | 589 | 403 | 69 | 0.3 | 110 |
| Starchy roots and tubers | 14 | 11 | 79 | 3 | 40 |
| Legumes, nuts and oilseeds | 179 | 175 | 98 | 5 | 90 |
| Fruit and fruit products | 87 | 69 | 79 | 0.04 | 100 |
| Meat and meat products (including edible offal) | 46 | 44 | 96 | 3.9 | 10 |
| Fish and other seafood | 938 | 546 | 58 | 0.002 | 90 |
| Milk and dairy products | 239 | 207 | 87 | 0.3 | 40 |
| Eggs and egg products | 6 | 6 | 100 | 10 | 10 |
| Sugar and confectionary | 37 | 37 | 100 | 10 | 20 |
| Animal and vegetable fats and oils | 27 | 27 | 100 | 40 | 40 |
| Fruit and vegetable juices | 70 | 52 | 74 | 1 | 40 |
| Non‐alcoholic beverages (excepting milk‐based beverages) | 1 | 1 | 100 | 20 | 20 |
| Alcoholic beverages | 45 | 44 | 98 | 4 | 20 |
| Drinking water | 7623 | 4574 | 60 | 0.0002 | 10 |
| Herbs, spices and condiments | 97 | 59 | 61 | 10 | 100 |
| Food for infants and young children | 482 | 241 | 50 | 0.04 | 100 |
| Products for special nutritional use | 108 | 59 | 55 | 0.3 | 100 |
| Composite food (including frozen products) | 72 | 19 | 26 | 2.2 | 77.5 |
| Snacks, desserts and other foods | 20 | 15 | 75 | 2 | 28 |
| TOTAL | 13,608 | 7,074 | 52 | ||
Grains and grain‐based products
Excluding drinking water, the food group ‘Grains and grain‐based products’ was the most represented with a total of 2,928 analytical data submitted on iAs. Within this food category, close to 1,800 samples of different types of rice were included together with rice‐based commodities known to contain relatively high levels of iAs. Examples of these commodities are the samples codified as ‘Rice cakes/rice waffles/rice crackers’ (n = 579) or samples of rice flakes (n = 120). Among the different cereals, it is well known that rice plants accumulate more iAs than similar cereal crops due to its capacity to uptake and translocate iAs to the grain, and to the semiaquatic anaerobic growing environment (paddy fields) which promotes the root uptake of iAs (Zhao et al., 2010; Punshon et al., 2017).
An overview of the distribution of the levels of iAs in the different types of rice is shown in Table 4. Those reporting the highest levels were red rice, although with a limited number of samples (n = 7; mean LB–UB = 233 μg/kg), and brown rice (n = 382, mean LB–UB = 128–131 μg/kg). It is well known that brown (husked) rice retains the bran layer that, apart from being of high nutritional value, contains high levels of iAs (Signes Pastor et al., 2017). In white rice, where the outer layers and the germ are removed during the whitening (milling) process, the mean levels of iAs were 74–79 μg/kg (LB–UB, n = 337), similar to those in long‐grain rice (n = 502; mean LB–UB = 77–87 μg/kg). Parboiled rice (n = 131; mean LB–UB = 98–102 μg/kg) contains, overall, slightly higher levels of iAs as compared to white rice. Parboiling is a process that traditionally involves boiling or steaming the rough rice to improve storage stability, and increases the nutritive value of the rice. As a side effect, when parboiling unhusked rice, the arsenic present in the husk can also be transferred to the grain. Recently, a novel parboiled method has been proposed starting from husked rice instead of rough rice which seems to decrease the iAs content by 25% in the final polished grain (Rahman et al., 2019).
Table 4.
Summary statistics (μg/kg) of iAs in different types of rice
| N | 25th percentile | 50th percentile | Mean | 75th percentile | 95th percentile | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | ||
| Rice, unspecified | 404 | 43 | 50 | 81 | 84 | 79 | 85 | 113 | 113 | 165 | 165 |
| Rice, brown | 382 | 89 | 93 | 117 | 118 | 128 | 131 | 151 | 152 | 236 | 236 |
| Rice, long‐grain | 502 | 51 | 60 | 75 | 81 | 77 | 87 | 110 | 110 | 150 | 152 |
| Rice, parboiled | 131 | 78 | 83 | 99 | 101 | 98 | 102 | 120 | 120 | 159 | 159 |
| Rice, white | 337 | 56 | 58 | 72 | 74 | 74 | 79 | 94 | 98 | 135 | 135 |
A higher number of rice samples with data on iAs is now available (n = 1,768) as compared to the 2014 EFSA scientific report (n = 706); the mean iAs levels in the different types of rice were in the same order of magnitude as those available in 2014 (EFSA, 2014).
Although iAs concentrations in rice can vary widely across and even within growing regions, the levels in this report for the different types of rice compare well to those described in the literature (Ruttens et al., 2018; US FDA, 2016). Recently, a scientific paper studied the variation of arsenic concentrations and speciation in rice grains across 29 representative growing regions in six continents; iAs values in white rice ranged between < 2 and 399 μg/kg (Carey et al., 2020). Similarly, a review describing levels of arsenic in different food showed a high variation of iAs in rice samples across countries: < 100 μg/kg in India, 130 μg/kg in Bangladesh, 140 μg/kg in Taiwan and China and 190 μg/kg in Japan (Upadhyay et al., 2019). In 2014, a comprehensive literature search to characterise the state of knowledge on speciated arsenic in food reported a mean iAs concentration of 130 μg/kg based on more than 1,000 samples (Lynch et al., 2014).
Apart from the samples of rice grain and still within the food group ‘Grains and grain‐based products’, the samples with the highest levels of iAs were processed products with rice in its composition, such as rice flakes and rice bread, among others (Table 5). These samples were all grouped under ‘Rice‐based products’ when assessing the contribution of the different food to the dietary exposure to iAs. Under ‘Rice‐based products’ is also the group ‘Rice cakes/Rice waffles/Rice crackers’ (n = 579; mean LB–UB = 146–148 μg/kg). The relatively high presence of iAs in this type of products was already identified in the 2014 report (EFSA, 2014) and is also profusely described in the literature (Islam et al., 2017, Domínguez‐González et al., 2020). These are the only rice‐based products with an ML for iAs provided by Commission Recommendation (EU) 2015/1006. Table 5 shows the distribution of iAs levels in the most relevant rice‐based products, including ‘Rice drink’ which is codified in FoodEx as milk imitate.
Grain‐based products not containing rice showed much lower levels of iAs as compared to those containing rice as an ingredient. Highly consumed food commodities such as ‘Wheat bread and rolls’ were all left‐censored data (n = 35). In other food groups such as ‘Wheat milling products’, quantified iAs levels were reported only for few samples leading to broad differences between LB and UB estimations (0.7−19.7 μg/kg with 95% of left‐censored data). All grain‐based products not containing rice were grouped under ‘Grains and grain‐based products (no rice)’ when assessing their contribution to the dietary exposure to iAs.
Drinking water
A total of 7,623 samples of different types of drinking water were included in the data set. Although only 63 samples of drinking water were analysed for iAs, the tAs levels reported for the remaining samples were assumed to be iAs (see Section 2.1.2).
Although most of the cases of relatively high levels of arsenic in drinking water are reported in Southern Asian countries such as India, Bangladesh and Cambodia (Uppal et al., 2019), countries in all continents are affected, including Europe. Several scientific publications have reported high arsenic levels in drinking water across Europe, typically in areas where complex biogeochemical interactions mobilise arsenic from volcanic rocks and sulfide mineral sediments into groundwater (Herath et al., 2016). A few examples include the mean arsenic levels between 16 and 21.2 μg/L in tap water reported in different areas of Central Italy (Cubadda et al., 2015), the median arsenic values between 7.7 and 28 μg/L in drinking water in diverse countries of Central Europe, in particular Hungary (Lindberg et al., 2006), tAs levels that ranged from < 0.5 to 240 μg/L in groundwater in the Pannonian Basin (Rowland et al., 2011), or the maximum levels up to 233 μg/L reported in private drinking water supplies in the UK (Middleton et al., 2016). In these areas, the additional exposure to iAs due to the relatively high levels of iAs in drinking water should be considered together with that coming from different locally produced/grown food (e.g. bread, vegetables etc.) that will also contain higher iAs than in other locations (Cubadda et al., 2015).
A total of 356 samples of different types of drinking water were reported with iAs levels above 10 μg/L,16 around 3% corresponding to bottled water (Table 6). Pooling these samples together with the rest of samples of drinking water results in a significant increase of the mean iAs content, due to the relatively high iAs concentrations reported for some of these samples (e.g. 183 μg/L for unspecified drinking water). Considering that: (i) these samples actually are non‐compliant with the current European ML/parametric value, and (ii) most of these samples (94%) were submitted exclusively by two countries, they were considered as not representative of the iAs concentrations present in drinking water consumed by the European population. Therefore, they were excluded to avoid introducing a bias when estimating dietary exposure to iAs. Detailed statistics for the iAs levels reported for these samples can be found in Table 6.
Table 6.
Summary statistics of the levels of iAs (μg/L)a in the samples of drinking water excludedb from the final data set used to estimate dietary exposure in the European population
| N | 25th percentile | 50th percentile | Mean | 75th percentile | 95th percentile | Max | |
|---|---|---|---|---|---|---|---|
| Drinking water, unspecified | 193 | 13 | 17 | 24 | 27 | 61 | 183 |
| Tap water | 63 | 12 | 15 | 20 | 23 | 44 | 93 |
| Well water | 89 | 15 | 21 | 39 | 30 | 139 | 388 |
| Bottled water, unspecified | 2 | – | – | 17 | – | – | – |
| Still mineral water | 5 | – | – | 14 | – | – | – |
| Carbonated mineral water | 4 | – | – | 19 | – | – | – |
Percentiles are only provided when the number of samples is sufficient to provide statistically robust values (EFSA, 2011a).
Samples excluded based on reported iAs levels above 10 μg/L (parametric value/ML).
Table 7 provides an overview of the distribution of the levels of iAs in the different samples of drinking water. In the samples considered for the dietary exposure estimations (n = 7,267), the mean LB levels of iAs reported for tap water and well water were higher than those reported for bottled water. The highest mean levels were reported for unspecified drinking water (mean = 2.0–2.4 μg/kg, LB–UB, n = 593) while the lowest were for carbonated mineral water (mean = 0.8–1.7 μg/kg, LB–UB, n = 593).
Table 7.
Summary statistics (μg/kg) of the levels iAs in different samples of drinking water
| N | 25th percentile | 50th percentile | Mean | 75th percentile | 95th percentile | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | ||
| Drinking water, unspecified | 1,715 | 0 | 1.0 | 0 | 2.0 | 2.0 | 2.9 | 3.5 | 3.5 | 8.2 | 8.2 |
| Tap water | 593 | 0 | 1.0 | 0 | 2.0 | 1.7 | 2.7 | 3.2 | 3.4 | 8.0 | 8.0 |
| Well water | 945 | 0 | 1.0 | 0 | 2.0 | 1.5 | 2.7 | 2.0 | 4.0 | 8.0 | 8.0 |
| Bottled water, unspecified | 522 | 0 | 0.7 | 0 | 1.0 | 0.6 | 1.2 | 0.2 | 2.9 | 4.0 | 4.9 |
| Still mineral water | 1,895 | 0 | 1.0 | 0 | 1.0 | 1.0 | 2.1 | 1.4 | 4.0 | 4.4 | 4.7 |
| Carbonated mineral water | 1,597 | 0 | 0.6 | 0 | 1.0 | 0.8 | 1.7 | 1.0 | 2.0 | 4.6 | 5.0 |
Food for infants and young children
A total of 482 samples of ‘Food for infants and young children’ were included with analytical results reported as iAs (see Annex B.2). Figure 8 shows the summary statistics of selected food groups within this category, with the samples of infant and follow‐on formulae provided as powder already converted into the liquid form (after applying a factor of eight, see Section 2.4.1).
Figure 8.
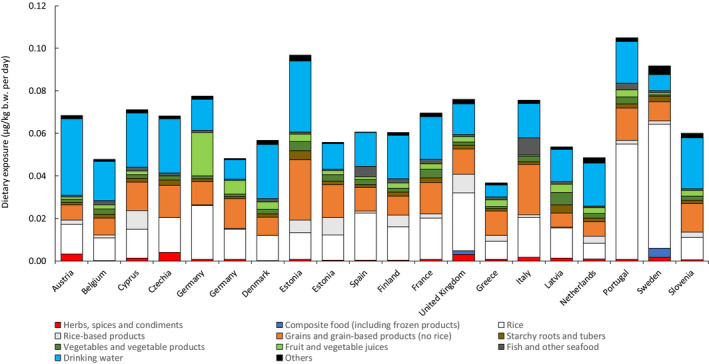
Average contribution of selected food groups to the mean dietary exposure to iAs (at the LB estimations) in the age class ‘Adolescents’ across different European countries
Several samples were codified as ‘Cereal‐based food for infants and young children’ (n = 224). The information provided allowed, in some cases, distinguishing among samples with and without rice as an ingredient. For the samples that did not report the presence of rice (n = 131), the iAs content was 31–38 μg/kg (LB–UB) (Table 8). Those reported as containing rice (n = 93) had mean iAs concentrations of 77–79 μg/kg (LB–UB), with a 95th percentile of 130 μg/kg (grouped as ‘Cereal‐based food for infants and young children with rice)’. Although it is a limited number of samples (n = 93), the distribution of the iAs levels among the samples with rice showed a relatively high variation. Overall, scientific literature reports relatively high iAs for rice‐based products for infants and young children that compares well with the levels reported to EFSA (Signes‐Pastor et al., 2016; Gu et al., 2020). It is also important to note that among the samples not declaring rice as an ingredient (grouped as ‘Cereal‐based food for infants and young children’), several reported values above 50 μg/kg (95th percentile = 110 μg/kg) that seems to indicate that rice might be present although not declared and not confirmed by data providers.
Table 8.
Summary statistics (μg/kg)a of the levels of iAs in different samples of ‘Food for infants and young children’
| N | 25th percentile | 50th percentile | Mean | 75th percentile | 95th percentile | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | ||
| Cereal‐based food for infants and young children with riceb | 93 | 45 | 47 | 80 | 80 | 77 | 79 | 101 | 101 | 130 | 130 |
| Cereal‐based food for infants and young children | 131 | 0 | 10 | 12 | 18 | 31 | 38 | 45 | 52 | 110 | 110 |
| Ready‐to‐eat meals for children, cereal‐based | 12 | 0 | 0.02 | 0 | 0.03 | 3.3 | 5.8 | 0.0 | 10 | – | – |
| Ready‐to‐eat meals for children, meat/fish‐based | 12 | 0 | 21 | 0 | 50 | 8.8 | 39 | 2.5 | 50 | – | – |
| Follow‐on formula, milk‐based | 47 | 0 | 0.8 | 0 | 0.8 | 0.08 | 2.1 | 0 | 1.3 | – | – |
| Infant formula, milk‐based | 38 | 0 | 0.8 | 0 | 0.8 | 0.02 | 4.0 | 0 | 1.3 | – | – |
| Biscuits, rusks and cookies for children | 52 | 53 | 60 | 76 | 83 | 83 | 94 | 106 | 106 | – | – |
Percentiles are only provided when the number of samples is sufficient to provide statistically robust values (EFSA, 2011a).
Samples that reported the presence of rice as an ingredient.
Samples under the food category ‘Ready‐to‐eat meals for infants and young children’ include products with heterogeneous ingredients (cereals, fish, meat, vegetables etc.) where cereals are not always the main ingredient (Table 8). Those reported as ‘Ready‐to‐eat meals for children, meat/fish‐based’ (n = 12) had the highest mean levels, 8.8–39 μg/kg (LB–UB), followed by ‘Ready‐to‐eat meals for children, cereal‐based’ (n = 12) with mean levels of 3.3–5.8 μg/kg (LB–UB). The rest of the food groups under the category ‘Ready‐to‐eat meals for infants and young children’, namely vegetable‐based products (n = 17), meat‐ and vegetable‐based products (n = 9), fruit purée for children (n = 7) and those reported as ready‐to‐eat meals for infants and young children unspecified (n = 42) were all reported as left‐censored data (Annex B.2). An ad hoc category was created grouping all samples of ready‐to‐eat meals for infants and young children (n = 99), with the aim of covering the eating occasions of this food group codified as unspecified in the EFSA Comprehensive database.
A total of 52 samples were reported as ‘Biscuits, rusks and cookies for children’ (Table 8). This food group shows relatively high levels of iAs, mean = 83–94 μg/kg (LB–UB), with only nine samples not quantified and many of them above 100 μg/kg of iAs denoting a likely presence of rice in its composition even if not reported. Under this category can be included typical snacks (rice cakes, rice crackers, rice biscuits) that are widely used to feed infants and young children. In a recent study, several samples of rice‐based products for infants in the Australian market, including rice crackers, were found to have levels of iAs above 100 μg/kg (Gu et al., 2020). The equivalent in adults would be the food group ‘Rice cakes/Rice waffles/Rice crackers’ described above; however, it cannot be discarded that these types of products are also consumed by infants and young population. The iAs levels in these food for adults seem to be higher than those indicated for infant consumption (mean = 146–148 μg/kg, LB–UB); similar results are reported in the scientific literature (Carey et al., 2018).
Other food groups under ‘Food for infants and young children’ were infant formula and follow‐on formulae. As shown in Annex B.2, most of the samples reported for these groups were left‐censored data (84 out of 91 samples). Two main groups were represented: ‘Follow‐on formula, milk‐based’ (n = 47) and ‘Infant formula, milk‐based’ (n = 38), in both cases with relatively low levels of iAs, 0.08–2.1 μg/kg (LB–UB) and 0.02–4.0 μg/kg (LB–UB), respectively (Table 8). It is reported that milk‐based formula contains low levels of iAs (Jackson et al., 2012; Gu et al., 2020) and, therefore, its contribution to the iAs intake is in general rather low. However, the contribution of infant and follow‐on formulae might drastically increase in certain areas due to the use of drinking water with relatively high levels of iAs to reconstitute the formulae. Almost no data were provided on other types of formulae, in particular on those codified as ‘Infant formula, hypoallergenic’. Within this food category are included rice‐based formulae, specially indicated to children who suffer milk allergy/lactose intolerance. As it is well known that these products contain relatively high levels of iAs (Meharg et al., 2008, Llorente‐Mirandes et al., 2011, Signes‐Pastor et al., 2016; Gu et al., 2020), a particular dietary exposure scenario was conducted (see below in Section 3.3.4).
Other food categories
Among the samples of ‘Vegetables and vegetable products (including fungi)’, attention was given to mushrooms, as they are well known to accumulate different arsenic species, including iAs, depending on the substrate and the fungal species (Mleczek et al., 2016; Braeuer and Goessler, 2019). A total of 76 samples were reported as ‘Fungi’ either cultivated or wild, in many cases without further details. Among those cultivated, iAs data were included for 18 samples of button mushrooms (Agaricus bisporus) with only one quantified sample (11 μg/kg). Overall, there were more quantified samples of wildly growing mushrooms as compared to those cultivated; a total of 14 samples of Cantharelle (Cantharellus cibarius) were analysed with six of them possessing quantifiable levels of iAs (mean LB = 16.9 μg/kg). Similar levels (mean LB = 17.2 μg /kg) were found for five samples of the genus Boletus.
A total of 279 samples of seaweed were submitted with data on iAs. Overall, seaweed possess high levels of arsenic although it is primarily found in the form of arsenosugars (Llorente‐Mirandes et al., 2011, Taylor et al., 2017a,b). Almost half of the samples were codified as unspecified seaweed, although based on the additional information provided three of them were identified as the brown seaweed Hiziki or Hijiki (Sargassum fusiforme, syn. Hizikia fusiformis). These brown seaweed are well documented as having high levels of iAs as compared to other seaweed (Rose et al., 2007; EFSA, 2014). The average mean of the three samples of Hijiki reported here was 40.2 mg/kg (see Table 9), in two of them with a ratio iAs/tAs of around 60% and in the other one of only 9%. Several of the unspecified seaweed with high levels of iAs (close to 100 mg/kg) and a ratio iAs/tAs above 85% are described as ‘brown algae’, so it cannot be discarded that they might also refer to samples of Hijiki. Few samples (n = 13) of another brown alga, Kombu (Laminaria spp.), were also included in the data set. Although this type of seaweed generally contains low levels of iAs, very high levels were reported for one sample (94 mg/kg) and with an unusual high iAs/tAs ratio (76.4%). For the other reported seaweed, Laver (Porphyra spp.) and Wakame (Undaria spp), the iAs mean levels (LB) were 62.5 and 182.3 μg /kg, respectively, and with very low iAs/tAs ratios.
Table 9.
Mean levels (μg/kg) of iAs in selected food categories
| N | %LC | Mean values (μg/kg)c | ||
|---|---|---|---|---|
| LB | UB | |||
| Grains for human consumption, except rice | 67 | 64 | 9 | 18 |
| Rice, unspecified | 405 | 15 | 79 | 85 |
| Rice, brown | 382 | 3 | 128 | 131 |
| Rice, long‐grain | 502 | 17 | 77 | 87 |
| Rice, parboiled | 131 | 7 | 98 | 102 |
| Rice, red | 7 | 0 | 232 | 232 |
| Rice, white | 337 | 10 | 74 | 79 |
| Wheat milling products | 41 | 95 | 1 | 20 |
| Wheat flour, white | 13 | 62 | 5 | 10 |
| Rice milling products | 6 | 0 | 81 | 81 |
| Rice flour | 19 | 5 | 104 | 109 |
| Rice flour white | 7 | 0 | 73 | 73 |
| Pasta (Raw) | 8 | 75 | 14 | 26 |
| Pasta, gluten free | 6 | 50 | 59 | 70 |
| Noodle, rice | 17 | 24 | 66 | 76 |
| Breakfast cereals | 16 | 31 | 17 | 21 |
| Mixed cereal flakes | 6 | 33 | 52 | 64 |
| Cereal bars | 7 | 86 | 4 | 21 |
| Corn flakes | 11 | 45 | 21 | 30 |
| Rice flakes | 113 | 5 | 123 | 126 |
| Rice flakes and chocolate | 7 | 0 | 46 | 46 |
| Breakfast rice, popped (loose) | 62 | 19 | 100 | 114 |
| Rice, popped with sugar | 8 | 0 | 94 | 94 |
| Rice porridge | 7 | 0 | 12 | 12 |
| ‘Rice cakes/Rice waffles/Rice crackers’ | 579 | 4 | 146 | 148 |
| Biscuits (cookies) | 22 | 82 | 8 | 26 |
| Rice bread | 10 | 10 | 75 | 80 |
| Follow‐on formula, milk‐based | 47 | 91 | 0.08 | 2 |
| Infant formula, milk‐based | 38 | 95 | 0.02 | 4 |
| Ready‐to‐eat meals for children, unspecified | 99 | 90 | 2 | 17 |
| Ready‐to‐eat meals for children, cereal‐based | 12 | 83 | 3 | 6 |
| Ready‐to‐eat meals for children, meat/fish‐based | 12 | 75 | 9 | 39 |
| Cereal‐based food for infants and young children | 131 | 33 | 31 | 38 |
| Cereal‐based food for infants and young children, with rice | 93 | 6 | 77 | 79 |
| Biscuits, rusks and cookies for children | 52 | 17 | 83 | 94 |
| Fungi, cultivated | 56 | 96 | 13 | 45 |
| Fungi, wild, edible | 20 | 60 | 16 | 25 |
| Sea weedsa , b | 138 | 41 | 2,803 | 2,837 |
| Kombub | 13 | 38 | 9,113 | 9,134 |
| Laverb | 66 | 56 | 63 | 97 |
| Wakameb | 62 | 45 | 182 | 201 |
| Fish meat | 451 | 90 | 4 | 16 |
| Crustaceans | 58 | 53 | 15 | 27 |
| Mussel (Mytilus edulis) | 244 | 13 | 30 | 32 |
| Oyster (Ostrea edulis) | 64 | 5 | 10 | 11 |
| Clam (Mya arenaria) | 20 | 10 | 108 | 110 |
| Rice‐based meals | 63 | 19 | 28 | 29 |
| Cow milk | 109 | 97 | 0.007 | 4 |
| Rice drink | 43 | 42 | 8 | 17 |
| Juice, Apple | 5 | 80 | 3 | 20 |
| Juice, Orange | 25 | 96 | 1 | 10 |
| Juice, multi‐fruit | 5 | 40 | 7 | 17 |
| Fruit nectar | 19 | 63 | 4 | 15 |
| Mixed fruit and vegetable juice | 5 | 40 | 1 | 1 |
| Drinking water, unspecified | 1,715 | 58 | 2 | 3 |
| Tap water | 593 | 60 | 2 | 3 |
| Well water | 945 | 65 | 1 | 3 |
| Bottled water | 522 | 68 | 0.6 | 2 |
| Still mineral water | 1,895 | 63 | 1 | 2 |
| Carbonated mineral water | 1,597 | 62 | 0.8 | 2 |
N = number of samples; LC = left‐censored data; LB = lower bound; UB = upper bound.
Includes three samples of brown seaweed Hiziki or Hijiki (Sargassum fusiforme, syn. Hizikia fusiformis) with iAs reported values of 56,000 μg/kg (two samples) and 8,500 μg/kg.
Apart from few samples either with no information provided or reported as ‘unprocessed’, samples of seaweed refer to dehydrated products.
Mean values are rounded to the nearest integer, except for mean values below 1 μg/kg that are shown with one significant figure.
For three samples of ‘Purslane (Portulaca spp.)’, a leaf vegetable typically consumed raw in salads, relatively high values of iAs as compared to other vegetables were reported, in particular for one sample (54 mg/kg). The ratio iAs/tAs varied significantly among the three samples (0.7–68%). These plants are described as used as part of bioremediation practices pursuing the elimination of heavy metals from soil (Tiwari et al., 2008).
Few samples of ‘Milk and dairy products’ (n = 239) were also included. The most relevant group in terms of iAs levels was ‘Rice, drink’ (n = 43), with reported mean levels of 8.2–17.4 μg/kg (LB–UB). In fact, rice drink is considered as part of the group ‘Rice‐based products’ when assessing the contribution of the different food to the dietary exposure (see Table 5). For the rest of the food groups under this category, only few samples were quantified (96.4% left‐censored data), among them three samples of cow milk all with iAs values below 0.3 μg/kg.
As regards ‘Fruit and vegetable juices’, a total of 70 samples were reported as analysed for iAs. Apart from one sample of ‘Champignon juice, concentrated’ that reported 120 μg/kg of iAs, most of the samples were left‐censored (74%). It is important to note the relatively high differences between LB and UB mean levels in those food groups with quantified samples. In the past, special attention was paid to the levels of arsenic in apple juice and the role of this beverage as a relevant source of dietary iAs exposure in particular in children (US FDA, 2013). Only few samples analysed iAs in apple juice (n = 5); among them only in one sample iAs was quantified (13 μg/kg). However, 381 samples of apple juice were analysed for tAs (see Annex B.4) with mean LB values of 1.5 μg/kg which are similar to those reported in the 2014 report (EFSA, 2014).
One of the food categories with the highest number of samples analysed for iAs was ‘Fish and other seafood’ (n = 938). Almost half of these samples were codified as ‘Fish meat’ (n = 451). The reported levels of iAs were relatively low (mean LB = 4 μg/kg) which is in agreement with the literature that shows that saltwater fish usually contains mainly arsenobetaine (Julshamn et al., 2012; Kalantzi et al., 2017; Upadhyay et al. 2019). The difference observed between LB and UB estimations (4–16 μg/kg) could have an important contribution to the uncertainty of the dietary exposure estimations considering the relatively high consumption of fish in certain populations. Higher levels of iAs were reported for crustaceans (mean 15–27 μg/kg, LB–UB, n = 58) and for molluscs, in particular in clams (mean 108–110 μg/kg, LB–UB, n = 20).
A selection of food groups as they were used for dietary exposure estimations is shown in Table 9. A detailed description, with the appropriate grouping of the food samples, the iAs levels adjusted after applying the corresponding dilution factors, as well as with the ad hoc categories created as needed is presented in Annex B.3.
3.2. Occurrence data on total arsenic
In the previous assessment of the dietary exposure to iAs, EFSA had to use the data on tAs due to the scarcity of data on iAs (EFSA, 2014). At that time, the step of deriving the concentration of iAs from the reported tAs values was identified as one of the main sources of uncertainties. Yet linked to the use of tAs data but mainly to the lack of sensitive analytical methods, the large difference between LB and UB levels for some food categories also impacted the dietary exposure estimations. As a consequence, and following the publication of the 2014 EFSA scientific report, the European Commission published a recommendation to the Member States to monitor the presence of arsenic in relevant food commodities, preferably by determining the content of both iAs and tAs.7
For the preparation of the current report, an exhaustive and detailed analysis of all reported analytical data was conducted before deciding to exclusively use the data on iAs. Still, the submitted data on tAs are presented and discussed in this report, trying to identify relevant food that might deserve attention in the future for speciation analysis.
The data set on tAs contains a total of 48,682 samples; detailed FoodEx classification of each food group and summary statistics of the reported tAs levels (LB–UB) are shown in Annex B.4.17 The three main food categories at FoodEx level 1 were ‘Fish and other seafood’ (n = 9,926), ‘Meat and meat products’ (n = 9,373) and ‘Grains and grain‐based products’ (n = 6,794), all three together representing more than 50% of the total number of samples.
One characteristic of the food category ‘Meat and meat products’ is the high number of left‐censored data (75%) that in many relevant food groups led to relatively high differences between mean LB and mean UB estimations, e.g. ‘Pork/piglet meat’ (n = 1,067, 89% left‐censored, LB = 1.2 μg/kg; UB = 14.0 μg/kg), ‘Beef meat’ (n = 1,048, 83% left‐censored, LB = 1.9 μg/kg; UB = 12.5 μg/kg), ‘Chicken meat’ (n = 590, 79% left‐censored, LB = 2.9 μg/kg; UB = 13.5 μg/kg). A similar situation is observed for the food category ‘Milk and dairy products’ where around 87% of the samples were left‐censored data, with very low tAs levels in most of the food groups (e.g. ‘Cow milk’, n = 665, mean LB = 0.7 μg/kg, mean UB = 9.7 μg/kg). Food of animal origin contains typically low levels of iAs as animals, similar to humans, extensively methylate the ingested iAs and the excess is excreted in the urine together with the methylated forms (Cubadda et al., 2017).
For the food category ‘Grains and grain‐based products’, out of rice samples and rice‐based products, around 80% of the analytical results were also left‐censored data. The levels of tAs reported for wheat and wheat‐based products were carefully considered taking into account the relevance they had in the dietary exposure estimations in the 2014 EFSA scientific report (EFSA, 2014). Food commodities such as ‘Wheat flour, white’ (n = 247) were mostly left censored (90%), resulting in wide mean LB–mean UB ranges (2.2–40.7 μg/kg). Similarly, more than 90% of the samples codified as ‘Wheat bread and rolls’ were not quantified; for 45 samples of ‘Wheat bread, white’ mean levels of 2.3 and 27.7 μg/kg were estimated (LB–UB). As mentioned above in Section 3.1.2, all the samples of ‘Wheat bread and rolls’ analysed for iAs (n = 35) were left censored.
Almost 1,500 samples of ‘Fruit and vegetable juices’ reported levels on tAs with the vast majority being left censored (85%), including samples of apple juice (n = 380; 87% left censored, mean = 1.5–15.2 μg/kg, LB–UB), a food traditionally linked to the presence of arsenic (US FDA, 2013). Only one out of the five samples analysed for iAs in apple juice was quantified (see Section 3.1.2), with mean LB levels similar to those reported for tAs (2.6 μg/kg) although in this particular case the UB estimate is also highly impacted by the left‐censored data (mean UB = 19.6 μg/kg).
Contrary to what was observed for the categories mentioned above, less than 3% of the samples codified as ‘Fish and other seafood’ (n = 9,926) were left‐censored data. Arsenobetaine is the major arsenic species in most fish, a compound considered as non‐toxic since it is not metabolised in humans and is excreted intact. However, a ‘potentially toxic fraction’ made up of other organic arsenic species (e.g. arsenolipids, arsenosugars) is also present (Taylor et al., 2017a). There is significant uncertainty in predicting iAs from analysed tAs levels in seafood as the relative proportion of iAs tends to decrease as the tAs content increases, and it varies depending on the seafood type (EFSA CONTAM Panel, 2009; Francesconi, 2010; EFSA, 2014). The general recommendation is that, when possible, the dietary exposure should be based on analytical data specific for iAs rather than using conversion factors from tAs (FAO/WHO, 2011, 2018; JECFA, 2011).
Two food categories were characterised by their relatively high levels of tAs, seaweed and mushrooms. Overall, for seaweed, the predominant species are organic (mainly arsenosugars, but also others), although as mentioned above there are particular seaweed that have been reported to contain from moderate to very high levels of iAs, e.g. the brown alga hiziki/hijiki (Taylor et al., 2017b; Cherry et al., 2019). Although the quantitation of iAs in seaweed seems to be challenging (de la Calle et al., 2012; Briscoe et al., 2016), this high variation in the levels of iAs in seaweed is a reason to advice the use of speciation analysis to better assess possible risks linked to its consumption and the presence of arsenic. In the case of mushrooms, they are known to accumulate different types of arsenic species. The content in different arsenic species depends on the fungal species with not a defined rule to predict arsenic speciation, where also the growth substrate seems to play a key role. In general, cultivated fungi (e.g. button mushrooms) contains lower levels of arsenic than wild fungi (Braeuer and Goessler, 2019), although different studies have reported that the arsenic quantified in samples of Shiitake and Oyster mushrooms was predominantly iAs (Chen et al., 2018; Li et al., 2018). As stated for seaweed, arsenic speciation analysis with both accurate quantification of iAs and the identification and characterisation of other arsenic species is highly recommended to properly assess the risks in consumers of mushrooms.
A total of 5,010 samples were analysed for both iAs and tAs; in 1,068 samples neither tAs nor iAs were quantified. In 1,098 of the samples, tAs was quantified, but no levels of iAs were reported even if speciation analysis was conducted. Among them, approximately half of the samples were fish (n = 358) and seaweed (n = 166), food groups that, as mentioned above, typically contain relatively low levels of iAs as compared to the other arsenic species. This is particularly evident for fish, where 94% of the samples were left censored for iAs despite reporting quantified levels for tAs.
Table 10 shows a selection of samples with quantified levels of both iAs and tAs divided by food group. For a more accurate and robust interpretation of the data only food with at least 10 samples were considered. For rice samples, the iAs levels represented on average around 70% of the value reported for tAs. This ratio is in agreement with arsenic speciation results published in the literature as well as with the data provided in the 2014 EFSA scientific report (EFSA, 2014; Domínguez‐González et al., 2020). A wide range of iAs/tAs ratios were found across the samples of rice grains (13–> 100%), with a trend to have lower ratios as the tAs concentrations increase. This is also in line with the reported iAs/tAs ratios for different types of rice available in the world market (FAO/WHO, 2014). Similar average iAs/tAs ratios were estimated for the different rice‐based products (e.g. popped rice, rice flour etc.). The two dominating arsenic species in rice are iAs and DMA; the presence of these species in the rice grain seems to depend on complex interactions of many different factors (plant physiology and genetics, soil characteristics, paddy management practices etc.) (Meharg and Zhao, 2008).
Table 10.
Estimated proportion of iAs as compared to reported levels of tAs in a selected group of food samplesa
| Number of samples | Average iAs/tAs ratio (%) | Range of iAs/tAs ratios (%) | |
|---|---|---|---|
| Rice | 257 | 70 | 13–116 |
| Rice, brown | 320 | 73 | 15–147 |
| Rice, long‐grain | 342 | 71 | 13–166 |
| Rice, parboiled | 115 | 73 | 38–136 |
| Rice, white | 246 | 68 | 28–133 |
| Rice flour | 17 | 70 | 37–110 |
| Unleavened bread, crisp bread and rusk | 12 | 91 | 64–111 |
| Noodle, rice | 12 | 61 | 32–75 |
| Rice flakes | 93 | 69 | 22–134 |
| Rice, popped | 434 | 74 | 9–163 |
| Sea weeds | 76 | 11 | 0.1–89 |
| Laver | 20 | 1 | 0.1–34 |
| Wakame | 27 | 1 | 0.1–12 |
| Prawns | 11 | 7 | 0.2–40 |
| Shrimps | 12 | 3 | 0.3–19 |
| Clam | 18 | 5 | 0.5–21 |
| Mussel | 213 | 2 | 0–31 |
| Oyster | 60 | 2 | 0–6 |
| Rice drink | 14 | 89 | 54–137 |
| Cereal‐based food for infants and young children | 41 | 63 | 5–113 |
| Simple cereals which are or have to be reconstituted with milk or other appropriate nutritious liquids | 50 | 70 | 42–110 |
| Biscuits, rusks and cookies for children | 12 | 61 | 36–81 |
| Dietary supplements | 16 | 42 | 3–93 |
| Algae formula (e.g. Spirulina, Chlorella) | 23 | 27 | 0.2–88 |
| Rice‐based meals | 49 | 63 | 27–92 |
For a more accurate and robust interpretation of the data only food with at least 10 samples analysed for both iAs and tAs were considered.
For the samples of seaweed with quantified iAs, the average iAs/tAs ratio was overall rather low. However, for few samples reported as unspecified seaweed, unspecified brown algae and/or hijiki, the contribution of iAs to the tAs varied between 61% and 89%. The iAs/tAs ratio estimated for molluscs and crustaceous confirmed what was discussed above regarding the relatively low levels of iAs as compared to organic species.
3.3. Dietary exposure assessment to iAs
3.3.1. Mean and high dietary exposure to iAs across European countries and age classes
Table 11 shows a summary of the chronic dietary exposure estimates to iAs across 44 different dietary surveys carried out in 23 different European countries. The exposure estimates calculated for each dietary survey together with detailed contribution of the different food groups are presented in Annexes B.5–B.8. Overall, mean UB estimates were two to three times higher than LB estimates, being this difference slightly smaller in the 95th percentile estimates. The highest dietary exposure was estimated in the young population (infant, toddlers and other children); in particular, for LB estimations, the highest mean estimate was 0.30 μg/kg bw per day in toddlers while for UB estimations, the maximum was 0.61 μg/kg bw per day for both infants and toddlers. The highest 95th percentile exposure at the LB was estimated in toddlers (0.58 μg/kg bw per day) and at the UB in infants (1.20 μg/kg bw per day).
Table 11.
Summary statistics of the dietary chronic exposure assessment (μg/kg bw per day) to iAs across European dietary surveys. Estimates were rounded to two decimal places
| Mean dietary exposure (μg/kg bw per day) | |||||||
|---|---|---|---|---|---|---|---|
| N | Lower bound (LB) | Upper bound (UB) | |||||
| Min | Median | Max | Min | Median | Max | ||
| Infants | 13 | 0.09 | 0.15 | 0.22 | 0.26 | 0.42 | 0.61 |
| Toddlers | 16 | 0.12 | 0.17 | 0.30 | 0.34 | 0.44 | 0.61 |
| Other children | 19 | 0.07 | 0.11 | 0.17 | 0.19 | 0.30 | 0.37 |
| Adolescents | 20 | 0.04 | 0.06 | 0.11 | 0.10 | 0.16 | 0.23 |
| Adults | 22 | 0.03 | 0.04 | 0.07 | 0.08 | 0.11 | 0.15 |
| Elderly | 20 | 0.03 | 0.03 | 0.06 | 0.06 | 0.10 | 0.14 |
| Very elderly | 15 | 0.03 | 0.03 | 0.05 | 0.08 | 0.10 | 0.14 |
| Pregnant women | 5 | 0.04 | 0.06 | 0.07 | 0.10 | 0.13 | 0.14 |
| Lactating women | 2 | 0.03 | a | 0.06 | 0.09 | a | 0.14 |
| 95th percentile dietary exposure (μg/kg bw per day) | |||||||
| Lower bound (LB) | Upper bound (UB) | ||||||
| N | Min | Median | Max | Min | Median | Max | |
| Infants | 13 | 0.21 | 0.36 | 0.52 | 0.76 | 0.84 | 1.20 |
| Toddlers | 16 | 0.24 | 0.37 | 0.58 | 0.62 | 0.75 | 0.99 |
| Other children | 19 | 0.17 | 0.26 | 0.37 | 0.41 | 0.54 | 0.67 |
| Adolescents | 20 | 0.10 | 0.14 | 0.26 | 0.21 | 0.30 | 0.44 |
| Adults | 22 | 0.07 | 0.10 | 0.19 | 0.16 | 0.21 | 0.33 |
| Elderly | 20 | 0.06 | 0.08 | 0.14 | 0.13 | 0.18 | 0.25 |
| Very elderly | 15 | 0.07 | 0.08 | 0.14 | 0.14 | 0.17 | 0.23 |
| Pregnant women | 5 | 0.09 | 0.12 | 0.19 | 0.17 | 0.22 | 0.28 |
| Lactating women | 2 | 0.08 | a | 0.14 | 0.16 | a | 0.25 |
Not calculated since estimates were only available from two dietary surveys.
In the adult population (adults, elderly and very elderly), mean dietary exposure estimates range between 0.03 and 0.15 μg/kg bw per day (min LB–max UB), and between 0.06 and 0.33 μg/kg bw (min LB–max UB) for the 95th percentile estimates. Dietary exposure to iAs in specific groups of the population, namely pregnant and lactating women, was within the range of exposure estimates in the adult population.
3.3.2. Distribution by age class and contributors to the dietary exposure
Infants
A total of 13 dietary surveys across European countries were considered. Mean dietary exposure estimates ranged from 0.09 to 0.61 μg/kg bw per day (min LB–max UB). The 95th percentile dietary exposure estimates ranged from 0.21 to 1.20 μg/kg bw per day (min LB–max UB).
Figure 5 shows the average contribution of selected food groups to the mean dietary exposure to iAs in the infant population at the LB estimations. The key contributors to the dietary exposure were ‘Cereal‐based food for infants and young children’ that in certain countries reached a contribution close to 60% of the total, and ‘Drinking water’ (9–52%, median = 19%). It is important to also mention the contribution of ‘Biscuits, rusks and cookies for children’ in five dietary surveys with mean contributions in these surveys between 8% and 30%. Other types of infant food such as ‘Ready‐to‐eat meals for infants and young children’ also played a relevant role as a source of iAs (up to 25%); the mean contribution of rice (all types of rice together) ranged from 2% to 21% (median = 13%). Different food groups contributed to the differences observed between LB and UB estimations depending on the dietary survey, among them ‘Ready‐to‐eat meals for infants and young children’, ‘Milk and dairy products’, and in particular ‘Infant formula and follow‐on formula’. For this latter food group, while the mean contribution to the exposure to iAs at the LB estimations (as shown in Figure 5) is almost negligible (0–4%), at the UB estimation it reached contributions up to 63% with a median contribution across countries of 22%. Undoubtedly, these differences are driven by the high amount of left‐censored data (95%) and the relatively high LOQs reported for some samples within this food category.
Figure 5.
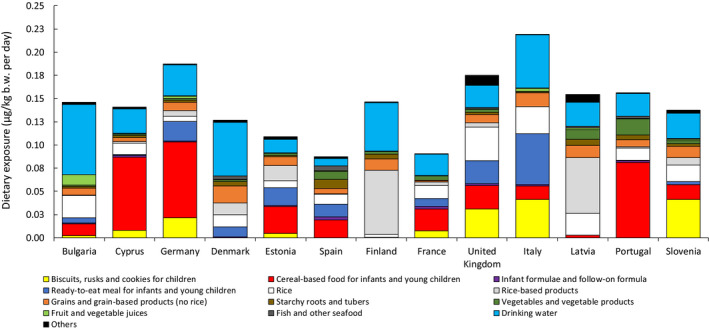
Average contribution of selected food groups to the mean dietary exposure to iAs (at the LB estimations) in the age class ‘Infants’ across different European countries
Toddlers
A total of 16 dietary surveys from 15 European countries were considered to estimate chronic dietary exposure to iAs. Overall, the dietary exposure to iAs in toddlers was the highest among the different age classes. Mean dietary exposure estimates ranged from 0.12 to 0.61 μg/kg bw per day (min LB–max UB). The 95th percentile dietary exposure estimates ranged from 0.24 to 0.99 μg/kg bw per day (min LB–max UB).
Figure 6 provides an overview of the most important dietary sources of iAs in the toddler population at the LB estimations; rice had an average contribution from 9% to 36% (median = 21%). The mean contribution of ‘Drinking water’ was between 6% and 39% (median = 22%); it is worth mentioning the contribution arising from the consumption of ‘Grains and grain‐based products (no rice)’ (9–30%, median = 14%). As in the infant population, the consumption of ‘Biscuits, rusks and cookies for children’ played an important role in the exposure to iAs in a few countries (see Figure 6).
Figure 6.
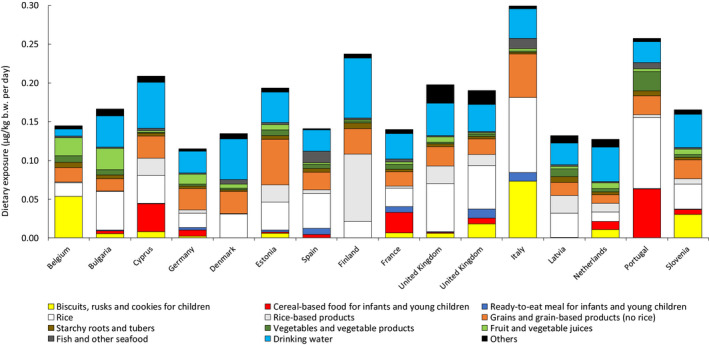
Average contribution of selected food groups to the mean dietary exposure to iAs (at the LB estimations) in the age class ‘Toddlers’ across different European countries
Among the food categories impacting on the observed differences among LB–UB estimations, the most important is ‘Milk and dairy products’, in particular the consumption of cow milk. For this food, the LB–UB mean iAs levels ranged from 0.007 to 4.4 μg/L (n = 109, 97% left‐censored data). These differences in the LB–UB occurrence values for cow milk have a strong impact in the contribution to the iAs exposure, from contributions at the LB that can be considered as negligible (< 0.2%) to contributions at the UB as high as 30% (median = 16%). Another food category impacting on the LB–UB estimations was ‘Fruit and vegetable juices’.
Other children
For this age class, a total of 19 dietary surveys covering 18 different European countries were considered. Mean dietary exposure estimates ranged from 0.07 to 0.37 μg/kg bw per day (min LB–max UB). The 95th percentile dietary exposure estimates ranged from 0.17 to 0.67 μg/kg bw per day (min LB–max UB).
Figure 7 provides an overview of the most important dietary sources of iAs across dietary surveys at the LB estimations. Overall, the main sources of exposure to iAs were rice (14–60%, median = 27%), ‘Grains and grain‐based products (no rice)’ (10–30%, median = 19%), and ‘Drinking water’ (8–44%,18 median = 27%). Among the food within ‘Grains and grain‐based products (no rice)’, the most relevant source was ‘Pasta’ followed by different types of breakfast cereals. The contribution of ‘Rice‐based products’ to the intake of iAs was rather important in a few countries (11–25%); the same as observed for ‘Fruit and vegetable juices’, with mean contributions in a few dietary surveys that reached around 16% of the total intake.
Figure 7.
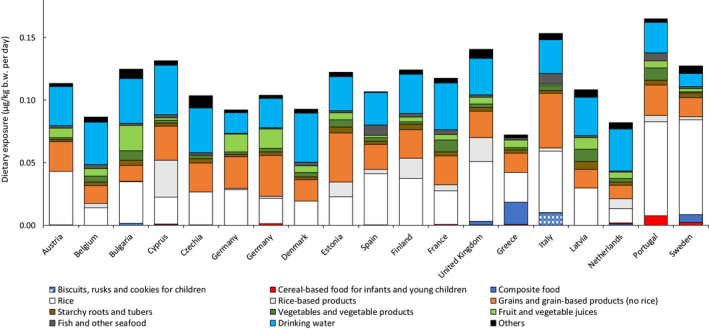
Average contribution of selected food groups to the mean dietary exposure to iAs (at the LB estimations) in the age class ‘Other children’ across different European countries
Adolescents
A total of 20 dietary surveys covering 18 different European countries were considered for this age class. Mean dietary exposure estimates ranged from 0.04 to 0.23 μg/kg bw per day (min LB–max UB). The 95th percentile dietary exposure estimates ranged from 0.10 to 0.44 μg/kg bw per day (min LB–max UB).
Figure 8 provides an overview of the most important dietary sources of iAs across dietary surveys at the LB estimations. Similar to what was observed in the other age classes, the main sources of exposure to iAs were rice (13–64%, median = 24%), ‘Grains and grain‐based products (no rice)’ (10–31%, median = 17%), and ‘Drinking water’ (8–53%, median = 29%).
Adults
A total of 22 dietary surveys across European countries were considered for this age class. Mean dietary exposure estimates ranged from 0.03 to 0.15 μg/kg bw per day (min LB–max UB). The 95th percentile dietary exposure estimates ranged from 0.07 to 0.33 μg/kg bw per day (min LB–max UB).
Figure 9 provides an overview of the most important dietary sources of iAs across dietary surveys at the LB estimations together with the food groups already mentioned in the other age classes, rice (11–49%, median = 24%), ‘Grains and grain‐based products (no rice)’ (6–26%, median = 11%), and ‘Drinking water’ (22–58%,19 median = 35%), it is important to mention the mean contribution of ‘Vegetables and vegetable products’ (2–16%, median = 5%) to the intake of iAs in the adult population. Although overall it was not an important source, in few surveys, the consumption of ‘Fish and other seafood’ was also relevant in the intake of iAs, with mean contributions representing up to 9%.
Figure 9.
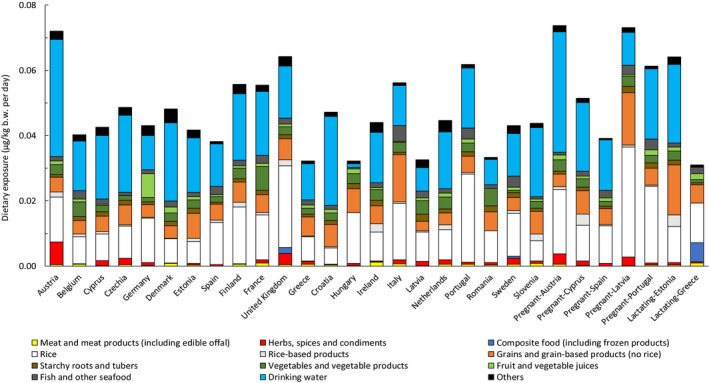
Average contribution of selected food groups to the mean dietary exposure to iAs (at the LB estimations) in the age class ‘Adults’ and on specific population groups (‘Lactating women’ and ‘Pregnant women’) across different European countries
The dietary exposure estimations to iAs in specific population groups namely ‘Lactating women’ and ‘Pregnant women’ were within the estimates reported in the adult population. For ‘Pregnant women’ mean estimates ranged from 0.04 to 0.14 μg/kg bw per day, and for ‘Lactating women’ between 0.03 and 0.06 μg/kg bw per day (min LB–max UB). The 95th percentile dietary exposure estimates were between 0.09 and 0.28 μg/kg bw per day in ‘Pregnant women’, and between 0.08 and 0.25 μg/kg bw per day (min LB–max UB) in ‘Lactating women’. The pattern of food contributing to the mean intake of iAs was also similar to the one described for the general adult population. To note that the dietary survey covering the lactating population in Greece did not report any consumption of drinking water; this is reflected in a relatively small iAs intake estimation as compared to other adult populations. Likewise, an important contribution of composite food was identified in this survey, in particular rice‐based meals.
Elderly and very elderly
Dietary exposure to iAs in the elderly population was estimated in a total of 20 dietary surveys while a total of 15 dietary surveys were used to estimate the exposure in the population ≥ 75 years old (‘Very elderly’). Overall, very similar dietary exposure was estimated for both elderly and very elderly populations, being the lowest across the different age classes. In both elderly and very elderly, mean dietary exposure estimates ranged from 0.03 to 0.14 μg/kg bw per day (min LB–max UB). The 95th percentile dietary exposure estimates ranged from 0.06 to 0.25 μg/kg bw per day for elderly, and between 0.07 and 0.23 μg/kg bw per day (min LB–max UB) for very elderly.
Figure 10 shows the most relevant sources of iAs across dietary surveys in each of the age classes. The sources of iAs were very similar within dietary surveys that cover both the elderly and very elderly population. As compared to other population groups, the relevance of rice was comparable (3–43%, median = 22%), while in the case of Grains and grain‐based products (no rice)’ (5–30%, median = 10%), there was a slight decrease although still very similar to the contribution identified in adults. In parallel, the group ‘Vegetables and vegetable products’ (2–32%, median = 7%) became even more important than in the adult population, as it also occurred with the group ‘Fish and other seafood’ (1–14%, median = 5%). The mean contribution of ‘Drinking water’ was, overall, slightly lower than the one described in the age class ‘Adults’, although in particular countries, it went up to 67% (median = 33%, range 18–67%18).
Figure 10.
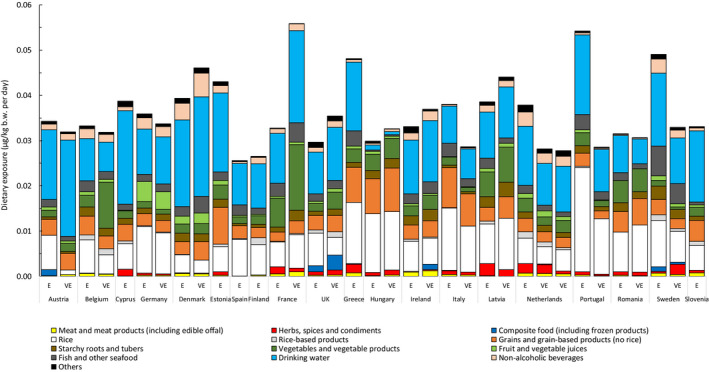
Average contribution of selected food groups to the mean dietary exposure to iAs (at the LB estimations) in the age class ‘Elderly’ and ‘Very elderly’ across different European countries. E = Elderly; VE = Very elderly
3.3.3. Comparison to the 2014 EFSA scientific report and to other dietary exposure assessments using measured iAs
As compared to the 2014 EFSA scientific report, the dietary exposure estimates to iAs were noticeably lower, with the maximum means and 95th percentile estimates across the different age classes around 1.5–3 times lower. However, although direct comparisons are always difficult due to the different occurrence/consumption data used and/or the different methodology, the dietary exposure estimates in this report are in good agreement with recent assessments using measured iAs.
As an example, Cubadda et al. (2016) estimated dietary exposure to iAs in the Italian population using the same consumption data as available in the EFSA Comprehensive database and occurrence data (measured iAs) collected using TDS methodology. For those age classes where a more direct comparison can be conducted (‘Other children’, ‘Adolescents’ and ‘Adults’), the mean estimations reported by Cubbada et al. compare well with the LB estimations in this report. Mean exposure estimates reported by Cubbada et al. were 0.152 μg/kg bw per day and 0.070 μg/kg bw per day for children and adults, while in this report, for the same age classes, they were 0.153 and 0.056 μg/kg bw per day. Another example refers to the recently published iAs intake assessment in the Swedish population (Kollander et al., 2019). As with the Italian assessment, the consumption data are those also used by EFSA in this report. In adults (18–80 years old), the mean iAs intake estimate for the Swedish population was 0.050 μg/kg bw per day while the 95th percentile was 0.086 μg/kg bw per day. The mean estimates of exposure are within the min LB–max UB estimations for the three adult populations (adults, elderly and very elderly) assessed separately in this report (0.03–0.11 μg/kg bw per day). Similarly, the 95th percentile exposure estimate was also within the EFSA estimates (0.07–0.20 μg/kg bw per day, min LB–max UB). For children (4–12 years old), the mean estimates were also comparable, although in the case of the highly exposed populations, EFSA estimates (LB–UB, 0.26–0.54 μg/kg bw per day) were higher than the literature data (95th percentile = 0.18 μg/kg bw per day). Similarly, other recent studies provided similar dietary exposure estimates to iAs (using measured iAs) to those reported here; in the region of Valencia, the average exposure in the adult population (6–95 years old) was between 0.08 and 0.09 μg/kg bw per day and between 0.16 and 0.19 μg/kg bw per day in young children (6–15 years old), for the so‐called optimistic and pessimistic scenarios, respectively (Marin et al., 2017).
The difference in the exposure estimates between the current and the 2014 EFSA scientific report is probably due to the sum of different factors, being difficult to stand out one above the rest. Changes in the methodology used to estimate the chronic dietary exposure in the current report were minor and mainly refer to an improvement of the linkage between consumption and occurrence thanks to the availability of additional information (e.g. facets for ingredients/processing in FoodEx2, FoodEx2 classification). This additional information allowed a refinement of the assessment, leading to more accurate exposure estimates. The consumption data used in this report were in a vast majority not available in 2014; there could be some differences in the consumption patterns as compared to the former surveys within the same country and age class. In the 2014 EFSA scientific report, almost all the occurrence data on iAs were derived from the tAs reported, while in the current report only measured data on iAs have been used (apart from the assumption that all tAs measured in drinking water is iAs). It is this different approach what likely plays the most important role in the differences observed in the dietary exposure estimations. Indeed, the approach followed in 2014 implied the use of many assumptions to derive iAs values from the available data on tAs (EFSA, 2014). That methodology is recognised to have significant limitations, with estimates surrounded by a high degree of uncertainty that, in general, leads to an overestimation of the exposure (Lynch et al., 2014; Cubadda et al., 2017). The main reason seems to be the high variability observed in the ratios iAs/tAs in different food but even within the same food; it has been observed in different food that the relative proportion of iAs as compared to tAs tends to decrease as the concentration of tAs increases (Francesconi, 2010; FAO/WHO, 2014). As mentioned above, FAO/WHO has repeatedly indicated that dietary exposures to iAs should be based on actual data rather than using generalised conversion factors from tAs measurements (FAO/WHO, 2011, 2018; JECFA, 2011), a view also supported in the scientific literature (Cubadda et al., 2017). In fact, in the last years, the number of scientific publications that made use of measured iAs to estimate dietary exposure to iAs has steadily increased (Cubadda et al., 2016; Marin et al., 2017; Kollander et al., 2019; Domínguez‐González et al., 2020).
3.3.4. Additional dietary exposure scenarios
Dietary exposure to iAs in breastfeeding infants
An additional scenario was carried out to estimate dietary exposure to iAs in breastfed infants. Default consumption of 800 mL as average daily milk consumption and 1,200 mL as high consumption were used, representative for a breastfed infant of three months and a body weight of 6.1 kg (IOM, 1991; EFSA, 2014; EFSA CONTAM Panel, 2020). With respect to arsenic levels in breast milk, the literature data are rather limited. A recent study has reported median levels around 0.31 μg/L of tAs in US mothers, with a maximum of 0.62 μg/L and detectable arsenic in only five of the nine samples of breast milk analysed (Carignan et al., 2015). These levels are in good agreement with other values reported in European populations, e.g. in breast milk from Swedish mothers (Björklund et al., 2012). It is important to note that in the Swedish study (n = 32), iAs was not detected in the breast milk samples with the highest concentrations of tAs. Different studies support the fact that even in areas with drinking water containing relatively high concentrations of arsenic, only small amounts appear to pass through mammary glands to breast milk (Carignan et al., 2015, Signes‐Pastor et al., 2018). Other studies have shown that following the consumption of fish, a small percentage of arsenosugars and arsenolipids are transported to the milk of nursing women (Xiong et al., 2020). In general, the information on the dominant species of arsenic in breast milk is currently unclear.
Based on the current available information, an average value of 0.3 μg/L arsenic in breast milk was used in the exposure scenario with the assumption that all arsenic present is iAs; this is also the value used in the 2014 EFSA scientific report (EFSA, 2014). Under this assumption, the iAs mean dietary exposure for an infant of 6.1 kg exclusively fed with breast milk would be 0.04 μg/kg bw per day; for an infant with high consumption, the dietary exposure would be 0.06 μg/kg bw per day.
Dietary exposure to iAs in infants consuming rice‐based formulae
It is accepted that during weaning, infants will have, in general, higher exposure to arsenic as compared to breastfeeding. The presence of arsenic in infant and follow‐on formulae is firstly influenced by its composition and secondly by the quality of the water used for reconstitution. In general, milk‐based formulae contain low levels of arsenic (Jackson et al., 2012); this in agreement with the data available for this report where mean LB values of iAs below 0.1 μg/kg are reported (see Table 9). On the other hand, infants or children who suffer from milk intolerance (e.g. lactose) and/or milk allergies (e.g. proteins) will replace milk‐based infant and follow‐on formulae by the so‐called ‘Foods for special medical purposes’ (FSMPs). Although soy formulae has been widely used as the alternative to milk‐based formula, rice‐based formulae is gaining acceptance (Bocquet et al., 2019). As shown in Section 3.1.2, occurrence data on formulae other than milk‐based were not available for this report.
Recently, Meyer et al. (2018) analysed the levels of iAs [reported as As(V)] in samples of dry formula powder from different countries (France, Belgium, Italy), including few samples consisting of hydrolysed rice suitable for infants from 0 to 6 months. Average levels of iAs in these samples (n = 7) were 12 μg /kg (range 9–20 μg /kg). To better understand the potential exposure to iAs following the consumption of these products, an exposure scenario was conducted using these occurrence values after applying a dilution factor of eight for the reconstitution of the dry formula powder (1.5 μg/L). The potential presence of iAs in the drinking water used for the reconstitution of the formulae was not considered in the dietary exposure estimations. As consumption data, values of 200 and 260 mL/kg bw per day as conservative mean and high level consumption were used, as recommended by the EFSA Scientific Committee for infants below 16 weeks of age (EFSA Scientific Committee, 2017). Dietary exposure to iAs was estimated as 0.30 and 0.39 μg/kg bw per day in mean and high consumers, respectively.
Dietary exposure to iAs following the consumption of ‘Cereal‐based food for infants and young children’
Another typical food consumed by the young population are ‘Cereal‐based food for infants and young children’; this type of products is becoming highly consumed among young children affected by either coeliac disease or gluten intolerance and following a gluten‐free diet. In the present report, a total of 93 samples codified under this group were identified as having rice as an ingredient (see Table 9). Mean values reported for these samples were 77–79 μg/kg (LB–UB), with a high variation of concentrations from few samples with non‐quantified iAs up to 389 μg/kg. This variation could be explained by the use of rice with different levels of iAs, rice with different processing (milled rice/husked rice) or the use of different amounts of rice in the formulation of the products (Carey et al., 2018). Median iAs levels around 120 μg/kg have been reported in samples of so‐called ‘baby rice’ (typically products based on rice flour with added vitamins/minerals intended for 4–6 months infants) (Meharg et al., 2008; US FDA 2016; Signes‐Pastor et al., 2016).
An exposure scenario was conducted to estimate the dietary exposure to iAs using the reported consumption of ‘Cereal‐based food for infants and young children’, and the occurrence data from the 93 samples codified under this group identified as having rice as an ingredient. Using the EFSA Comprehensive database, average and 95th percentile consumption data were extracted for this commodity for consumers only; as occurrence values, the average and the 95th percentiles were used (mean LB = 77 μg/kg, 95th percentile = 130 μg/kg), the highest percentile to account for population that might regularly consumed products with high content of rice. Table 12 shows the dietary exposure estimations to iAs and each of the values used as occurrence and consumption data. Dietary exposure estimates to iAs as high as 0.62 and 0.70 μg/kg bw day were calculated for toddlers and infants, respectively. These values would correspond to exposure estimates considering high consumers of products with relatively high levels of iAs (P95 × P95). The relevance of these estimates is even higher when one considers that this type of commodities is generally used as a complement in the progressive adaptation of young children to ordinary food and, therefore, additional intake of iAs from the diet might occur.
Table 12.
Estimated dietary exposure to iAs (μg/kg bw day) for young population (infants and toddlers, consumers only) following the consumption of ‘Cereal‐based food for infants and young children’ with rice as an ingredient
| Consumption (grams/day) | Occurrence (LB, μg/kg) | Dietary exposure (μg/kg bw day) | ||||||
|---|---|---|---|---|---|---|---|---|
| Average | 95th percentile | Average | 95th percentile | Average consumers | High consumers | |||
| Infants | 17 | 45 | 77 | 130 | 0.15a | 0.26b | 0.41c | 0.70d |
| Toddlers | 18 | 50 | 77 | 130 | 0.13a | 0.22b | 0.36c | 0.62d |
Average consumption and average occurrence.
Average consumption and 95th percentile occurrence.
95th percentile consumption and average occurrence.
95th percentile consumption and 95th percentile occurrence.
Dietary exposure to iAs through the consumption of rice and ‘Rice cakes/Rice waffles/Rice crackers’
Considering their contribution to the dietary exposure to iAs across the different age classes, particular attention was paid to ‘consumers only’ of rice and rice‐based snacks codified as ‘Rice cakes/Rice waffles/Rice crackers’ (see Section 2.3). These scenarios will also help to better understand the magnitude of the exposure to iAs in consumers of these commodities without the possible ‘dilution effect’ when taking into account the whole population; this effect could be particularly relevant for the food commodities codified under ‘Rice cakes/Rice waffles/Rice crackers’ as the number of consumers is relatively low.
Table 13 shows the dietary exposure estimates to iAs in ‘consumers only’ of ‘Rice cakes/Rice waffles/Rice crackers’ divided by age class; the occurrence value reported in Table 5 was used (n = 579, mean LB = 146 μg/kg). Average daily consumption of ‘Rice cakes/Rice waffles/Rice crackers’ leads to mean dietary exposures to iAs as high as 0.25 μg/kg bw per day in ‘Adolescents’ while in high consumers, the dietary exposure estimates peaked up to 0.17 μg/kg bw per day in ‘Toddlers’. It is important to note that these exposure estimates should be carefully interpreted due to the very low number of consumers available (e.g. the highest mean dietary exposure refers to a dietary survey with just one consumer).
Table 13.
Dietary exposure estimates to iAs (LB, mean and 95th percentile, μg/kg bw per day) in ‘consumers only’ of ‘Rice cakes/Rice waffles/Rice crackers’
| Number of surveys (range of consumers) | Dietary exposure estimates (μg/kg bw per day) | ||
|---|---|---|---|
| Mean | 95th percentilea | ||
| Min–Max | Min–Max | ||
| Infants | 5 (2–43) | 0.03–0.20 | – |
| Toddlers | 9 (2–66) | 0.03–0.16 | 0.17 |
| Other children | 9 (2–111) | 0.02–0.18 | 0.15 |
| Adolescents | 13 (1–60) | 0.02–0.25 | 0.08 |
| Adults | 13 (1–58) | 0.005–0.09 | – |
| Elderly | 8 (1–17) | 0.005–0.06 | – |
| Very elderly | 4 (1–6) | 0.01–0.02 | – |
| Pregnant women | 4 (1–4) | 0.02–0.05 | – |
| Lactating women | 1 (14) | 0.04 | – |
95th percentile estimates with less than 60 consumers were not calculated since they may not be statistically robust (EFSA, 2011a).
Although not shown in Table 13, when looking to ‘consumers only’ of ‘Biscuits, rusks and cookies for children’ (n = 52, LB = 83 μg/kg) that basically codifies for similar food commodities but specifically dedicated to infants/young children, the dietary exposure to iAs (LB) was as high as 0.17 and 0.49 μg/kg bw per day in average and high consumers,20 respectively (both estimates in infants).
When looking at ‘consumers only’ of rice, the attention was put on the consumption of white rice which represents more than 50% of the eating occasions of rice in the EFSA Comprehensive database. The occurrence value reported in Table 4 for ‘Rice, white’ was used (n = 337, mean LB = 74 μg/kg). The highest chronic dietary exposure in average consumers was estimated in infants (0.23 μg/kg bw per day) while for high consumers of white rice the highest exposure was 0.27 μg/kg bw per day in toddlers (Table 14). As certain ethnic groups living in different European countries seem to have around 30‐fold higher consumption of rice than white Caucasians (Cascio et al., 2011), a higher consumption of rice than that considered in this report and, therefore, higher exposure to iAs, cannot be discarded.
Table 14.
Dietary exposure estimates to iAs (LB, mean and 95th percentile, μg/kg bw per day) in ‘consumers only’ of ‘Rice, white’
| Consumptiona (g/day) | Dietary exposure estimates (μg/kg bw per day) | |||
|---|---|---|---|---|
| Average | 95th percentile | Mean | 95th percentileb | |
| Min–Max | Min–Max | |||
| Infants | 1–63 | 4–20 | 0.01–0.46 | 0.04–0.17 |
| Toddlers | 4–42 | 10–48 | 0.02–0.25 | 0.07–0.27 |
| Other children | 4–50 | 13–133 | 0.01–0.17 | 0.04–0.41 |
| Adolescents | 1–66 | 15–142 | 0.001–0.08 | 0.02–0.19 |
| Adults | 5–60 | 14–116 | 0.01–0.06 | 0.01–0.13 |
| Elderly | 4–79 | 9–154 | 0.004–0.08 | 0.01–0.13 |
| Very elderly | 5–64 | 107–112 | 0.005–0.07 | 0.10–0.14 |
| Pregnant women | 18–32 | 61 | 0.01–0.03 | 0.07 |
| Lactating women | 23–28 | 50 | 0.03 | 0.06 |
Consumption in ‘consumers only’.
95th percentile estimates with less than 60 consumers were not calculated since they may not be statistically robust (EFSA, 2011a).
There are few other food groups on which attention should also be put when assessing dietary exposure to iAs. One of these food groups are rice drinks; in the occurrence data set used in this report data were available for this commodity (n = 43; mean LB–UB = 8–17 μg/L). Only few consumers of this commodity are included in the EFSA Comprehensive database and therefore its impact on the overall dietary exposure to iAs was negligible. Still, the levels of iAs present in these rice drinks make them not suitable alternatives for breast milk or formulae at any stage of infancy or early childhood (FSA, 2009; COT, 2016). Another food commodity that can be relevant for some groups of population when assessing dietary exposure to iAs are certain types of seaweed. Although the overall consumption of seaweed in Europe is small, certain seaweed can contain relatively high values of iAs (see Section 3.1.2). This mainly refers to the brown seaweed known as Hiziki or Hijiki (Sargassum fusiforme, syn. Hizikia fusiformis), for which only three samples were present in the occurrence data set, but two of them with iAs levels of 56 mg/kg. In fact, this led several authorities in the past (FSA, FSANZ) to advise consumers to avoid the consumption of hijiki seaweed.
3.4. Uncertainties
A qualitative uncertainty assessment was conducted to better understand the strengths and limitations of the dietary exposure assessment to iAs, and how the different uncertainties can affect the exposure estimates. The main uncertainties are associated with the characteristics of both the occurrence data and the consumption data as well as their linkage to estimate dietary exposure (Table 15).
Table 15.
Summary of the qualitative evaluation of the impact of uncertainties on the dietary exposure to iAs
| Sources of uncertainty | Directiona |
|---|---|
| Occurrence data | |
| Measurement uncertainty of analytical results | +/– |
| Exclusion of analytical results due to poor quality in one of its parameters (e.g. high LOD/LOQ, analytical method not adequate, etc.) | +/– |
| Use of the substitution method to handle left‐censored data (LB–UB) | +/– |
| Limited occurrence data for some food categories that could potentially contribute to iAs dietary exposure | – |
| Possible presence of iAs‐containing ingredients in particular food not reported (e.g. rice in cereal‐based food) | + |
| Effect of household preparation on the concentration of iAs not considered | + |
| Consumption data | |
| Possible presence of iAs‐containing ingredients in particular consumed food not reported (e.g. rice in cereal‐based food) | – |
| Underestimation of the consumption data of drinking water in few dietary surveys | – |
| Contribution of water used in the food preparation not always considered | – |
| Dietary exposure | |
| Linkage between occurrence and consumption data | +/– |
| Use of factors in the preparation of food as consumed (e.g. dilution factors) | +/– |
+ = uncertainty with potential to cause over‐estimation of exposure; – = uncertainty with potential to cause under‐estimation of exposure.
Several uncertainties were identified linked to the occurrence data used in this assessment. Few uncertainties are linked to the generation of the analytical data; as an example, for around 25% of the samples, no information on the analytical method used was provided which adds uncertainty to the values provided. For a few samples, the iAs values were derived adding the concentrations reported for As(III) and As(V); there is uncertainty linked to the use of the left‐censoring limits reported for As(III) and As(V) when deriving the iAs from left‐censored data.
There are important contributors to the exposure to iAs (mainly due to their relatively high consumption) with a high number of left‐censored data. In these food groups (e.g. ‘Grains and grain‐based products (no rice)’), the use in certain occasions of analytical methods with low sensitivity led to relatively substantial differences between LB and UB values. These LB–UB differences add uncertainty to the dietary exposure estimations. Even though all described and well‐known dietary sources of iAs are covered by the available occurrence data set, the use of only occurrence data on iAs might lead to some particular food categories not being considered in the assessment (e.g. soft drinks, alcoholic beverages) and, therefore, to underestimation of the exposure to iAs. The contribution from breast milk and infant and follow‐on formulae other than milk‐based was not considered as no occurrence data were available; this leads to underestimate the dietary exposure in the general scenario for the infant population.
Still related to the occurrence data used in the assessment, for some samples reporting relatively high levels of iAs there is uncertainty on whether rice is present or not as an ingredient. As an example, several samples of ‘Cereal‐based food for infants and young children’ with relatively high levels of iAs were grouped as not containing rice leading to an increase of the mean occurrence value in this food group. Their linkage to consumption data of food supposedly with no rice in their composition might lead to overestimate the exposure. On the other hand, on the side of the consumption data, there is also uncertainty on whether the presence of rice is reported on some eating occasions, in particular for eating occasions of ‘Cereal‐based food for infants and young children’. When the presence of rice was reported in the eating occasions, ad hoc linkage was made with the corresponding occurrence data; if rice was present but not reported in the food consumed there could be an underestimation of the dietary exposure.
A relevant source of uncertainty relates to the effect of food preparation on the iAs levels, affecting in particular to rice, but also to vegetables and to other cereal‐based products such as pasta. Although the information provided in the EFSA Comprehensive database allows in most of the cases an accurate linkage between consumption and occurrence, food preparation is highly dependent on consumers’ preferences and it is not always possible to reflect all relevant details in the dietary surveys (e.g. pre‐rinsing rice before cooking). The water used during the food preparation also plays a crucial role on the final levels of iAs that are ingested. Household preparation in areas with highly contaminated water will lead to an increase in the iAs concentration in the prepared food, and might also imply higher iAs levels in certain locally produced and locally grown food (Cubadda et al., 2015; Mandal et al., 2019). In contrast, the use of water with relatively low iAs levels as typically found in Europe could, in general, lead to a decrease of the arsenic levels during food preparation. An example is pre‐rinsing/washing rice before cooking or boiling the raw rice using a large water–rice ratio and draining the excess of water afterwards (Jitaru et al., 2016). Overall, certain overestimation of the iAs intake could be expected in the dietary exposure assessment.
The lack of consumption data on drinking water in a few dietary surveys leads to an underestimation of the dietary exposure in those surveys. There may be an underestimation of the dietary exposure to iAs since in a few dietary surveys the water used to prepare certain food (infusions, coffee, infant formulae etc.) could have not been reported. This underestimation could be even more important if drinking water with relatively high levels of iAs as have been reported in some European countries, particularly in areas identified as hotspots (e.g. the Pannonian Basin), is used. Other uncertainties and limitations related to the use of the EFSA Comprehensive Food Consumption Database, as described by EFSA (EFSA, 2011a) and applicable in the present assessment are not further described in this report.
4. Conclusions
Chronic dietary exposure to iAs was assessed in 23 different European countries using a total of 44 different dietary surveys (87,945 subjects).
A total of 13,608 analytical results on iAs were considered in the current assessment, with 7,623 corresponding to drinking water15 and 5,985 to different types of food. Overall, the most represented food category was ‘Grain and grain‐based products’, in particular rice and rice‐based products. Samples were collected across Europe between 2013 and 2018.
The highest dietary exposure was estimated in the young population (infant, toddlers and other children). The highest mean dietary exposure estimates at the LB were in toddlers (0.30 μg/kg bw per day), and in both infants and toddlers (0.61 μg/kg bw per day) at the UB. At the 95th percentile, the highest exposure estimates (LB–UB) were 0.58 and 1.20 μg/kg bw per day in toddlers and infants, respectively. In general, UB estimates were two to three times higher than LB estimates.
The mean dietary exposure estimates at the LB were, overall, below the range of benchmark dose lower confidence limit (BMDL01) values of 0.3–8 μg/kg bw per day established by the CONTAM Panel in 2009. However, for the 95th percentile dietary exposure (LB), the maximum estimates for infant, toddlers and other children were within this range of BMDL01 values.
Different food categories, such as ‘Infant formula and follow‐on formula’, ‘Milk and dairy products’, ‘Grains and grain‐based products (no rice)’) and ‘Fruit and vegetables juices’ with low LB values, a relatively high number of left‐censored data and relatively high consumption across different age classes, were the main contributors to these LB–UB differences.
Across the different age classes, the main contributors to the dietary exposure to iAs (LB) were ‘Rice’, ‘Rice‐based products’, ‘Grains and grain‐based products (no rice)’ and ‘Drinking water’. Apart from the food categories mentioned above, particular foodstuffs indicated for the young population played a relevant role in the dietary exposure to iAs in this population. This mainly refers to the contribution of ‘Cereal‐based food for infants and young children’ in the infant population (up to 60%), and to that of ‘Biscuits, rusks and cookies for children’. The latter food group includes, among other products, rice‐based snacks (rice waffles, rice wafers, rice crackers and rice cakes) that contributed up to 30–37% in infants and toddlers, respectively. Overall, the contribution of milk‐based infant and follow‐on formulae to the intake of iAs is rather low due to its relatively low iAs levels, although the final levels could be strongly affected by the iAs present in the drinking water used for reconstitution. In the adult population, food groups such as ‘Vegetables and vegetable products’ and ‘Fish and other seafood’ were also apparent sources of iAs in certain countries. The main uncertainties associated with the dietary exposure estimations refer to the impact of using the substitution method to treat the left‐censored data (LB–UB differences), to the lack of information (consumption and occurrence) on some iAs‐containing ingredients in specific food groups, and to the effect of food preparation on the iAs levels.
As compared to the 2014 EFSA scientific report, the dietary exposure estimates to iAs were noticeably lower, with the maximum mean and 95th percentile estimates across the different age classes around 1.5–3 times lower. This difference is probably due to the sum of different factors related to the occurrence and consumption data used. Among these factors, the use of measured iAs allowed a more accurate and realistic dietary exposure assessment as compared to assessments that make use of assumptions and modelling to derive iAs values from tAs data which are affected by high inherent uncertainty. Likewise, the improvement of the linkage between consumption and occurrence, thanks to the availability of additional information (e.g. facets for ingredients/processing in FoodEx2, FoodEx2 classification), led to more accurate exposure estimations. Dietary exposure estimates in the current report are in good agreement with recently published scientific literature that also made use of measured iAs to estimate dietary exposure to iAs.
Different ad hoc dietary exposure scenarios were conducted to complement the general exposure scenario. It was shown that breastfeeding results in much lower exposure to iAs compared to the consumption of rice‐based formulae; the consumption of this type of formulae could lead to dietary exposure estimates of 0.30 and 0.39 μg/kg bw per day in mean and high consumers, respectively. Several of the ad hoc dietary exposure scenarios in average and for high consumers produce estimates close to or within the range of BMDL01 values (0.3–8 μg/kg bw per day) established by the CONTAM Panel in 2009.
5. Recommendations
Analytical data submitted to EFSA should follow the requirements as specified in the annually updated Chemical monitoring reporting guidance, including information on ingredients (e.g. rice, seaweed, etc.), to better describe the samples analysed.
-
Validated analytical methods with adequate sensitivity are needed to quantify iAs, together with extraction methods that guarantee minimal (change in valency) or no changes to the original species. This should result in more accurate and precise dietary exposure estimates:
-
—
By minimising the uncertainty arising at the UB dietary exposure estimates due to the presence of left‐censored data in particular food groups (e.g. ‘Infant formula’, ‘Follow‐on formula’, ‘Milk and dairy products’, ‘Grains and grain‐based products (no rice)’), ‘Fruit and vegetables juices’).
-
—
By obtaining better speciation data in complex food matrices such as mushrooms and seaweed.
-
—
By collecting reliable data on iAs content in different food, in particular grain and grain‐based commodities but also in less‐studied food such as non‐dairy based formulae (rice‐based formulae), mushrooms, seaweed, soft‐drinks, alcoholic beverages etc.
-
—
Further research is needed to better understand the effect of processing/food preparation on the different arsenic species present in food.
Consumption data of specific populations (e.g. people with coeliac disease and/or gluten intolerance) that might have a higher consumption of rice and/or rice‐based products are needed to better assess their dietary exposure to iAs.
Detailed consumption data on rarely consumed food (e.g. seaweed, mushrooms, rice‐based snacks) are needed to obtain more accurate and precise dietary exposure estimates in population groups consuming these commodities.
Abbreviations
- AAS
Atomic absorption spectrometry
- AB
Arsenobetaine
- AC
Arsenocholine
- AES
Atomic Emission Spectrometry
- AFS
Atomic Fluorescence Spectrometry
- As(III)
Arsenite/arsenous acid
- As(V)
Arsenate/arsenic acid
- BMDL
Benchmark dose lower confidence limit
- BMDS
Benchmark dose software
- bw
Body weight
- CONTAM
Panel on Contaminants in the Food Chain
- COT
Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment
- CRM
Certified reference materials
- DMA
Dimethylarsinic acid
- FAO/WHO
Food and Agriculture Organization/ World Health Organization
- FDA
Food and Drug Administration (United States)
- FSA
Food Standards Agency (United Kingdom)
- GC
Gas chromatography
- HG
Hydride generation
- HPLC
High performance liquid chromatography
- iAs
Inorganic arsenic
- IARC
International Agency for Research on Cancer
- ICP
Inductively coupled plasma
- IOM
Institute of Medicine
- IT
Ion trap
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- LB
Lower bound
- LOD
Limit of detection
- LOQ
Limit of quantification
- MA
Methylarsonic acid
- ML
Maximum level
- MS
Mass spectrometry
- tAs
Total arsenic
- UB
Upper bound
- WHO/ICPS
World Health Organization/International Programme on Chemical Safety
Annex A – Raw occurrence data set for food samples (2013–2018) as extracted from EFSA DWH on 30 April 2020
1.
Available as Excel file on EFSA Knowledge Junction on Zenodo at: https://doi.org/10.5281/zenodo.4322963
Annex B – Summary statistics on occurrence data, consumption data and dietary exposure assessment results
1.
Available as Excel file on EFSA Knowledge Junction on Zenodo at: https://doi.org/10.5281/zenodo.4322963
Suggested citation: EFSA (European Food Safety Authority) , Arcella D, Cascio C and Gómez Ruiz JÁ, 2021. Scientific report on the chronic dietary exposure to inorganic arsenic. EFSA Journal 2021;19(1):6380, 50 pp. 10.2903/j.efsa.2021.6380
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00076
Acknowledgements: EFSA specially thanks Jean‐Charles Leblanc, Annette Petersen and Tanja Schwerdtle for providing valuable comments during the preparation of the report and for reviewing the final version, and the EFSA staff members Alessandro Delfino for the support provided and Mary Gilsenan for her final revision of the report. EFSA also wishes to acknowledge all European countries that provided the consumption and occurrence data used in the preparation of this scientific output. This scientific report was endorsed by the EFSA Panel on Contaminants in the Food Chain during its 112th Plenary meeting on 24–25 November 2020.
Approved: 14 December 2020
Notes
Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. OJ L 330, 5.12.1998, p. 1–28.
Commission Directive 2003/40/EC of 16 May 2003 establishing the list, concentration limits and labelling requirements for the constituents of natural mineral waters and the conditions for using ozone‐enriched air for the treatment of natural mineral waters and spring waters. OJ L126, 22.5.2003, p. 34–39.
Commission Recommendation (EU) 2015/1006 of 25 June 2015 amending Regulation (EC) No 1881/2006 as regards maximum levels of inorganic arsenic in foodstuffs. OJ L161, 26.6.2015, p. 14–16.
Milled rice (white rice) is husked rice from which all or part of the bran and germ have been removed by milling (CODEX standard for rice CXS 198‐1995, amended in 2019).
Husked rice (brown rice or cargo rice) is paddy rice from which the husk only has been removed. The process of husking and handling may result in some loss of bran (CODEX standard for rice CXS 198‐1995, amended in 2019).
Parboiled rice may be husked or milled rice processed from paddy or husked rice that has been soaked in water and subjected to a heat treatment so that the starch is fully gelatinised, followed by a drying process (CODEX standard for rice CXS 198‐1995, amended in 2019).
Commission Recommendation (EU) 2015/1381 of 10 August 2015 on the monitoring of arsenic in food. OJ L213, 12.8.2015, p. 9–10.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
Commission Recommendation (EU) 2018/464 of 19 March 2018 on the monitoring of metals and iodine in seaweed, halophytes and products based on seaweed. OJ L78, 21.3.2018, p. 16–18.
Few samples were also available with data reported on different arsenic species: 21 as ‘Organic arsenic’, 649 as MA, 653 as DMA, 607 as As(V), 600 as As(III), and 317 as Arsenobetaine.
Samples collected in 2017: two samples of rice unspecified, four samples of rice brown and two samples of rice popped. Samples collected in 2018: two samples of rice brown and one sample of rice popped.
https://www.efsa.europa.eu/en/food-consumption/comprehensive-database. Dietary data included in the assessment were submitted to EFSA when the UK was a member of the EU.
Almost all samples reported as drinking water were analysed for total arsenic (7,560). It was assumed that the reported levels on tAs correspond to iAs (see Section 2.1.2).
Parametric value established by the Council Directive 98/83/EC for water intended for human consumption and ML established for natural mineral waters by Commission Directive 2003/40/EC.
Although most of the samples of drinking water were analysed for tAs, these samples are not included in Annex B.4 since in this scientific report it was assumed that the reported levels on tAs correspond to inorganic arsenic (see section 2.1.2).
In the dietary survey from Greece, only a small number of individuals reported water consumption since the survey was mainly focused on nutrient intake. This most probably leads to an underestimation of the dietary exposure to iAs; the contribution in this country was not considered when reporting the contribution of drinking water across the European countries.
In the dietary survey from Hungary (adults, elderly and very elderly), only a small number of individuals reported water consumption since the survey was mainly focused on nutrient intake. This most probably leads to an underestimation of the dietary exposure to iA; the contribution in this country was not considered when reporting the contribution of drinking water across the other European countries.
For the 95th exposure estimates only those dietary surveys with at least 60 consumers were considered.
References
- Al Amin MH, Xiong C, Francesconi KA, Itahashi Y, Yoneda M and Yoshinaga J, 2020. Variation in arsenolipid concentrations in seafood consumed in Japan. Chemosphere, 239, 124781. [DOI] [PubMed] [Google Scholar]
- Banerjee K, Helwick RP and Gupta S, 1999. A treatment process for removal of mixed inorganic and organic arsenic species from groundwater. Environmental Progress, 18, 280–284. [Google Scholar]
- Björklund KL, Vahter M, Palm B, Grandér M, Lignell S and Berglund M, 2012. Metals and trace element concentrations in breast milk of first time healthy mothers: a biological monitoring study. Environmental Health, 11, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocquet A, Dupont C, Chouraqui JP, Darmaun D, Feillet F, Frelut ML, Girardet JP, Hankard R, Lapillonne A, Rozé JC and Simeoni U, 2019. Efficacy and safety of hydrolyzed rice‐protein formulas for the treatment of cow's milk protein allergy. Archives de Pédiatrie, 26, 238–246. [DOI] [PubMed] [Google Scholar]
- Braeuer S and Goessler W, 2019. Arsenic species in mushrooms, with a focus on analytical methods for their determination – a critical review. Analytica Chimica Acta, 27, 1–21. 10.1016/j.aca.2019.04.004 Epub 2019 Apr 9 PMID: 31146831. [DOI] [PubMed] [Google Scholar]
- Briscoe ML, Ugrai TM, Creswell J and Carter AT, 2016. An interlaboratory comparison study for the determination of arsenic and arsenic species in rice, kelp, and apple juice. Spectroscopy, 30, 20–30. [Google Scholar]
- de la Calle MB, Baer I, Robouch P, Cordeiro F, Emteborg H, Baxter MJ, Brereton N, Raber G, Velez D, Devesa V, Rubio R, Llorente‐Mirandes T, Raab A, Feldmann J, Sloth JJ, Rasmussen RR, D'Amato M and Cubadda F, 2012. Analytical and Bioanalytical Chemistry, 404, 2475–2488. [DOI] [PubMed] [Google Scholar]
- Carey M, Donaldson E, Signes‐Pastor AJ and Meharg AA, 2018. Dilution of rice with other gluten free grains to lower inorganic arsenic in foods for young children in response to European Union regulations provides impetus to setting stricter standards. PLoS ONE, 13, e0194700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey M, Meharg C, Williams P, Marwa E, Jiujin X, Gomes Farias J, De Silva PMCS, Signes‐Pastor A, Lu Y, Nicoloso FT, Savage L, Campbell K, Elliott C, Adomako E, Green AJ, Moreno‐Jiménez E, Carbonell‐Barrachina AA, Triwardhani EA, Pandiangan FI, Haris PI, Lawgali YF, Sommella A, Pigna M, Brabet C, Montet D, Njira K, Watts MJ and Meharg AA, 2020. Global sourcing of low‐inorganic arsenic rice grain. Exposure and Health. 10.1007/s12403-019-00330-y [DOI] [Google Scholar]
- Carignan CC, Cottingham KL, Jackson BP, Farzan SF, Gandolfi AJ, Punshon T, Folt CL and Karagas MR, 2015. Estimated exposure to arsenic in breastfed and formula‐fed infants in a United States cohort. Environmental Health Perspectives, 123, 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio C, Raab A, Jenkins RO, Feldmann J, Meharg AA and Haris PI, 2011. The impact of a rice based diet on urinary arsenic. Journal of Environmental Monitoring, 13, 257–265. [DOI] [PubMed] [Google Scholar]
- Chen S, Yuan B, Xu J, Chen G, Hu Q and Zhao L, 2018. Simultaneous separation and determination of six arsenic species in Shiitake (Lentinus edodes) mushrooms: method development and applications. Food Chemistry, 1, 134–141. [DOI] [PubMed] [Google Scholar]
- Cherry P, O'Hara C, Magee PJ, McSorley EM and Allsopp PJ, 2019. Risks and benefits of consuming edible seaweeds. Nutrition Reviews, 77, 307–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COT (Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment), 2016. Statement on potential risks from arsenic in the diet of infants aged 0 to 12 months and children aged 1 to 5 years. COT Meeting, 8 April 2016, 1–38.
- Cubadda F, D'Amato M, Mancini FR, Aureli F, Raggi A, Busani L and Mantovani A, 2015. Assessing human exposure to inorganic arsenic in high‐arsenic areas of Latium: a biomonitoring study integrated with indicators of dietary intake. Annali di Igiene, 27, 39–51. [DOI] [PubMed] [Google Scholar]
- Cubadda F, D'Amato M, Aureli F, Raggi A and Mantovani A, 2016. Dietary exposure of the Italian population to inorganic arsenic: The 2012–2014 Total Diet Study. Food and Chemical Toxicology, 1, 148–158. [DOI] [PubMed] [Google Scholar]
- Cubadda F, Jackson BP, Cottingham KL, Van Horne YO and Kurzius‐Spencer M, 2017. Human exposure to dietary inorganic arsenic and other arsenic species: State of knowledge, gaps and uncertainties. Science of the Total Environment, 1, 1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez‐González MR, Barciela‐Alonso MC, Calvo‐Millán VG, Herbello‐Hermelo P and Bermejo‐Barrera P, 2020. The bioavailability of arsenic species in rice. Analytical and Bioanalytical Chemistry. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2010. Standard sample description for food and feed. EFSA Journal 2010;8(1):1457, 54 pp. 10.2903/j.efsa.2010.1457 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Report on the development of a Food Classification and Description System for exposure assessment and guidance on its implementation and use. EFSA Journal 2011;9(12):2489, 84 pp. 10.2903/j.efsa.2011.2489 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Scientific report of on the dietary exposure to inorganic arsenic in the European population. EFSA Journal 2014;12(3):3597 pp. 10.2903/sp.efsa.2014.3579 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. The food classification and description system FoodEx2 (revision 2). EFSA supporting publication 2015;EN‐804 90 pp. 10.2903/sp.efsa.2015.en-804 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Arcella D and Gómez Ruiz JA, 2018. Use of cut‐off values on the limits of quantification reported in datasets used to estimate dietary exposure to chemical contaminants. EFSA Journal 2018;15:1452E, 11 pp. 10.2903/sp.efsa.2018.en-1452 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2020. Chemical monitoring reporting guidance: 2020 data collection. EFSA Supporting publication 2020;EN‐1796, 102 pp. 10.2903/sp.efsa.2020.EN-1796 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2009. Scientific Opinion on arsenic in food. EFSA Journal 2009;7(10):1351 pp. 10.2903/sp.efsa.2009.1351 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), Schrenk D, Bignami M, Bodin L, Chipman JK, del Mazo J, Grasl‐Kraupp B, Hogstrand C, Hoogenboom LR, Leblanc J‐C, Nebbia CS, Nielsen E, Ntzani E, Petersen A, Sand S, Vleminckx C, Wallace H, Barreg ard L, Ceccatelli S, Cravedi J‐P, Halldorsson TI, Haug LS, Johansson N, Knutsen HK, Rose M, Roudot A‐C, Van Loveren H, Vollmer G, Mackay K, Riolo F and Schwerdtle T, 2020. Scientific Opinion on the risk to human health related to the presence of perfluoroalkyl substances in food. EFSA Journal 2020;18(9):6223, 391 pp. 10.2903/j.efsa.2020.6223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Bresson J‐L, Dusemund B, Gundert‐Remy U, Kersting M, Lambr e C, Penninks A, Tritscher A, Waalkens‐Berendsen I, Woutersen R, Arcella D, Court Marques D, Dorne J‐L, Kass GEN and Mortensen A, 2017. Guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age. EFSA Journal 2017;15(5):4849, 58 pp. 10.2903/j.efsa.2017.4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO (Food and Agriculture Organization/ World Health Organization), 2011. Food Standards Programme Codex Committee on Contaminants in Foods. Fifth session. Working document for information and use in discussions related to contaminants and toxins in the GSCTFF. CF/5 INF/1. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-735-05%252FREP11_CFe.pdf
- FAO/WHO (Food and Agriculture Organization/ World Health Organization), 2014. Food Standards Programme Codex Committee on Contaminants in Foods. Eight session. Proposed draft maximum levels for arsenic in rice (raw and polished rice). Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FShared%2BDocuments%252FArchive%252FMeetings%252FCCCF%252Fcccf8%252Fcf08_06e.pdf
- FAO/WHO (Food and Agriculture Organization/ World Health Organization), 2018. Food Standards Programme Codex Committee on Contaminants in Foods. 12th session. Working document for information and use in discussions related to contaminants and toxins in the GSCTFF. CF/12 INF/1. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-735-12%252FREPORT%252520%2528FINAL%2529%252FREP18_CFe.pdf
- Francesconi KA, 2010. Arsenic species in seafood: origin and human health implications. Pure Applied Chemistry, 82, 373–381. [Google Scholar]
- FSA (Food Standards Agency) , 2009. ‘Arsenic in rice’. Available at: http://www.food.gov.uk/science/arsenic-in-rice
- Gu Z, de Silva S and Reichman SM, 2020. Arsenic concentrations and dietary exposure in rice‐based infant food in Australia. International Journal of Environmental Research and Public Health, 17, 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herath I, Vithanage M, Bundschuh J, Maity JP and Bhattacharya P, 2016. Natural arsenic in global groundwaters: distribution and geochemical triggers for mobilization. Current Pollution Reports, 2, 68–89. [Google Scholar]
- Hughes MF, Beck BD, Chen Y, Lewis AS and Thomas DJ, 2011. Arsenic exposure and toxicology: a historical perspective. Toxicological Sciences, 123, 305–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer), 1973. Arsenic and inorganic arsenic compounds. In Some Inorganic and Organometallic Compounds. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans, vol. 2. Lyon, France: International Agency for Research on Cancer. pp. 48–73.
- IARC (International Agency for Research on Cancer), 1980. Arsenic and arsenic compounds. In Some Metals and Metallic Compounds. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans, vol. 23. Lyon, France: International Agency for Research on Cancer. pp. 39–141.
- IARC (International Agency for Research on Cancer), 2012. Monographs on the Evaluation of Carcinogenic Risks to Humans. A Review of Human Carcinogens: Arsenic, Metals, Fibres, and Dusts, vol. 100C. Lyon, France: International Agency for Research on Cancer, pp. 41–93.
- IOM (Institute of Medicine), 1991. Nutrition during Lactation. National Academies Press, Washington, DC. [Google Scholar]
- Islam S, Rahman MM, Rahman MA and Naidu R, 2017. Inorganic arsenic in rice and rice‐based diets: health risk assessment. Food Control, 82, 196–202. [Google Scholar]
- Jackson BP, Taylor VF, Punshon T and Cottingham KL, 2012. Arsenic concentration and speciation in infant formulas and first foods. Pure and Applied Chemistry, 84, 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JECFA (Joint FAO, WHO Expert Committee on Food Additives), 2011. Evaluation of certain contaminants in food: seventy‐second report of the joint FAO/WHO expert committee on food additives. World Health Organization, Geneva: 115 pp. [Google Scholar]
- Jitaru P, Millour S, Roman M, El Koulali K, Noël L and Guérin T, 2016. Exposure assessment of arsenic speciation in different rice types depending on the cooking mode. Journal of Food Composition and Analysis, 1, 37–47. [Google Scholar]
- Julshamn K, Nilsen BM, Frantzen S, Valdersnes S, Maage A, Nedreaas K and Sloth JJ, 2012. Total and inorganic arsenic in fish samples from Norwegian waters. Food Additives and Contaminants: Part B, 5, 229–235. [DOI] [PubMed] [Google Scholar]
- Kalantzi I, Mylona K, Sofoulaki K, Tsapakis M and Pergantis SA, 2017. Arsenic speciation in fish from Greek coastal areas. Journal of Environmental Sciences, 1, 300–312. [DOI] [PubMed] [Google Scholar]
- Kollander B, Sand S, Almerud P, Ankarberg EH, Concha G, Barregård L and Darnerud PO, 2019. Inorganic arsenic in food products on the Swedish market and a risk‐based intake assessment. Science of The Total Environment, 1, 525–535. [DOI] [PubMed] [Google Scholar]
- Li MY, Wang P, Wang JY, Chen XQ, Zhao D, Yin DX, Luo J, Juhasz AL, Li HB and Ma LQ, 2018. Arsenic concentrations, speciation, and localization in 141 cultivated market mushrooms: implications for arsenic exposure to humans. Environmental Science and Technology, 53, 503–511. [DOI] [PubMed] [Google Scholar]
- Lindberg AL, Goessler W, Gurzau E, Koppova K, Rudnai P, Kumar R, Fletcher T, Leonardi G, Slotova K, Gheorghiu E and Vahter M, 2006. Arsenic exposure in Hungary, Romania and Slovakia. Journal of Environmental Monitoring, 8, 203–208. [DOI] [PubMed] [Google Scholar]
- Llorente‐Mirandes T, Ruiz‐Chancho MJ, Barbero M, Rubio R and López‐Sánchez JF, 2011. Determination of water‐soluble arsenic compounds in commercial edible seaweed by LC‐ICPMS. Journal of Agricultural and Food Chemistry, 59, 12963–12968. [DOI] [PubMed] [Google Scholar]
- Lynch HN, Greenberg GI, Pollock MC and Lewis AS, 2014. A comprehensive evaluation of inorganic arsenic in food and considerations for dietary intake analyses. The Science of the Total Environment, 496, 299–313. [DOI] [PubMed] [Google Scholar]
- Mandal U, Singh P, Kundu AK, Chatterjee D, Nriagu J and Bhowmick S, 2019. Arsenic retention in cooked rice: Effects of rice type, cooking water, and indigenous cooking methods in West Bengal, India. Science of the Total Environment, 648, 720–727. [DOI] [PubMed] [Google Scholar]
- Marin S, Pardo O, Baguena R, Font G and Yusa V, 2017. Dietary exposure to trace elements and health risk assessment in the region of Valencia, Spain: a total diet study. Food Additives & Contaminants: Part A, 34, 228–240. [DOI] [PubMed] [Google Scholar]
- Meharg AA and Zhao FJ. Arsenic & rice. Springer Science & Business Media; 2012 Feb 28.
- Meharg AA, Sun G, Williams PN, Adomako E, Deacon C, Zhu YG, Feldmann J and Raab A, 2008. Inorganic arsenic levels in baby rice are of concern. Environmental Pollution, 152, 746–749. [DOI] [PubMed] [Google Scholar]
- Meyer R, Carey MP, Turner PJ and Meharg AA, 2018. Low inorganic arsenic in hydrolysed rice formula used for cow's milk protein allergy. Pediatric Allergy and Immunology, 29, 561–563. 10.1111/pai.12913.29701313 [DOI] [PubMed] [Google Scholar]
- Middleton DR, Watts MJ, Hamilton EM, Ander EL, Close RM, Exley KS, Crabbe H, Leonardi GS, Fletcher T and Polya DA, 2016. Urinary arsenic profiles reveal exposures to inorganic arsenic from private drinking water supplies in Cornwall, UK. Scientific Reports, 9, 25656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mleczek M, Niedzielski P, Siwulski M, Rzymski P, Gąsecka M, Goliński P, Kozak L and Kozubik T, 2016. Importance of low substrate arsenic content in mushroom cultivation and safety of final food product. European Food Research and Technology, 242, 355–362. 10.1007/s00217-015-2545-4 [DOI] [Google Scholar]
- Punshon T, Jackson BP, Meharg AA, Warczack T, Scheckel K and Guerinot ML, 2017. Understanding arsenic dynamics in agronomic systems to predict and prevent uptake by crop plants. The Science of the Total Environment, 581–582, 209–220. 10.1016/j.scitotenv.2016.12.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman H, Carey M, Hossain M, Savage L, Islam MR and Meharg AA, 2019. Modifying the Parboiling of Rice to Remove Inorganic Arsenic, While Fortifying with Calcium. Environmental Science & Technology, 53, 5249–5255. [DOI] [PubMed] [Google Scholar]
- Rose M, Lewis J, Langford N, Baxter M, Origgi S, Barber M, MacBain H and Thomas K, 2007. Arsenic in seaweed—forms, concentration and dietary exposure. Food and Chemical Toxicology, 45, 1263–1267. [DOI] [PubMed] [Google Scholar]
- Rowland HA, Omoregie EO, Millot R, Jimenez C, Mertens J, Baciu C, Hug SJ and Berg M, 2011. Geochemistry and arsenic behaviour in groundwater resources of the Pannonian Basin (Hungary and Romania). Applied Geochemistry, 26, 1–7. [Google Scholar]
- Ruttens A, Cheyns K, Blanpain AC, De Temmerman L and Waegeneers N, 2018. Arsenic speciation in food in Belgium. Part 2: Cereals and cereal products. Food and Chemical Toxicology, 1, 32–41. [DOI] [PubMed] [Google Scholar]
- Signes Pastor A, Carey M and Meharg AA, 2017. Inorganic arsenic removal in rice bran by percolating cooking water. Food Chemistry, 234 10.1016/j.foodchem.2017.04.140 [DOI] [PubMed] [Google Scholar]
- Signes‐Pastor AJ, Carey M and Meharg AA, 2016. Inorganic arsenic in rice‐based products for infants and young children. Food Chemistry, 15, 128–134. [DOI] [PubMed] [Google Scholar]
- Signes‐Pastor AJ, Cottingham KL, Carey M, Sayarath V, Palys T, Meharg AA, Folt CL and Karagas MR, 2018. Infants’ dietary arsenic exposure during transition to solid food. Scientific Reports, 8, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor V, Goodale B, Raab A, Schwerdtle T, Reimer K, Conklin S, Karagas MR and Francesconi KA, 2017a. Human exposure to organic arsenic species from seafood. Science of the Total Environment, 15, 266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor VF, Li Z, Sayarath V, Palys TJ, Morse KR, Scholz‐Bright RA and Karagas MR, 2017b. Distinct arsenic metabolites following seaweed consumption in humans. Scientific Reports, 7, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirunavukkarasu OS, Viraraghavan T, Subramanian KS and Tanjore S, 2002. Organic arsenic removal from drinking water. Urban Water, 4, 415–421. [Google Scholar]
- Tiwari KK, Dwivedi S, Mishra S, Srivastava S, Tripathi RD, Singh NK and Chakraborty S, 2008. Phytoremediation efficiency of Partulaca tuberosa Rox and Partulaca oleracea L., naturally growing in an industrial effluent irrigated area in Vadodra, Gujrat, India. Environmental Monitoring and Assessment, 147, 15–22. [DOI] [PubMed] [Google Scholar]
- Upadhyay MK, Shukla A, Yadav P and Srivastava S, 2019. A review of arsenic in crops, vegetables, animals and food products. Food Chemistry, 276, 608–618. [DOI] [PubMed] [Google Scholar]
- Uppal JS, Zheng Q and Le XC, 2019. Arsenic in drinking water—recent examples and updates from Southeast Asia. Current Opinion in Environmental Science & Health, 1, 126–135. [Google Scholar]
- US EPA (United States Environmental Protection Agency), 2001. National primary drinking water regulations; arsenic and clarifications to compliance and new source contaminants monitoring. Federal Register, 66, 6976–7066. [Google Scholar]
- US FDA (U.S. Food and Drug Administration), 2013. Supporting Document for Action Level for Arsenic in Apple Juice. July 2013. Available online https://www.fda.gov/food/chemical-metals-natural-toxins-pesticides-guidance-documents-regulations/supporting-document-action-level-arsenic-apple-juice
- US FDA (U.S. Food and Drug Administration), 2016. Arsenic in Rice and Rice Products Risk Assessment Report. Available online: https://www.fda.gov/files/food/published/Arsenic-in-Rice-and-Rice-Products-Risk-Assessment-Report-PDF.pdf
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety), 2009. Principles and Methods for the Risk Assessment of Chemicals in Food, International Programme on Chemical Safety, Environmental Health Criteria 240. Chapter 6: Dietary Exposure Assessment of Chemicals in Food. Available online: http://www.who.int/ipcs/food/principles/en/index1.htm
- Xiong C, Stiboller M, Glabonjat RA, Rieger J, Paton L and Francesconi KA, 2020. Transport of arsenolipids to the milk of a nursing mother after consuming salmon fish. Journal of Trace Elements in Medicine and Biology, 9, 126502. [DOI] [PubMed] [Google Scholar]
- Zhao FJ, McGrath SP and Meharg AA, 2010. Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annual Review of Plant Biology, 61, 535–559. [DOI] [PubMed] [Google Scholar]


