Abstract
COVID-19 is an emerging pandemic. The course and management of the disease in the liver transplant setting may be difficult due to a long-standing immunosuppressive state. In Egypt, the only available option is living donor liver transplantation (LDLT). In our centre, we have transplanted 440 livers since 2008. In this study, we report a single-centre experience with COVID-19 infection in long-term liver transplant recipients. A total of 25 recipients (5.7 %) had COVID-19 infections since March 2020. Among these recipients, two developed COVID-19 infections twice, approximately three and two months apart, respectively.
Keywords: COVID-19, Liver transplant, SARS-CoV-2, Pandemic
1. Background
COVID-19 is a novel viral infection pandemic of international concern. The emerging virus has affected more than 30 million people in the 6 months since its initial identification [1]. Liver transplant recipients are under long-term immunosuppression and are considered one of the most vulnerable groups for COVID-19 infection. However, data regarding the course and outcome of the disease in organ transplantation recipients are scarce.
2. Case report
During the current pandemic, our centre continued to follow up post-transplant recipients using telemedicine or hospital visits as needed. Any cases with suspected symptoms of COVID-19 infection were referred to our hospital triage and underwent routine work-ups, including measures of chest CT, CBC, CRP, serum ferritin, D-dimer, serum creatinine, ABG, and liver function. In this study, we report 2 cases of COVID-19 reinfection in liver transplant recipients. In both cases, the patients were discharged from the hospital after the first infection episode when the results of two successive COVID-19 PCR tests 48−72 h apart were negative.
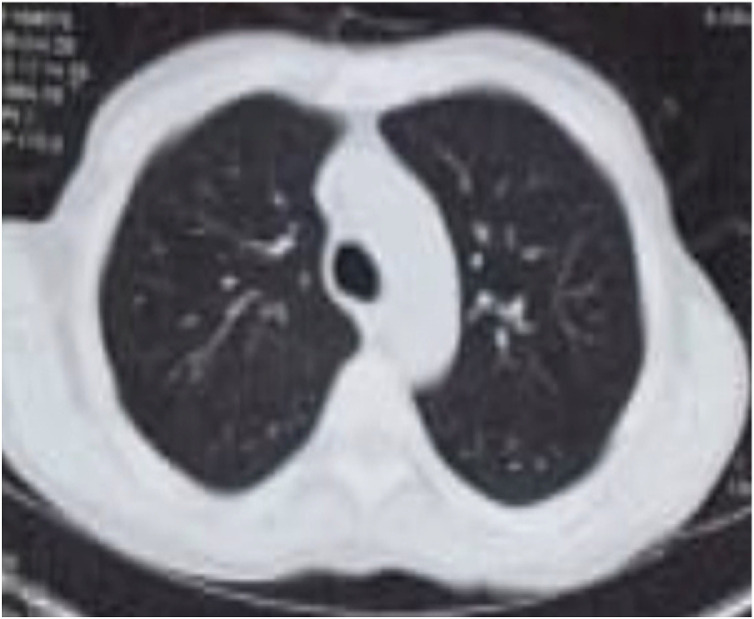
The first case, a 49-year-old male, was transplanted on December 2019 due to cryptogenic cirrhosis. In both instances of COVID-19 infection, he was completely asymptomatic and was discovered to be COVID-19-positive by a nasopharyngeal swab taken during a routine work-up before ERCP for biliary problems. The first bout of COVID-19 infection was on 11 May 2020, and the second was on 17 August 2020 (96 days apart) (Fig. 1, Fig. 2, Fig. 3 ). The laboratory data are summarized in Table 1 .
Fig. 1.
CT chest on May 2020 showing bilateral reticular pattern (first attack).
Fig. 2.
CT chest on May 2020 showing faint ground glass appearance (second attack).
Fig. 3.
CT chest on October 2020 showing complete resolution of radiological abnormalities.
Table 1.
Laboratory values of first case during the first and second episode of COVID-19 infection.
| Lab values | 1st attack | 2nd attack |
|---|---|---|
| HB (gm/dl) | 9 | 8.5 |
| WBCs 109 cells/L | 2.8 | 2.2 |
| Platelets109 cells/L | 108 | 95 |
| Ferritin(ng/dl) | 523 | 465 |
| D Dimer | 2 | 0.7 |
| Bilirubin (Total/Direct) | 4/3.3 | 3/2 |
| ALK Ph(U/L) | 678 | 642 |
| GGT (U/L) | 1067 | 620 |
| ALT (U/L) | 45 | 60 |
| AST (U/L) | 35 | 54 |
The other case, a 61-year-old male, underwent LDLT in December 2017 due to HCV-related cirrhosis. After COVID infection, this patient presented with severe respiratory symptoms, fever, and acute kidney injury (AKI). The first bout of COVID-19 infection was on 13 June 2020, and the second was on 7 August 2020 (<2 months apart). The patient presented with COVID-19 infection with high-grade fever, cough, dyspnoea, diarrhoea and hypotension (second attack). He was admitted to intermediate care with a diagnosis of COVID-19 and sepsis.
Both patients were admitted to an isolation hospital and received symptomatic treatment according to their clinical conditions, with temporary MMF suspension in both cases and temporary cyclosporine suspension in the second case due to severe sepsis and acute kidney injury. Both patients responded well to symptomatic treatment. The first case had a negative nasopharyngeal swab as measured by PCR and was discharged from the hospital 48 h later and resumed taking his usual immunosuppressant drugs.
The second case was more severe, with lung and kidney infection. ABG showed metabolic acidosis, serum creatinine of 7 mg/dl, and potassium of 6 mg/dl (Table 2 ). The patient required 11 days of hospitalization. His chest CT showed multiple bilateral peripheral variable-sized ground glass opacities involving both lung lobes. Multiple consolidation opacities were observed in both the lingula and the base of the left lower lung lobe. No pulmonary nodules, lymph nodes, or pleural effusion were detected (Fig. 4 ). His renal function returned to normal baseline after fluid therapy and symptomatic treatment. He did not require dialysis.
Table 2.
Laboratory values of second case during the first and second episode of COVID-19 infection.
| Lab values | 1st attack | 2nd attack |
|---|---|---|
| HB (gm/dl) | 11 | 11.2 |
| WBCs 109 cells/L | 9.5 | 16 |
| Platelets109 cells/L | 150 | 224 |
| Ferritin(ng/dl) | 546 | 670 |
| D Dimer | 5 | 1.2 |
| Bilirubin (Total/Direct) | 0.5/0.2 | 0.6/0.3 |
| ALK Ph(U/L) | 100 | 92 |
| GGT (U/L) | 35 | 38 |
| ALT (U/L) | 20 | 23 |
| AST (U/L) | 30 | 19 |
| Creatinine(mg/dl) | 3 | 7 |
| Potassium(mg/dl) | 3.6 | 6 |
Fig. 4.
CT multiple bilateral peripheral variable-sized ground glass opacities involving all lung lobes and multiple consolidation opacities.
According to the National Ministry of Health protocol, the patients received oral hydroxychloroquine (400 mg BID for 24 h, then 200 mg BID), oseltamivir (75 mg BID) the first time (this was subsequently removed from the national guidelines), azithromycin (500 mg QD; plus imipenem in the second case) and prophylactic anticoagulation with enoxaparin (40 mg QD).Neither case required ICU admission or mechanical ventilation.
3. Discussion
In late December 2019, an outbreak of a novel coronavirus disease (COVID-19) was reported in Wuhan, China [2], and subsequently became a global pandemic.
This novel virus was named by the International Committee on Taxonomy of Viruses (ICTV) as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [3].
The pathological features of COVID-19 are not yet well understood, but the data suggest similarities to those observed in severe acute respiratory syndrome (SARS) and Middle Eastern respiratory syndrome (MERS) coronavirus infections [4].
Tissue damage after SARS-CoV-2 infection is mediated either by a direct virus-induced cytopathogenic effect or through an immune-mediated inflammatory response. [5]
Recent studies on COVID-19 have reported that the incidence of liver injury ranges from 14.8%–53% [6]. These percentages raise concerns regarding liver transplant recipients: are they more susceptible to SARS-CoV-2 infection due to their immunosuppressive state? If they are infected, is the transplanted liver at risk? Data from different liver transplant centres are scarce, and there is no consensus on managing the disease with immunosuppressant drugs. To date, there have been no specific recommendations in terms of the course or management of liver transplant patients from major societies.
During the pandemic, the Ministry of Health in Egypt increased medical health service capacity and assigned 23 hospitals to manage COVID-19 in each governorate as quarantine hospitals [7]. Ain Shams University Hospital (ASCOT) temporarily held an LDLT programme from 2 March to 13 July 2020. Most government and university centres have temporarily discontinued transplant activities based on an unacceptable donor risk, uncertain data on the impact of COVID-19 on recipients, and to reserve resources to manage the pandemic [7]. At our centre, although operations were temporarily restricted, outpatient clinics and follow-up continued. Our staff followed up recipients using telemedicine and/or hospital visits as needed. This may explain the relatively higher number of patients we treated with COVID-19 infection compared to other centres in Egypt that completely suspended their activities or were serving as isolation hospitals.
Overall, our patients’ clinical characteristics (symptoms, laboratory examinations, and chest CT) were similar to those of other non-transplant adult patients with COVID-19. Twenty-five patients presented to the Ain Shams Center for Organ Transplantation (ASCOT) with fever (96 %), dry cough (78 %), and loss of smell and taste (22 %). This symptomology was consistent with the empirical data and some publications that reported these COVID-19 symptoms [8], [9].
Managing immunosuppression is crucial in these cases. Our centre had mild cases (92 %), and we did not decrease or suspend the usual CNI doses with temporary suspension of MMF. Three patients required ICU admission with complete suspension of CNI and MMF and started methylprednisolone at 1 mg/kg/day for 3–5 days. No patients required mechanical ventilation.
In a report from Italy, three COVID-19-related diseases occurred at an Italian transplant centre in Lombardy. They were long-term patients on minimal immunosuppressive regimens, not recent transplant recipients or fully immunosuppressed patients [10]. In contrast to the report of Bhoori et al., other cohort deaths included four patients transplanted within the past 2 years. Among the patients who died, four (44 %) had diabetes, four (44 %) had hypertension, and three (33 %) had obesity. Although the numbers were low, the frequencies of these comorbidities were not significantly different between the fatal and nonfatal COVID-19 cases. The authors recommend that those caring for patients with previous liver transplantation and other forms of chronic liver disease use registries to pool details on COVID-19 cases to permit rapid large-scale collaborative analyses that are required to inform clinical care [11]. In 2020, Zhang et al. claimed that, although early data suggest that the effects of COVID-19 on the liver might be modest and be similar to the infection severity among patients without pre-existing liver disease, the effects of COVID-19 on those with liver transplants or established liver disease remain unclear [12].
Notably, reinfection occurred after 3 months in the first patient and after 55 days in the second patient after primary infection, although both patients were discharged from the hospital after 2 successive COVID-19 negative tests by PCR following the first COVID-19 infection. To date, reports of reinfection have been infrequent. The risk of reinfection may be lower in the first 3 months after initial infection, according to limited evidence from another beta-coronavirus (HCoV-OC43), the genus to which SARS-CoV-2 belongs. Similar to other human coronaviruses for which studies have demonstrated reinfection, the probability of SARS-CoV-2 reinfection is expected to increase with time after recovery from initial infection due to waning immunity and possible genetic drift [13], [14], [15], [16].
The degree of protective immunity conferred by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is currently unknown. The possibility of reinfection with SARS-CoV-2 is not well understood [17]. To date, no reported cases of COVID-19 reinfection in the post-transplant setting have been published.
On the other hand, Li Anchoret al., 2020 reported that 36 of 378 patients had COVID-19 RNA shedding longer than 30 days. SARS COV IgM & IgG levels remained persistently high in these patients even to the ninth week. They postulated that the high levels of IgM indicated the duration of viral infection and may be related to prolonged viral RNA shedding [15].
Akiko Iwasaki commented on the four published cases of reinfection in the general population (as of the writing of this report). It is important to keep in mind that reinfection cases are being tabulated because of symptoms and are biased towards the detection of symptomatic cases. Asymptomatic reinfection cases can only be identified by routine community testing or at an airport, and the number of asymptomatic reinfections is probably significantly underestimated [18].
To the best of our knowledge, this is the first report of COVID-19 reinfection in liver transplantation recipients in Africa and the Middle East. COVID-19 reinfection can occur in liver transplant recipients with varying severity. In this paper, we recorded two cases of COVID-19 reinfection in liver transplant recipients with good outcomes. This could add to reference cases for managing these cases but cannot be extrapolated to other of cases in which different immunosuppression regimens are used. Why do some reinfections result in milder disease, whereas others are more severe? The question remains unanswered.
Author contributions
Iman Montasser, Hany Dabbous, Manar Salah and Samer Atef were responsible for patient follow up, manuscript preparation, reference collection and final editing; Yassmine Massoud, Hend Ebada and Mohamed Amin Sakr contributed to this paper with drafting and critical revision and editing; Mohammed Bahaa, Mahmoud El Meteini done the final revision.
Statement of Informed Consent
This case report was retrospective study; the consent form was therefore waived.
Funding
This research did not receive any grant from any funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors like to acknowldge all staff members in Ain Shams Centre for Organ Transplantation(ASCOT).
References
- 1.World Health Organization Coronavirus disease 2019 (COVID-19) situation report – 44. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200415-sitrep-86-covid-19.pdf?sfvrsn=c615ea20_6
- 2.Wu F., Zhao S., Yu B., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020 doi: 10.1038/s41586-020-2008-3. published online Feb 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorbalenya A.E. Severe acute respiratory syndrome-related coronavirus–the species and its viruses, a statement of the Coronavirus Study Group. BioRxiv. 2020 [Google Scholar]
- 4.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin Y., Yang H., Ji W., Wu W., Chen S., Zhang W., et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12(4):372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zidan Ahmed, Alabbad Saleh, Ali Tariq, Nizami Imran, Haberal Mehmet, Tokat Yaman, et al. Position statement of transplant activity in the Middle East in era of COVID-19. Pandemic Transplant. 2020 doi: 10.1097/TP.0000000000003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hjelmesæth J., Skaare D. Loss of smell or taste as the only symptom of COVID-19. Tidsskrift for Den norskelegeforening. 2020 doi: 10.4045/tidsskr.20.0287. [DOI] [PubMed] [Google Scholar]
- 10.Bhoori S., Rossi R.E., Citterio D., Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;(20) doi: 10.1016/S2468-1253(20)30116-3. 30116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webb GwilymJ., Moon Andrew M., Barnes Eleanor, Sidney Barritt A. Thomas Marjot Determining risk factors for mortality in liver transplant patients with COVID-19. Lancet Gastroenterol Hepatol. 2020;(7):643–644. doi: 10.1016/S2468-1253(20)30125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang C., Shi L., Wang F.-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–443. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korea C.D.C. 2020. Findings from Investigation and Analysis of re-positive cases. May 19. [Google Scholar]
- 14.Xiao F., Sun J., Xu Y., Li F., Huang X., Li H., et al. Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerg Infect Dis. 2020 doi: 10.3201/eid2608.200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li N., Wang X., Lv Tangfeng. Prolonged SARS-CoV-2 RNA shedding: not a rare phenomenon. J Med Virol. 2020 doi: 10.1002/jmv.25952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiyuka P.K., Agoti C.N., Munywoki P.K., Njeru R., Bett A., Otieno J.R., et al. Human coronavirus NL63 molecular epidemiology and evolutionary patterns in rural coastal Kenya. J Infect Dis. 2018 doi: 10.1093/infdis/jiy098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tillett Richard L., Sevinsky Joel R., Hartley Paul D., Kerwin Heather, Crawford Natalie, Gorzalski Andrew, et al. Genomic evidence for reinfection with SARS-CoV-2: a case study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30764-30767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwasaki Akiko. What reinfections mean for COVID-19. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30783-0. Published online October 12, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]