Abstract
Humeral shaft fractures are relatively common, representing approximately 1% to 5% of all fractures.
Conservative management is the treatment of choice for most humeral shaft fractures and offers functional results and union rates that are not inferior to surgical management.
Age and oblique fractures of the proximal third are risk factors for nonunion. Surgical indication threshold should be lower in patients older than 55 years presenting with this type of fracture.
Functional outcomes and union rates after plating and intramedullary nailing are comparable, but the likelihood of shoulder complications is higher with intramedullary nailing.
There is no advantage to early exploration of the radial nerve even in secondary radial nerve palsy.
Cite this article: EFORT Open Rev 2021;6:24-34. DOI: 10.1302/2058-5241.6.200033
Keywords: fracture, humeral shaft, treatment
Introduction
Humeral shaft fractures (HSF) are relatively common, representing approximately 1% to 5% of all fractures.1–3 The annual incidence ranges from 13 to 20 per 100,000 persons and has been found to be higher with age.4–6 HSF have a bimodal age distribution with the first peak seen in men aged 21 to 30 years following high-energy trauma, commonly resulting in comminuted fractures with associated soft tissue injuries.7 The second peak is witnessed in women aged 60 to 80 years, typically following low-energy trauma.7
The objectives of this article are to review the evaluation of patients presenting with HSF, delineate the relative indications of conservative and surgical management, summarize treatment-related outcomes and complications, and to provide some technical pearls to facilitate management. Paediatric and periprosthetic fractures are beyond the scope of this article and thus will not be addressed.
Patient evaluation
History and physical examination
It is essential to obtain a detailed history from the patient including the mechanism of injury, neurological symptoms, and any associated injuries. Fractures caused by low-energy trauma, such as fall from standing height, should raise the suspicion of poor bone quality associated with osteoporosis or oncologic disease. Past medical history is also required to help guide the management plan.
Often, patients with HSF present with pain, disability, a swollen upper extremity and visible deformity. The deformity is usually a varus angulation for most fractures located distal to the deltoid tuberosity but a valgus deformity is also possible for fracture lines between the pectoralis major insertion and the deltoid tuberosity. The skin must be carefully inspected to rule out an open wound. The initial neurological examination is then carried out, specifically focusing on radial nerve function. It is critical to establish and record the baseline function of the radial nerve. The first examination can be limited by pain and therefore it is essential to repeat this after pain control has been achieved. It is also mandatory to reassess the radial nerve function before and after any manipulation and/or treatment (cast, surgery, etc). One should be mindful of extending the wrist to neutral before examining finger extension as lumbrical muscular function can be mistaken for intact radial nerve function.
The radial and ulnar pulses should be assessed because brachial artery injuries can be associated with high-velocity HSF.
Imaging studies
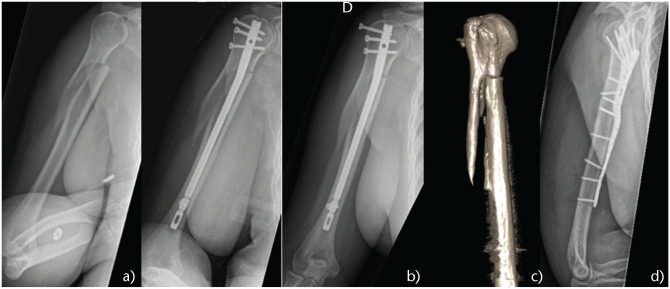
Generally, plain radiographs are sufficient to confirm the diagnosis and plan the treatment for HSF. Anteroposterior (AP) and lateral radiographs are required to visualize and make a full assessment of the fracture. Additional radiographs of the shoulder and elbow are highly recommended in cases where there is suspected injury to these joints (pain around the shoulder or elbow), or shoulder/elbow not clearly visible. O’Donnell et al8 performed bilateral shoulder magnetic resonance imaging (MRI) in 33 patients with a HSF and found abnormalities in 63% of the shoulders on the injured side, most commonly consisting of a subacromial bursitis (30%) or a partial tear of the rotator cuff (15%), although in common practice MRIs should not be performed routinely. Computed tomography (CT) has no role in the acute setting, but CT-angiograms should be conducted if there is any doubt about the quality of pulses (Fig. 1). CT scan can aid in management when used to confirm a nonunion, especially in spiral fractures.
Fig. 1.
(a) 27-year-old patient, motor vehicle accident, weak but palpable radial pulse. (b) CT-angiogram confirmed brachial artery injury. (c) Fracture was urgently fixed through a medial approach. Vascular surgeon performed a venous bypass.
Management
Decision making
Several options are possible for the management of HSF: conservative management, open reduction and internal fixation (ORIF) with a plate, or closed reduction and intramedullary nailing (IMN). An external fixator is also an option, however rarely indicated. Undisplaced or minimally displaced HSF are routinely treated conservatively. In fact, anterior angulation of 20°, a varus or valgus of 30°, 15° of malrotation and 3 cm of shortening have been shown to adequately maintain the upper limb function.9,10 For this reason, fractures that are displaced within these values following immobilization are good candidates for conservative management.
Regarding surgical indications (see Table 1), these are divided into three groups:11,12
Table 1.
Surgical indications
| Indications | Relative indications |
|---|---|
| Acceptable alignment cannot be achieved with brace | Multiple trauma (patient might need weight bearing with crutches) |
| Conservative treatment failure | Bilateral humeral shaft fracture |
| Intra-articular extension* | Open fractures (except with severe soft tissue injury)* |
| Soft tissue condition precludes bracing (burns, open fractures with Gustilo III, obese patients, gunshot wound, etc.) | Segmental fractures** |
| Pathological fracture (metastase)** | Comminuted fractures |
| Brachial plexus injury | Delay in radial nerve recovery* |
| Floating elbow | |
| Vascular injury requiring repair* |
More suitable for open reduction and internal fixation with plating.
More suitable for intramedullary nailing.
1) Local conditions: [soft tissues] i.e. burns, open fractures Gustilo III, obese patient (these conditions preclude the use of a brace) or [fracture configuration] i.e. pathological fracture, segmental fracture.
2) Associated injuries: poly-trauma (for general care, ambulation, use of crutches), bilateral HSF, floating elbow, arterial injury, brachial plexus (conservative treatment with brace requires active muscle contraction, i.e. shoulder and elbow function to be intact).
3) Conservative treatment failure: patient not comfortable in the brace, unmanageable pain, secondary displacement or absence of an acceptable alignment, and delayed or nonunion.
It is important to highlight the fact that there is an increased tendency to choose surgical management of HSF as an option although this is not supported by the literature. This was reported by Huttunen et al,13 using the Finland National Hospital Discharge Registry from 1987 to 2009, which showed a dramatic increase in the rate of surgical treatment for these patients. Over this 23-year period, the rate of men treated surgically for HSF doubled to 46% and nearly tripled for women to 54%. The trend towards a more operative approach could be explained by the increased demand of patients and achievement of earlier mobilization. Innovations in surgical techniques may also play an important role.
Conservative treatment
Non-operative treatment is considered by many surgeons to be the gold standard for the management of HSF. The functional brace introduced by Sarmiento in 197714 uses incompressible fluids of soft tissue around the fracture site to create a rigid envelope around the fragments, preventing angular and rotational deforming forces. With muscles contracting inside the rigid plastic valve, the bony alignment usually improves. Shortening, on the other hand, is more dependent on the type of fracture and cannot be corrected fully using this technique.
Conservative treatment is a stepwise process. At the time of the fracture, the upper limb is immobilized in an above-elbow hanging cast or U-shaped coaptation splint, for a duration of 1–2 weeks. Further treatment proceeds with immobilization in a prefabricated functional brace (Fig. 2) consisting of a part around the arm, possibly over the shoulder and straps to tighten on a daily basis to accommodate the decreasing soft tissue swelling and muscle atrophy encountered during the course of treatment. The patient is asked to mobilize their shoulder and elbow,15 to help align the fractures further. Physical therapy might be beneficial in the beginning to aid with shoulder and elbow motion. Active and active assisted mobilization of both elbow and shoulder according to pain tolerance is recommended from the beginning of treatment. In regard to follow-up visits, plain radiographs (AP and lateral) should be obtained one week after brace application to make sure the alignment is correct or even improving. Subsequent radiographs should be obtained every two to six weeks, depending on the fracture evolution. The brace should be kept until union, which on average is at 10 to 12 weeks. It is crucial to note that patient compliance with the treatment is necessary for optimal results.16
Fig. 2.
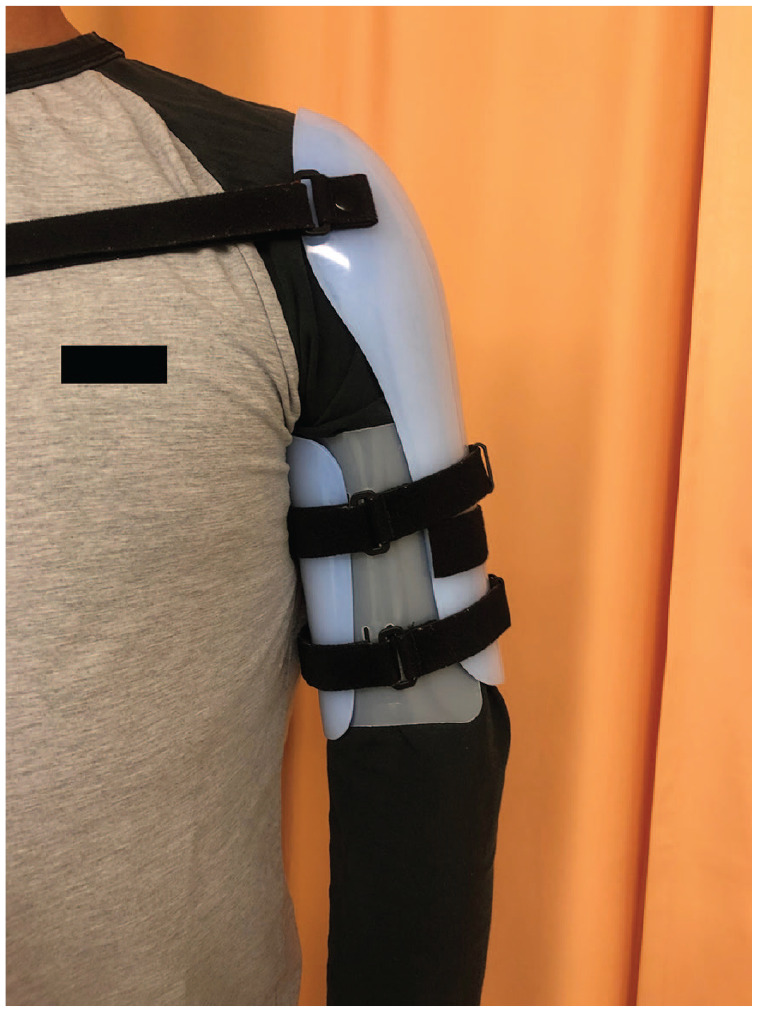
Functional bracing.
Reported outcomes
Union rate with this technique ranges between 77.4% and 100%.17–20 In a review including almost 2000 patients, Papasoulis et al17 reported a union rate of 94.5% and a mean time to union of 10.7 weeks.
Several studies have shown proximal third humerus fractures treated conservatively had lower union rates than those involving the distal two-thirds.19,21,22 Ali et al21 observed an overall union rate of 83% on a series of 138 fractures of the humeral diaphysis treated with functional bracing. Subgroup analysis showed a lower union rate for the proximal third of 76%, whereas the rates for the middle and distal thirds were 88% and 85% respectively. They also found that oblique fractures of the proximal third had a lower union rate than other fracture types and locations. Ring et al23 also found lower union rates for proximal and middle third fractures, and lower union rates for oblique/spiral fractures.
Alternatively, several other studies have shown a higher risk of nonunion for transverse fractures.11,18,20 It is unclear at this time what the main factor for these differences is; however, a small area of contact between the fragments could explain the lower union rate seen in transverse fractures. For oblique/spiral fractures on the other hand, the reason for this is likely due to incarceration of muscle and soft tissues in the fracture site. Comminuted fractures show a higher union rate than simple fractures regardless of their location.11,21 Ekholm et al11 showed an 82% union rate for type A fractures (according to the AO/OTA classification),24 while type B and C fractures showed 96% and 100% nonunion rates respectively. In addition, in a retrospective study of 79 patients with conservatively treated humeral shaft fracture, Neuhaus et al observed that smoking (odds ratio [OR] = 5.8, 95% CI = 1.4 to 25.0), female sex (OR = 5.3, 95% CI = 1.2 to 23.0) and initial displacement (OR = 1.4, 95% [CI] = 1.1 to 1.7 for each millimetre of gap), increase the risk of secondary displacement at six weeks with conservative treatment.25 Lastly, in a retrospective study evaluating union rates after conservative treatment of HSF in elderly patients, Pollock et al26 found that the incidence of nonunion was significantly higher in patients older than 55 years.
In regard to alignment, Papasoulis et al17 reported in their review, no impairment of function or decrease in clinical scores with a residual deformity of less than 10° of varus in more than 85% of the patients.
The functional results following treatment with functional bracing appear to be equivalent to that of surgical treatment, thus can be used successfully to manage HSF.16,20,27,28 Papasoulis et al17 found full range of motion recovery of shoulder and elbow for 80% and 85% of patients respectively. However, it is important to inform patients that the risk of short-term shoulder and elbow stiffness might be higher with bracing.
Surgical treatment: open reduction and internal fixation with a plate
Indications
Surgical indications are summarized in Table 1. Radial nerve palsy (RNP) in HSF is not an indication for surgery as it is associated with a high rate of spontaneous recovery (see also – special considerations/radial nerve, below). Alternatively, any vascular injury requiring repair or bypass is an absolute indication for surgical management of the fracture as the rigid fixation protects the vascular anastomosis.29 In this particular case, as the vascular repair is performed through a direct approach (usually medial approach), internal fixation with a plate is faster and more reliable than IMN.
HSF with a proximal or distal intra-articular extension is another scenario where ORIF with plate is the preferable option.
Surgical exposures
Fractures located in the proximal and/or middle third are addressed with the classic anterolateral approach. When required, this approach is extended distally to expose the entire humerus. However, this approach is not recommended for distal intra-articular fractures.
Fractures of the distal third are usually exposed through a triceps-splitting approach. For fractures of the distal and middle third, the modified posterior approach described by Gerwin et al30 enables exposure of 76–94% of the humerus (depending on the radial nerve mobilization and intermuscular septum release).
Surgical technique
The patient is positioned in the beach chair position for an anterolateral approach. The use of an arm-holder is helpful to maintain humeral shaft alignment. Whereas for posterior exposure, lateral decubitus is the preferred position.
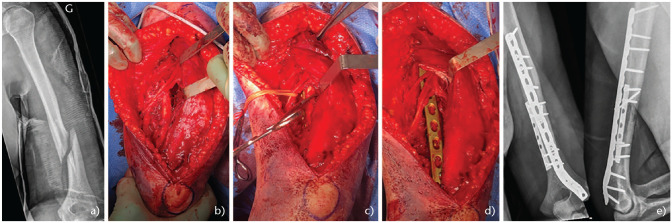
The optimal plate construct includes a 4.5 mm plate or equivalent and should cover a minimum of six cortices above and below the fracture site, although eight cortices are preferable.31 When needed it is recommended to combine a small and a large fragment plate, for example using a short third tubular plate to maintain the reduction (transverse fracture or butterfly fragment), and then supplementing this with a narrow 4.5 mm plate to definitively fix the fracture (Fig. 3). For distal third fractures, the use of posterolateral column precontoured plates (3.5/4.5) are recommended allowing strong metaphyseal fixation (Fig. 3).
Fig. 3.
(a) 75-year-old female. Fall from own height. Poor bone quality. (b) Combination of third tubular and 3.5/4.5 mm plate (three-month radiographs).
The use of locking screws in HSF plating remains controversial. When comparing locking versus non-locking plates for comminuted fractures with good bone quality, there is no biomechanical benefit with respect to torsion, flexion or axial stiffness between the two constructs.32,33 On the other hand, when faced with poor bone quality, the use of locking plates can be advantageous. In a biomechanical study by Gardner et al looking specifically at an osteoporotic fracture model,34 non-locking constructs showed significantly lower stability than either locking or hybrid constructs.
Minimally invasive plate osteosynthesis is a surgical option that appears to offer a high rate of success as well as a low complication rate.35,36 However, in a retrospective study involving 76 patients, van de Wall et al37 demonstrated that absolute stability for simple humeral shaft fractures leads to a significantly shorter time to radiological union compared to relative stability.
Postoperative management
Generally, it is possible to obtain a stable fixation with the use of a plate. For this reason, the patient is permitted to perform active and active-assisted mobilization with no shoulder or elbow range of motion restrictions. A sling can be utilized for a few days for pain management. Postoperative weight restriction should be maintained at a maximum of one kilogram until fracture healing is evident (usually three months). Weight bearing as tolerated is allowed (for example the need to walk with crutches) for young patients, but this should be discussed on a case-by-case basis for elderly patients.
Reported outcomes
Union rate after plating ranges from 87% to 96%,38–43 with an average time to union of 12 weeks. The complication rate ranges from 5% to 25%,44–49 most commonly found to be non-specific complications such as infection, nonunion and malunion. Iatrogenic RNP is a risk with most approaches to the humeral shaft, and Streufert et al50 reviewed 261 HSF treated with ORIF, finding iatrogenic RNP occurred in 7.1% of anterolateral, 11.7% of triceps-splitting and 17.9% of triceps-sparing approaches. For this reason, it is vital that the radial nerve is identified and protected in all open dissections.
Surgical treatment: intramedullary nailing
Indications
In theory, IMN can offer biomechanical and surgical advantages over plating. From a biomechanical point of view, the intramedullary positioning of the device aligns with the mechanical axis of the humeral diaphysis. For this reason the implant experiences lower bending forces and allows for better load sharing.51,52 Surgical indications for nailing are the same as for plating; however, as mentioned previously, some fractures are more amenable to plating than to nailing. Fracture characteristics and patterns in which IMN has been found to be superior are pathological and impending fractures, segmental lesions and fractures in osteopenic bone (Table 1).11,12 A simple middle third transverse fracture is also a good indication for IMN. Furthermore, the nail can be inserted through a smaller incision, which allows for less soft tissue stripping compared to plating techniques. This is particularly true for middle third humerus fractures.
Surgical technique
The optimal patient position for this surgery is in the beach chair. The use of an arm-holder is extremely useful in maintaining the shaft alignment as well as when performing the distal free-hand locking screw. The entry point does depend on the nail design but generally it is located at the junction of the greater tuberosity and the articular surface of the humeral head, which means one has to penetrate the rotator cuff muscles. For this procedure, it is recommended to perform a deltoid-splitting approach to visualize the supraspinatus tendon. In fact, upon entering the humeral head in the middle of the supraspinatus tendon, one will find themselves in the centre of the head in the sagittal plane. Using a K-wire under fluoroscopy, it is important to ensure that the entry point is in an acceptable position in the sagittal and coronal plane. Following this, the wire should be advanced further prior to opening the supraspinatus tendon longitudinally above it and under direct visualization. The next step involves opening the canal over the K-wire, ensuring fracture alignment with traction and/or external manoeuvres, followed by advancing the guide in the intramedullary canal down to the elbow. Reaming has been found to be superior in younger patients and not always necessary in the elderly patient. Great care needs to be taken with the length of nail chosen as too long a nail can lead to two technical errors: (1) distraction at the fracture site when impacting the nail, and/or (2) protrusion of the nail into the subacromial space. For distal bolt placement, AP locking is safer and requires a small 2–3 cm approach to lower the risk of musculocutaneous nerve injury. Finally, the supraspinatus tendon is carefully closed to minimize shoulder complications.
The anterograde IMN is preferred over retrograde IMN because of specific complications of the latter which include iatrogenic supracondylar fracture, loss of elbow extension and heterotopic ossification.53,54
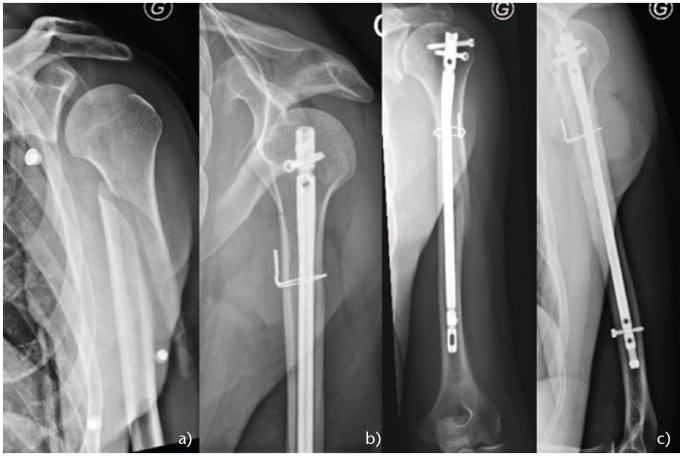
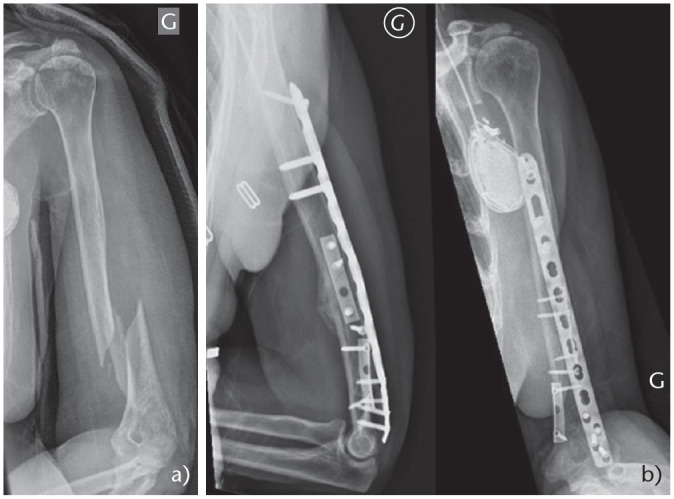
For proximal third spiral or long oblique fractures, authors recommend a mini-open approach to reduce the fracture and then fix it with cerclage wires. In fact, with this subtype of fractures, the deltoid has the tendency to abduct the proximal fragment whereas the pectoralis major pulls the distal fragment medially, which can increase the risk of nonunion or delayed union (Fig. 4 and Fig. 5).
Fig. 4.
(a) Proximal third oblique fracture. (b) Nail without cerclage. (c) Nonunion. (d) ORIF with precontoured plate.
Fig. 5.
(a) Proximal third oblique fracture. (b) Nail with cerclage wire. (c) Fracture union.
Postoperative management
The patient is encouraged to perform active and active-assisted motions as tolerated for both shoulder and elbow. A sling may be used for a few days for pain management. Postoperative weight lifting restriction is kept at a maximum one kilogram until fracture healing is evident (usually three months). Weight bearing as tolerated is allowed in most cases.
Reported outcomes
The literature regarding the management of HSF with the locking nail device has been inconsistent. On one hand, highly variable nonunion rates (between 0% and 14%) have been reported,12,38–43,55–57 with the highest rates found with old generation nails. On the other hand, in previous literature, the incidence of shoulder complications (includes pain, impingement, loss of motion or strength) (ranges from 6% to 100%).58–60 Part of the problem can be explained by subacromial trauma due to a prominent nail, scar tissue and/or rotator cuff damage in this hypovascularized critical area causing chronic tendon dysfunction. Several authors have described different approaches avoiding this hypovascular area and repairing the tendon in a careful manner that have shown lower shoulder dysfunction rates.55–57
Reported outcomes are summarized in Table 2.
Table 2.
Reported outcomes of humeral shaft fracture treatments
| First author | Year | Type | Cohort (n) | Union rate (%) | Complication | Functional |
|---|---|---|---|---|---|---|
| Conservative | ||||||
| Ekholm11 | 2006 | retro | 78 | 89.7 | NA | NA |
| Sarmiento14 | 1977 | retro | 51 | 98.0 | 16% > 5° angular deformity | 82% full ROM elbow and shoulder |
| Sarmiento15 | 1990 | retro | 72 (distal third) | 95.8 | 81% varus angulation (without precision), 3% valgus angulation (without precision) 39% posterior angulation from 3–22°, 41% anterior angulation from 1–30°, 36% from 2–15 mm shortening |
45% loss 5–45° ER, 15% loss 10–60°ABD, 13% loss 5–20° F, 24% loss 5–25° elbow extension, 26% loss 5–25° elbow flexion |
| Denard16 | 2010 | retro | 63 | 79.4 | 12.7% malunion (> 20° any plane), 3.2% infection | Elbow ROM 136.25 ± 28.63 (80–180) |
| Sarmiento18 | 2000 | retro | 620 | 97.4 | NA | 8% loss > 10° elbow ROM |
| Rutgers19 | 2006 | retro | 49 | 89.8 | 4% skin breakdown | NA |
| Koch20 | 2002 | retro | 67 | 86.6 | 41.7% deformity > 10° | 4.2% unsatisfactory |
| Ali21 | 2015 | retro | 138 | 83.0 | NA | NA |
| Toivanen22 | 2005 | retro | 93 | 77.4 | NA | NA |
| Neuhaus25 | 2014 | retro | 79 | 80.0 | NA | NA |
| Pollock26 | 2020 | retro | 31 | 68.0 | NA | NA |
| Intramedullary nailing | ||||||
| Dimakopoulos55 | 2005 | retro | 29 | 100.0 | 3% extension of fracture line into the distal metaphysis | Average constant score 16 w FU 96, average Mayo Elbow Score 95.8/100 |
| Park56 | 2008 | pro | 34 | 94.0 | 6% proximal protrusions | Mean ROM at final FU: elevation 144 ± 23.4, ER 66 ± 18, IR 17 ± 4, Neer’s score 91 ± 10, ASES score 84.5 ± 12.4, Costant score 84 ± 14 |
| Rommens57 | 2008 | retro | 99 | 97.0 | 3% secondary RNP, 2% insertion point fracture, 1% implant malposition |
N = 92 Constant score: 91.3% excellent, 5.4% good, 2.2% fair, 1.1% poor Mayo Elbow Score: 81.5% excellent, 14.1% good, 2.2% fair, 2.2% poor |
| Putti38 | 2009 | pro | 16 | 100.0 | 6% proximal impingement, 12.5% iatrogenic fracture, 12.5% secondary RNP, 18.75% adhesive capsulitis | Mean ASES score 45.2 |
| Singisetti39 | 2010 | pro | 20 | 95.0 | 5% deep infection | Rodriguez-Merchan criteria: 20% excellent, 45% good, 25% fair, 10% poor |
| Changulani40 | 2007 | pro | 21 | 85.7 | 4.7% deep infection, 33.3% 1.5–4.0 cm shortening, 4.7% axillary nerve injury | Mean ASES score 44 |
| Benegas41 | 2014 | pro | 19 | 94.7 | 5.2% superficial infection | Mean UCLA score 31.2 points Mean Broberg-Morrey score 94.8 points |
| McCormac42 | 2000 | pro | 19 | 89.0 | 15% secondary RNP, 5% late fracture, 10% intraoperative comminution, 5% infection, 15% impingement, 5% adhesive capsulitis (shoulder) | Mean ASES score 47 points |
| Chapman43 | 2000 | pro | 38 | 95.0 | 2.6% malunion (> 10° any plane), 5% secondary RNP, 10% hardware removal | 16% decreased shoulder ROM (> 10° compared with contralateral side) |
| Plate | ||||||
| Denard16 | 2010 | retro | 150 | 91.3 | 1.3% malunion (> 20° any plane), 4.7% infection | 130.12 ± 17.01 (25–150) |
| Putti38 | 2009 | pro | 18 | 94.0 | 6% adhesive capsulitis | Mean ASES score 45.1 |
| Singisetti39 | 2010 | pro | 16 | 94.0 | 6.25% secondary RNP, 6.5% deep infection | Rodriguez-Merchan criteria: 25% excellent, 68.75% good, 0% fair, 6.25% poor |
| Changulani40 | 2007 | pro | 24 | 87.5 | 12.5% deep infection, 4.1% arm shortening (without precision), 4.1% secondary RNP | Mean ASES score 45 |
| Benegas41 | 2014 | pro | 21 | 100.0 | 4.7% deep infection | Mean UCLA score 31.4 points, Mean Broberg-Morrey score 94.1 points |
| McCormac42 | 2000 | pro | 22 | 95.0 | 4.5% intraoperative comminution, 4.5% minimal loss of fixation | Mean ASES score 48 points |
| Chapman43 | 2000 | pro | 46 | 93.0 | 4% malunion (> 10° any plane), 6.5% deep infection, 2% secondary RNP, 2% hardware removal | 8.6% decreased elbow ROM (> 10° compared with contralateral side) |
Nail versus plate?
There are many studies comparing IMN and plating outcomes for HSF. A prospective study by Putti et al,38 comparing modern locking nails with direct compression plating, found no significant difference regarding union rates and functional outcomes, but noted a higher complication rate after nailing of up to 50%. In a systematic review, Kurup et al61 found an increased risk of shoulder impingement, restriction of shoulder movement and need for hardware removal associated with nailing. In a meta-analysis by Ouyang et al62 which includes 10 studies (439 patients), they confirmed the likelihood of shoulder complications associated with IMN. The risk ratio (RR) for restriction of shoulder motion (201 patients) was 9.91, p = 0.006. The risk ratio for shoulder impingement (305 patients) was 7.38, p = 0.0003. They did not find any other significant differences regarding infection, nonunion, radial nerve injury or implant failure.
Surgical treatment: external fixation
Indications
External fixation remains an option in rare cases such as polytrauma patients with severe soft tissue damage, open fracture with significant contamination, or associated vascular injury requiring rapid stabilization prior to vascular repair.63–65
Surgical technique
Excellent knowledge of neurovascular structures which are potentially at risk of injury, notably the radial nerve, is required for this surgical procedure. Several authors have described the safe positioning of pins.66,67 Proximal pins have to be inserted at the level of the deltoid tuberosity or higher (avoiding up to 8 cm distal to the acromion to prevent axillary nerve injury), starting directly lateral or slightly anterolateral (maximum 30°). Due to the high number of structures at risk (radial, musculocutaneous, ulnar and median nerves, and the brachial artery), pin insertion in the mid-diaphysis should be avoided. If a pin in this location is absolutely necessary, a mini-open as opposed to a percutaneous approach should be used to avoid any complications. In the distal aspect, it is possible to insert pins lateromedial or antero-posterior, but a small opening is advised (2–3 cm) to reduce the risk of injury to the radial (lateral) or musculocutaneous nerve (antero-posterior).
Reported outcomes
Studies reporting external fixators outcomes are rare. In a retrospective series of 85 patients with HSF treated using external fixation, Scaglione et al showed a union rate of 97.6% at an average of 12 weeks.68
Special considerations
Holstein-Lewis fracture
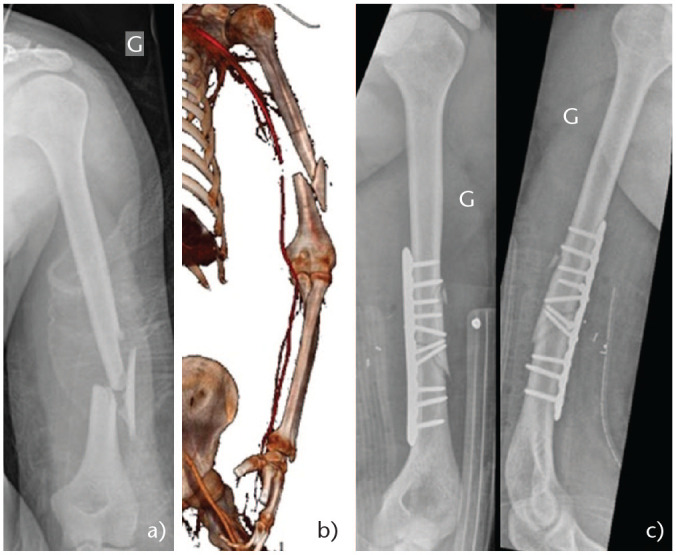
The Holstein-Lewis (HL) subtype fracture58 is a spiral fracture of the distal third of the humeral diaphysis with proximal and radial displacement of the distal fragment. This fracture pattern is likely to injure the radial nerve during its passage through the lateral intramuscular septum (Fig. 6). HL fractures are typically encountered with arm wrestling (torsional force). A study formed from the Stockholm registry including 361 HSF, they found a 7.5% HL fracture incidence. RNP was associated with 22% (6 of 27) of these patients.69 Radial nerve involvement occurred in 8% of the non HL HSF. Of patients with HL fractures, 26% were treated surgically and 74% conservatively. All conservatively managed fractures achieved union and all patients with associated RNP had eventual resolution.
Fig. 6.
(a) Young patient, arm wrestling injury. Holstein-Lewis fracture type. (b) Modified posterior approach. (c) Radial nerve under tension prior to reduction (bone spike) and (d) after reduction. (e) Combination of third tubular and postero-lateral anatomic 3.5/4.5 mm plate.
At mean follow-up of 6.3 years, there was no difference concerning functional outcomes between non-operative and surgical management for HL fractures. Therefore, an HL fracture is not an absolute indication for surgical treatment, regardless of associated RNP. Having said this, distal third fractures are difficult to manage non-operatively, firstly because of their tendency to displace into varus and secondly due to the difficulty of obtaining immobilization in a brace.
Radial nerve palsy
RNP is the most common nerve injury accompanying a long bone fracture with an incidence in HSF of 7–17%.70 In a systematic review including 4517 patients with HSF, Shao et al71 observed a prevalence of RNP of 11.8% with an overall rate of recovery of 88.1%. They found spontaneous recovery of the radial nerve in 70.7% of patients treated conservatively. Additionally, no significant difference was found when comparing radial nerve resolution between groups managed by observation and delayed nerve exploration (87.6% nerve recovery) and those who underwent early nerve exploration (87.9% recovery), suggesting that the initial expectant treatment had no negative effects on the extent of nerve recovery. A recent review72 has identified that secondary radial nerve injury can be treated the same as for primary radial nerve injury. However, early nerve exploration is recommended when fixing a HSF with internal fixation with associated open fracture or vascular injury.72–74
In an updated review including Shao et al,71 combined with 23 other recent articles, Ilyas et al75 reported the results of 1423 patients with RNP. Primary RNP was present in 890 of 7262 HSF, demonstrating an overall prevalence of 12.3%. Moreover, the prevalence of RNP in fractures of the proximal/middle/distal third was 1.8%, 15.2% and 23.6%, respectively. Regarding fracture type, the prevalence was 6.8% in comminuted, 8.4% in oblique, 19.8% in spiral and 21.2% in transverse fractures. Secondary RNP was found to have a significantly higher recovery rate than primary RNP (93.9% vs. 88.2%). In the spontaneously recovered cases, the duration from initial injury until the onset of nerve recovery was 8.29 weeks and full recovery 4.91 months.
Regarding the waiting time, Shao et al71 suggested calculating the estimated time to onset of recovery by measuring the distance between the suspected site of nerve injury to the brachioradialis muscle (2 cm above the lateral epicondyle). Assuming a nerve regenerates at the rate of approximately 1 mm a day,76 and adding 30 days, as Seddon77 had suggested, the maximum length of time which may be required for the onset of motor recovery can be calculated easily. Thus, because spontaneous recovery does occur most of the time, radial nerve palsy has to be initially monitored clinically. If no recovery is observed at 10 to 12 weeks, an electroneuromyography (EMG) should be performed and the possibility of a nerve exploration should be discussed with the patient.
Summary
The conservative treatment of HSF offers good functional outcomes and high union rates in at least 80% of patients. For this reason, it remains the treatment of choice for most HSF. If the alignment is not acceptable, surgery is to be considered. This is particularly true for patients older than 55 years presenting with an oblique fracture of the proximal third (lower union rate).
Regarding surgical management, the literature does not show any significant difference in terms of union rates or radial nerve complications between plating and IMN, but the likelihood of shoulder complications (impingement and decreased range of motion) is higher with IMN. Thus, the cuff has to be managed with great care both at the entry point and during closing.
Footnotes
ICMJE Conflict of interest statement: The authors declare no conflict of interest relevant to this work.
OA licence text: This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) licence (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Brinker MR, O’Connor DP. The incidence of fractures and dislocations referred for orthopaedic services in a capitated population. J Bone Joint Surg Am 2004;86:290–297. [PubMed] [Google Scholar]
- 2. Schemitsch EH, Bhandari M. Fractures of the diaphyseal humerus. In: Browner BD, Jupiter JB, Levine AM, Trafton PG, eds. Skeletal trauma. Third ed. Toronto: WB Saunders, 2001:1481–1511. [Google Scholar]
- 3. Spiguel AR, Steffner RJ. Humeral shaft fractures. Curr Rev Musculoskelet Med 2012;5:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ekholm R, Adami J, Tidermark J, Hansson K, Törnkvist H, Ponzer S. Fractures of the shaft of the humerus: an epidemiological study of 401 fractures. J Bone Joint Surg Br 2006;88:1469–1473. [DOI] [PubMed] [Google Scholar]
- 5. Kim SH, Szabo RM, Marder RA. Epidemiology of humerus fractures in the United States: nationwide emergency department sample, 2008. Arthritis Care Res (Hoboken) 2012;64:407–414. [DOI] [PubMed] [Google Scholar]
- 6. Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury 2006;37:691–697. [DOI] [PubMed] [Google Scholar]
- 7. Tytherleigh-Strong G, Walls N, McQueen MM. The epidemiology of humeral shaft fractures. J Bone Joint Surg Br 1998;80:249–253. [DOI] [PubMed] [Google Scholar]
- 8. O’Donnell TMP, McKenna JV, Kenny P, Keogh P, O’Flanagan SJ. Concomitant injuries to the ipsilateral shoulder in patients with a fracture of the diaphysis of the humerus. J Bone Joint Surg Br 2008;90:61–65. [DOI] [PubMed] [Google Scholar]
- 9. Klenerman L. Fractures of the shaft of the humerus. J Bone Joint Surg Br 1966;48:105–111. [PubMed] [Google Scholar]
- 10. Shields E, Sundem L, Childs S, et al. The impact of residual angulation on patient reported functional outcome scores after non-operative treatment for humeral shaft fractures. Injury 2016;47:914–918. [DOI] [PubMed] [Google Scholar]
- 11. Ekholm R, Tidermark J, Törnkvist H, Adami J, Ponzer S. Outcome after closed functional treatment of humeral shaft fractures. J Orthop Trauma 2006;20:591–596. [DOI] [PubMed] [Google Scholar]
- 12. Walker M, Palumbo B, Badman B, Brooks J, Van Gelderen J, Mighell M. Humeral shaft fractures: a review. J Shoulder Elbow Surg 2011;20:833–844. [DOI] [PubMed] [Google Scholar]
- 13. Huttunen TT, Kannus P, Lepola V, Pihlajamäki H, Mattila VM. Surgical treatment of humeral-shaft fractures: a register-based study in Finland between 1987 and 2009. Injury 2012;43:1704–1708. [DOI] [PubMed] [Google Scholar]
- 14. Sarmiento A, Kinman PB, Galvin EG, Schmitt RH, Phillips JG. Functional bracing of fractures of the shaft of the humerus. J Bone Joint Surg Am 1977;59:596–601. [PubMed] [Google Scholar]
- 15. Sarmiento A, Horowitch A, Aboulafia A, Vangsness CT., Jr Functional bracing for comminuted extra-articular fractures of the distal third of the humerus. J Bone Joint Surg Br 1990;72:283–287. [DOI] [PubMed] [Google Scholar]
- 16. Denard A, Jr, Richards JE, Obremskey WT, Tucker MC, Floyd M, Herzog GA. Outcome of nonoperative vs operative treatment of humeral shaft fractures: a retrospective study of 213 patients. Orthopedics 2010;33. [DOI] [PubMed] [Google Scholar]
- 17. Papasoulis E, Drosos GI, Ververidis AN, Verettas D-A. Functional bracing of humeral shaft fractures: a review of clinical studies. Injury 2010;41:e21–e27. [DOI] [PubMed] [Google Scholar]
- 18. Sarmiento A, Zagorski JB, Zych GA, Latta LL, Capps CA. Functional bracing for the treatment of fractures of the humeral diaphysis. J Bone Joint Surg Am 2000;82:478–486. [DOI] [PubMed] [Google Scholar]
- 19. Rutgers M, Ring D. Treatment of diaphyseal fractures of the humerus using a functional brace. J Orthop Trauma 2006;20:597–601. [DOI] [PubMed] [Google Scholar]
- 20. Koch PP, Gross DFL, Gerber C. The results of functional (Sarmiento) bracing of humeral shaft fractures. J Shoulder Elbow Surg 2002;11:143–150. [DOI] [PubMed] [Google Scholar]
- 21. Ali E, Griffiths D, Obi N, Tytherleigh-Strong G, Van Rensburg L. Nonoperative treatment of humeral shaft fractures revisited. J Shoulder Elbow Surg 2015;24:210–214. [DOI] [PubMed] [Google Scholar]
- 22. Toivanen JA, Nieminen J, Laine HJ, Honkonen SE, Järvinen MJ. Functional treatment of closed humeral shaft fractures. Int Orthop 2005;29:10–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ring D, Chin K, Taghinia AH, Jupiter JB. Nonunion after functional brace treatment of diaphyseal humerus fractures. J Trauma 2007;62:1157–1158. [DOI] [PubMed] [Google Scholar]
- 24. Meinberg EG, Agel J, Roberts CS, Karam MD, Kellam JF. Fracture and dislocation classification compendium-2018. J Orthop Trauma 2018;32:S1–S170. [DOI] [PubMed] [Google Scholar]
- 25. Neuhaus V, Menendez M, Kurylo JC, Dyer GS, Jawa A, Ring D. Risk factors for fracture mobility six weeks after initiation of brace treatment of mid-diaphyseal humeral fractures. J Bone Joint Surg Am 2014;96:403–407. [DOI] [PubMed] [Google Scholar]
- 26. Pollock FH, Maurer JP, Sop A, et al. Humeral shaft fracture healing rates in older patients. Orthopedics 2020;43:168–172. [DOI] [PubMed] [Google Scholar]
- 27. Shields E, Sundem L, Childs S, et al. Factors predicting patient-reported functional outcome scores after humeral shaft fractures. Injury 2015;46:693–698. [DOI] [PubMed] [Google Scholar]
- 28. Matsunaga FT, Tamaoki MJS, Matsumoto MH, Netto NA, Faloppa F, Belloti JC. Minimally invasive osteosynthesis with a bridge plate versus a functional brace for humeral shaft fractures: a randomized controlled trial. J Bone Joint Surg Am 2017;99:583–592. [DOI] [PubMed] [Google Scholar]
- 29. Paryavi E, Pensy RA, Higgins TF, Chia B, Eglseder WA. Salvage of upper extremities with humeral fracture and associated brachial artery injury. Injury 2014;45:1870–1875. [DOI] [PubMed] [Google Scholar]
- 30. Gerwin M, Hotchkiss RN, Weiland AJ. Alternative operative exposures of the posterior aspect of the humeral diaphysis with reference to the radial nerve. J Bone Joint Surg Am 1996;78:1690–1695. [DOI] [PubMed] [Google Scholar]
- 31. Updegrove GF, Mourad W, Abboud JA. Humeral shaft fractures. J Shoulder Elbow Surg 2018;27:e87–e97. [DOI] [PubMed] [Google Scholar]
- 32. O’Toole RV, Andersen RC, Vesnovsky O, et al. Are locking screws advantageous with plate fixation of humeral shaft fractures? A biomechanical analysis of synthetic and cadaveric bone. J Orthop Trauma 2008;22:709–715. [DOI] [PubMed] [Google Scholar]
- 33. Hak DJ, Althausen P, Hazelwood SJ. Locked plate fixation of osteoporotic humeral shaft fractures: are two locking screws per segment enough? J Orthop Trauma 2010;24:207–211. [DOI] [PubMed] [Google Scholar]
- 34. Gardner MJ, Griffith MH, Demetrakopoulos D, et al. Hybrid locked plating of osteoporotic fractures of the humerus. J Bone Joint Surg Am 2006;88:1962–1967. [DOI] [PubMed] [Google Scholar]
- 35. Hohmann E, Glatt V, Tetsworth K. Minimally invasive plating versus either open reduction and plate fixation or intramedullary nailing of humeral shaft fractures: a systematic review and meta-analysis of randomized controlled trials. J Shoulder Elbow Surg 2016;25:1634–1642. [DOI] [PubMed] [Google Scholar]
- 36. Kulkarni VS, Kulkarni MS, Kulkarni GS, Goyal V, Kulkarni MG. Comparison between antegrade intramedullary nailing (IMN), open reduction plate osteosynthesis (ORPO) and minimally invasive plate osteosynthesis (MIPO) in treatment of humerus diaphyseal fractures. Injury 2017;48:S8–S13. [DOI] [PubMed] [Google Scholar]
- 37. vande Wall BJM, Theus C, Link BC, et al. Absolute or relative stability in plate fixation for simple humeral shaft fractures. Injury 2019;50:1986–1991. [DOI] [PubMed] [Google Scholar]
- 38. Putti AB, Uppin RB, Putti BB. Locked intramedullary nailing versus dynamic compression plating for humeral shaft fractures. J Orthop Surg (Hong Kong) 2009;17:139–141. [DOI] [PubMed] [Google Scholar]
- 39. Singisetti K, Ambedkar M. Nailing versus plating in humerus shaft fractures: a prospective comparative study. Int Orthop 2010;34:571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Changulani M, Jain UK, Keswani T. Comparison of the use of the humerus intramedullary nail and dynamic compression plate for the management of diaphyseal fractures of the humerus: a randomised controlled study. Int Orthop 2007;31:391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Benegas E, Ferreira Neto AA, Gracitelli MEC, et al. Shoulder function after surgical treatment of displaced fractures of the humeral shaft: a randomized trial comparing antegrade intramedullary nailing with minimally invasive plate osteosynthesis. J Shoulder Elbow Surg 2014;23:767–774. [DOI] [PubMed] [Google Scholar]
- 42. McCormack RG, Brien D, Buckley RE, McKee MD, Powell J, Schemitsch EH. Fixation of fractures of the shaft of the humerus by dynamic compression plate or intramedullary nail: a prospective, randomised trial. J Bone Joint Surg Br 2000;82:336–339. [DOI] [PubMed] [Google Scholar]
- 43. Chapman JR, Henley MB, Agel J, Benca PJ. Randomized prospective study of humeral shaft fracture fixation: intramedullary nails versus plates. J Orthop Trauma 2000;14:162–166. [DOI] [PubMed] [Google Scholar]
- 44. Chiu FY, Chen CM, Lin CF, Lo WH, Huang YL, Chen TH. Closed humeral shaft fractures: a prospective evaluation of surgical treatment. J Trauma 1997;43:947–951. [DOI] [PubMed] [Google Scholar]
- 45. Hee HT, Low BY, See HF. Surgical results of open reduction and plating of humeral shaft fractures. Ann Acad Med Singap 1998;27:772–775. [PubMed] [Google Scholar]
- 46. Heim D, Herkert F, Hess P, Regazzoni P. Surgical treatment of humeral shaft fractures: the Basel experience. J Trauma 1993;35:226–232. [PubMed] [Google Scholar]
- 47. Modabber MR, Jupiter JB. Operative management of diaphyseal fractures of the humerus: plate versus nail. Clin Orthop Relat Res 1998;347:93–104. [PubMed] [Google Scholar]
- 48. Niall DM, O’Mahony J, McElwain JP. Plating of humeral shaft fractures: has the pendulum swung back? Injury 2004;35:580–586. [DOI] [PubMed] [Google Scholar]
- 49. Wade RH. Closed humeral shaft fractures: a prospective evaluation of surgical treatment. J Trauma 1998;44:1115. [DOI] [PubMed] [Google Scholar]
- 50. Streufert BD, Eaford I, Sellers TR, et al. Iatrogenic nerve palsy occurs with anterior and posterior approaches for humeral shaft fixation. J Orthop Trauma 2020;34:163–168. [DOI] [PubMed] [Google Scholar]
- 51. Ekholm R, Ponzer S, Törnkvist H, Adami J, Tidermark J. Primary radial nerve palsy in patients with acute humeral shaft fractures. J Orthop Trauma 2008;22:408–414. [DOI] [PubMed] [Google Scholar]
- 52. Hughes RE, Schneeberger AG, An KN, Morrey BF, O’Driscoll SW. Reduction of triceps muscle force after shortening of the distal humerus: a computational model. J Shoulder Elbow Surg 1997;6:444–448. [DOI] [PubMed] [Google Scholar]
- 53. Farragos AF, Schemitsch EH, McKee MD. Complications of intramedullary nailing for fractures of the humeral shaft: a review. J Orthop Trauma 1999;13:258–267. [DOI] [PubMed] [Google Scholar]
- 54. Rommens PM, Verbruggen J, Broos PL. Retrograde locked nailing of humeral shaft fractures: a review of 39 patients. J Bone Joint Surg Br 1995;77:84–89. [PubMed] [Google Scholar]
- 55. Dimakopoulos P, Papadopoulos AX, Papas M, Panagopoulos A, Lambiris E. Modified extra rotator-cuff entry point in antegrade humeral nailing. Arch Orthop Trauma Surg 2005;125:27–32. [DOI] [PubMed] [Google Scholar]
- 56. Park J-Y, Pandher DS, Chun J-Y, Md STL. Antegrade humeral nailing through the rotator cuff interval: a new entry portal. J Orthop Trauma 2008;22:419–425. [DOI] [PubMed] [Google Scholar]
- 57. Rommens PM, Kuechle R, Bord T, Lewens T, Engelmann R, Blum J. Humeral nailing revisited. Injury 2008;39:1319–1328. [DOI] [PubMed] [Google Scholar]
- 58. Holstein A, Lewis GM. Fractures of the humerus with radial-nerve paralysis. J Bone Joint Surg Am 1963;45:1382–1388. [PubMed] [Google Scholar]
- 59. Ingman AM, Waters DA. Locked intramedullary nailing of humeral shaft fractures: implant design, surgical technique, and clinical results. J Bone Joint Surg Br 1994;76:23–29. [PubMed] [Google Scholar]
- 60. Robinson CM, Bell KM, Court-Brown CM, McQueen MM. Locked nailing of humeral shaft fractures: experience in Edinburgh over a two-year period. J Bone Joint Surg Br 1992;74:558–562. [DOI] [PubMed] [Google Scholar]
- 61. Kurup H, Hossain M, Andrew JG. Dynamic compression plating versus locked intramedullary nailing for humeral shaft fractures in adults. Cochrane Database Syst Rev 2011;6:CD005959. [DOI] [PubMed] [Google Scholar]
- 62. Ouyang H, Xiong J, Xiang P, Cui Z, Chen L, Yu B. Plate versus intramedullary nail fixation in the treatment of humeral shaft fractures: an updated meta-analysis. J Shoulder Elbow Surg 2013;22:387–395. [DOI] [PubMed] [Google Scholar]
- 63. Marsh JL, Mahoney CR, Steinbronn D. External fixation of open humerus fractures. Iowa Orthop J 1999;19:35–42. [PMC free article] [PubMed] [Google Scholar]
- 64. Mostafavi HR, Tornetta P., III Open fractures of the humerus treated with external fixation. Clin Orthop Relat Res 1997;337:187–197. [DOI] [PubMed] [Google Scholar]
- 65. Zinman C, Norman D, Hamoud K, Reis ND. External fixation for severe open fractures of the humerus caused by missiles. J Orthop Trauma 1997;11:536–539. [DOI] [PubMed] [Google Scholar]
- 66. Gausepohl T, Koebke J, Pennig D, Hobrecker S, Mader K. The anatomical base of unilateral external fixation in the upper limb. Injury 2000;31:11–20. [DOI] [PubMed] [Google Scholar]
- 67. Bloom T, Zhao C, Mehta A, Thakur U, Koerner J, Sabharwal S. Safe zone for superolateral entry pin into the distal humerus in children: an MRI analysis. Clin Orthop Relat Res 2014;472:3779–3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Scaglione M, Fabbri L, Dell’Omo D, Goffi A, Guido G. The role of external fixation in the treatment of humeral shaft fractures: a retrospective case study review on 85 humeral fractures. Injury 2015;46:265–269. [DOI] [PubMed] [Google Scholar]
- 69. Ekholm R, Ponzer S, Törnkvist H, Adami J, Tidermark J. The Holstein-Lewis humeral shaft fracture: aspects of radial nerve injury, primary treatment, and outcome. J Orthop Trauma 2008;22:693–697. [DOI] [PubMed] [Google Scholar]
- 70. Schwab TR, Stillhard PF, Schibli S, Furrer M, Sommer C. Radial nerve palsy in humeral shaft fractures with internal fixation: analysis of management and outcome. Eur J Trauma Emerg Surg 2018;44:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shao YC, Harwood P, Grotz MRW, Limb D, Giannoudis PV. Radial nerve palsy associated with fractures of the shaft of the humerus: a systematic review. J Bone Joint Surg Br 2005;87:1647–1652. [DOI] [PubMed] [Google Scholar]
- 72. Vaishya R, Kandel IS, Agarwal AK, Vijay V, Vaish A, Acharya K. Is early exploration of secondary radial nerve injury in patients with humerus shaft fracture justified? J Clin Orthop Trauma 2019;10:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bishop J, Ring D. Management of radial nerve palsy associated with humeral shaft fracture: a decision analysis model. J Hand Surg Am 2009;34:991–996.e1. [DOI] [PubMed] [Google Scholar]
- 74. Korompilias AV, Lykissas MG, Kostas-Agnantis IP, Vekris MD, Soucacos PN, Beris AE. Approach to radial nerve palsy caused by humerus shaft fracture: is primary exploration necessary? Injury 2013;44:323–326. [DOI] [PubMed] [Google Scholar]
- 75. Ilyas AM, Mangan JJ, Graham J. Radial nerve palsy recovery with fractures of the humerus: an updated systematic review. J Am Acad Orthop Surg 2020;28:e263–e269. [DOI] [PubMed] [Google Scholar]
- 76. Green DP, Hotchkiss RN, Pederson WC. Green’s operative hand surgery. Fourth ed. New York: Churchill Livingstone, 1999:1492. [Google Scholar]
- 77. Seddon HJ. Nerve grafting. J Bone Joint Surg Br 1963;45:447–461. [PubMed] [Google Scholar]