Abstract
This report is part of the Echinococcus multilocularis surveillance scientific reports which are presented annually by EFSA to the European Commission and are intended to assess the sampling strategy, data collection and detection methods used by Finland, Ireland, the UK and Norway in their respective surveillance programmes. The surveillance programmes of these four countries were evaluated by checking the information submitted by each of them and verifying that the technical requirements were fulfilled as laid down in Commission Delegated Regulation (EU) 2018/772 of 21 November 2017 supplementing Regulation (EU) No 576/2013 of the European Parliament and of the Council with regard to preventive health measures for the control of E. multilocularis infection in dogs, and repealing Delegated Regulation (EU) No 1152/2011. Due to the UK exiting the European Union and under the Withdrawal Act, the data submitted by the UK after the 31 January 2020 are excluded from this assessment. The information was divided in four different categories for assessment: the type and sensitivity of the detection method, the selection of the target population, the sampling strategy and the methodology. For each category, the main aspects that need to be considered in order to accomplish the technical requirements of the legislation were checked against compliance of several criteria. All the countries participating in this surveillance (Finland, the UK, Ireland and Norway) succeeded in the fulfilment of the technical legal requirements foreseen in Commission Delegated Regulation (EU) 2018/772 concerning these four different categories. Within the UK, Northern Ireland fulfils those requirements only when assuming a diagnostic test sensitivity value of 0.99, provided by the national reference laboratory, which is higher than the sensitivity value suggested by EFSA (conservative value of 0.78) and not supported by adequate scientific evidence. None of the four countries recorded positive samples in the 12‐month reporting period.
Keywords: Echinococcus multilocularis, absence of infection, freedom from disease, surveillance
Summary
Following a request from the European Commission and, indirectly, from the European Free Trade Association (EFTA) Surveillance Authority, the Animal and Plant Health Unit (ALPHA) at the European Food Safety Authority (EFSA) was asked – in the context of Article 31 of Regulation (EC) No 178/2002 – to annually evaluate the surveillance programme on Echinococcus multilocularis infection in animals carried on by the Member States listed in the Annex to Commission Implementing Regulation (EU) 2018/878: Malta, Finland, the UK and Ireland and Norway.
In order to be included in the Annex to Commission Implementing Regulation (EU) 2018/878, Member States must comply with the rules laid down in Article 2 of Commission Delegated Regulation (EU) 2018/772 on ‘rules for categorisation of Member States in view of their eligibility for preventive health measures for the control of E. multilocularis infection in dogs entering their territory. In accordance with this Article, Malta falls under the category described in paragraph 2, i.e. it is in the position of demonstrating that the infection with E. multilocularis parasite has not been established because of the absence of the definitive host (wild red fox) in the whole of its territory. Article 4(1) provides details on the conditions to be fulfilled in order to remain eligible for preventive health measures. For Member States like Malta, in the absence of a definitive host, the conditions to be met are:
having a national observation programme in place to detect the presence of wild red foxes;
immediate notification to the Commission and the other Member States of the detection of the presence of wild red foxes during each 12‐month observation period;
report to the Commission on the results of the national programme referred to in point (a) by 31 May following the end of each 12‐month observation period. The evaluation of the observation programme and its results are out of the remit of the mandate received by EFSA and this related scientific report.1
Also, in accordance with Article 2, Ireland, Finland and the UK, fall under the category described in paragraph 3, i.e. they are in the position to demonstrate that the occurrence of the infection with this parasite has not been recorded in wild definitive host animals. Article 4(2) provides details on the conditions to be fulfilled in order to remain eligible for preventive health measures.
In this report, EFSA assesses the pathogen‐specific surveillance programmes implemented by the three concerned Member States and by Norway.
The surveillance programmes performed by Finland, Ireland, the UK and Norway as reported in 2020 were assessed by checking the reports for completeness against relevant elements that need to be addressed when performing an E. multilocularis surveillance in the context of Commission Delegated Regulation (EU) 2018/772 and analysing the raw data collected by these countries. In order to facilitate the assessment, the information given by the different countries was divided into four different categories corresponding to the critical points that are addressed in the legislation in the ‘requirements for the pathogen‐specific surveillance programme provided for in point c) of Article 4(2): (i) the type and sensitivity of the detection method, (ii) the selection of the target population, (iii) the sampling strategy and (iv) the methodology.
The three Member States and Norway (i) used appropriate techniques for the detection of E. multilocularis in intestinal contents or faeces, (ii) performed a 12‐month surveillance period of data collection and (iii) designed an appropriate sampling strategy for the detection of the parasite, if present in any part of the country, at the design prevalence of less than 1% (0.01), with a 95% confidence level.
All the countries selected adequate wild definitive hosts in order to perform the surveillance. In the UK, Northern Ireland fulfils the requirements of Commission Delegated Regulation (EU) 2018/772 related to the desired confidence level of 95% only when assuming a test sensitivity of 0.99, provided by the national reference laboratory, i.e. a value higher than the one recommended by EFSA in 2015 (0.78), though not supported by appropriate scientific literature.
None of the three Member States nor Norway recorded positive samples in the 12‐month surveillance period.
1. Introduction
Overall, at any time, more than 1 million people are affected by one of the four forms of human echinococcosis: alveolar (caused by Echinococcus multilocularis (EM)), cystic (caused by Echinococcus granulosus senso lato), polycystic (caused by Echinococcus vogeli) or unicystic (caused by Echinococcus oligarthra). The WHO assists countries to develop and implement pilot projects leading to the validation of effective cystic echinococcosis control strategies.2
Human alveolar echinococcosis (AE), caused by the larval stage of the fox tapeworm EM, is a serious parasitic zoonosis (Torgerson et al., 2010; EFSA AHAW Panel, 2015; EFSA and ECDC, 2017). AE is confined to the northern hemisphere, in particular to regions of China, the Russian Federation and countries in continental Europe and North America.2 E. multilocularis is an emerging parasite in Hungary. Because of its still low incidence, differential diagnosis and therapy of AE is a new challenge in clinical practice in Hungary (Dezsényi et al., 2019).
Affected humans show clinical signs that include fatigue, loss of weight, abdominal pain, general malaise and signs of hepatitis or hepatomegaly. In untreated patients, the disease can develop to a severe form associated with liver failure, splenomegaly, portal hypertension and acidosis which can be fatal. Even treated patients can experience a reduction in their quality of life (Mihmanli et al., 2016; WHO, 2017). Indeed, AE is thought to be responsible for about 666,434 disability‐adjusted life‐years (DALYs) globally per year (Torgerson et al., 2010).
The transmission cycle of E. multilocularis occurs when the adult stage (strobilar stage) of the cestode residing in the small intestine of the definitive hosts release the eggs into the environment via faeces (Peregrine et al., 2012; EFSA AHAW Panel, 2015). The infective eggs are ingested by the intermediate hosts and the oncosphere migrates inside them until reaching organs, especially the liver (Peregrine et al., 2012; CDC, online). In the liver, the oncosphere develops into an encysted larval (metacestode stage) which resembles a malignancy in appearance and behaviour, because it proliferates indefinitely by exogenous budding and invades the surrounding tissues. In rodents, hydatid cysts contain numerous small vesicles with multiple protoscoleces (infective stages), while in humans protoscoleces are rarely observed (Moro and Schantz, 2009). The cycle continues when the definitive host consumes an infected intermediate host (Torgerson et al., 2010). Humans may be infected directly through close contact with the definitive host or indirectly through ingestion of food or water contaminated with eggs of the parasite (Torgerson et al., 2010).
In Europe, several animal species can maintain the cycle of E. multilocularis in the nature. A scientific opinion on E. multilocularis performed by EFSA in 2015, revised the potential hosts (definitive and intermediate) of the parasite for this continent (Table 1; See EFSA AHAW Panel, 2015 for more detailed information).
Table 1.
Potential definitive and intermediate hosts of E. multilocularis in Europe (EFSA AHAW Panel, 2015)
| Definitive hosts | |
| Red fox (Vulpes vulpes) | Considered the main DH |
| Arctic fox (Vulpes lagopus) | In Europe, only relevant in Svalbard (Norway) |
| Raccoon dog (Nyctereutes procyonoides), Wolf (Canis lupus), Golden jackal (Canis aureus) | In the presence of the red fox, they can act as DHs. There is no evidence supporting their ability to maintain the lifecycle in absence of the red fox |
| Domestic dog and wild cat (Felis s. silvestris) | Overall, the prevalence of dogs with the parasite is low. However, in experimental surveys, they become infected easily. On the contrary, cats hardly get infected experimentally, but their natural infection has been reported in numerous occasions. For both species, further information is needed |
| Intermediate hosts | |
| Common vole (Microtus arvalis), field vole (Microtus agrestis), common pine vole (Microtus subterraneus), sibling vole (Microtus levis), bank voles (Myodes spp.), water voles (Arvicola spp.), snow vole (Chionomys nivalis), lemming (Lemmus lemmus) | Various species of voles are confirmed as suitable hosts. However, factors such as their population densities and predation rates may influence in their role in the cycle |
| Muridae (Apodemus spp., Mus spp., Rattus spp.), brown hare (Lepus europaeus), shrew (Sorex sp.) | Although some murid rodents, hares and shrews are susceptible, natural infections occur only sporadically |
| Muskrat (Ondatra zibethicus), beaver (Castor spp.), nutria (Myocastor coypu), Alpine marmot (Marmota marmota) | Large rodents are susceptible hosts. Their role seems to be related to the dispersion of the parasite; e.g. through translocations (beaver) |
| Suids, horses and domestic dogs | Only accidental or refractory intermediate hosts |
The distribution of the parasite seems to expand over time. The uncertainty is linked to the fact that no baseline study has ever been performed at European level. The data relate to scientific literature. Until the 1980s, only four countries (France, Germany, Switzerland and Austria) were known to be endemic for the disease (Eckert and Deplazes, 1999). Since then, EM infections in animals have been increasingly reported in countries previously thought to be free (Davidson et al., 2012). The latest available information indicates that at least twenty‐four European countries have found the presence of E. multilocularis in the main definitive host, the red fox (EFSA and ECDC, 2018). In addition, human cases of AE are notified every year in some of these countries. Although human AE is a notifiable disease in some Member States, in practice, this parasitic disease (together with the cystic echinococcosis (CE)) is largely underreported in Europe. In 2017, 827 confirmed human echinococcosis cases were reported in the EU. The EU notification rate was 0.19 cases per 100,000 population, a decrease by 13.6% compared with 2016. Species information was provided for the majority (71.4%) of cases and E. multilocularis accounted for 146 cases (26.3%). A high proportion (> 75%) of the human echinococcosis cases were reported where no information on importation and travel destination were available. The proportion of cases who were hospitalised continues to decrease, compared to 2016, with higher hospitalisation rates for AE than for CE. One fatal case (species not specified) was reported in 2017 (ECDC, 2017).
The prevalence of the parasite is not homogeneous and may vary depending on multiple elements such as for example microclimatic conditions, geographical location, host population dynamics and amount of IHs (Casulli et al., 2015; EFSA AHAW Panel, 2015). A systematic review of the geographical distribution of E. multilocularis in definitive and intermediate hosts in the European Union and adjacent countries found differences between countries (Oksanen et al., 2016; Table 2). The prevalence has been reported to range from 0% to more than 50% (EFSA AHAW Panel, 2015).
Table 2.
Table based on Oksanen's suggested prevalence classes (Oksanen et al., 2016) of countries in which E. multilocularis has been reported in foxes (see also EFSA AHAW panel, 2015; ECDC, 2016; Lalošević et al., 2016)
| Countries | Prevalence in foxes | Human AE casesa |
|---|---|---|
| Finland, Ireland, Malta, UK, Norwayb | 0 | Austria, Belarus, Belgium, Bulgaria, Czech Republic, Denmark, Estonia, France, FYR Macedonia, Germany, Greece, Hungary, Latvia, Lithuania, Moldova, Poland, Romania, Slovakia, Slovenia, Switzerland, Netherlands, Turkey and Ukraine |
| Denmark, Slovenia and Sweden | ≤ 1% | |
| Austria, Belarus, Belgium, Croatia, Hungary, Italy, Netherlands, Romania and Ukraine | > 1% to < 10% | |
| Czech Republic, Estonia, France, Germany, Latvia, Lithuania, Luxembourg, Poland, Serbia, Slovakia, Liechtenstein and Switzerland | > 10% |
Only included the confirmed E. multilocularis species.
Excluding Svalbard.
The EU adopted Commission Delegated Regulation (EU) 2018/772 supplementing Regulation (EU) No 576/2013 of the European Parliament and of the Council with regard to preventive health measures for the control of E. multilocularis infection in dogs, and repealing Delegated Regulation (EU) No 1152/2011. Article 2 lays down the pathways for a Member State to become eligible for the implementation of preventive health measures for the prevention of introduction of E. multilocularis through dogs in Member States, or parts thereof. The concerned Member State may (i) demonstrate that the infection with the E. multilocularis parasite has not been established because of the absence of wild red foxes in the whole of its territory; (ii) demonstrate that wild definitive host animals likely to harbour the E. multilocularis parasite are present in the whole or parts of its territory and that occurrence of the infection with this parasite has not been recorded in those animals during the ongoing surveillance activities; or (iii) is implementing a compulsory eradication programme.
This Regulation gives to those Member States (or parts thereof) the right to apply preventive health measures (see Article 6) to dogs intended for non‐commercial movements prior to their introduction. It should be noted that the same preventive health measures are to be implemented for the import and commercial trade of dogs. In order to remain eligible for applying preventive health measures, the Regulation also requires certain obligations for those Member States (see Art. 4), regarding the implementation of pathogen‐specific surveillance programmes, in accordance with Annex I, to provide evidence for absence of E. multilocularis infection in definitive or intermediate hosts. The requirements for the pathogen specific surveillance programme are reported and summarised below:
The pathogen‐specific surveillance programme, using appropriate risk‐based or representative sampling, shall be designed to detect, per epidemiologically relevant geographical unit in the Member State or part thereof, the E. multilocularis parasite in the wild definitive host population, if present in any part of the Member State at a prevalence of not more than 1% at confidence level of at least 95%;
The pathogen‐specific surveillance programme shall describe the target wild definitive host population, including density, age structure, geographical and gender distribution, taking into account the relative risk of infection with the E. multilocularis parasite in different species and subpopulation of the target wild definitive host population;
-
The pathogen‐specific surveillance programme shall consist in the ongoing collection, during the 12‐month surveillance period, of samples from wild definitive hosts, to be analysed using:
the sedimentation and counting technique (SCT), or a technique of equivalent sensitivity and specificity, by examination of intestinal contents for the detection of the E. multilocularis parasite; or
polymerase chain reaction (PCR) methods, or a technique of equivalent sensitivity and specificity, by examination of intestinal contents or faeces for the detection of species‐specific deoxyribonucleic acid (DNA) from tissue or eggs of the E. multilocularis parasite.
The outcomes of the pathogen‐specific surveillance programme of each Member State and of Norway need to be annually submitted to the Commission by the 31 of May.
At the moment, only four Member States (Finland, Ireland, Malta and the UK) are listed in the Annex to Commission Implementing Regulation (EU) 2018/878 as complying with the rules for categorisation laid down either in Article 2(2) or (3) of Commission Delegated Regulation (EU) 2018/772. The Decision of the EEA Joint Committee No 183/2019 of 10 July 2019 also added the whole territory of Norway to the list of countries mentioned in the Annex to Commission Delegated Regulation (EU) 2018/878 as complying with the rules for categorisation laid down in Article 2(3) of Commission Delegated Regulation (EU) 2018/772.
This report follows previous annual reports (EFSA, 2013, 2014, 2015, 2016, 2017, 2018) presented by EFSA to the European Commission and aims to analyse and assess the sampling strategy, data collection and detection methods used by these five countries in the context of Commission Delegated Regulation (EU) 2018/772 in their respective E. multilocularis (pathogen‐specific) surveillance programmes, and verify that the requirements laid down in this regulation are being complied with.
Based on the ‘rules for categorisation of Member States in view of their eligibility for preventive health measures’ (Art. 2), Malta falls under the category described in paragraph 2 of the same article, i.e. it is in the position of demonstrating that the infection with E. multilocularis parasite has not been established because of the absence of wild red foxes in the whole of its territory. Article 4 provides details on the conditions to be fulfilled in order to remain eligible for preventive health measures. For Member States like Malta, in absence of definitive host, the conditions to be met are: (a) having a national observation programme in place to detect the presence of wild red foxes; (b) immediate notification to the Commission and the other Member States of the detection of the presence of wild red foxes during each 12‐month observation period; (c) report to the Commission on the results of the national programme referred to in point (a) by 31 May following the end of each 12‐month observation period. The evaluation of the observation programme and its results is out of the remit of this assessment.
1.1. Background and Terms of Reference as provided by the European Commission and the EFTA surveillance authority
The Commission adopted Commission Regulation (EU) No 1152/2011 of 14 July 2011, as regards preventive health measures for the control of E. multilocularis infection in dogs. This was in order to ensure continuous protection of Finland, Ireland, Malta and the United Kingdom that claim to have remained free of the parasite E. multilocularis as a result of applying national rules until 31 December 2011. The Decision of the EEA Joint Committee No 103/2012 of 15 June 2012 added the whole territory of Norway[^2] to the list of countries complying with the conditions of Article 3 of the Regulation. For the purposes of Norway's obligations under the EEA Agreement, including those under Regulation (EU) No 1152/2011, the territory of Norway does not include Svalbard, cf. Protocol 40 to the EEA Agreement.
This Regulation includes certain obligations for these Member States and Norway in order to implement a pathogen‐specific surveillance programme aimed at detecting the parasite, if present in any part of those Member States, in accordance with certain requirements regarding the sampling, the detection techniques and the reporting.
EFSA is asked, in the context of Article 31 of Regulation (EC) No 178/2002, to provide the following scientific and technical assistance to the Commission:
Regular follow‐up of the literature regarding E. multilocularis infection in animals in the European Union and adjacent countries, including its geographical distribution and prevalence;
Analysis and critical assessment, in the context of Regulation (EU) No 1152/2011, of (i) the sampling strategy considered for the programmes of the countries concerned; (ii) the data collected in the framework of these programmes; (iii) the detection methods used.
1.2. Interpretation of the Terms of Reference
This report addresses Term of Reference (ToR) 2 of the mandates M‐2012‐0200 and M‐2014‐0287 submitted to EFSA by the European Commission and the EFTA Surveillance Authority, respectively, and applies the principles and procedures established in the EFSA reports ‘Scientific and technical assistance on E. multilocularis infection in animals’ (EFSA, 2012a) and ‘A framework to substantiate absence of disease: the risk‐based estimate of system sensitivity tool (RiBESS) using data collated according to the EFSA Standard Sample Description ‐ An example on E. multilocularis’ (EFSA, 2012b).
Commission Delegated Regulation (EU) 2018/772, repealing Regulation (EU) No 1152/2011, gives a description of the requirements for the surveillance programme (Annex I). The methodology adopted by EFSA for the previous assessments does not require changes to fit the new requirements which remain the same in their substantial traits.
1.3. Additional information (if appropriate)
Following an update of the relevant regulation, Malta has been exempted from the obligation of running a surveillance exercise on the domestic dog population. For this reason, in this report the data of Malta are not presented.
2. Data and methodologies
To address ToR 2, EFSA developed a scientific and a technical report in 2012 (EFSA, 2012a,b). The principles and procedures that were established there have been applied in the assessment of each of the subsequent annual national surveillance reports submitted to the Commission, including this report.
As a first step, the quality of the 2019 surveillance reports of the three Member States and Norway was assessed by checking the description of the surveillance system for completeness against the relevant elements that need to be addressed in the context of Commission Delegated Regulation (EU) 2018/772.
In order to facilitate the assessment, we divided the information into four different categories (see Table 3) corresponding to the critical points of the three paragraphs addressed in the legislation in the ‘requirements for the pathogen‐specific surveillance programme (Annex I).
Table 3.
Assessment categories and their equivalence in the Commission Delegated Regulation (EU) 2018/772 (Annex I)
| Information category | Main points considered in the assessment | Delegated Regulation (EU) 2018/772 |
|---|---|---|
| 1 | The type and sensitivity of the detection method was evaluated to ensure the fulfilment of the technical legal requirements regarding appropriate techniques for the detection of E. multilocularis in intestinal contents (sedimentation and counting technique –SCT‐ or a technique of equivalent sensitivity and specificity) or intestinal contents/faeces (detection of species‐specific DNA from tissue or eggs of the E. multilocularis parasite by polymerase chain reaction –PCR‐or a technique of equivalent sensitivity and specificity) | Annex I – Point 3 |
| 2 | The selection of the target population was evaluated to ensure the fulfilment of the technical legal requirements regarding the collection of samples from wild definitive hosts or domestic definitive hosts in the absence of the first | Annex I – Point 2 |
| 3 | The sampling strategy was evaluated to ensure the fulfilment of the technical legal requirements regarding appropriate sampling for detection of the E. multilocularis parasite, if present in any part of the Member State, at the design prevalence of less than 1% (0.01) | Annex I – Point 1 |
| The sampling strategy was also evaluated to ensure the fulfilment of the technical legal requirements regarding the 12‐month surveillance period of data collection | Annex I – Point 3 | |
| 4 | The Methodology was evaluated to ensure the fulfilment of the technical legal requirements regarding a confidence level of at least 0.95 against a design prevalence of 1% (0.01) | Annex I – Point 1, 2, 3 |
For each of the four evaluation parts, the most relevant elements were extracted from the reports submitted by the Member State and checked against the criteria described below (Table 4).
Table 4.
Relevant elements checked for compliance of the technical requirements of Annex I of Commission Delegated Regulation (EU) 2018/772
| Points addressed in the Annex II | Element | Description of element |
|---|---|---|
| Type and sensitivity of the detection method | Type of test | The diagnostic test used for the detection of EM must be defined. Modifications of the original method should be indicated |
| Test sensitivity | The sensitivity and specificity of the test used in the surveillance system must be reported. This would ideally be estimates from each participating laboratory reported as a point estimate (average) of the values across the country with minimum and maximum values or a probability distribution. Alternatively, a value of 0.78, as recommended by EFSA (2015), shall be used | |
| Selection of the target population | Definition of susceptible host population targeted by the system | The susceptible wild definitive host population(s) (red foxes, raccoon dogs) targeted by the surveillance system should be described and the choice justified. If domestic host species (dogs or cats) are sampled, evidence for the absence of wild definitive hosts and for these domestic animals having had access to outdoors should be provided |
| Size of susceptible host population targeted by the system | The size of the targeted (wildlife) population should be reported, together with the evidence for this. Historical population data should be updated since these may not reflect current populations | |
| Sampling strategy | Epidemiological unit | It should be clearly defined if individual animals or individual faecal samples collected from the environment constitute the epidemiological unit. If individual faecal samples are collected from the environment, the method applied to establish the species from which the faeces originated has to be reported |
| Sample size calculation | The applied survey design should be fully documented, including considerations regarding potential biases inherent in the survey design. The method and the formula used to calculate the sample size should be fully documented | |
| Implementation of the sampling activity | The sampling methods used should be fully documented including the related assumptions and uncertainties, and a justification for choosing the approach should be provided. Timeframe of the surveillance data and geographical clustering of the infection must be reported. The sample collection period must comprise the whole year and the spatial distribution of the sampling must be representative | |
| Methodology | Design prevalence (DP) | DP is specified in Annex I to Regulation (EU) No 2018/772 and must be 1% (0.01) or lower |
| Geographic epidemiological unit | The geographic epidemiological unit(s) identified as target for the surveillance activity has to be clearly indicated and supported by justification | |
| Methodology for calculation of area sensitivity | For the calculation of the area sensitivity, the diagnostic sensitivity should be set conservatively to the lowest value, excluding the lowest 20th percentile, from the ones reported in the scientific literature and related to the diagnostic tests implemented by the countries listed in Annex I of the Commission Delegated Regulation (EU) No 2018/772. In this case, it is 78% (EFSA AHAW Panel, 2015) |
A summary of the assessment of the relative elements of the different countries is given at the end of the document (see Annexes Annex A – Finland. Assessment tables of the surveillance report, Annex B – Ireland. Assessment tables of the surveillance report, Annex C – United Kingdom. Assessment tables of the surveillance report, Annex D – Norway. Assessment tables of the surveillance report). As a second step, the raw data on individual samples submitted by the five countries via the EFSA Data Collection Framework (DCF) were analysed. For the purpose, the software R (R core Team, 2019) was used to compute descriptive statistics. Table 5 lists and describes all the parameters that were extracted from the data submitted.
Table 5.
List of the parameters extracted from the raw data submitted by the Member States via the Data Collection Framework
| Parameter | Description | |
|---|---|---|
| 1 | Theoretical Sampling period | The 12‐month reporting period. It may go from January to December, but this is not a restriction: the reporting period can also include 12 consecutive months over 2 years |
| 2 | Actual Sampling Period | Range. Date of the first sampling date and date of the last sampling within the theoretical sampling period |
| 3 | Summary dates | Descriptive statistics of the sampling period |
| 4 | Sampling period | Total number of days sampled within the actual sampling period |
| 5 | Number of samples | Total number of samples collected during the theoretical sampling period |
| 6 | Number of test results | Total number of test results. If the number of test results is equal to the number of samples, none of the latter required further investigations (i.e. were negative at the first test) |
| 7 | Laboratory test completion | Comparison between the year when the samples are collected and the year when the test was completed |
| 8 | Sensitivity | Sensitivity of the diagnostic test |
| 9 | Host | Target population size (N); additional information on the host species |
| 10 | Animal sample | Type of sample collected |
| 11 | Sampling Strategy and Design | As reported (e.g. representative sample, risk‐based) |
| 12 | Sampling point | Activity adopted for the sample collection (e.g. hunting, veterinary activity, etc.) |
3. Assessment
3.1. Finland
3.1.1. Information as submitted in the report by the Member State
The Finnish Food Authority, which was formed in 2019 when the former Finnish Food Safety Authority Evira merged with two other governmental organisations, used a PCR method (PCR 12S rRNA) for the detection of E. multilocularis eggs in rectal content. The PCR method was described by Isaksson et al. (2014), with a modification in the magnetic beads washing step (manual instead of automatic). To estimate the actual sensitivity of the test developed by Isaksson et al. (2014), internal validations were performed yearly in Evira/Finnish Food Authority from 2014 to 2019. In this validation procedure, positive (spiked) samples were tested blindly. As positive control in DNA isolation, own spiked specimens have been used: 10 inactivated (–80°C) E. multilocularis eggs/3 mL of intestinal content. Negative control is water sample in PCR. In routine analyses, a positive control was always analysed parallel to actual samples. If a positive control was found negative, the analysis of the whole batch of samples was repeated. The latest (and so far, the only) proficiency test on detection of E. multilocularis in faeces (PCR) was conducted in May 2015. The results of the Finnish Food Authority were correct. The report of the results was provided to the EC.
For the whole country of Finland, the entire wild small canid population(s) of the country was defined as the geographical epidemiological unit (even though the population is a continuum of the north‐western taiga population). The epidemiological and sampling unit was defined as the individual animal (red fox or raccoon dog).
The targeted host species were the raccoon dog (Nyctereutes procyonoides) and red fox (Vulpes vulpes). The justifications reported for choosing these target species were the facts that the red fox is the primary host of E. multilocularis in Europe (Deplazes, 2006), and that raccoon dogs have been shown to be good definitive hosts for E. multilocularis (Kapel et al., 2006).
Population size estimates are based on hunting bag statistics provided by the Natural Resources Institute Finland (available on line: http://statdb.luke.fi/PXWeb/pxweb/en/). Kauhala (2007) estimated that annual hunting bag is ca. 50% of the autumn population of the raccoon dog and ca. 40% of the autumn population of the red fox. The average annual hunting bag in the 5‐year period 2014–2018 (latest available data) was 175,600 raccoon dogs and 48,300 red foxes. Therefore, FI estimated the population sizes of the raccoon dog and the red fox to be 2 × 175,600 = 351,200 individuals and 2.5 × 48,300 = 120,750 individuals, respectively. The estimated size of the susceptible population is therefore 471,950.
The population densities for both species are highest in the southern part of the country. See maps in Figure 1. These maps are from year 2007 but the relative densities most probably still apply: population densities of the raccoon dog are highest in the southern part, especially in the south‐eastern part, of the country and decrease towards the north.
Figure 1.

Finland – Raccoon dog densities (left) and red fox densities (right) according to Kauhala (2007) (Yks./km2 = individuals/km2)
The hunting bag of the raccoon dog has been biggest in the four south‐eastern regions in 2016–2018. As for the red fox, the largest regional hunting bag in recent years has been achieved in Lapland where hunting effort has been high.
No information on age or gender structure of the target population was available.
The sample size was calculated by Finland using an overall sensitivity of the diagnostic approach of 0.78 and the design prevalence (DP) of 1% prescribed in Regulation (EU) No 1152/2011 using the RiBESS tool. As size for the target population, a fixed value of 471,950 was used. The RiBESS tool returned a sample size equal to 383 to achieve the required confidence.
The samples were collected by hunters on a voluntary basis. Hunters were informed of the sample collection by press releases in the Finnish Food Authority website3 and e‐mails and personal contacts to the Finnish Wildlife Agency which in turn informed local hunting associations. To motivate hunters, they received by post a written report of the results of the health status of the animals they sent in. A total of 325 and 198 samples were collected from raccoon dogs and foxes respectively (N = 523).
The majority of the samples originated from Southeast Finland as this is the region where active monitoring of rabies control programme has taken place since 1990 (Pohjois‐Karjala, Etelä‐Karjala, Etelä‐Savo, Kymenlaakso, 64% of the samples). The same area can be considered having an elevated risk of introduction of E. multilocularis due to geographical closeness of infected areas in the south. Also, Southeast Finland has the highest density of raccoon dogs in Finland (Kauhala, 2007), but in general, the population densities for both species are highest in the southern part of the country. The red fox inhabits the whole country including the northernmost ‘fjeld’ regions where densities can be locally high. The raccoon dog is continuously spreading towards north and nowadays a few hundred individuals are hunted yearly even in southern Lapland. Gender ratio was male‐biased in foxes (1:1.20) and in raccoon dogs (1:1.17). Of the animals that could be classified by age (N‐age = 496), 53% were juveniles. The proportion of juveniles was 61% in raccoon dogs and 39% in foxes. A large sample of foxes (22% of all animals) was received from Lappi (Lapland) where active red fox population reduction to protect the arctic fox was on‐going (see Figure 2).
Figure 2.

Finland – Geographical distribution of samples
Samples were collected throughout 2019 (see Figure 3). Sampling is mostly done in the cold season. Nearly all the foxes from Lapland were hunted in January–March. In May, June and July, the sample sizes decreased since the fox and female raccoon dogs with pups are protected, and consequently, hunting is only focused on diseased or injured individuals. However, the raccoon dog was recently (1 June 2019) classified in the Finnish law as an alien invasive species with no protection seasons.
Figure 3.

Finland – Temporal distribution of samples
All 523 samples were negative in PCR. Thus, no sample was found positive for E. multilocularis.
3.1.2. EFSA comments and considerations
3.1.2.1. Type and sensitivity of the detection method
Type of the detection method: The diagnostic test used by Finland for the detection of E. multilocularis consists of a PCR method (PCR targeting 12S rRNA gene) described by Isaksson et al. (2014). The technique has been well described. A slight modification of the technique has been realised and it has been indicated in the report.
Test sensitivity: The test sensitivity (TSe) used for the estimation of the sample size was 0.78, as suggested by EFSA (EFSA, 2015). However, an overall system sensitivity of 0.87 (0.83–0.90) has been estimated based on internal validations performed by Evira/Finnish Food Authority (EFSA, 2019). The additional positive (spiked) samples tested in 2019 help in narrowing the uncertainty around the sensitivity of the test in use.
An exact binomial test shows a ‘probability of success’ (‘best guess’ of the sensitivity) equal to 0.87, with a confidence interval going from 0.84 to 0.91 (bottom row of Table 6) and a Bayesian approach leads substantially to the same results.
Table 6.
Results of the internal validation round of tests performed by Finland over time
| Year | Spiked samples (n, positive controls) | Samples testing positive (s) | Estimated sensitivity for each trial (exact binomial test) | Bayesian cumulativea |
|---|---|---|---|---|
| 2014 | 131 | 102 | 0.78 (0.70–0.85) | 0.78 (0.7–0.84) |
| 2015 | 38 | 32 | 0.84 (0.69–0.94) | 0.79 (0.73–0.85) |
| 2016 | 32 | 31 | 0.97 (0.84–1) | 0.82 (0.76–0.87) |
| 2017 | 76 | 72 | 0.95 (0.87–0.99) | 0.85 (0.81–0.89) |
| 2018 | 31 | 31 | 1 (0.89–1) | 0.87 (0.83–0.90) |
| 2019 | 24 | 24 | 1 (0.86–1) | 0.88 (0.84–0.91) |
| Total | 332 | 292 | 0.87 (0.84–0.91) |
Estimated as , where y is the number of years/rounds of test.
3.1.2.2. Selection of the target population
Definition of susceptible host population target by the system: The selection of raccoon dogs and red fox species as target populations was based on their role as definitive hosts in the cycle. This is an assumption also confirmed by the EFSA Scientific opinion on E. multilocularis infection in animals (EFSA AHAW Panel, 2015).
It is not possible to conclude on the role of the age and gender composition of the target population in the epidemiology and the lifecycle of EM, due to lack of appropriate data and studies (EFSA AHAW Panel, 2015).
Size of susceptible host population targeted by the system: Host population sizes were based on a scientific study performed in 2007. Although population data have not been updated since 2007, new information regarding annual hunting bags has been included in the report. The decision to accept the size of the population as published by Kauhala (2007) and adjusting for the change of the size of the hunting bag is scientifically sound, particularly considering that the sample size calculation is not heavily affected when the population size has these dimensions (~ infinite population) (see EFSA AHAW Panel, 2015). The fact of considering the sum of the red fox and raccoon dog populations as the target population size seems to be correct, as raccoon dogs can act as DHs in conjunction with the red fox (EFSA AHAW Panel, 2015).
3.1.2.3. Sampling strategy
Epidemiological unit: The epidemiological unit appears in the report and is defined as the individual animal. Individual rectal contents were collected directly by hunters.
Sample size calculation: The method used to calculate the sample size of Finland was the RiBESS tool. The sample size was calculated with an overall sensitivity of the diagnostic approach of 0.78 (see Section 3.1.2.1) and a population size of 471,950 (sum of red fox and raccoon dog population). The sample size required in this case is 383. The sample size collected (N = 523) is sufficient to satisfy the legal requirements.
Implementation of the sampling activity: The geographical information shows that, in 2019, 12 (15 in 2018) out of 20 NUTS 3 regions were included in the sampling activity (see Figure 4). There was a higher intensity of the sampling in the south‐east of the country.
Figure 4.
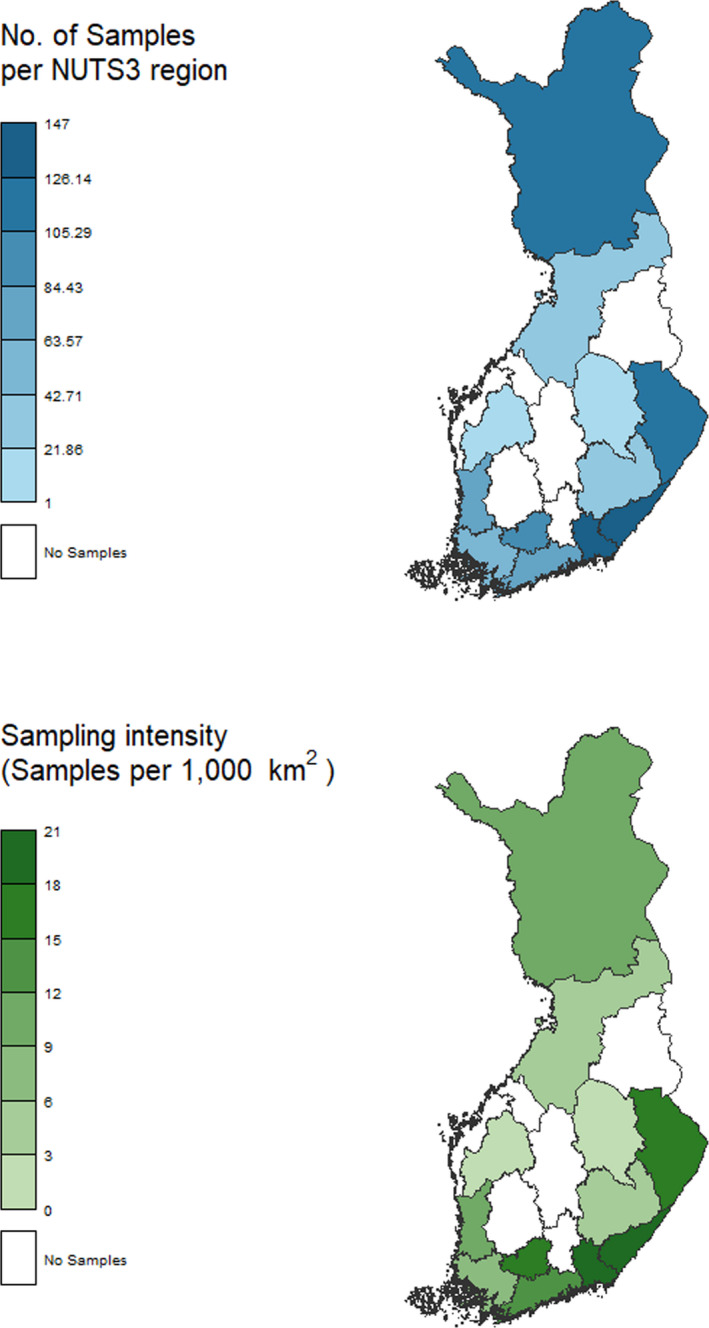
Finland – Sampling activity and intensity by NUTS 3 region
The surveillance strategy as described in the Finnish report cannot be considered a simple random sample, but rather a ‘convenience sample’. Most of the samples were collected by hunters and efforts were concentrated in the north and south‐east of the country. However, in the case of wildlife animals, ‘convenience sampling’ is the most frequently used method. To mitigate the potential bias caused by this sampling activity, more samples than required were collected. Samples were collected during a period of 12 months as established in the relevant Regulation. The reduction of the intensity of the sampling during the summer months (May, June and July) is well justified and may not compromise the success of the detection of the parasite. A previous EFSA assessment suggested that a sampling distribution concentrated in the second half of the year – in a Freedom from Disease framework – could be more effective than a sampling distributed over the whole year; however, a quantitative evaluation was not performed (EFSA, 2013).
3.1.2.4. Methodology
Design prevalence: The DP was equal to 1% (0.01), as it is specified in Annex I to Commission Delegated Regulation (EU) 2018/772.
Epidemiological geographical unit: The geographical unit was specified to be the entire territory of Finland. The choice is sound as no risk factors were reported to justify the identification of subareas within the Finnish territory.
Methodology for calculation of the area sensitivity: The area sensitivity was estimated by Finland using the RiBESS tool. The parameters included for the calculation were the following, all fully documented:
DP of 1% (0.01),
TSe of 0.78,
population size of 471,950 (raccoon dogs + red foxes) and
sample size of 523
The value of the area sensitivity (0.983) exceeded the established minimum value of 0.95 needed to fulfil the technical legal requirements of Commission Delegated Regulation (EU) 2018/772.
In summary, the set of data relative to the surveillance activity in 2019 ensures the fulfilment of the technical legal requirements of Annex I of Commission Delegated Regulation (EU) 2018/772.
3.2. Ireland
3.2.1. Information as submitted in the report by the Member State
Rectal contents from foxes were examined according to the method of Trachsel et al. (2007) referred to as PCR Cest1‐Cest2 NAD1. The DNA nucleotide sequences of primers were: Cest1 = TGCTGATTTGTTAAAGTTAGTGATC and Cest2 = CATAAATCAATGGAAACAACAACAAG. The positive control that was used was an extract of DNA from adult E. multilocularis worms which was supplied by the EU Reference Laboratory for Parasites (EURPL). The negative control used was sterile saline solution.
The estimation of the TSe (of 0.78) was based on the most recent advice arising from scientific opinion by EFSA (EFSA AHAW Panel, 2015). In addition, the Irish National Reference Laboratory for Parasites is amenable to participating in any study in order to re‐evaluate the TSe estimate, provided a sufficient number of E. multilocularis positive samples are supplied by the EURLP or a similar laboratory.
The Irish National Reference Laboratory for Parasites successfully passed both Echinococcus‐related proficiency tests that it participated in this year. These proficiency tests were organized by the European Union Reference Laboratory for Parasites (ISS, Rome) and were titled as follows; ‘Detection of Echinococcus spp. worms in the intestinal mucosa of the definitive host’ and ‘Molecular identification of Echinococcus at the species level’.
The epidemiological unit used was the same geographical area as that of the EU member state Ireland. The rationale for selecting this area as the epidemiological unit was in order to comply with the conditions of the Regulation 2018/772 for Member States listed in Annex 1.
The animal level epidemiological unit was the individual animal (that is, the red fox (Vulpes vulpes)).
In accordance with the requirements for pathogen‐specific surveillance for E. multilocularis outlined in Commission Delegated Regulation (EU) 2018/772, the most suitable host species to survey is a wildlife definitive host species. In Ireland, because of the occurrence of red foxes throughout the country and no known occurrence of raccoon dogs (Hayden and Harrington, 2000; Marnell et al., 2009), the former was selected as the wildlife definitive host species to survey for the presence of E. multilocularis. The red fox population has been estimated to be between 150,000 and 200,000 (Hayden and Harrington, 2000; Marnell et al., 2009).
The red fox is a seasonal breeder, whereby cubs are born in the spring and are almost fully grown by seven months of age (Hayden and Harrington, 2000). Therefore, the age structure of the population between young and adult foxes varies depending on the time of year. There is little published scientific evidence of the gender structure of the Irish red fox population.
The red fox is distributed throughout Ireland (Hayden and Harrington, 2000; Marnell et al., 2009). Further information about the distribution of the red fox population within Ireland has been produced in a report by Dr Tomás Murray from the National Biodiversity Data Centre in 2015 (see also Figure 5).
Figure 5.
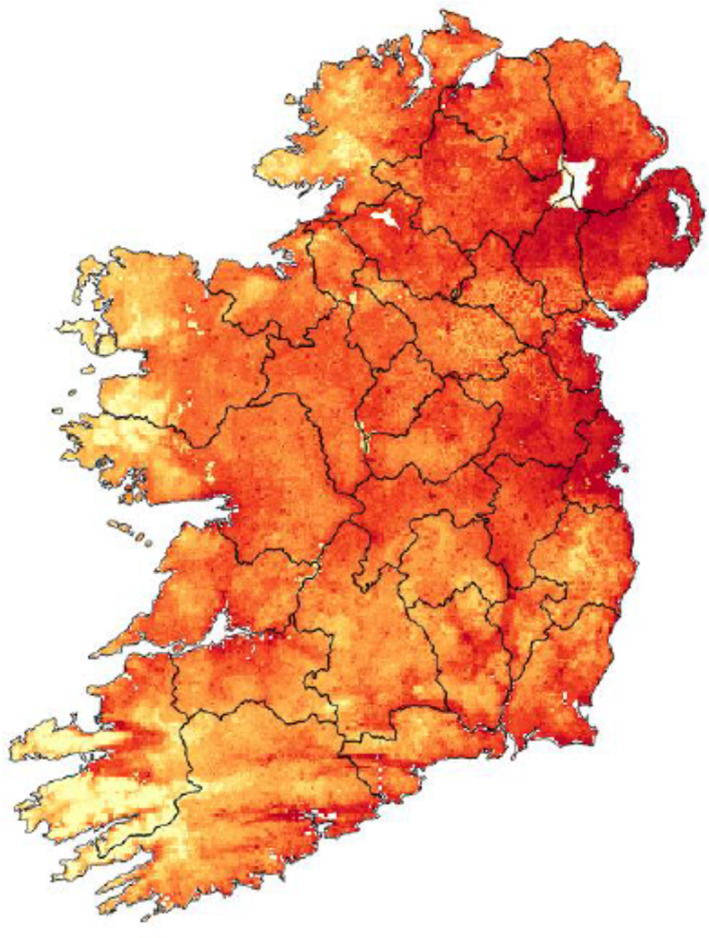
Ireland – Probability of presence per 1 km2 from the final Maxent species distribution model (Phillips et al., 2006) for red fox (Vulpes vulpes). Source: data up to 2015 provided by Dr. Tomás Murray, from National Biodiversity Data Centre (Ireland)
The survey was designed to detect E. multilocularis, if present, in red foxes in Ireland by taking a representative sample of the red fox population based on a DP of 0.01, a target survey sensitivity of 0.95, fox population size of 150,000 and TSe of 0.78.
The animal samples were obtained from foxes which were culled (by shooting) for pest and predator control reasons and foxes that were inadvertently captured in traps set for other wildlife as part of wildlife disease control measures. Each of the 16 Regional Veterinary Offices in Ireland was requested to obtain a number of wild foxes, based on their respective area size and the fox population density to obtain a total number for that region which reflected the number calculated in the ‘Red fox (Vulpes vulpes) Species Distribution Model’ for each area.
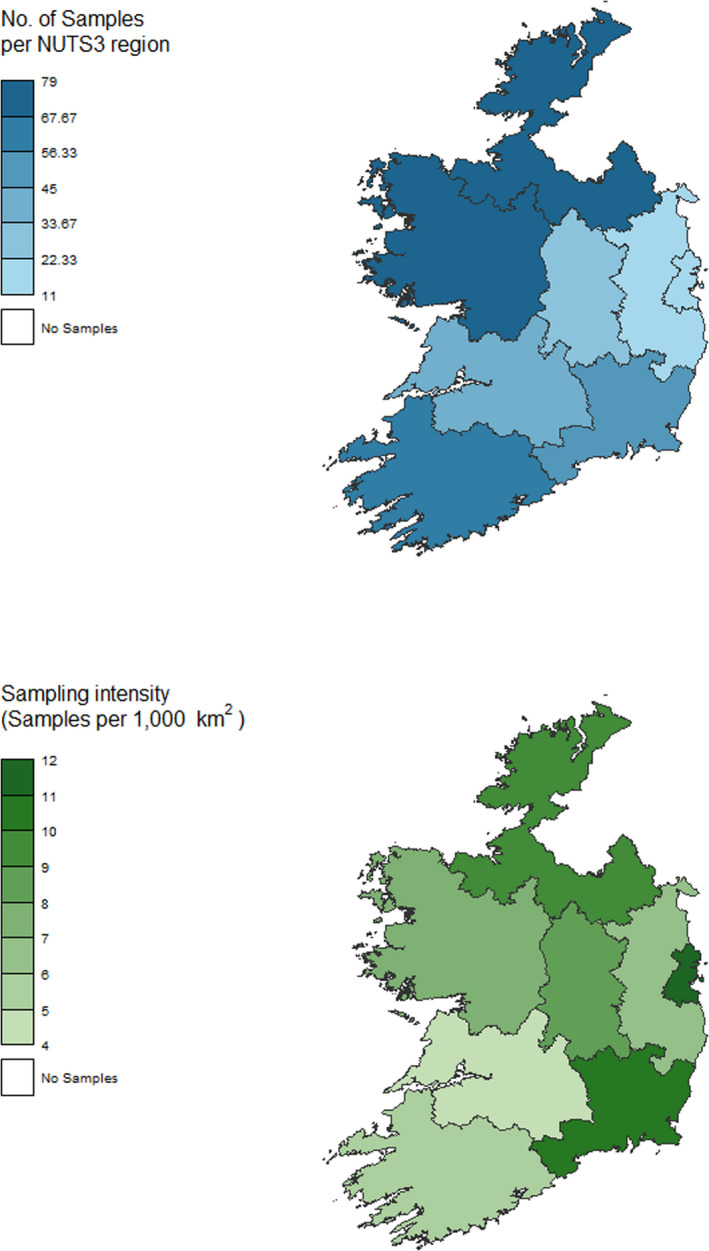
Samples were collected through the work of the 16 Regional Veterinary Office personnel and from all 8 NUTS 3 regions. A slightly greater number than the minimum required to achieve the desired survey sensitivity for the entire survey were tested. In total, a collection of 400 samples was reported by Ireland.
The sampling intensity was undertaken to reflect the distribution throughout Ireland and further adjusted to reflect the geographical variation in the density of the fox population distribution (Figure 6). Samples were obtained during 10 months of the year (see Figure 7). A greater number of samples were collected from culling during October and November, in order to avoid the culling of adult female foxes during the nursing period. Collection of samples predominantly during the winter months should not adversely affect the sensitivity of the survey, based on a study from an endemic urban area in Switzerland, which found a greater prevalence of E. multilocularis in foxes in winter months (Hofer et al., 2000).
Figure 6.
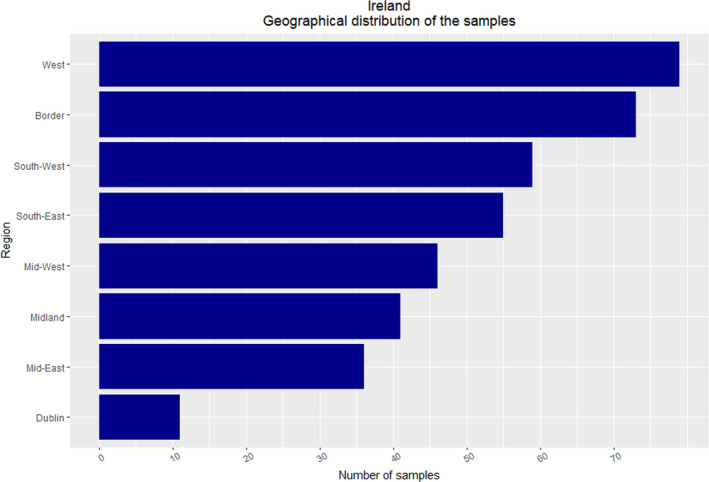
Ireland – Geographical distribution of samples
Figure 7.
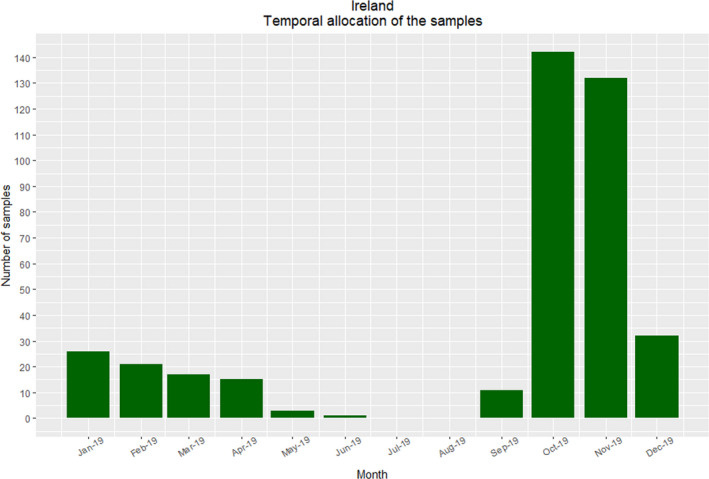
Ireland – Temporal distribution of samples
3.2.2. EFSA comments and considerations
3.2.2.1. Type and sensitivity of the detection method
Type of the detection method: The diagnostic test chosen by Ireland is well described (PCR Cest1‐Cest2 NAD1) and is based on a peer‐reviewed method with a correct reference included in the report.
Test sensitivity: Ireland followed EFSA′s advice regarding the setting of the conservative, lowest value of the sensitivity (0.78) (EFSA, 2015).
3.2.2.2. Selection of the target population
Definition of susceptible host population target by the system: The red fox, has been recognized as the main wildlife definitive host species for this parasite (EFSA AHAW Panel, 2015). The selection of this species to perform the pathogen surveillance is well explained and referenced. The absence of other important definitive wild hosts (raccoon dogs and wolves) is also supported by scientific literature. Regarding the age or gender of the target population, their role in the epidemiology and in the lifecycle of EM is not known due to the lack of appropriate data and studies (EFSA AHAW Panel, 2015).
Size of susceptible host population targeted by the system: Although the original information regarding the red fox population size was published in 2000 and 2009 (Hayden and Harrington, 2000; Marnell et al., 2009), Dr Tomás Murray, of the National Biodiversity Data Centre, Ireland, specifically provided additional information regarding the Irish fox population in 2015, including more recent data on the relative population density distribution based on ongoing observation records. Nevertheless, at a population size greater than 10,000, moderate fluctuations in the population size would not significantly change the sample size required to achieve the same confidence. Therefore, fluctuations in the previous population size of ~ 150,000 do not significantly alter the sample size required (EFSA, 2014).
3.2.2.3. Sampling strategy
Epidemiological unit: The epidemiological unit is defined in the report as the individual animal. Faeces samples were obtained post‐mortem from culled (control programmes) or animals trapped inadvertently.
Sample size calculation: The method used to calculate the sample size for Ireland was the RIBESS tool. The sample size was calculated with: (a) an overall sensitivity of 0.78 (as recommended by EFSA AHAW Panel, 2015) and (b) a population size of 150,000 (red fox population). With these conditions, the minimum number of samples to collect in order to obtain a minimum of 0.95 of area sensitivity is 383. The total number of samples collected by Ireland was 400, which ensures the fulfilment of the technical legal requirements in Commission Delegated Regulation (EU) 2018/772 concerning a confidence level of at least 0.95 against a DP of 1% (0.01). Although EFSA would recommend considering the population size as the maximum value of the range instead of the minimum number (200,000 instead of 150,000), the minimum sample size thus calculated to achieve the same confidence would not differ significantly.
Implementation of the sampling activity: The geographical information shows that all regions were included in the sampling activity (see Figure 9). The sampling activity per 1000 km2 shows a homogenous intensity, i.e. the target sample size is distributed across the territory as a function of the area size, adjusted for the density of the population. Such a sampling strategy, leading to a so‐called proportional sample, is more likely to be representative compared to other strategies. Samples were obtained during the whole year excluding July and August (see Figure 8). The reduction in collection of samples during spring and summer is justified to avoid culling adult female foxes during the nursing period. This fact might not influence the representativeness of the sample, as suggested in a previous EFSA assessment (EFSA, 2013). A sampling distribution concentrated in the second half of the year – in a Freedom from Disease framework – could be more effective than a sampling distributed across the whole year (EFSA, 2013).
Figure 9.
Great Britain – Map estimating fox density in the UK. This is a systematic approach using NBN presence data and published density data and provides a confidence interval of 120–280,000 foxes. Some areas have few data as permission was not given to use the records. For more information, see Croft et al. (2017)
Figure 8.
Ireland – Sampling activity and intensity by NUTS 3 region
3.2.2.4. Methodology
Design prevalence: The DP was equal to 1% (0.01), as it is specified in Annex I Commission Delegated Regulation (EU) 2018/772.
Epidemiological geographical unit: The geographical unit was specified to be the entire territory of Ireland. The choice is sound as no risk factors were reported to justify the identification of sub‐areas within the Irish territory.
Methodology for calculation of the area sensitivity: The area sensitivity was estimated by Ireland using the RiBESS tool. The parameters included for the calculation were the following:
DP of 1%,
TSe of 0.78,
population size of 150,000 and
sample size of 400.
The value of the area sensitivity is 0.957, i.e. exceeding the established minimum value of 0.95 needed to fulfil the technical legal requirements described in Commission Delegated Regulation (EU) 2018/772. With a population size of 200,000, the value of the area sensitivity would also reach this CL; 0.956 (> 0.95).
In summary, the set of data relative to the surveillance activity in 2019 ensures the fulfilment of the technical legal requirements of Annex I of Commission Delegated Regulation (EU) 2018/772.
3.3. United Kingdom
3.3.1. Information as submitted in the report by the Member State
The sample size was calculated using the EFSA RiBESS tool. Random sampling – not risk‐based – sampling, is carried out at certain times of the year – the target is the wild population and therefore hunting is not permitted during the breeding season.
In Great Britain (GB), a PCR test (PCR Cest1‐Cest2 NAD1) was used to detect E. multilocularis DNA in rectal content (post‐mortem sampling) (Mathis et al., 1996; Dinkel et al., 1998). The method is based on the concentration of helminth eggs by a combination of sequential sieving of faecal samples and flotation of the eggs in zinc chloride solution. DNA of the taeniid eggs retained in the 20 microns sieve was obtained after alkaline lysis and nested PCR was performed using E. multilocularis species‐specific primers against the mitochondrial 12S rRNA gene.
TSe for the PCR is between 85% and 99% depending on the laboratory. The sensitivity of the proposed method is further determined using spiked faecal samples and the specificity is tested with other taeniid species. In the case of the APHA/FERA laboratory, 78% sensitivity was used as the lowest possible sensitivity, based on successful ring trial participation. In GB, the APHA/FERA laboratory completed the ring trial and were given UKAS status for their testing proficiency (2018).
In Northern Ireland (NI), a sedimentation and counting technique (SCT) test was used to detect E. multilocularis eggs from individual intestinal content (Eckert, 2003). The analyses were performed at the Agri‐Food and Biosciences Institute (AFBI) which is the national reference laboratory for the Department of Agriculture, Environment and Rural Affairs (DAERA).
The egg counting method sensitivity varies between laboratories. Eckert's suggestion to consider a Se of 99% was used (Eckert, 2003). In NI, AFBI participated in the last proficiency testing in 2015.
The UK was divided into two surveillance regions for the purpose of this report: NI and GB (England, Scotland and Wales).
The epidemiological unit was the individual animal. As animal carcasses rather than fox scat were collected, the results could be reported at the individual fox level.
The red fox (Vulpes vulpes) is the only wild definitive host for E. multilocularis in the UK (both GB and NI). No other wild definitive host is present. GB and NI are island populations with no access for other wild carnivores from other parts of Europe. A review of the populations of wild mammals in GB has been published and there are not recognized breeding population of raccoon dogs or wolves.
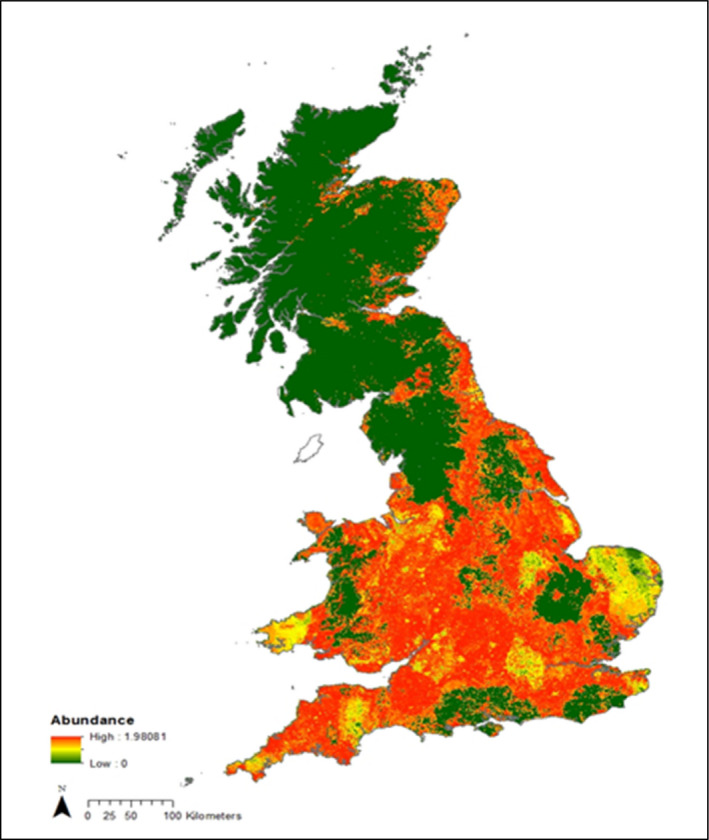
The fox population size in GB (pre‐breeding adults) has been estimated at 240,000 by wildlife experts, and the numbers were published in 2013 (Defra, 2013). The population does fluctuate from year to year, but is believed to be relatively stable, if marginally increasing. The urban/sub‐urban fox population is now estimated at ~ 33,000 (up from 15,000) (~ 13%). The variation in abundance is likely correlated with food resources, so while the density in hill areas of Scotland have been estimated at one breeding pair every 40 km2, the highest density recorded was in the urban areas of 30 foxes in a single km2 (http://www.lhnet.org/red-fox/; Croft et al., 2017). The rapid spread of sarcoptic mange in the red fox and the population genetic structure according to microsatellite analysis (Atterby et al., 2015) demonstrates that there is considerable mixing of the red fox population within GB and within the island of Ireland, despite the variation in abundance. The average range of a red fox in the UK in open farm land is considered to be ~ 200–600 ha (2–6 km2). There is good evidence that the total abundance has not changed in the last decade (Wright et al., 2014; Croft et al., 2017) as measured on BTO survey squares (mostly rural), and as predicted. The urban fox distribution has changed in recent years with almost all urban areas now having foxes present (Scott et al., 2014). A map of systematically estimated fox distribution and abundance using NBN data and published density information and a small project using public sighting data to estimate fox abundance in all urban areas was provided (see Figure 9).
The map in Figure 9 shows that there is an uneven distribution of the wild host population – some areas have less dense fox populations than others – for example, the highest density is in urban areas in the south‐west of England, the least dense are rural areas in Northern Scotland (see map) and that this distribution has not changed significantly in the last 10 years. This uneven distribution means sampling of animals is also uneven. GB consists of islands, surrounded by sea with no land bridges for foxes to arrive; therefore, there is a constant population (which varies during the year according to whether the females have given birth). Population size is based on numbers of breeding females.
For NI, an estimate of 14,000 is given, which is equivalent of 1 fox/km2 and accounts for the large area of rural land in contrast to the urban land use (Conserve Ireland, 2009). This probability of presence per 1 km2 originates from the final Maxent species distribution model (Phillips et al., 2006) for red fox (Vulpes vulpes). The input data go up to 2015 and were provided by Dr. Tom as Murray, from National Biodiversity Data Centre (Ireland). There is a single land border with another EU Member State, which is the Republic of Ireland. This border is porous for wildlife; however, Ireland also has official disease free status for E. multilocularis. The fox is found throughout Ireland, although the density of fox populations is highly variable. They are most abundant in areas that offer a wide variety of food and cover. In contrast areas of uniform land, such as moorland or open plains, generally carry much lower densities. At high population densities, foxes generally have small home ranges and disperse over short distances. Some foxes become resident in an area and form stable home ranges, whilst others are nomadic and appear to wander from one place to another. Two crucial factors determining the size of a fox territory are the availability of food and the cost of defending the territory.
Wild animal carcasses were collected from hunting, road kills or research stations, therefore only an approximate location of the animal can be used. Hunters and gamekeepers who shoot foxes as part of pest population control were contracted to collect carcasses. Carcasses were delivered to field stations and frozen until sampling was undertaken. Road kills were only occasionally suitable for testing, therefore the number was low. No issues resulted in deviation from the sampling plan.
Reports were made at NUTS 3 level (the lowest level of NUTS; in GB individual counties or upper‐tier authorities, unitary authorities, or districts; districts in NI). The NUTS boundaries are only rarely amended and therefore comparisons could be made from one year to the next in terms of distribution.
In GB, 464 samples were collected and tested. In NI, 302 samples were collected and tested. The sampling activity targeted the regions with higher fox density, according to the red fox population density map provided (See Figure 10 and Figure 14 for GB; Figure 11 and Figure 15 for NI).
Figure 10.

Great Britain – Geographical distribution of samples
Figure 14.
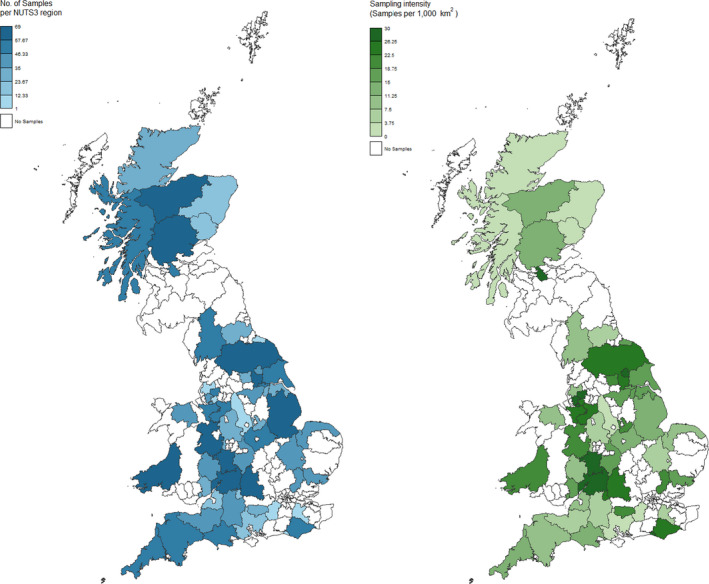
Great Britain – Sampling activity and intensity by NUTS 3 region
Figure 11.
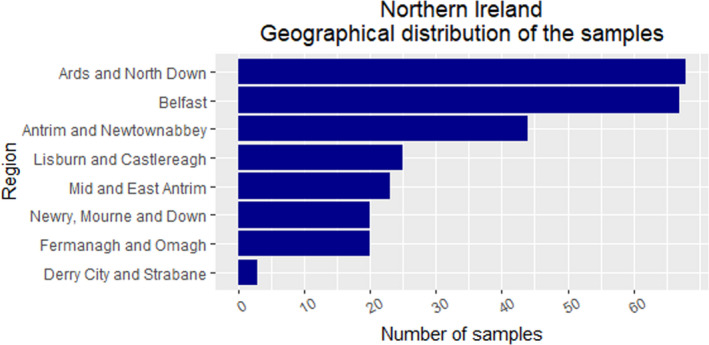
Northern Ireland – Geographical distribution of samples
Figure 15.
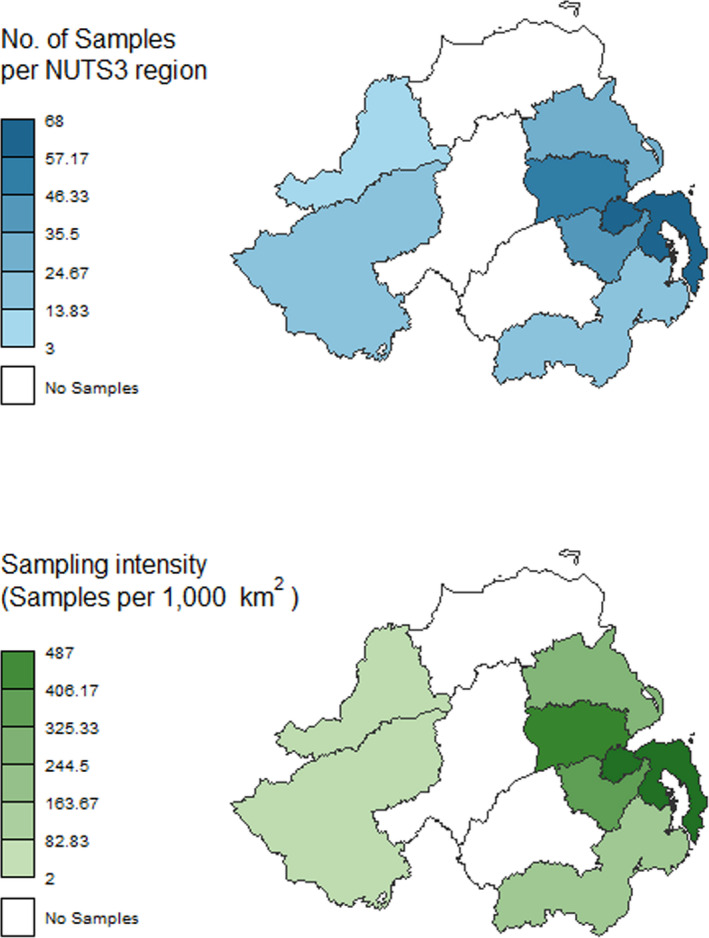
Northern Ireland – Sampling activity and intensity by NUTS 3 region
Note from EFSA: the maps and the plots from Figures 10, 11, 12, 13, 14, 15, generated by EFSA, do not reflect the data reported by the UK. In fact, due to the UK exiting the EU, EFSA was not allowed to accept data referring to data generated after the 31 January 2020. Therefore, the analysis was conducted on data up to the 31 January 2020.
Figure 12.
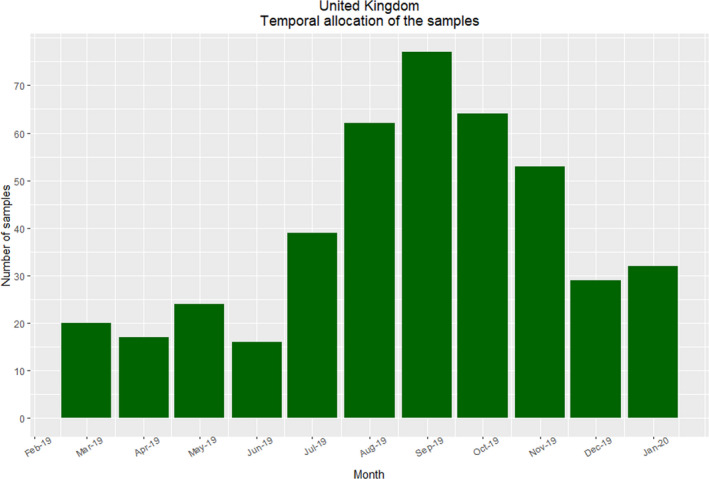
Great Britain – Temporal distribution of samples
Figure 13.

Northern Ireland – Temporal distribution of samples
Sampling was carried out at certain times of the year; the target was the wild population and hunting was not permitted during the breeding season (See Figures 13–14).
3.3.2. EFSA comments and considerations
3.3.2.1. Type and sensitivity of the detection method
Type of test: Both methods used for detection of E. multilocularis in the UK were well described. GB selected a PCR Cest1‐Cest2 NAD1 test (Mathis et al., 1996; Dinkel et al., 1998) for detection of E. multilocularis in rectal content. In NI, the SCT test (Eckert, 2003), considered as the reference standard for detection of E. multilocularis eggs from individual intestinal content, was used.
Test sensitivity: The diagnostic technique used by GB has been found to range from 88% to 95.7% (Casulli et al., 2015). APHA/FERA laboratory used a sensitivity of 78% considering the lowest possible sensitivity based on successful ring trial participation (EFSA, 2017). This value also corresponds with the EFSA′s recommended value of the sensitivity.
According to Casulli et al. (2015) and Conraths and Deplazes (2015), the SCT method selected by NI has a sensitivity of 98% and 83.8%, respectively. The analyses performed at the AFBI considered a Se of 99% (Eckert, 2003). The considerations about the appropriateness of the TSe value chosen are the same as last year: the evidence provided to support the TSe value for the SCT (Eckert, 2003) actually refers to a previous work (Hofer et al., 2000). However, the aim of the latter study was not to estimate the sensitivity of the SCT test, but rather to estimate the prevalence in the target population. In the paper of Hofer, it is reported that ‘no sample classified as negative by the SCT was detected positive by the intestinal scraping technique (IST)’. This observation could falsely lead to the conclusion that the SCT has a sensitivity close to 100%, but in reality, the only possible conclusion is that the IST sensitivity is not higher than the one of the SCT, but both of them are unknown. To estimate the diagnostic sensitivity of a test it is essential to know the real conditions of the samples that are examined, i.e. if they are truly infected or if they are negative controls. In the absence of this information, it is impossible to estimate the probability of the test to detect a positive sample given that the sample is truly infected (as the latter condition is not known). Note that this procedure was not followed to estimate the diagnostic sensitivity of the IST technique neither. As a conclusion, the almost perfect sensitivity of the SCT is, in reality, an assumption not supported by an adequate scientific evidence. EFSA recommends using a TSe of 0.78 as a more conservative option: an overestimation of the performance of the test can lead to wrong conclusions on the area sensitivity achieved.
3.3.2.2. Selection of the target population
Definition of susceptible host population target by the system: The selection of the red fox to perform the pathogen surveillance seems appropriate, as this species has been recognized as the main wildlife definitive host species for this parasite (EFSA AHAW Panel, 2015). Regarding the absence of other potential wild definitive hosts (raccoon dogs, wolves) the information is consistent with the report of Ireland. A reference has been provided on the population of British mammals and raccoon dogs and wolves are not recognized or recorded as breeding populations.
Size of susceptible host population targeted by the system: Data of fox population size is well documented for GB and NI.
3.3.2.3. Sampling strategy
Epidemiological and sampling unit: For GB, the epidemiological unit (post‐mortem faecal samples from individual animals of research stations) was well defined and ensures individuality. The same is agreed for NI, where intestinal contents from hunted or road kill individual animals were sampled.
Sample size calculation: The method used to calculate the sample size of GB was the RIBESS tool. The sample size was calculated with an overall sensitivity of the diagnostic approach of 0.78 and a population size of 240,000(red fox population). With these conditions, the minimum number of samples to collect in order to obtain a minimum of 0.95 of area sensitivity is 383. The total number of samples considered by EFSA (collected and tested before the 31 January 2020) from GB was 433, which ensures the fulfilment of the technical legal requirements of Commission Delegated Regulation (EU) 2018/772 regarding a confidence level of at least 0.95 against a DP of 1% (0.01). The method used to calculate the sample size of NI was the RIBESS tool. The sample size was calculated with an overall sensitivity of the diagnostic approach of 0.99 and a population size of 14,000 (red fox population). With these conditions, the minimum number of samples to collect in order to obtain a minimum of 0.95 of area sensitivity is 298. The total number of samples considered by EFSA (collected and tested before the 31 January 2020) from NI was 270. If a sensitivity of 0.78 is considered, as suggested by EFSA as a worse‐case scenario (EFSA, 2015), the required samples to fulfil the technical legal requirements regarding a confidence level of at least 0.95 against a DP of 1% (0.01) increase to 379. As an internal validation of the TSe has not been made with a large number of samples year over year (ideally it should be determined by each lab for the protocol used in‐house), a value of 0.78 would be the most suitable value in order to calculate the sample size. The total number of samples collected by NI and considered for this assessment (270), assuming the theoretical value of 0.78 as TSe, returns a confidence level equal to 0.88, lower than the value indicated among the technical legal requirements of Commission Delegated Regulation (EU) 2018/772 regarding a confidence level of at least 0.95 against a DP of 1% (0.01). Even considering the total number of samples collected and tested until the end of March 2020 (302, as declared by NI), and adopting a more conservative approach (i.e. assuming a TSe of 0.78) the target confidence of 95% is not achieved.
On the other hand, the sampling carried out in the Republic of Ireland, given the lack of geographical barrier between the two regions, would provide additional guarantees that NI, as part of Isle of Ireland, remains disease free this year.
Implementation of the sampling activity: The sampling process has more of the characteristics of a convenience sampling, rather than a simple random sample. The difficulties in performing a simple random sampling technique, however, are well known and are broadly discussed in previous reports. See also Figure 14.
3.3.2.4. Methodology
Design prevalence: The DP used was equal to 1% (0.01), as it is specified in Annex I to Commission Delegated Regulation (EU) 2018/772.
Epidemiological geographical unit: UK was divided in two geographical epidemiological units, the whole territory of GB and NI.
Methodology for calculation of the area sensitivity: The area sensitivity was estimated by GB using the RiBESS tool. The parameters included for the calculation were the following:
DP of 1%,
TSe of 0.78,
population size of 240,000 and
sample size of 433 (considered by EFSA).
The value of the area sensitivity (0.966) exceeded the established minimum value of 0.95 needed to fulfil the technical legal requirements included in Commission Delegated Regulation (EU) 2018/772.
The area sensitivity for NI considering the following parameters:
DP of 1%,
TSe of 0.99,
population size of 14,000 and
sample size of 270 (From April 2019 to January 2020),
With these conditions, area sensitivity is lower than 0.95 (0.934). If a TSe of 0.78 is assumed, as suggested by EFSA (2015), the area sensitivity (0.881) assumes even lower values. Only considering the sample size declared by NI (302) and adopting a less conservative approach (TSe = 0.99) NI achieves the required confidence of freedom.
In summary, the set of data from the surveillance activity in 2019/2020 for the UK, due to the use of a TSe value not supported by adequate scientific evidence by NI, does not ensure the fulfilment of the technical legal requirements of Commission Delegated Regulation (EU) 2018/772 regarding a confidence level of at least 0.95, against a DP of 1% (0.01) for one of its geographical epidemiological units (NI), unless the TSe reported by the laboratory on a small set of spiked samples is used.
Apart from the legal requirements and from a geographical point of view, the whole island of Ireland (i.e. combining NI (UK) and Ireland) can be considered as one epidemiological unit as the fox population is widely distributed in the island of Ireland and individual animals move freely throughout the territory without physical barriers. EFSA performed therefore a theoretical analysis considering the population of foxes of the whole territory of Ireland by combining the results of NI and Ireland. The global area sensitivity achieved would be 0.995, i.e. above the confidence required by the legislation.
| Surveillance component | Component sensitivity | Overall area sensitivity |
|---|---|---|
| IE | 0.957 | 0.995 |
| NI | 0.881 (TSe = 0.78) |
3.4. Norway
3.4.1. Information as submitted in the report by the country
In the Norwegian E. multilocularis surveillance system, a DNA‐fishing technique was used (Isaksson et al., 2014), referred to as PCR 12S rRNA, which involves magnetic capture mtDNA extraction from samples applying specific DNA‐hybridisation (Isaksson et al., 2014), followed by real‐time PCR (CO1rtPCR) (Øines et al., 2014). Samples are also analysed in duplicates in the detection step to increase sensitivity, and to reduce the risk of errors introduced by the operator. Results from samples with very low target DNA have shown some false negative which are minimized by running detection in duplicates (Øines et al., 2014). Primers were ‘EMrtCO1F’ (5′‐TGGTATAAAGGTGTTTACTTGG‐3′), ‘EMrtCO1Rew’ (5′‐ACGTAAACAACACTATAAAAGA‐3′) and ‘Zen probe’ 5′‐56‐FAM/TCTAGTGTA/Zen/AATAAGAGTGATCCTATTTTGTGGTGGGT/3IABkFq/‐3′. Following a positive signal, samples are verified by PCR/sequencing confirmation of NAD1 (Trachsel et al., 2007) and an independent real‐time PCR (Taq PCR/12S rDNA real‐time by Isaksson et al., 2014).
TSe was assumed to be at least 63% and the specificity 100% (see Øines et al., 2014 for details). However, the true TSe is probably higher (see Figure 16 on results of spiked samples).
Figure 16.
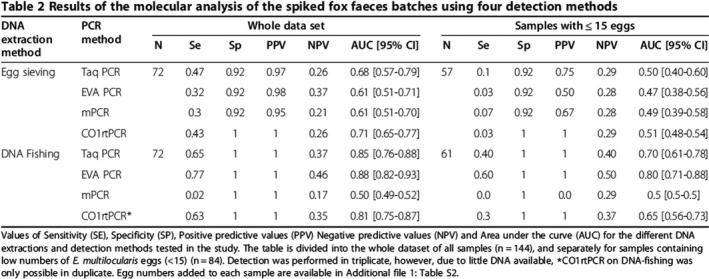
Table extracted from Øines et al., 2014 on the results of the molecular analysis of the spiked fox faeces batches using four detection methods
During the routine testing, eggs/DNA extracted from whole worms (E. multilocularis provided by the EURL) and MilliQ water is included as positive and negative control, respectively.
Prior to analysis of the surveillance samples, we test the new reagents each year by spiking faeces or water with known numbers of E. multilocularis eggs or worms. The results are listed in the table below, which demonstrates an overall sensitivity of 0.76. The sensitivity is positively correlated with the amount of DNA in the samples. In samples with ≥ 10 eggs the sensitivity is > 0.9. For samples with ≥ 5 eggs, the sensitivity is > 0.8. (see table in Figure 17).
Figure 17.
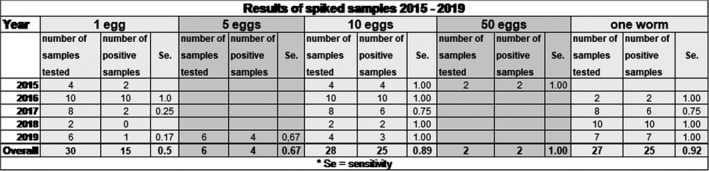
Table reporting the results from testing spiked samples (2016–2019 data)
Specificity: Negative controls (MQ water) were included for all reactions. None were positive by RT‐PCR.
The final evaluation of the Proficiency Testing (PT) 2019 was positive. The reporting and results for the results of the Echinococcus sp. PT from EURLP 2020 on identification to species level is due until autumn because of the COVID‐19 situation.
Red fox is the target species and practically, the only wild definitive host for E. multilocularis in Norway. There are only small populations of wolves and artic foxes, whereas raccoon dogs are only occasionally reported. Artic fox is a critically endangered species in Mainland Norway and in 2019 there was an estimated 280 adult animals.4 In winter 2019–2020 there was registered 56 wolves in Norwegian territories and 47–50 wolves living in territories that is located partly in Norway and partly in Sweden.5
In addition to the 543 red fox tested in 2019, as part of the Norwegian official surveillance program (not reported in the E. multilocularis annual report), samples from small number (< 20) of wolves (Canis lupus), submitted for forensic post‐mortem examination, were also tested analysed for E. multilocularis; all tested negative.
There are no scientific studies describing the Norwegian red fox population size. However, around 21,000 red foxes are hunted annually in Norway (Statistics Norway) and in the absence of better alternatives, an updated estimated Norwegian red fox population of 151,000 was used in the surveillance programme. This updated population estimate was provided by professor emeritus Olav Hjeljord at the Norwegian University of Life Sciences and was partly based on the spatial distribution of preferred fox habitat and hunting statistics. Prof. Hjeljord confirmed that the estimate of a population size of 151,000 red foxes in Norway are still valid (personal communication, 29/6/2020).
The red fox is geographically distributed all over Norway, but the population densities during spring are (roughly estimated) varying from 1 red fox/10 km2 (mountain areas), 3 red foxes/10 km2 (forest/marsh) and 10 red foxes/10 km2 (urban/agricultural areas; e.g. Akershus, Vestfold, Østfold) (pers.com. prof. Olav Hjeljord) (See also Figure 18).
Figure 18.
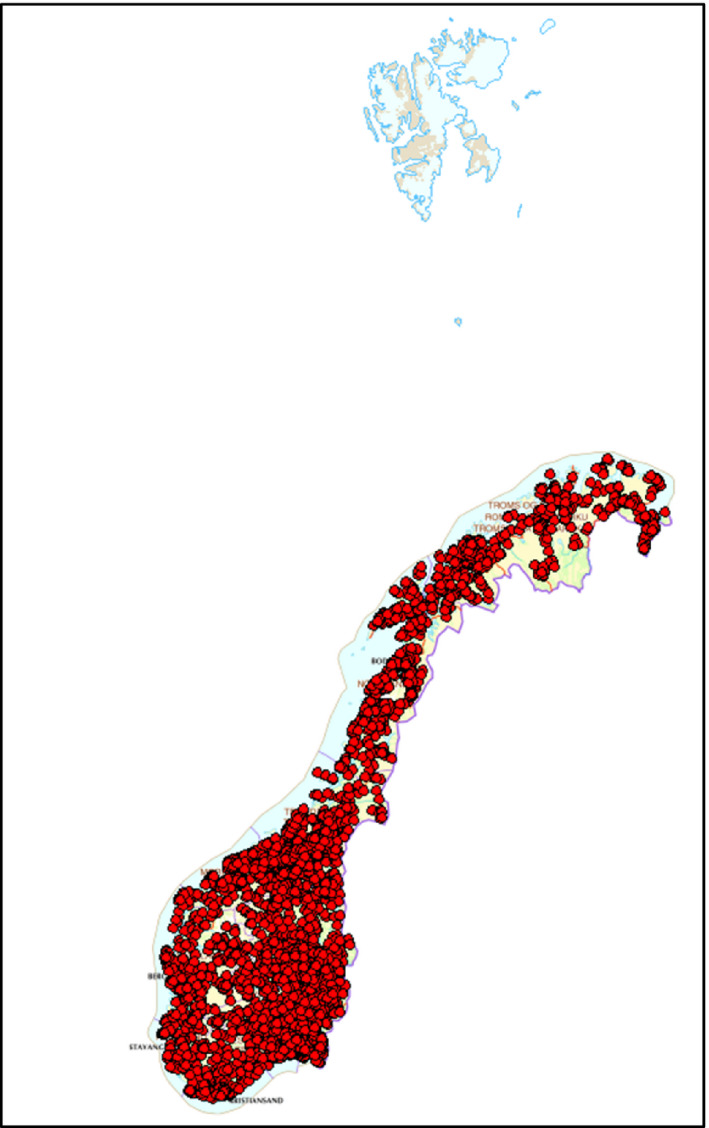
Map showing observations of red fox in Norway. Online service where citizens can logon and register their observations of fauna and flora in Norway. Source: Norwegian Biodiversity Information Centre. https://artsdatabanken.no/Pages/180936
The RiBESS tool (https://shiny-efsa.openanalytics.eu/app/ribess) was used to estimate the sample size required to substantiate the absence of the parasite from the target population with a confidence level of 95%. The goal was approximately 474 samples from red foxes in 2019, i.e. the epidemiological unit is the red fox.
In the Norwegian neighbouring country, Sweden, the first case of E. multilocularis was reported in late 2011 in a red fox from the southern part of the country. Consequently, foxhunters along the Swedish Norwegian border in the south‐eastern part of Norway were encouraged to increase their hunting and to submit more samples. The presence of E. multilocularis in Sweden may entail an increased risk of introduction of the parasite to Norway via migrating foxes. However, habitat use and extent of migration of red foxes in Sweden is not known. This lack of knowledge makes it complicated to assess the potential threat from Swedish foxes. Additionally, increasing prevalence of E. multilocularis has been observed in other nearby regions such as the Baltics and Denmark. We therefore consider the risk of introduction to be relatively high, and for calculation of the sample size needed to achieve the desired target confidence of freedom we used a probability of introduction of 0.5.
Initially, red foxhunters from across the country were invited to participate based on a list obtained from The Norwegian Register of Hunters. After a few years, it became very popular to participate in the surveillance program. Therefore, the last 4–5 years we have had an online registration at the NVI's Web pages to register as a (potential) hunter for the following years sampling. This registration is usually open for 3–4 weeks in November. The hunters enter their name and municipality via the webpages of the Norwegian Veterinary Institute (https://www.vetinst.no/nyheter/registrering-som-provetaker-av-rodrev). This registration is announced on NVI's web page and at the NVI's Facebook page. Those that have contributed to the programme previous years are invited by e‐mail to register, but the registration is also open for new hunters. The selection of foxhunters has then been based on residence and previous quality of their submitted samples. In addition, the selection also includes some hunters that are new to the program and therefore covers some new regions.
Sample containers and detailed instructions for sampling were forwarded to the hunters who participated in the program. The foxes were mainly killed with firearms (shotgun or rifle), but occasionally caught in traps or road killed. To secure, that the samples originated from individual animals the hunters also had to submit the tongue from each fox. The samples together with information concerning origin of the fox, date of the hunt, sex (male or female) and estimated age of the animal (juvenile or adult) were submitted to the laboratory in pre‐paid envelopes. In addition to samples from foxes, samples from wolves killed legally or illegally during 2019 were tested for E. multilocularis. For safety reasons, all samples were frozen at –80°C for at least three days before analysis. All counties in Norway were represented in the sampling regime.
543 samples were collected from red foxes in 2019 and all were negative in PCR.
Samples were collected throughout 2019. The spatial distribution of samples is somewhat uneven since the topography of Norway (large areas with mountains) entails scattered settlements, and hunters do the fox sampling voluntarily in the proximity of their homes. The temporal distribution of samples is also somewhat uneven due to preferred hunting conditions during winter and banned hunting between 15 April and 15 July (and between 24 and 31 December). In September and October, it is also hunting season for wild cervids such as moose and red deer (and in which many Norwegian hunters participate), which might be an explanation for the low numbers of red fox samples from these months.
Figure 19.

Norway – Geographical distribution of samples
Figure 20.
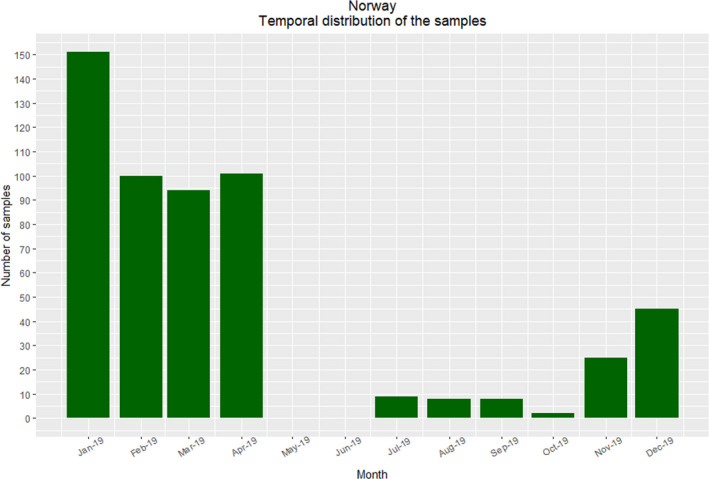
Norway – Temporal distribution of samples
Figure 21.

Norway – Sampling activity and intensity by NUTS 3 region
3.4.2. EFSA comments and considerations
3.4.2.1. Type and sensitivity of the detection method
Type of the detection method: Norway used a DNA‐fishing technique, the PCR 12S rRNA (Isaksson et al., 2014), which is well described and appropriately referenced in the report.
Test sensitivity: Internal trials performed by Norway (see Figure 17) seem to indicate a better performance of the test which is correlated to the amount of eggs/worms in the sample. The choice of Norway to use a TSe equal to 0.63 appears to be a conservative choice, in line with the results obtained in the Norwegian laboratory.
Table 7 summarises the results of the set of trials performed in Norway on samples spiked with different concentrations of eggs and worms.
Table 7.
Summary of the number of tested spiked samples (n) and number of samples testing positive (s) for each concentration of egg/worm. The last column reports the outcome of an exact binomial test (R Core Team, 2019)6
| Spike | s | n | Test sensitivity 50th perc (95% CI) |
|---|---|---|---|
| 1 egg | 15 | 30 | 0.5 (0.31–0.69) |
| 5 egg | 4 | 6 | 0.67 (0.22–0.96) |
| 10 egg | 25 | 28 | 0.89 (0.72–0.98) |
| 50 egg | 2 | 2 | 1 (0.16–1) |
| 1 worm | 25 | 27 | 0.93 (0.76–0.99) |
| Overall | 71 | 93 | 0.76 (0.66–0.84) |
Taken individually and looking at the 50th percentile there is a positive correlation between the concentration of the parasite in the sample and the sensitivity. Unfortunately, the small number of samples used to test high concentrations (50 eggs) brings a huge uncertainty around the estimate associated to the results (95% CI: 0.15–0.98). This uncertainty also affects the estimation of the overall performance of the test: pooling all the results together allows to estimate the performance of the test in a situation close to reality, i.e. where the amount of the parasite or its eggs is unknown. The bottom line in the table shows the result of this estimation: based on the available data, the test appears to have a sensitivity greater than 0.76 in 50% of the cases; however, the lower bound of the confidence interval suggests that a more conservative value would be 0.66. This low value, as said, is data driven and affected by the sample size: additional test will contribute to narrow the uncertainty around the 50th percentile.
For precautionary reasons the diagnostic sensitivity was set to the sensitivity obtained by Øines et al., 2014 (0.63), a lower value than the minimum recommended by EFSA (0.78) and lower than the lower bound of the estimated performance based on internal trials (0.66). Such a low TSe implies a much higher effort to reach the 95% of confidence stated in the legislation, as a large sample size is required.
3.4.2.2. Selection of the target population
Definition of susceptible host population target by the system: Red fox was considered the target species for Norway, and only few numbers of wolves were also included in the surveillance, but not reported. The reasons put forward by Norway to justify its decision of not including other wild definitive hosts (artic foxes and raccoon dogs) are valid.
Size of susceptible host population targeted by the system: In absence of data on fox populations in Norway, the size was estimated considering the annual hunted foxes.
3.4.2.3. Sampling strategy
Epidemiological unit: The epidemiological unit appears in the report and is defined as the red fox (Vulpes vulpes). Individual rectal contents were collected directly by hunters.
Sample size calculation: the EFSA RiBESS tool, was used to verify that the sample size was sufficient to claim a prevalence of not more than 1% at a confidence level of at least 95%. Considering DP of 1% (0.01), a TSe of 0.63 and a population size of 151,000, the sample sized required is 474. The number of samples collected by Norway in 2019 (543 samples) is more than required.
Implementation of the sampling activity: Samples were collected from all the Norwegian NUTS3 regions with an increase of the sampling in the south‐east and north‐west of the country. The differences of sampling intensities among the different areas have also been justified in the report.
3.4.2.4. Methodology
Design prevalence: The DP was equal to 1% (0.01), as it is specified in Annex I to Commission Delegated Regulation (EU) 2018/772.
Epidemiological geographical unit: The geographical unit is deduced to be the entire territory of Norway. The choice is sound as no risk factors were reported to justify the identification of sub‐areas within the Norwegian territory.
Methodology for calculation of the area sensitivity: The area sensitivity was estimated for Norway using the RiBESS tool and considering the following parameters:
DP of 1%,
TSe of 0.63,
population size of 151,000 and
sample size of 543,
The area sensitivity value is 0.968 (> 0.95), which exceeds the established minimum value of 0.95 needed to fulfil the technical legal requirements of Commission Delegated Regulation (EU) 2018/772.
In summary, the set of data relative to the surveillance activity in 2019 ensures the fulfilment of the technical legal requirements of Annex I of Commission Delegated Regulation (EU) 2018/772.
4. Conclusions
E. multilocularis was not detected in any of the samples from the four countries (Finland, the UK, Ireland, and Norway) collected in the 12‐month reporting period (2019 or 2019–2020).
All the countries participating in this surveillance (Finland, the UK, Ireland, and Norway) fulfil the technical legal requirements regarding the use of appropriate techniques for the detection of E. multilocularis in intestinal contents or faeces. All these countries use different methods for detection of the parasite as described in the report. Sensitivity (and specificity) values of the techniques have been reported for a proper assessment of the surveillance performance.
All the countries participating in this surveillance (Finland, the UK, Ireland, and Norway) fulfil the technical legal requirements regarding the collection of samples from wild definitive hosts. The four countries selected adequate wild definitive hosts in order to perform the surveillance.
All the countries participating in this surveillance (Finland, the UK, Ireland and Norway) fulfil the technical legal requirements concerning an appropriate sampling for detection of the E. multilocularis parasite, if present in any part of the Member State, at the DP of less than 1% (0.01).
The sampling strategies performed by Finland, the UK, Ireland and Norway cannot be considered ‘based on a simple random sample’: the sampling strategy in wild live animals is not random sampling but rather convenience sampling. Also, obtaining representative samples from wildlife populations is often hampered by the lack of precise knowledge on the distribution of wild host populations (EFSA, 2015), although some countries demonstrated that they had such information, based on combining sampling activity results and modelling.
All the countries participating in this surveillance (Finland, the UK, Ireland and Norway) fulfil the technical legal requirements regarding the 12‐month surveillance period collection. In general, the lower number of wild animal samples during spring and summer was well justified and historical data show that this lower number does not compromise the success of the detection of the parasite.
All the countries participating in this surveillance (Finland, the UK, Ireland and Norway) fulfil the technical legal requirements regarding the confidence level of at least 0.95 against a DP of 1%.
Assuming a TSe of 0.78 as per EFSA recommendation in the absence of scientific evidence supporting other values (as in the case of NI), the number of samples collected by NI is not sufficient to achieve the confidence level of at least 0.95 against a DP of 1% as required in the relevant legislation. The area sensitivity (or confidence level) achieved is equal to 0.88, lower than the value indicated among the technical legal requirements of the Regulation. The UK should provide scientific evidence to support the TSe value proposed in the surveillance.
5. Recommendations
Norway and Finland are recommended to publish the results of their internal trials performed in order to estimate the sensitivity of the diagnostic assays used. The scientific publication(s) may serve as a basis for an overall project that enable a sound scientific approach in order to validate and estimate the diagnostic sensitivity (and specificity) of the diagnostic assays used for E. multilocularis at EU level. This project could be set up in collaboration with EFSA and the EURL.
NI shall consider the critical appraisal provided in this report on the study performed by Eckert (2003) related to the TSe of the SCT and reconsider the value used to substantiate their freedom of E. multilocularis. An internal study to estimate the performance of the SCT in NI is recommended.
Glossary
- Alveolar echinococcosis
The human disease caused by infection with the larval stage (metacestode) of E. multilocularis. It is characterised by infiltrative, tumour‐like growth, initially in the liver, potentially causing high fatality rates
- EFSA Data Collection Framework (DCF)
The EFSA web interface accessible by most common web browsers through which data providers can submit their files. The system provides automatic feedback on errors in structure and content, and confirmation of successful submissions
- Enzyme‐linked immunosorbent assay (ELISA)
The test that applies the immunological concept of an antigen binding to its specific antibody, which allows detection of very small quantities of antigens such as proteins, peptides, hormones, or antibody in a fluid sample, utilizing enzyme‐labelled antibodies or antigens and a chromogenic substrate for the enzyme to detect the target molecules
- Geographical epidemiological unit
The portion of territory within a given Member State characterised by a specific risk of presence which differs from other portions, if any. An example could be the portion of territory within a defined distance from the border. In this assessment, all countries have assumed the entire territory as a unique geographical epidemiological unit
- NUTS
The Nomenclature of Territorial Units for Statistics (NUTS), or in French Nomenclature Unités Territoriales Statistiques, is a geocode standard for referencing the administrative divisions of countries for statistical purposes. The standard was developed by the European Union and subdivides the territory of the European Union into regions at three different levels (NUTS 1, 2 and 3, moving from larger to smaller territorial units (see also http://epp.eurostat.ec.europa.eu/statistics_explained/index.php/Glossary:NUTS)
- Odds Ratio (OR)
The ratio of the odds of an event occurring in one group to the odds of it occurring in another group. It estimates the probability of the event given exposure to a specific factor by measuring the probability of exposure given the presence of the event
- RiBESS
Risk Based Estimate of System Sensitivity and Sample Size: The R‐based tool developed by EFSA for the calculation of the sample size needed to substantiate absence of a given disease and/or to calculate the survey sensitivity (confidence) once the samples have been collected. Freely available at www.efsa.openanalytics.eu
- SCT
Sedimentation and counting technique The technique for the quantitative assessment of the E. multilocularis burden of foxes or other definitive hosts, where intestinal material is washed and sedimented several times and the resulting sediment is examined under a stereomicroscope for the presence of the parasite
Abbreviations
- AFBI
Agri‐Food and Biosciences Institute
- ASe
area sensitivity
- CI–CL
confidence interval–confidence level
- DALY
disability‐adjusted life‐year
- DAERA
Department of Agriculture, Environment and Rural Affairs
- Defra
Department for Environment Food and Rural Affairs
- DH
definitive host
- EFTA
European Free Trade Association
- EM
Echinococcus multilocularis
- GB
Great Britain (including England, Wales and Scotland)
- IST
intestinal scraping technique
- N
Target population size
- NI
Northern Ireland
- PCR
polymerase chain reaction
- RR
relative risk
- Se
Sensitivity
- SSe
System sensitivity
- Sp
Specificity
- ToR
Term of Reference
- TSe
test sensitivity
- UK
United Kingdom (including Great Britain and Northern Ireland)
Annex A – Finland. Assessment tables of the surveillance report
A.1. Finland – Part I of surveillance report: checklist on the surveillance system for a representative sample survey and comments
| Points addressed in Annex II | Element | Description of Element | Information provided in surveillance report | Requirement fulfilled | Comments |
|---|---|---|---|---|---|
| Type and sensitivity of the detection method | Type of test | The diagnostic test used for the detection of EM must be defined. Modifications of the original method should be indicated | Yes | Yes | Technique well described. The modification to the original technique is well described |
| Test sensitivity | The sensitivity and specificity of the test used in the surveillance system must be reported. This would ideally be estimates from each participating laboratory reported as a point estimate (average) of the values across the country with minimum and maximum values or a probability distribution. Alternatively, a value of 0.78, as recommended by EFSA (2015), shall be used | Yes | Yes |
Based on the results of internal trials, an exact binomial test indicates that the actual value of the test used in Finland may lie between 0.84 and 0.91 (95% CL). A Bayesian approach gives similar results. Therefore, the lowest value (0.84) may be the most conservative choice for estimating the overall system sensitivity considering a worst‐case scenario. However, these results should be published on a scientific journal. Finland used a more conservative approach assuming a test sensitivity of 0.78 |
|
| Selection of the target population | Definition of susceptible host population targeted by the system | The susceptible wild definitive host population(s) targeted by the surveillance system should be described and the choice justified. If domestic host species are sampled, evidence for the absence of wild definitive hosts and for these domestic animals having had access to outdoors should be provided | Yes | Yes | NA |
| Size of susceptible host population targeted by the system | The size of the targeted (wildlife) population should be reported, together with the evidence for this. Historical population data should be updated since these may not reflect current populations | Yes | Yes | Although population data have not been updated since 2007, new information regarding annual hunting bags has been included in the report. The decision to use the size of the population as published by Kauhala in the estimations is scientifically sound, considering that the sample size calculation is not heavily affected when the population size has large dimensions (see EFSA AHAW Panel, 2015). The fact of considering the sum of the red fox and raccoon dog populations as the target population size seems to be correct, as raccoon dogs can act as DHs in conjunction with the red fox (EFSA AHAW Panel, 2015) | |
| Sampling strategy | Epidemiological unit | It should be clearly defined if individual animals or individual faeces samples collected from the environment constitute the epidemiological unit. If individual faeces samples are collected from the environment, the method applied to establish the species from which the faeces originated has to be reported | Yes | Yes | NA |
| Sample size calculation | The applied survey design should be fully documented, including considerations regarding potential biases inherent in the survey design. The method and the formula used to calculate the sample size should be fully documented | Yes | Yes | NA | |
| Implementation of the sampling activity | The sampling methods used should be fully documented including the related assumptions and uncertainties, and a justification for choosing the approach should be provided. Timeframe of the surveillance data and geographical clustering of the infection must to be reported. The sample collection period must comprise the whole year and the spatial distribution of the sampling must be homogeneous | Yes | Yes | NA | |
| Methodology | Design prevalence (DP) | DP is specified in Annex II to Regulation (EU) No 1152/2011 and must be 1% or lower | Yes | Yes | NA |
| Geographical epidemiologic unit | The geographic epidemiological unit(s) identified as target for the surveillance activity has to be clearly indicated and supported by justification | Yes | Yes | NA | |
| Methodology for calculation of area sensitivity | For the calculation of the area sensitivity, the diagnostic sensitivity should be set conservatively to the lowest value, excluding the lowest 20th percentile, from the ones reported in the scientific literature and related to the diagnostic tests implemented by the countries listed in Annex I of the Commission Delegated Regulation (EU) No 1152/2011. In this case, is 78% (EFSA AHAW Panel, 2015) | Yes | Yes | NA |
A.2. Finland – Part II of surveillance report: descriptive statistics for a representative survey
| Parameter | Evidence | Requirement fulfilled | Action/Comments |
|---|---|---|---|
| Theoretical Sampling period | From 1 January 2019 to 31 December 2019 | Yes | NA |
| Actual Sampling Period | January 2019 to December 2019 | Yes | Exact sampling date not reported (day is missing). Explain, if possible, what the technical issue is or report the day od sampling |
| Number of samples | 523 | Yes | The sample size achieves an area sensitivity of 0.983 (> 0.95) |
| Number of test results | 523 | Yes | NA |
| Sensitivity | 0.78 | Yes | NA |
| Host | Raccoon dog and Red fox | Yes | N A |
| Animal sample | Yes | Yes | NA |
| Sampling Strategy and Design Objective sampling | Objective sampling and Simple random sample | Yes | The sampling strategy is actually a convenience sampling, biologically driven. The latter, in wildlife, is considered adequate |
| Sampling point | Wild (Hunting) | Yes | NA |
Annex B – Ireland. Assessment tables of the surveillance report
B.1. Ireland – Part I of surveillance report: checklist of the description of the surveillance system for a representative sample survey
| Points addressed in Annex II | Element | Description of Element | Information provided in surveillance report | Requirement fulfilled | Comments |
|---|---|---|---|---|---|
| Type and sensitivity of the detection method | Type of test | The diagnostic test used for the detection of EM must be defined. Modifications of the original method should be indicated | Yes | Yes | The diagnostic test chosen by Ireland is well described (PCR Cest1‐Cest2 NAD1) and a reference for this peer‐reviewed published method is provided |
| Test sensitivity | The sensitivity and specificity of the test used in the surveillance system must be reported. This would ideally be estimates from each participating laboratory reported as a point estimate (average) of the values across the country with minimum and maximum values or a probability distribution. Alternatively, a value of 0.78, as recommended by EFSA (2015), shall be used | Yes | Yes | NA | |
| Selection of the target population | Definition of susceptible host population targeted by the system | The susceptible wild definitive host population(s) targeted by the surveillance system should be described and the choice justified. If domestic host species are sampled, evidence for the absence of wild definitive hosts and for these domestic animals having had access to outdoors should be provided | Yes | Yes | The absence of other important definitive wild hosts is also supported by scientific literature |
| Size of susceptible host population targeted by the system | The size of the targeted (wildlife) population should be reported, together with the evidence for this. Historical population data should be updated since these may not reflect current populations | Yes | Yes | The last update on the population size is from 2015. However, with a population size greater than 10,000, moderate fluctuations in the population size would not significantly change the sample size required | |
| Sampling strategy | Epidemiological unit | It should be clearly defined if individual animals or individual faeces samples collected from the environment constitute the epidemiological unit. If individual faeces samples are collected from the environment, the method applied to establish the species from which the faeces originated has to be reported | Yes | Yes | NA |
| Sample size calculation | The applied survey design should be fully documented, including considerations regarding potential biases inherent in the survey design. The method and the formula used to calculate the sample size should be fully documented | Yes | Yes | NA | |
| Implementation of the sampling activity | The sampling methods used should be fully documented including the related assumptions and uncertainties, and a justification for choosing the approach should be provided. Timeframe of the surveillance data and geographical clustering of the infection must to be reported. The sample collection period must comprise the whole year and the spatial distribution of the sampling must be homogeneous | Yes | Yes | NA | |
| Methodology | Design prevalence (DP) | DP is specified in Annex II to Regulation (EU) No 1152/2011 and must be 1% or lower | Yes | Yes | NA |
| Geographical epidemiologic unit | The geographic epidemiological unit(s) identified as target for the surveillance activity has to be clearly indicated and supported by justification | Yes | Yes | NA | |
| Methodology for calculation of area sensitivity | For the calculation of the area sensitivity, the diagnostic sensitivity should be set conservatively to the lowest value, excluding the lowest 20th percentile, from the ones reported in the scientific literature and related to the diagnostic tests implemented by the countries listed in Annex I of the Commission Delegated Regulation (EU) No 1152/2011. In this case, is 78% (EFSA AHAW Panel, 2015) | Yes | Yes | NA |
B.2. Ireland – Part II of surveillance report: descriptive statistics for a representative survey
| Parameter | Evidence | Requirement fulfilled | Action/Comments |
|---|---|---|---|
| Theoretical Sampling period | From 1 January 2019 to 31 December 2019 | Yes | NA |
| Actual Sampling Period | 6 January 2019 to 18 December 2019 | Yes | NA |
| Number of samples | 400 | Yes | The sample size achieves an area sensitivity of 0.957 (> 0.95) |
| Number of test results | 400 | Yes | NA |
| Sensitivity | 0.78 | Yes | NA |
| Host | Red fox | Yes | NA |
| Animal sample | Yes | Yes | NA |
| Sampling Strategy and Design Objective sampling | Objective sampling and Simple random sample | Yes | The sampling strategy is actually a convenience sampling, biologically driven. The latter, in wildlife, is considered adequate |
| Sampling point | Hunting and Wildlife research h stations | Yes | NA |
Annex C – United Kingdom. Assessment tables of the surveillance report
C.1. Great Britain – Part I of surveillance report: checklist of the description of the surveillance system for a representative sample survey
| Points addressed in Annex II | Element | Description of Element | Information provided in surveillance report | Requirement fulfilled | Comments |
|---|---|---|---|---|---|
| Type and sensitivity of the detection method | Type of test | The diagnostic test used for the detection of EM must be defined. Modifications of the original method should be indicated | Yes | Yes | NA |
| Test sensitivity | The sensitivity and specificity of the test used in the surveillance system must be reported. This would ideally be estimates from each participating laboratory reported as a point estimate (average) of the values across the country with minimum and maximum values or a probability distribution. Alternatively, a value of 0.78, as recommended by EFSA (2015), shall be used | Yes | Yes | NA | |
| Selection of the target population | Definition of susceptible host population targeted by the system | The susceptible wild definitive host population(s) targeted by the surveillance system should be described and the choice justified. If domestic host species are sampled, evidence for the absence of wild definitive hosts and for these domestic animals having had access to outdoors should be provided | Yes | Yes | NA |
| Size of susceptible host population targeted by the system | The size of the targeted (wildlife) population should be reported, together with the evidence for this. Historical population data should be updated since these may not reflect current populations | Yes | Yes | NA | |
| Sampling strategy | Epidemiological unit | It should be clearly defined if individual animals or individual faeces samples collected from the environment constitute the epidemiological unit. If individual faeces samples are collected from the environment, the method applied to establish the species from which the faeces originated has to be reported | Yes | Yes | NA |
| Sample size calculation | The applied survey design should be fully documented, including considerations regarding potential biases inherent in the survey design. The method and the formula used to calculate the sample size should be fully documented | Yes | Yes | NA | |
| Implementation of the sampling activity | The sampling methods used should be fully documented including the related assumptions and uncertainties, and a justification for choosing the approach should be provided. Timeframe of the surveillance data and geographical clustering of the infection must to be reported. The sample collection period must comprise the whole year and the spatial distribution of the sampling must be homogeneous | Yes | Yes | NA | |
| Methodology | Design prevalence (DP) | DP is specified in Annex II to Regulation (EU) No 1152/2011 and must be 1% or lower | Yes | Yes | NA |
| Geographical epidemiologic unit | The geographic epidemiological unit(s) identified as target for the surveillance activity has to be clearly indicated and supported by justification | Yes | Yes | The whole territory of GB and NI were correctly considered each as one epidemiological unit in their respective analysis | |
| Methodology for calculation of area sensitivity | For the calculation of the area sensitivity, the diagnostic sensitivity should be set conservatively to the lowest value, excluding the lowest 20th percentile, from the ones reported in the scientific literature and related to the diagnostic tests implemented by the countries listed in Annex I of the Commission Delegated Regulation (EU) No 1152/2011. In this case, is 78% (EFSA AHAW Panel, 2015) | Yes | Yes | NA |
C.2. Great Britain ‐ Part II of surveillance report: descriptive statistics for a representative survey
| Parameter | Evidence | Requirement fulfilled | Action/Comments |
|---|---|---|---|
| Theoretical Sampling period | From 1 March 2019 to 29 February 2020 | Yes | NA |
| Actual Sampling Period | 2 March 2019 to 29 January 2020 | Yes | Note: due to the UK exiting the EU, EFSA rejected all data related to activities performed after the 31 January 2020 |
| Number of samples | 464 (433 accepted by EFSA due to the Brexit) | Yes | The sample size achieves an area sensitivity of 0.966 (> 0.95) |
| Number of test results | 464 (433 accepted by EFSA due to the Brexit) | Yes | NA |
| Sensitivity | 0.78 | Yes | NA |
| Host | Red fox | Yes | NA |
| Animal sample | Yes | Yes | NA |
| Sampling Strategy and Design Objective sampling | Objective sampling and Simple random sample | Yes | The sampling strategy is actually a convenient sampling based on biological considerations. Considered adequate in wildlife |
| Sampling point | Hunting | Yes | NA |
C.3. Northern Ireland ‐ Part I of surveillance report: checklist of the description of the surveillance system for a representative sample survey
| Points addressed in Annex II | Element | Description of Element | Information provided in surveillance report | Requirement fulfilled | Comments |
|---|---|---|---|---|---|
| Type and sensitivity of the detection method | Type of test | The diagnostic test used for the detection of EM must be defined. Modifications of the original method should be indicated | Yes | Yes | NA |
| Test sensitivity | The sensitivity and specificity of the test used in the surveillance system must be reported. This would ideally be estimates from each participating laboratory reported as a point estimate (average) of the values across the country with minimum and maximum values or a probability distribution. Alternatively, a value of 0.78, as recommended by EFSA (2015), shall be used | Yes | Impossible to evaluate | The evidence provided to support the test sensitivity value for the SCT (Eckert, 2003) actually refers to a previous work (Hofer et al., 2000) which focusses on the prevalence in the target population and not in the sensitivity of the SCT. The almost perfect sensitivity of the SCT (0.99) is actually an assumption. A safer option would be to follow the EFSA recommendation (Test Se = 0.78). As an alternative, NI should provide evidence to support the suggested test sensitivity value of 0.99 | |
| Selection of the target population | Definition of susceptible host population targeted by the system | The susceptible wild definitive host population(s) targeted by the surveillance system should be described and the choice justified. If domestic host species are sampled, evidence for the absence of wild definitive hosts and for these domestic animals having had access to outdoors should be provided | Yes | Yes | NA |
| Size of susceptible host population targeted by the system | The size of the targeted (wildlife) population should be reported, together with the evidence for this. Historical population data should be updated since these may not reflect current populations | Yes | Yes | NA | |
| Sampling strategy | Epidemiological unit | It should be clearly defined if individual animals or individual faeces samples collected from the environment constitute the epidemiological unit. If individual faeces samples are collected from the environment, the method applied to establish the species from which the faeces originated has to be reported | Yes | Yes | NA |
| Sample size calculation | The applied survey design should be fully documented, including considerations regarding potential biases inherent in the survey design. The method and the formula used to calculate the sample size should be fully documented | Yes | Impossible to evaluate | The use of a test sensitivity (0.99) not supported by adequate scientific evidence makes impossible to evaluate the adequacy of the output from the RiBESS tool | |
| Implementation of the sampling activity | The sampling methods used should be fully documented including the related assumptions and uncertainties, and a justification for choosing the approach should be provided. Timeframe of the surveillance data and geographical clustering of the infection must to be reported. The sample collection period must comprise the whole year and the spatial distribution of the sampling must be homogeneous | Yes | Yes | NA | |
| Methodology | Design prevalence (DP) | DP is specified in Annex II to Regulation (EU) No 1152/2011 and must be 1% or lower | Yes | Yes | NA |
| Geographical epidemiologic unit | The geographic epidemiological unit(s) identified as target for the surveillance activity has to be clearly indicated and supported by justification | Yes | Yes | GB and NI were correctly considered each as one epidemiological unit in their respective analysis | |
| Methodology for calculation of area sensitivity | For the calculation of the area sensitivity, the diagnostic sensitivity should be set conservatively to the lowest value, excluding the lowest 20th percentile, from the ones reported in the scientific literature and related to the diagnostic tests implemented by the countries listed in Annex I of the Commission Delegated Regulation (EU) No 1152/2011. In this case, is 78% (EFSA AHAW Panel, 2015) | Yes | Impossible to evaluate |
If a test sensitivity of 0.78 is assumed (as suggested by EFSA in case no adequate scientific evidence is provided supporting other values), even considering the sample size as declared by NI (302) the area sensitivity (0.908) is not sufficient to comply with the technical legal requirements of the EU regulation in force. Only assuming the test sensitivity value proposed by NI (0.99) the area sensitivity achieved satisfies the legal requirements (0.952). Note: the absence of scientific evidence does not imply that the proposed test sensitivity (0.99) is wrong. EFSA, however, in the absence of adequate scientific evidence cannot assess the performance of the surveillance activity |
C.4. Northern Ireland ‐ Part II of surveillance report: descriptive statistics for a representative survey
| Parameter | Evidence | Requirement fulfilled | Action/Comments |
|---|---|---|---|
| Theoretical Sampling period | From 1 April 2019 to 31 March 2020 | NA | NA |
| Actual Sampling Period | 7 April 2019 to 31 January 2020 | Yes | Note: due to the UK exiting the EU, EFSA rejected all data related to activities performed after the 31 January 2020 |
| Number of samples | 302 (270 accepted by EFSA due to the Brexit) | Impossible to evaluate | The sample size is sufficient only assuming a test sensitivity value of 0.99 and a sample size of 302. The test sensitivity value, however, is not supported by adequate scientific evidence |
| Number of test results | 302 (270 accepted by EFSA due to the Brexit) | Yes | NA |
| Sensitivity | 0.99 | Impossible to evaluate | The value is not supported by adequate scientific evidence. |
| Host | Red fox | Yes | NA |
| Animal sample | Yes | Yes | NA |
| Sampling Strategy and Design Objective sampling | Objective sampling and Simple random sample | Yes | The sampling strategy is actually a convenient sampling based on biological considerations. Considered adequate in wildlife |
| Sampling point | Hunting and Road transport | Yes | NA |
Annex D – Norway. Assessment tables of the surveillance report
D.1. Norway – Part I of surveillance report: checklist of the description of the surveillance system for a representative sample survey
| Points addressed in Annex II | Element | Description of Element | Information provided in surveillance report | Requirement fulfilled | Comments |
|---|---|---|---|---|---|
| Type and sensitivity of the detection method | Type of test | The diagnostic test used for the detection of EM must be defined. Modifications of the original method should be indicated | Yes | Yes | NA |
| Test sensitivity | The sensitivity and specificity of the test used in the surveillance system must be reported. This would ideally be estimates from each participating laboratory reported as a point estimate (average) of the values across the country with minimum and maximum values or a probability distribution. Alternatively, a value of 0.78, as recommended by EFSA (2015), shall be used | Yes | Yes | Despite internal trials seem to indicate a better performance of the test (Test Se = 0.76, with a 95% CI = 0.66‐0.84), a more conservative value was set (0.63, Øines et al., 2014). This value is lower than the minimum recommended by EFSA (0.78). Such a low test sensitivity implies a much higher effort to reach the 95% of confidence stated in the legislation, as a large sample size is required | |
| Selection of the target population | Definition of susceptible host population targeted by the system | The susceptible wild definitive host population(s) targeted by the surveillance system should be described and the choice justified. If domestic host species are sampled, evidence for the absence of wild definitive hosts and for these domestic animals having had access to outdoors should be provided | Yes | Yes | NA |
| Size of susceptible host population targeted by the system | The size of the targeted (wildlife) population should be reported, together with the evidence for this. Historical population data should be updated since these may not reflect current populations | Yes | Yes | NA | |
| Sampling strategy | Epidemiological unit | It should be clearly defined if individual animals or individual faeces samples collected from the environment constitute the epidemiological unit. If individual faeces samples are collected from the environment, the method applied to establish the species from which the faeces originated has to be reported | Yes | Yes | NA |
| Sample size calculation | The applied survey design should be fully documented, including considerations regarding potential biases inherent in the survey design. The method and the formula used to calculate the sample size should be fully documented | Yes | Yes | NA | |
| Implementation of the sampling activity | The sampling methods used should be fully documented including the related assumptions and uncertainties, and a justification for choosing the approach should be provided. Timeframe of the surveillance data and geographical clustering of the infection must to be reported. The sample collection period must comprise the whole year and the spatial distribution of the sampling must be homogeneous | Yes | Yes | NA | |
| Methodology | Design prevalence (DP) | DP is specified in Annex II to Regulation (EU) No 1152/2011 and must be 1% or lower | Yes | Yes | NA |
| Geographical epidemiologic unit | The geographic epidemiological unit(s) identified as target for the surveillance activity has to be clearly indicated and supported by justification | Yes | Yes | NA | |
| Methodology for calculation of area sensitivity | For the calculation of the area sensitivity, the diagnostic sensitivity should be set conservatively to the lowest value, excluding the lowest 20th percentile, from the ones reported in the scientific literature and related to the diagnostic tests implemented by the countries listed in Annex I of the Commission Delegated Regulation (EU) No 1152/2011. In this case, is 78% (EFSA AHAW Panel, 2015) | Yes | Yes | NA |
D.2. Norway ‐ Part II of surveillance report: descriptive statistics for a representative survey
| Parameter | Evidence | Requirement fulfilled | Action/Comments |
|---|---|---|---|
| Theoretical Sampling period | From 1 January 2019 to 31 December 2019 | NA | NA |
| Actual Sampling Period | 4 January 2019 to 23 December 2019 | Yes | NA |
| Number of samples | 543 | Yes | The sample size achieves an area sensitivity of 0.968 (> 0.95) |
| Number of test results | 543 | Yes | NA |
| Sensitivity | 0.63 | Yes | NA |
| Host | Red fox | Yes | NA |
| Animal sample | Yes | Yes | NA |
| Sampling Strategy and Design Objective sampling | Objective sampling and Simple random sample | Yes | The sampling strategy is actually a convenient sampling based on biological considerations. Considered adequate in wildlife |
| Sampling point | Hunting | Yes | NA |
Suggested citation: EFSA (European Food Safety Authority) and Zancanaro G, 2021. Annual assessment of Echinococcus multilocularis surveillance reports submitted in 2020 in the context of Commission Delegated Regulation (EU) 2018/772. EFSA Journal 2021;19(1):6382, 63 pp. 10.2903/j.efsa.2021.6382
Requestor: European Commission
Question numbers: EFSA‐Q‐2020‐00780, EFSA‐Q‐2020‐00781
Acknowledgements: EFSA wishes to thank the members of the EFSA Scientific Network for Risk Assessment in Animal Health and Welfare: Antti Oksanen, Belgees Boufana, Craig Simpson, Inger Sofie Hamnes, Helen Roberts, James O'Shaughnessy, Marja Isomursu, and William Byrne for their cooperation; Claire Donohue and Alexandra Papanikolaou for their technical and scientific support.
Competing interests: In line with EFSA's policy on declarations of interest, network experts Belgees Boufana and Helen Roberts did not participate in the development and endorsement of this scientific output if not on the sections of concern, i.e. Section 3.3.1 of this document.
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 1: © Kaarina Kauhala, Natural Resources Institute Finland; Figure 5: © Tomás Murray, Biodiversity Ireland; Figure 9: DEFRA, UK.
Approved: 14 December 2020
Notes
This year, due to the Covid19 pandemic and the consequences of the UK exiting the EU, in agreement with the European Commission, the deadline for the submission of the national reports was postponed to the 30 June 2020.
R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
References
- Atterby H, Allnutt TR, MacNicoll AD, Jones EP and Smith GC, 2015. Population genetic structure of the red fox (Vulpes vulpes) in the UK. Mammal Research, 60, 9–19. [Google Scholar]
- Casulli A, Possenti A, La Torre G, Boue F, Busani L, Colamesta V, Conraths FJ, D'Aguanno S, De Giusti M, De Vito C, Karamon J, Maas M, Mannocci A, Maffongelli E, Mipatrini D, Oksanen A, Probst C, Saulle R, Siles‐Lucas M, Umhang G, van den End S, van der Giessen J and Villari P, 2015. E. multilocularis infection in animals (GP/EFSA/AHAW/2012/01). EFSA Supporting Publication 2015;12(12):EN‐882, 168 pp. 10.2903/sp.efsa.2015.EN-882 [DOI] [Google Scholar]
- CDC (Centers for disease control and prevention), online. Parasites‐Echinococcosis. Available online: https://www.cdc.gov/parasites/echinococcosis/biology.html [Accessed: 5 July 2017].
- Conraths FJ and Deplazes P, 2015. E. multilocularis: epidemiology, surveillance and state‐of‐the‐art diagnostics from a veterinary public health perspective. Veterinary Parasitology, 213, 149–161. [DOI] [PubMed] [Google Scholar]
- Conserve Ireland , 2009. Red Fox. Available online: http://www.lhnet.org/red-fox/
- Croft S, Chauvenet A and Smith G, 2017. A systematic approach to estimate the distribution and total abundance of British mammals. PLoS ONE, 12, e0176399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RK, Romig T, Jenkins E, Tryland M and Robertson LJ, 2012. The impact of globalisation on the distribution of Echinococcus multilocularis. Trends in Parasitology, 28, 239–247. [DOI] [PubMed] [Google Scholar]
- Defra (Department for Environment Food and Rural Affairs), 2013. Request for information: bovine TB hotspots and population sizes of badgers and foxes. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/243894/5577.pdf
- Deplazes P, 2006. Ecology and epidemiology of Echinococcus multilocularis in Europe. Parassitologia, 48, 37–39. [PubMed] [Google Scholar]
- Dezsényi B, Tóth S, Horváth A, Szlávik J, Makrai Z, Strausz T, Nagy T, Dubóczki Z, Mersich T, Csomor J, Somorácz A, Nehéz L, Patonai A, Doros A, Danka J, Kucsera I, Auer H, Rezza G, Barth TF and Casulli A, 2019. Emerging human alveolar echinococcosis in Hungary. Early experiences in clinical management in a single center study from 2005‐2018. Journal of Infectious Diseases, 79, 118–119. [Google Scholar]
- Dinkel A, von Nickisch‐Rosenegk M, Bilger B, Merli M, Lucius R and Romig T, 1998. Detection of Echinococcus multilocularis in the definitive host: coprodiagnosis by PCR as an alternative to necropsy. Journal of Clinical Microbiology, 36, 1871–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control), 2016. Annual Epidemiological Report 2016 – Echinococcosis. Stockholm, Sweden. Available online: https://ecdc.europa.eu/en/publicationsdata/echinococcosis-annual-epidemiological-report-2016-2014-data
- ECDC (European Centre for Disease Prevention and Control), 2017. Echinococcosis. In: ECDC. Annual epidemiological report for 2015. Stockholm.
- Eckert J, 2003. Predictive values and quality control of techniques for the diagnosis of Echinococcus multilocularis in definitive hosts. Acta Tropica, 85, 157–163. [DOI] [PubMed] [Google Scholar]
- Eckert J and Deplazes P, 1999. Alveolar Echinococcosis in humans: the current situation in Central Europe and the Need for Countermeasures. Parasitology Today, 15, 315–319. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2012a. Scientific and technical assistance on Echinococcus multilocularis infection in animals. EFSA Journal 2012;10(11):2973, 22 pp. 10.2903/j.efsa.2012.2973 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2012b. A framework to substantiate absence of disease: the risk based estimate of system sensitivity tool (RiBESS) using data collated according to the EFSA Standard Sample Description ‐ An example on Echinococcus multilocularis. EFSA Supporting Publications 2012;9(12):EN‐366, 44 pp. 10.2903/sp.efsa.2012.EN-366 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013. Assessment of Echinococcus multilocularis surveillance reports submitted 2013 in the context of Commission Regulation (EU) No 1152/2011. EFSA Journal 2013;11(11):3465, 41 pp. 10.2903/j.efsa.2013.3465 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Assessment of Echinococcus multilocularis surveillance reports submitted in 2014 in the context of Commission Regulation (EU) No 1152/2011. EFSA Journal 2014;12(10):3875, 44 pp. 10.2903/j.efsa.2014.3875 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. Assessment of Echinococcus multilocularis surveillance reports submitted in 2015 in the context of Commission Regulation (EU) No 1152/2011. EFSA Journal 2015;13(11):4310, 58 pp. 10.2903/j.efsa.2015.4310 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Bau A, Palumbo R, Richardson J and Zancanaro G, 2016. Assessment of Echinococcus multilocularis surveillance reports submitted in 2016 in the context of Commission Regulation (EU) No 1152/2011. EFSA Journal 2016;14(12):4649, 55 pp. 10.2903/j.efsa.2016.4649 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Beltran‐Beck B and Zancanaro G, 2017. Scientific report on the assessment of Echinococcus multilocularis surveillance reports submitted in 2017 in the context of Commission Regulation (EU) No 1152/2011. EFSA Journal 2017;15(11):5051, 75 pp. 10.2903/j.efsa.2017.5051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) and Zancanaro G, 2019. Scientific report on the annual assessment of Echinococcus multilocularis surveillance reports submitted in 2019 in the context of Commission Delegated Regulation (EU) 2018/772. EFSA Journal 2019;17(11):5906, 58 pp. 10.2903/j.efsa.2019.5906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2015. Scientific opinion on Echinococcus multilocularis infection in animals. EFSA Journal 2015;13(12):4373, 129 pp. 10.2903/j.efsa.2015.4373 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2017. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2016. EFSA Journal 2017;15(12):5077, 228 pp. 10.2903/j.efsa.2017.5077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2018. The European Union summary report on trends and sources of zoonoses, zoonotic agents and foodborne outbreaks in 2017. EFSA Journal 2018;16(12):5500, 262 pp. 10.2903/j.efsa.2018.5500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden T and Harrington R, 2000. Exploring Irish mammals. Town House & Country House Ltd, Dublin, Ireland, 340 pp. [Google Scholar]
- Hofer S, Gloor S, Muller U, Mathis A, Hegglin D and Deplazes P, 2000. High prevalence of Echinococcus multilocularis in urban red foxes (Vulpes vulpes) and voles (Arvicola terrestris) in the city of Zurich, Switzerland. Parasitology, 120, 135–142. [DOI] [PubMed] [Google Scholar]
- Isaksson M, Hagström Å, Armua‐Fernandez MT, Wahlström H, Ågren EO, Miller A, Holmberg A, Lukacs M, Casulli A, Deplazes P and Juremalm M, 2014. A semi‐automated magnetic capture probe based DNA extraction and real‐time PCR method applied in the Swedish surveillance of Echinococcus multilocularis in red fox (Vulpes vulpes) faecal samples. Parasites & Vectors, 7, 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapel CMO, Torgerson PR, Thompson RCA and Deplazes P, 2006. Reproductive potential of Echinococcus multilocularis in experimentally infected foxes, dogs, raccoon dogs and cats. International Journal for Parasitology, 36, 79–86. [DOI] [PubMed] [Google Scholar]
- Kauhala K, 2007. Paljonko Suomessa on pienpetoja? Riista‐ja kalatalous Selvityksiä 1. Riista‐ja kalatalouden tutkimuslaitos, Helsinki 2007. Available online: http://jukuri.luke.fi/bitstream/handle/10024/532812/paljonko_suomessa_on_pienpetoja_julkaisu_nettiin.pdf?sequence=1
- Lalošević D, Lalošević V, Simin V, Miljević M, Čabrilo B and Čabrilo OB, 2016. Spreading of multilocular echinococcosis in southern Europe: the first record in foxes and jackals in Serbia, Vojvodina Province. European Journal of Wildlife Research, 62, 793–796. [Google Scholar]
- Marnell F, Kingston N and Looney D, 2009. Irish Red List No. 3 ‐ Terrestrial Mammals. National Parks & Wildlife Service, Dublin, Ireland, Available online: https://www.npws.ie/content/publications/irishred-list-no-3-terrestrial-mammals
- Mathis A, Deplazes P and Eckert J, 1996. An improved test system for PCR‐based specific detection of Echinococcus multilocularis eggs. Jornal of Helminthology, 70, 219–222. [DOI] [PubMed] [Google Scholar]
- Mihmanli M, Idiz UO, Kaya C, Demir U, Bostanci O, Omeroglu S and Bozkurt E, 2016. Current status of diagnosis and treatment of hepatic echinococcosis. World Journal of Hepatology, 8, 1169–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro P and Schantz PM, 2009. Echinococcosis: a review. International Journal of Infectious Diseases, 13, 125–133. [DOI] [PubMed] [Google Scholar]
- Øines Ø, Isaksson M, Hagström Å, Tavornpanich S and Davidson RK, 2014. Laboratory assessment of sensitive molecular tools for detection of low levels of Echinococcus multilocularis‐eggs in fox (Vulpes vulpes) faeces. Parasites & Vectors, 7, 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen A, Siles‐Lucas M, Karamon J, Possenti A, Conraths FJ, Romig T, Wysocki P, Mannocci A, Mipatrini D, La Torre G, Boufana B and Casulli A, 2016. The geographical distribution and prevalence of Echinococcus multilocularis in animals in the European Union and adjacent countries: a systematic review and meta‐analysis. Parasites & Vectors, 9, 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peregrine AS, Jenkins EJ, Barnes B, Johnson S, Polley L, Barker IK, De Wolf B and Gottstein B, 2012. Alveolar hydatid disease (Echinococcus multilocularis) in the liver of a Canadian dog in British Columbia, a newly endemic region. The Canadian Veterinary Journal, 53, 870–874. [PMC free article] [PubMed] [Google Scholar]
- Phillips SJ, Anderson RP and Schapire RE, 2006. Maximum entropy modeling of species geographic distributions. Ecological Modelling, 190, 231–259. [Google Scholar]
- R Core Team , 2019. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/
- Scott DM, Berg MJ, Tolhurst BA, Chauvenet ALM, Smith GC, Neaves K, Lochhead J and Baker PJ, 2014. Changes in the distribution of Red Foxes (Vulpes vulpes) in Urban Areas in Great Britain: findings and limitations of a media‐driven nationwide survey. PLoS ONE, 9, e99059 10.1371/journal.pone.0099059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgerson PR, Keller K, Magnotta M and Ragland N, 2010. The Global Burden of Alveolar Echinococcosis. PLOS Neglected Tropical Diseases, 4, e722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel D, Deplazes P and Mathis A, 2007. Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology, 134, 911–920. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization), 2017. Echinococcosis‐ Fact sheet 2017. WHO, Geneva, Switzerland. Available online: http://www.who.int/mediacentre/factsheets/fs377/en/
- Wright LJ, Newson SE and Noble DG, 2014. The value of a random sampling design for annual monitoring of national populations of larger British terrestrial mammals. European Journal of Wildlife Research, 60, 213–221. [Google Scholar]


