Abstract
MicroRNAs (miRs) are essential regulators of atherosclerosis (AS) development; however, the pathogenic roles of miR-140-5p during AS development are not completely understood. The present study investigated the effects of miR-140-5p on human vascular smooth muscle cells (VSMCs) and its target gene. miR-140-5p and roundabout guidance receptor 4 (ROBO4) mRNA expression levels were determined by performing reverse transcription-quantitative PCR. ROBO4 protein expression levels were analyzed via western blotting. Cell viability, migration, invasion and apoptosis were evaluated by conducting Cell Counting Kit-8, Transwell and flow cytometry assays, respectively. The binding of miR-140-5p to ROBO4 mRNA was verified using the dual-luciferase reporter assay. miR-140-5p was highly expressed in the plaque-containing artery tissues of patients with AS compared with healthy control tissues. Oxidized-low density lipoprotein (ox-LDL) treatment increased miR-140-5p expression and decreased ROBO4 expression in human VSMCs, which promoted VSMC viability, migration and invasion, but suppressed apoptosis compared with the control group. The effects of ox-LDL treatment on VSMCs were attenuated by miR-140-5p inhibitor. miR-140-5p directly bound to the 3′-untranslated region of ROBO4 mRNA. ROBO4 overexpression mitigated the effects of ox-LDL treatment on VSMC viability, migration, invasion and apoptosis. Therefore, the present study suggested that high level miR-140-5p expression promoted VSMC viability, migration, and invasion, and suppressed VSMC apoptosis by reducing ROBO4 gene expression. The present study provided novel insights into AS pathogenesis that may aid the development of new strategies for the treatment and prevention of AS.
Keywords: atherosclerosis, microRNA-140-5p, roundabout guidance receptor 4, vascular smooth muscle cell, viability, migration, apoptosis
Introduction
Atherosclerosis (AS) is a common vascular pathogenic condition characterized by artery wall thickening and reduced elasticity due to the formation of atherosclerotic plaques (fatty deposits) in arterial intima. The plaques are primarily composed of cholesterol, fat, calcium and other constitutes of human blood (1,2). The hardening of plaques with time usually results in arterial stenosis and reduces tissue blood supply, which can lead to a number of severe conditions, such as coronary heart disease, carotid artery disease, peripheral artery disease, chronic kidney disease, stroke, angina and even death (2,3). Currently, the clinical management of AS greatly depends on altering the patient's lifestyle, the use of statin medications and even surgical treatments, such as percutaneous coronary intervention and coronary artery bypass grafting, for patients with severe AS (2–4). A previous study demonstrated that major risk factors for AS include high low density lipoprotein (LDL) levels, hypertension, insulin resistance, obesity, diabetes, inflammation and unhealthy lifestyle habits, such as smoking and a high-saturated fat diet (5). However, the molecular mechanisms underlying plaque formation and AS pathogenesis are not completely understood.
The abnormal function of vascular smooth muscle cells (VSMCs) is closely associated with AS development (6–8). Accumulating evidence has suggested that VSMCs undergo significant phenotypic alterations that drive plaque formation and AS progression. Among those alterations are increased capacities for cell proliferation and migration, a reduced rate of VSMC apoptosis, and alterations in various extracellular matrix proteins and secreted cytokines (6,9). For example, VSMCs in healthy vascular vessel walls usually have extremely low turnover and proliferation rates; however, VSMC proliferation is greatly increased during the early stage of atherogenesis and in response to a vascular injury (6). A previous report revealed that high glucose-induced advanced glycosylation end products could remarkably promote VSMC proliferation and apoptosis, resulting in vein graft AS via activation of MAPK signaling pathways (10). Moreover, a previous investigation suggested that suppression of VSMC proliferation, inflammatory responses and adhesion by vasostatin-2 overexpression might serve as a novel therapeutic strategy for AS (11). Collectively, the aforementioned studies indicated that VSMC phenotypic switching is linked to plaque formation and AS development, suggesting that inhibiting the phenotype switch of VSMCs might serve as a promising therapeutic strategy for AS.
MicroRNAs (miRNAs/miRs) are a large group of single-stranded non-coding RNA molecules that consist of 19–25 nucleotides, and serve as regulators of gene expression by modulating mRNA degradation and interfering with protein translation (12–14). miRNAs are closely linked to various pathogenic processes, such as cancer, neural degeneration, diabetic nephropathy and vascular diseases (13–18). More importantly, miRNAs also serve essential pathogenic roles during AS development and progression (19,20). A previous study demonstrated that miR-140-5p could inhibit the angiogenesis process in a rat model of middle cerebral artery occlusion by regulating vascular endothelial growth factor (VEGF)A expression (21). Furthermore, miR-140-5p inhibits glioma cell proliferation and invasion by modulating the VEGF and matrix metallopeptidase 2 (MMP2) signaling pathways (22). Liu et al (23) reported that miR-140-5p aggravates AS by mediating oxidative stress responses by targeting nuclear factor, erythroid 2 like 2 and sirtuin 2 via the kelch like ECH associated protein 1/heme oxygenase 1 signaling pathway (23). Another study demonstrated that miR-140-5p serves a key role in pulmonary arterial hypertension by targeting SMAD-specific E3 ubiquitin protein ligase 1 (24). Despite the widespread expression of microRNAs, the current understanding of the association between microRNAs and AS pathogenesis is limited.
A bioinformatics analysis predicted that miR-140-5p might target the roundabout guidance receptor 4 (ROBO4) gene, which codes for a member of the neuronal Robo family that serve as vascular-specific receptors, and are involved in angiogenesis and vascular permeability regulation (25–27). ROBO4 expression is mediated by methylation of its promoter, and has been demonstrated to serve a key role in endothelial cell differentiation (28). Moreover, a previous study indicated that ROBO4 could modulate microvascular endothelial cell migration during angiogenesis (29,30). However, how miR-140-5p might affect ROBO4 gene expression during the AS pathogenesis process, and the roles served by miR-140-5p in VSMCs and AS development are not completely understood.
The present study aimed to investigated the role of ROBO4 and miR-140-5p in an oxidized LDL (ox-LDL)-induced cell model. The mechanism was identified by investigating the expression levels of miR-140-5p and ROBO4 expression in AS tissues and human VSMCs treated with ox-LDLs, as well as the effects of ox-LDL treatment on VSMC viability, migration, invasion and apoptosis. This study provided a novel perspective on the molecular pathogenesis of AS, and may aid in developing novel strategies for the prevention and treatment of the disease.
Materials and methods
Tissues, cell culture and treatments
Artery tissues containing AS plaque were collected from patients who had been diagnosed with AS (n=20; age, 48–71 years; 55% men and 45% women) and undergone surgical treatment in the Department of Vascular Surgery of The First Affiliated Hospital of Chongqing Medical University between July 2017 and December 2018. Samples of AS tissue were obtained from patients with lower extremity atherosclerotic occlusive disease who were undergoing surgery, and samples of healthy control tissue were obtained from individuals who had suffered an injury, such as amputation (20%), fracture (70%) or other (10%). The present study was reviewed and approved by the Medical Ethics Committee of The First Affiliated Hospital of Chongqing Medical University. Written informed consent was obtained from each participant.
Human VSMCs were purchased from the BeNa Culture Collection (cat. no. BNCC340087) and cultured in high-glucose DMEM (cat. no. 12100046; Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS (cat. no. 10099-141; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin, and 100 U/ml streptomycin at 37°C in a humidified cell culture chamber containing 5% CO2. All cells were used within 7 passages and stored in liquid nitrogen prior to use. Cells (5×105 cells/ml) were treated with 50 or 100 µg/ml ox-LDL (cat. no. YB-002; Guangzhou Yiyuan Biological Technology Co., Ltd.) for 24 h at 37°C. Cells treated with PBS were set as the control group.
Cell transfection
An miR-140-5p inhibitor (3′-GUCACCAAAACGGGAUACCAUC-5′) and its negative control (NC, 5′-UUCUCCGAACGUGUCACGUTT-3′) were synthesized by Shanghai GenePharma Co., Ltd. and used to induce miR-140-5p knockdown. VSMCs (1×105 cells/ml) were seeded into 96-well plates and cultured overnight at 37°C. Subsequently, VSMCs were incubated with 25 µl serum-free DMEM containing 5 pmol miR-140-5p inhibitor or NC and 0.25 µl Lipofectamine® 2000 Transfection Reagent (cat. no. 11668027; Thermo Fisher Scientific, Inc.) at 37°C according to the manufacturer's protocol. To induce ROBO4 overexpression, ROBO4 genomic sequences were amplified by PCR using specific primers (forward, 5′-GGGGTACCATGGTGGCTGTGGTGGGTGAGC-3′ and reverse, 5′-CCAAGCTTTCAGGAGTAATCTACAGGAGAAGCACCAGC-3′) and inserted into pcDNA3.0 plasmids (Biovector Science Lab, Inc.). VSMCs were transfected with recombinant pcDNA3.0-ROBO4 plasmids (1.5 µg) using Lipofectamine 2000 Transfection Reagent at 37°C. At 24 h post-transfection, transfection efficiency was assessed via reverse transcription-quantitative PCR (RT-qPCR). An empty vector was transfected into VSMCs as a negative control.
RT-qPCR
The relative expression levels of miR-140-5p and ROBO4 mRNA in cultured VSMCs and artery tissues were analyzed via RT-qPCR. Total RNA was isolated from cultured cells or tissues using TRIzol® reagent (cat. no. 9109; Takara Bio, Inc.) according to the manufacturer's protocol. Subsequently, total RNA (2.0 µg) was reverse transcribed into cDNA using the Bestar qPCR RT kit (cat. no. 2220; DBI Bioscience) according to the manufacturer's protocol. Subsequently, qPCR was performed using a Stratagene Mx3000P real-time PCR system (Agilent Technologies, Inc.) and Bestar™ qPCR MasterMix (cat. no. 2043; DBI Bioscience) according to the manufacturer's instructions. The following thermocycling conditions were used for qPCR: 40 cycles of 94°C for 2 min, followed by 94°C for 20 sec, 58°C for 20 sec and 72°C for 20 sec. The sequences of the primers used for qPCR are presented in Table I. miRNA and mRNA expression levels were quantified using the 2−∆∆Cq method (31) and normalized to the internal reference genes U6 and GAPDH, respectively.
Table I.
Primer sequences used for reverse transcription-quantitative PCR.
| Gene | Sequences (5′→3′) | Product length (bp) |
|---|---|---|
| GAPDH | F: TGTTCGTCATGGGTGTGAAC | 131 |
| R: ATGGCATGGACTGTGGTCAT | ||
| Bcl-2 | F: GAGGATTGTGGCCTTCTTTG | 170 |
| R: ACAGTTCCACAAAGGCATCC | ||
| MMP2 | F: ATGACAGCTGCACCACTGAG | 174 |
| R: ATTTGTTGCCCAGGAAAGTG | ||
| ROBO4 | F: GTGGTGGGTGAGCAGTTTAC | 270 |
| R: CATGTAGGTCCCTTCGTCAC | ||
| miR-140-5p | F: ACACTCCAGCTGGGTGGTGGTTTTACCCTATGGT | N/A |
| R: CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCTACCAT | ||
| U6 | F: CTCGCTTCGGCAGCACA | N/A |
| R: AACGCTTCACGAATTTGCGT |
MMP2, matrix metallopeptidase 2; ROBO4, roundabout guidance receptor 4; miR, microRNA; F, forward; R, reverse; N/A, not applicable.
Western blotting
Total protein was extracted from cultured VSMC cells using RIPA Lysis Buffer (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Total protein was quantified using the bicinchoninic acid method. Protein (~20 µg) was separated via 10% SDS-PAGE and transferred onto PVDF membranes (EMD Millipore), which were then blocked with 5% lipid-free milk solution for 2 h at room temperature. Subsequently, the membranes were incubated for 1–2 h at room temperature with primary antibodies targeted against: ROBO4 (cat. no. ab180824; 1:1,000; Abcam) and GAPDH (cat. no. ab8245; 1:5,000; Abcam). Following primary incubation, the membranes were incubated with rabbit anti-mouse HPR-conjugated secondary antibodies (cat. no. ab6728; 1:3,000; Abcam) for 2 h at room temperature. Immunoreactive bands were visualized using an Enhanced Chemiluminescence Substrate kit (cat. no. KLS0500; Merck KGaA). GAPDH was used as the loading control. Image-Pro Plus software (version 6.0; Media Cybernetics, Inc.) was used for semi-quantification.
Cell Counting Kit-8 (CCK-8) assay
VSMC viability was evaluated using a CCK-8 assay (cat. no. CK04; Dojindo Molecular Technologies, Inc.) according to the manufacturer's protocol. Briefly, cells (1×105 cells/well) in a 96-well plate were incubated in a humidified incubator at 37°C for 24 h. Subsequently, 10 µl CCK-8 solution was added to each well and incubated for 24, 48 or 72 h at 37°C. The absorbance of each well was measured at a wavelength of 450 nm (optical density450) using a microplate reader to calculate the cell viability rate.
Cell migration and invasion
VSMC migration and invasion were detected using a Transwell culture system. To assess cell migration, VSMCs (2×104) were re-suspended in serum-free DMEM containing 0.2% BSA (cat. no. ST025; Beyotime Institute of Biotechnology) and seeded into the upper chambers. The lower chambers were filled with 700 µl complete DMEM containing 10% FBS. Following incubation at 37°C for 48 h, cells in the lower chambers were fixed with 4% paraformaldehyde (cat. no. P0099; Beyotime Institute of Biotechnology), stained with crystal violet staining solution for 5–10 min at room temperature and counted using a CX41 light microscope (Olympus Corporation). To detect cell invasion, the inner sides of the Transwell chambers were pre-coated with Matrigel® Basement Membrane Matrix (cat. no. 356234BD; Biocoat, Inc.) at room temperature, after which, the aforementioned procedure was performed to assess cell invasion.
Cell apoptosis
VSMC apoptosis was detected by using an Annexin V-FITC/PI kit (Nanjing KeyGen Biotech Co., Ltd.) in combination with flow cytometry. Briefly, cultured VSMCs were seeded (4×105 cells/well) into a 6-well plate and treated with ox-LDL. After treatment, cells were collected and incubated with Annexin V-FITC solution (1 ml) for 12 min in the dark at room temperature, and incubated with propidium iodide solution (5 µl) for 5 min in the dark. Subsequently, the percentage of apoptotic VSMCs was determined by flow cytometry (BD FACSCalibur™; BD Biosciences) using FlowJo™ version 10 software (FlowJo LLC). Early + late apoptosis rate was regarded as total apoptosis percentage.
Immunofluorescence
VSMCs were fixed with 4% paraformaldehyde for 30 min at room temperature followed by permeabilization with 0.3% Triton X-100 for 15 min. After permeabilization, cells were washed with PBS and blocked with 5% goat serum (cat. no. C0265; Beyotime Institute of Biotechnology) for 30 min at room temperature, and subsequently incubated with an anti-α-smooth muscle actin (SMA) primary antibody (cat no. BM3902; 1:100; Boster Biological Technology) overnight at 4°C. Subsequently, cells were incubated with a secondary Cy5-labeled goat anti-rabbit antibody (cat no. ab97077; 1:300; Abcam). Cells were stained with DAPI at room temperature for 10 min for nuclear staining and observed using a fluorescence microscope (magnification, ×400).
Construction of vectors and transfection
The potential binding site of miR-140-5p on the 3′UTR of ROBO4 was predicted using TargetScan (http://www.targetscan.org/vert_72/) (32). siRNA against ROBO4 (5′-CCAAGACUACGAGUUCAAA-3′) and the NC (5′-AUUCGACGCAUGACACAAA-3′) were purchased from Shanghai GenePharma Co., Ltd. siRNA and NC were used at concentration of 20 µM diluted with diethyl pyrocarbonate H2O. DNA was extracted from the VSMCs using PrimeSTAR® HS DNA Polymerase (Takara Bio, Inc.). The following primers were used to amplify the 3′-untranslated region (UTR) sequences from VSMCs by PCR: Wild-type (WT) 3′UTR forward, 5′-CCCTCGAGACCGTGTCCCTGAGACTTCCC-3′ and reverse, 5′-ATTTGCGGCCGCTAGGAGTCAGGTGGAGATGATGTTT-3′. The PCR process was according to the information included in Table II. The resulting PCR product was ligated into psi-CHECK2 vectors (cat. no. 97157; Addgene, Inc.). Point mutations were introduced by performing PCR with the following primers: forward, 5′-CTTCCCAGACGGGAATCAGCTTACTGTCTCCTGTCCACCCACAAG-3′ and reverse, 5′-ACTTGTGGGTGGACAGGAGACAGTAAGCTGATTCCCGTCTGGGAAG-3′. Similarly, the resulting PCR products were inserted into psi-CHECK2 vectors. The coding sequences of ROBO4 were cloned from VSMCs using the following primers: forward, 5′-GGGGTACCATGGTGGCTGTGGTGGGTGAGC-3′ and reverse, 5′-CCAAGCTTTCAGGAGTAATCTACAGGAGAAGCACCAGC-3′. All PCR products were identified by sequencing conducted by Sangon Biotech Co., Ltd. Plasmid (~10 µg) was transfected into VSMCs (1×105 per ml). Transfections were performed using Lipofectamine 3000 (Thermo Fisher Scientific, Inc.) according to manufacturer's instructions. All primers used were synthesized by Sangon Biotech Co., Ltd. Subsequent assays were performed 24 h after transfection.
Table II.
PCR amplification procedure.
| Reagent | Volume (µl) |
|---|---|
| PrimeSTAR® HS DNA Polymerase | 0.5 |
| 5X PrimeSTAR buffer | 5.0 |
| dNTP Mix (2.5 mM each) | 2.0 |
| F (10 µM) | 0.5 |
| R (10 µM) | 0.5 |
| cDNA | 1.0 |
| ddH2O | ≤25 |
| Total volume | 25 |
F, forward; R, reverse.
Dual-luciferase reporter assay
The binding of miR-140-5p to ROBO4 mRNA was verified by performing a dual-luciferase reporter assay using a Dual-Luciferase Reporter Assay System (cat. no. E1910; Promega Corporation) according to the manufacturer's instructions. Briefly, the WT and mutant (MUT) 3′UTR ROBO4 gene sequences were separately inserted into psi-CHECK2 plasmids (cat. no. 97157; Addgene, Inc.). miR-140-5p mimic (5′-CAGUGUUUUACCCUAUGGUAG-3′) and its NC (5′-UUCUCCGAACGUGUCACGUTT-3′) were synthesized by Shanghai GenePharma Co., Ltd. VSMCs were transfected with the WT or MUT recombinant plasmids (8 µg) and miR-140-5p mimic or the NC (100 ppm) using Lipofectamine 2000 Transfection Reagent (Thermo Fisher Scientific, Inc.). Luciferase activity was detected using a GloMax 20/20 Luminometer (Promega Corporation). Renilla luciferase activity was set as the internal reference.
Statistical analysis
Statistical analyses were performed using SPSS software (version 20.0; IBM Corp). Data are presented as the mean ± SD. All experiments were repeated three times. Comparisons among groups were analyzed using the Student's t test or non-parametric Kruskal-Wallis followed by the Dunn's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
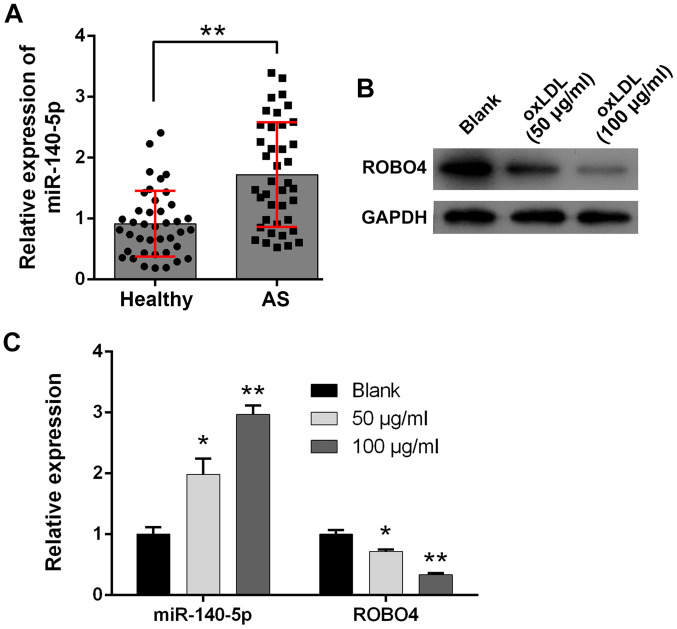
Increased miR-140-5p expression and decreased ROBO4 expression during AS
To investigate the potential pathogenic roles of miR-140-5p during AS development, artery tissues containing plaque were collected from 20 patients who had been diagnosed with AS and healthy artery tissues were collected from 20 healthy volunteers who served as healthy control subjects. miR-140-5p was expressed at significantly higher levels in the artery tissues of patients with AS compared with healthy control subjects (Fig. 1A). Moreover, cultured human VSMCs were treated with ox-LDL to establish a cellular model of AS development. A bioinformatics analysis predicted ROBO4 as a target of miR-140-5p. In the present study, ROBO4 protein expression levels in human VSMCs were notably reduced by ox-LDL treatment in a concentration-dependent manner compared with the blank control group (Fig. 1B). Furthermore, the RT-qPCR results indicated that miR-140-5p expression was significantly increased and ROBO4 gene expression was significantly decreased in ox-LDL-treated human VSMCs compared with blank control VSMCs (Fig. 1C).
Figure 1.
Increase in miR-140-5p expression and decrease in ROBO4 expression associated with AS pathogenesis. (A) miR-140-5p expression levels in healthy artery tissues and artery tissues obtained from patients with AS. **P<0.01 vs. normal. (B) ROBO4 protein expression levels in ox-LDL-treated human VSMCs. (C) miR-140-5p and ROBO4 gene expression levels in ox-LDL-treated human VSMCs. *P<0.05, **P<0.01 vs. blank group. miR, microRNA; ROBO4, roundabout guidance receptor 4; AS, atherosclerosis; ox-LDL, oxidized-low density lipoprotein; VMSC, vascular smooth muscle cell.
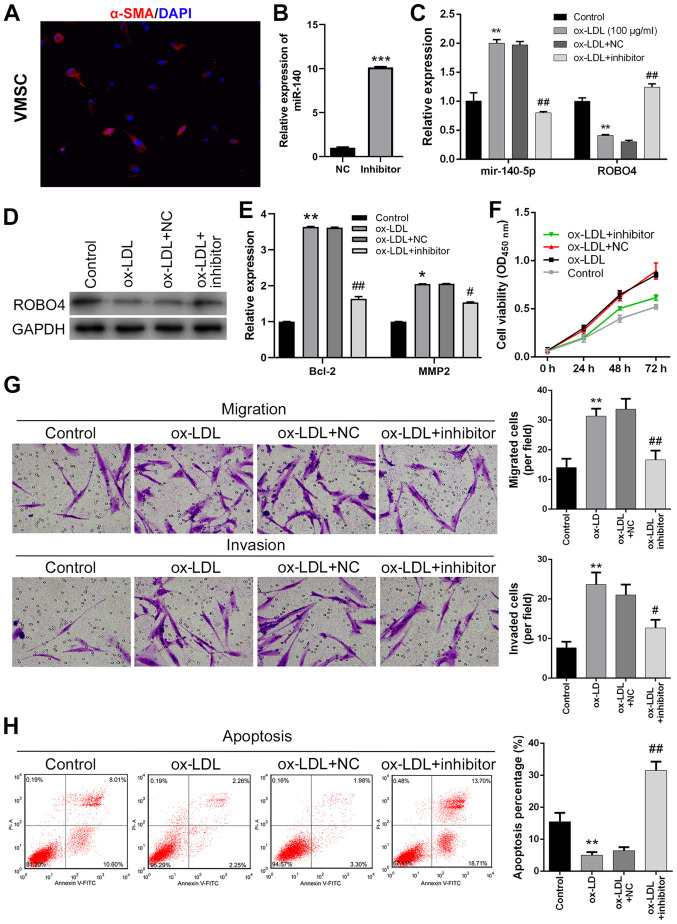
miR-140-5p inhibition suppresses VSMC viability, migration and invasion, and promotes apoptosis following ox-LDL treatment
To analyze the cellular functions of miR-140-5p during AS development, cultured human VSMCs were transfected with miR-140-5p inhibitor. VSMCs were identified by detecting α-SMA expression via immunofluorescence. The results suggested that the VSMCs expressed α-SMA (Fig. 2A). miR-140-5p expression levels in ox-LDL-treated human VSMCs were significantly downregulated by miR-140-5p inhibitor compared with the NC group (Fig. 2B). Furthermore, ROBO4 protein expression levels in ox-LDL-treated VSMCs were significantly downregulated compared with control VSMCs (Fig. 2C). ROBO4 protein expression levels in ox-LDL-treated human VSMCs were upregulated by transfection with miR-140-5p inhibitor (Fig. 2D). The RT-qPCR results indicated that Bcl-2 and MMP2 expression levels were significantly higher in ox-LDL-treated VSMCs compared with control VSMCs, which was reversed by miR-140 inhibitor (Fig. 2E). Moreover, human VSMC viability was greatly increased by ox-LDL treatment, but ox-LDL-mediated alterations to cell viability were reversed by miR-140-5p inhibitor (Fig. 2F). Similarly, the number of migratory and invading VSMCs was significantly increased following ox-LDL treatment compared with control VSMCs, and ox-LDL-mediated increases were attenuated by miR-140-5p inhibitor (Fig. 2G). By contrast, the number of apoptotic VSMCs was significantly decreased by ox-LDL treatment compared with control VSMCs, but restored by transfection with miR-140-5p inhibitor (Fig. 2H). Collectively, the results indicated that human VSMC viability, migration and invasion were enhanced, whereas VSMC apoptosis was suppressed in ox-LDL-treated VSMCs; however, miR-140-5p inhibitor reversed ox-LDL-mediated effects.
Figure 2.
Effect of miR-140-5p knockdown and ox-LDL treatment on VSMC viability, migration, invasion and apoptosis. (A) VSMCs were identified via immunofluorescence (magnification, ×200). (B) Transfection efficiency of miR-140-5p inhibitor. (C) miR-140-5p and ROBO4 expression levels in miR-140-5p inhibitor-transfected and ox-LDL-treated VMSCs. (D) ROBO4 protein expression levels in miR-140-5p inhibitor-transfected and ox-LDL-treated human VSMCs (E) Bcl-2 and MMP2 expression levels were measured by reverse transcription-quantitative PCR. Human VSMC (F) viability, (G) migration, invasion (magnification, ×200) and (H) apoptosis following miR-140-5p inhibitor transfection and ox-LDL treatment. *P<0.05, **P<0.01, ***P<0.001 vs. NC or control; #P<0.05, ##P<0.01 vs. ox-LDL + NC. miR, microRNA; ox-LDL, oxidized-low density lipoprotein; VSMC, vascular smooth muscle cell; ROBO4, roundabout guidance receptor 4; MMP2, matrix metallopeptidase 2; α-SMA, α-smooth muscle actin; NC, negative control; OD, optical density.
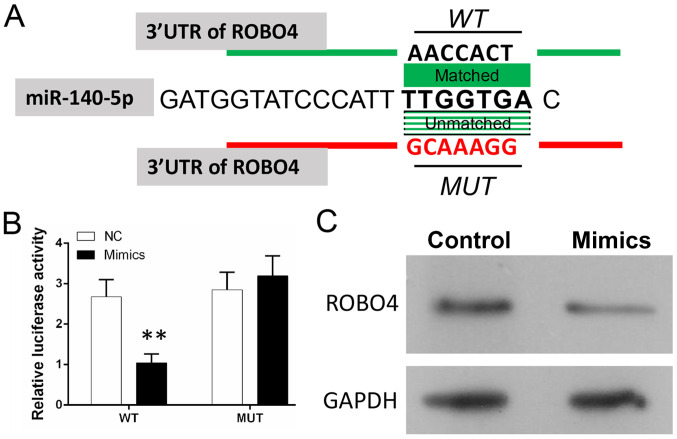
miR-140 directly targets the ROBO4 gene in VSMCs
A bioinformatics analysis predicted that ROBO4 might be a target gene of miR-140-5p by binding to the 3′-UTR of ROBO4 mRNA (Fig. 3A). To investigate the relationship between miR-140-5p and ROBO4, a dual-luciferase reporter assay was conducted to assess the binding between miR-140-5p and the 3′-UTR of ROBO4 mRNA (Fig. 3B). The results suggested that the luciferase activity of WT-VSMCs was significantly decreased in miR-140-5p mimic-transfected cells compared with the NC group (Fig. 3B). However, the luciferase activity of MUT-VSMCs was not significantly altered in miR-140-5p mimic-transfected cells compared with the NC group (Fig. 3B). The results indicated that miR-140-5p could bind to the 3′UTR of ROBO4 mRNA in VSMCs and verified ROBO4 as a target of miR-140-5p. Furthermore, miR-140-5p mimic downregulated ROBO4 expression levels compared with the NC group (Fig. 3C). Therefore, the results indicated that the relationship between ROBO4 and miR-140-5p may be associated with the ability of miR-140-5p to regulate AS pathogenesis.
Figure 3.
Investigation of miR-140-5p binding to ROBO4 mRNA in VSMCs. (A) The predicted binding site between miR-140-5p and the 3′-UTR sequences of ROBO4 mRNA. (B) Verification of miR-140-5p binding to the 3′-UTR sequences of ROBO4 mRNA in human VSMCs. (C) Expression of ROBO4 in VSMCs following transfection with miR-140 mimic. **P<0.01, vs. NC. miR, microRNA; ROBO4, roundabout guidance receptor 4; VSMC, vascular smooth muscle cell; 3′-UTR, 3′-untranslated region; WT, wild-type; MUT, mutant; NC, negative control; ox-LDL, oxidized-low density lipoprotein.
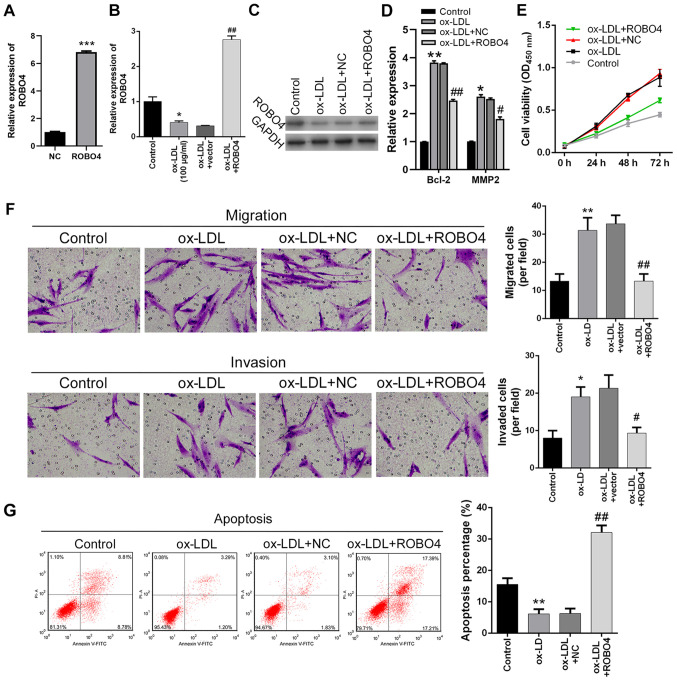
ROBO4 overexpression reverses ox-LDL-mediated effects on VSMCs
To explore the pathogenic roles of miR-140-5p-mediated inhibition of ROBO4 gene expression, ROBO4-overexpression human VSMCs were established. The results indicated that ROBO4 expression was significantly increased by transfection with the ROBO4-overexpression vector compared with the NC group (Fig. 4A). ROBO4 expression levels were markedly increased in ROBO4-overexpression ox-LDL-treated human VSMCs compared with empty vector-transfected ox-LDL-treated human VSMCs (Fig. 4B and C). Bcl-2 and MMP2 expression levels were significantly increased by ox-LDL treatment compared with control cells, but ROBO4 overexpression reversed ox-LDL-mediated alterations (Fig. 4D). In addition, ox-LDL-induced VSMC viability was notably decreased by ROBO4 overexpression (Fig. 4E). Similarly, ox-LDL-induced VSMC migration and invasion were significantly reduced by ROBO4 overexpression (Fig. 4F). Furthermore, the inhibitory effect of ox-LDL treatment on human VSMC apoptosis was also reversed by ROBO4 overexpression, which resulted in significantly increased VSMC apoptosis compared with ox-LDL-treated cells (Fig. 4G). Collectively, the results indicated that ox-LDL-mediated effects on human VSMC viability, migration, invasion and apoptosis could be attenuated by ROBO4 gene overexpression.
Figure 4.
Effects of ROBO4 overexpression and ox-LDL treatment on VSMC viability, migration, invasion and apoptosis. (A) Transfection efficiency of ROBO4 overexpression. ROBO4 (B) mRNA and (C) protein expression levels in human VSMCs following ROBO4 overexpression and ox-LDL treatment. (D) Bcl-2 and MMP2 expression levels were measured by reverse transcription-quantitative PCR. Effect of ROBO4 overexpression and ox-LDL treatment on human VSMC (E) viability, (F) migration, invasion (magnification, ×200) and (G) apoptosis. *P<0.05, **P<0.01, ***P<0.001 vs. NC or control; #P<0.05, ##P<0.01 vs. ox-LDL + NC. ROBO4, roundabout guidance receptor 4; ox-LDL, oxidized-low density lipoprotein; VSMC, vascular smooth muscle cell; MMP2, matrix metallopeptidase 2; NC, negative control; OD, optical density.
miR-140-5p mediates ox-LDL-induced VSMC phonotypes by targeting ROBO4
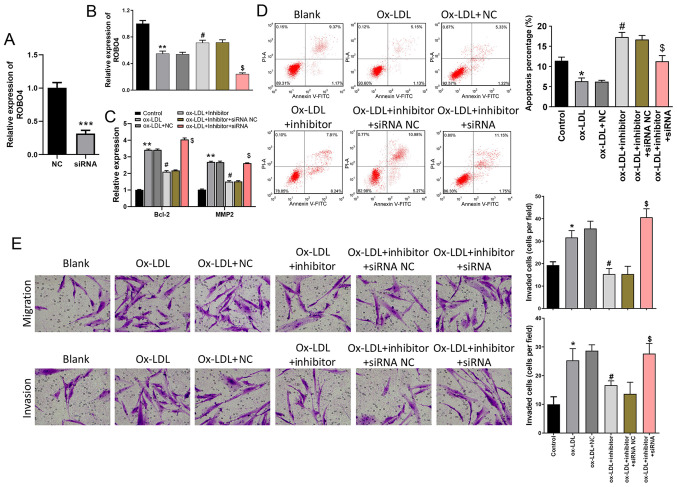
miR-140-5p inhibitor and ROBO4 siRNA were used to further investigate the role of miR-140-5p in VSMCs. The transfection efficiency of ROBO4 siRNA was assessed via RT-qPCR, which indicated that ROBO4 siRNA significantly downregulated ROBO4 expression levels compared with the NC group (Fig. 5A). ROBO4 expression was significantly reduced by treatment with ox-LDL compared with the control group, an effect that was partially reversed by miR-140-5p inhibitor. Moreover, ROBO4 expression in ox-LDL- and inhibitor-treated VSMCs was significantly decreased by treatment with ROBO4 siRNA (Fig. 5B). Bcl-2 and MMP2 expression levels were significantly increased in ox-LDL-treated cells compared with control cells, but treatment with miR-140-5p inhibitor significantly decreased ox-LDL-induced expression. In addition, Bcl-2 and MMP2 expression levels in ox-LDL- and inhibitor-treated VSMCs were significantly increased by ROBO4 siRNA (Fig. 5C). Compared with the control group, VSMC apoptosis was significantly decreased by ox-LDL treatment, but rescued by treatment with miR-140-5p inhibitor. ROBO4 siRNA significantly decreased the rate of apoptosis in ox-LDL and inhibitor-treated cells (Fig. 5D). The Transwell assays indicated that the migration and invasion of ox-LDL-treated VSMCs were significantly enhanced compared with the control group, but miR-140-5p inhibitor suppressed ox-LDL-induced migration and invasion. By contrast, ROBO4 siRNA significantly enhanced the migration and invasion of ox-LDL- and inhibitor-treated cells (Fig. 5E).
Figure 5.
miR-140-5p mediates VSMC apoptosis, migration and invasion by targeting ROBO4. (A) Transfection efficiency of ROBO4 siRNA. (B) Effects of ox-LDL, miR-140-5p inhibitor and ROBO4 siRNA on ROBO4 expression levels in VSMCs. (C) Bcl-2 and MMP2 expression levels were measured by reverse transcription-quantitative PCR. Effect of ox-LDL, miR-140-5p inhibitor and ROBO4 siRNA on VSMC (D) apoptosis, (E) migration and invasion (magnification, ×200). *P<0.05, **P<0.01, ***P<0.001 vs. NC or control; #P<0.05 vs. ox-LDL + NC; $P<0.05 vs. ox-LDL + inhibitor + siRNA NC. miR, microRNA; VSMC, vascular smooth muscle cell; ROBO4, roundabout guidance receptor 4; siRNA, small interfering RNA; ox-LDL, oxidized-low density lipoprotein; MMP2, matrix metallopeptidase 2; NC, negative control.
Discussion
Increasing evidence has revealed that non-coding RNA molecules, including miRNAs, are closely associated with various vascular disorders, such as AS, and their related complications (19,20,33). Despite previous reports describing the pathogenic roles served by miR-140-5p in angiogenesis and glioma cell proliferation/invasion (21,22), the roles of miR-R140-5p in AS development and progression are not completely understood. The present study indicated that miR-140-5p expression levels were significantly increased in artery plaque tissues collected from patients with AS and in ox-LDL-treated human VSMCs compared with healthy control tissues and control VSMCs, respectively. Moreover, ROBO4 gene expression was markedly decreased in ox-LDL-treated VSMCs compared with control VSMCs. The transfection experiments indicated that miR-140-5p promoted cell viability, migration, and invasion, but decreased apoptosis in ox-LDL-treated human VSMCs, which also inhibited ROBO4 gene expression. Additionally, the direct binding of miR-140-5p to the 3′-UTR of ROBO mRNA was validated using a dual luciferase reporter assay. Finally, the results indicated that the regulatory effects of ox-LDL treatment on human VSMCs were markedly abrogated by ROBO4 overexpression. Collectively, the results indicated that miR-140-5p served as an AS-promoting factor in human VSMCs by directly targeting and repressing ROBO4 gene expression.
The functions and molecular mechanisms underlying miR-140-5p have been extensively investigated due to its widespread and essential roles in various biological processes and pathogenic conditions. For instance, a recent study demonstrated that miR-140-5p could serve as a potent tumor suppressor and inhibit pediatric renal cancer cell proliferation by targeting and suppressing the expression of TGF-β receptor type 1 and insulin-like growth factor 1 receptor (34). Besides its widespread involvement in the development and progression of various human malignancies, miR-140-5p has been reported to serve as a key non-coding RNA molecule during the development of other human disorders, such as skeletal dysplasia, pulmonary arterial hypertension, inflammation-induced acute lung injuries and angiogenesis (35–37). On the other hand, several miRNAs, including miR-155, miR-33, miR-296 and miR-302a, have also been identified as essential regulators of AS development (38–41). Considering the functions of the microRNAs during AS pathogenesis, it was hypothesized that miR-140-5p may be involved in AS. The high levels of miR-140-5p expression detected in artery tissues from patients with AS and in ox-LDL-treated VSMCs, as well as the cellular functions of miR-140-5p in ox-LDL-treated VSMCs, were further indicated in the present study by transfecting human VSMCs with miR-140-5p inhibitor. The results provided a novel perspective for the molecular processes underlying AS development.
Previous studies indicated that human VSMCs undergo significant alterations in their cellular processes during AS pathogenesis, as demonstrated by increased rates of proliferation, migration and invasion, and a lower rate of apoptosis (6–8). For instance, VSMC proliferation was demonstrated to be severely limited by CD98, a deficiency of which greatly altered the number of smooth muscle cells in the plaque tissues of an AS mouse model (42). Wang et al (43) suggested that ox-LDL exerts biphasic effects on VSMC proliferation and apoptosis via an ox-LDL/β2-glycoprotein (GP)I/anti-β2GP complex. In the present study, ox-LDL treatment significantly promoted human VSMC viability, migration and invasion, but suppressed apoptosis, which was consistent with previously reported effects (6). The discrepancy between studies may be due to the sensitivity of VSMCs and the length of exposure to ox-LDLs. For instance, the NF-κB and MAPK signaling pathways send a variety of signals that regulate cell apoptosis and proliferation, and the net balance between cell death and survival is determined by the signaling pathway involved (43). Moreover, the present study indicated that miR-140-5p inhibitor transfection could almost completely reverse ox-LDL-induced alterations in VSMC viability, migration, invasion and apoptosis. However, inhibition of AS development via miR-140-5p inhibition requires further investigation in animal AS models. The cellular assays conducted in the present study characterized miR-140-5p as a novel AS-promoting miRNA that could possibly serve as biomarker for diagnosing AS, and be useful for the development of novel drugs for the treatment of AS.
ROBO4 is a major homolog of the neuronal Robo family, and was previously identified as a critical vascular-specific receptor that was specifically expressed in the vascular endothelium and inhibited endothelial migration (26). Subsequent investigations indicated that ROBO4 could interact with and activate the vascular Netrin receptor Unc-5 homolog B to inhibit angiogenesis and maintain vessel integrity by inhibiting the VEGF signaling pathway (25). A previous study also demonstrated the essential roles served by the ROBO4 gene in regulating vascular permeability and neovascularization (27). However, the pathogenic function of the ROBO4 gene during artery stiffening and AS development/progression is not completely understood. In the present study, ROBO4 gene expression was downregulated in the artery plaque tissues from patients with AS and in ox-LDL-treated human VSMCs compared with healthy artery tissues and control VSMCs, respectively. Consistent with a bioinformatics prediction, the dual-luciferase reporter assay indicated that miR-140-5p bound to the 3′-UTR regions of ROBO4 mRNA. Moreover, the results indicated that the effects of ox-LDL treatment on human VSMCs were mitigated by ROBO4 overexpression. The results characterized the ROBO4 gene as the target of miR-140-5p in VSMCs during AS development.
In summary, the present study indicated that miR-140-5p was highly expressed in human VSMCs during AS. The results suggested that miR-140-5p might promote the AS process by directly binding to ROBO4 mRNA and suppressing ROBO4 gene expression. ROBO4 overexpression effectively alleviated the cellular phenotypic alterations in VSMCs associated with AS pathogenesis. The present study provided novel insights into the pathogenic mechanisms underlying artery stiffening and AS development, and also established a rationale for further investigation into the use of miR-140-5p and ROBO4 as target molecules for use in AS diagnosis and treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by the North Sichuan Medical College City School Cooperation Program (grant nos. 18sxhz0364 and 18sxhz0408).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
YL and YZ designed the experiments. YL, YML and HP performed the experiments and collected the data. YL and YZ confirm the authenticity of all the raw data. HP analyzed the experimental data. YML described the results. YL drafted the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was reviewed and approved by the Medical Ethics Committee of The First Affiliated Hospital of Chongqing Medical University. Written informed consent was obtained from each participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Schwertani A, Choi HY, Genest J. HDLs and the pathogenesis of atherosclerosis. Curr Opin Cardiol. 2018;33:311–316. doi: 10.1097/HCO.0000000000000508. [DOI] [PubMed] [Google Scholar]
- 2.Weber C, Noels H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 3.Spence JD. Recent advances in pathogenesis, assessment, and treatment of atherosclerosis. F1000 Res. 2016;5:1880. doi: 10.12688/f1000research.8459.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicorescu I, Dallinga GM, de Winther MPJ, Stroes ESG, Bahjat M. Potential epigenetic therapeutics for atherosclerosis treatment. Atherosclerosis. 2019;281:189–197. doi: 10.1016/j.atherosclerosis.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Musunuru K, Kathiresan S. Surprises from genetic analyses of lipid risk factors for atherosclerosis. Circ Res. 2016;118:579–585. doi: 10.1161/CIRCRESAHA.115.306398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016;118:692–702. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu D, Yin C, Luo S, Habenicht AJR, Mohanta SK. Vascular smooth muscle cells contribute to atherosclerosis immunity. Front Immunol. 2019;10:1101. doi: 10.3389/fimmu.2019.01101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacolley P, Regnault V, Segers P, Laurent S. Vascular smooth muscle cells and arterial stiffening: Relevance in development, aging, and disease. Physiol Rev. 2017;97:1555–1617. doi: 10.1152/physrev.00003.2017. [DOI] [PubMed] [Google Scholar]
- 9.Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol. 2012;74:13–40. doi: 10.1146/annurev-physiol-012110-142315. [DOI] [PubMed] [Google Scholar]
- 10.Ping S, Li Y, Liu S, Zhang Z, Wang J, Zhou Y, Liu K, Huang J, Chen D, Wang J, et al. Simultaneous increases in proliferation and apoptosis of vascular smooth muscle cells accelerate diabetic mouse venous atherosclerosis. PLoS One. 2015;10:e0141375. doi: 10.1371/journal.pone.0141375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou J, Xue X, Li J. Vasostatin-2 inhibits cell proliferation and adhesion in vascular smooth muscle cells, which are associated with the progression of atherosclerosis. Biochem Biophys Res Commun. 2016;469:948–953. doi: 10.1016/j.bbrc.2015.12.097. [DOI] [PubMed] [Google Scholar]
- 12.Eulalio A, Huntzinger E, Izaurralde E. Getting to the root of miRNA-mediated gene silencing. Cell. 2008;132:9–14. doi: 10.1016/j.cell.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 13.Farazi TA, Spitzer JI, Morozov P, Tuschl T. miRNAs in human cancer. J Pathol. 2011;223:102–115. doi: 10.1002/path.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu J, Zhu W, Wu W. MicroRNAs change the landscape of cancer resistance. Methods Mol Biol. 2018;1699:83–89. doi: 10.1007/978-1-4939-7435-1_6. [DOI] [PubMed] [Google Scholar]
- 15.Kato M, Wang M, Chen Z, Bhatt K, Oh HJ, Lanting L, Deshpande S, Jia Y, Lai JY, O'Connor CL, et al. An endoplasmic reticulum stress-regulated lncRNA hosting a microRNA megacluster induces early features of diabetic nephropathy. Nat Commun. 2016;7:12864. doi: 10.1038/ncomms12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 17.Salta E, De Strooper B. microRNA-132: A key noncoding RNA operating in the cellular phase of Alzheimer's disease. FASEB J. 2017;31:424–433. doi: 10.1096/fj.201601308. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Liu Z, Zhou M, Liu C. MicroRNA-129-5p inhibits vascular smooth muscle cell proliferation by targeting Wnt5a. Exp Ther Med. 2016;12:2651–2656. doi: 10.3892/etm.2016.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feinberg MW, Moore KJ. MicroRNA Regulation of Atherosclerosis. Circ Res. 2016;118:703–720. doi: 10.1161/CIRCRESAHA.115.306300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu X, Li Z. MicroRNAs regulate vascular smooth muscle cell functions in atherosclerosis (Review) Int J Mol Med. 2014;34:923–933. doi: 10.3892/ijmm.2014.1853. [DOI] [PubMed] [Google Scholar]
- 21.Sun J, Tao S, Liu L, Guo D, Xia Z, Huang M. miR-140-5p regulates angiogenesis following ischemic stroke by targeting VEGFA. Mol Med Rep. 2016;13:4499–4505. doi: 10.3892/mmr.2016.5066. [DOI] [PubMed] [Google Scholar]
- 22.Hu Y, Li Y, Wu C, Zhou L, Han X, Wang Q, Xie X, Zhou Y, Du Z. MicroRNA-140-5p inhibits cell proliferation and invasion by regulating VEGFA/MMP2 signaling in glioma. Tumour Biol. 2017;39:1010428317697558. doi: 10.1177/1010428317697558. [DOI] [PubMed] [Google Scholar]
- 23.Liu Q-Q, Ren K, Liu S-H, Li W-M, Huang C-J, Yang X-H. MicroRNA-140-5p aggravates hypertension and oxidative stress of atherosclerosis via targeting Nrf2 and Sirt2. Int J Mol Med. 2019;43:839–849. doi: 10.3892/ijmm.2018.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothman AMK, Arnold ND, Pickworth JA, Iremonger J, Ciuclan L, Allen RMH, Guth-Gundel S, Southwood M, Morrell NW, Thomas M, et al. MicroRNA-140-5p and SMURF1 regulate pulmonary arterial hypertension. J Clin Invest. 2016;126:2495–2508. doi: 10.1172/JCI83361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koch AW, Mathivet T, Larrivée B, Tong RK, Kowalski J, Pibouin-Fragner L, Bouvrée K, Stawicki S, Nicholes K, Rathore N, et al. Robo4 maintains vessel integrity and inhibits angiogenesis by interacting with UNC5B. Dev Cell. 2011;20:33–46. doi: 10.1016/j.devcel.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Park KW, Morrison CM, Sorensen LK, Jones CA, Rao Y, Chien CB, Wu JY, Urness LD, Li DY. Robo4 is a vascular-specific receptor that inhibits endothelial migration. Dev Biol. 2003;261:251–267. doi: 10.1016/S0012-1606(03)00258-6. [DOI] [PubMed] [Google Scholar]
- 27.Zhang F, Prahst C, Mathivet T, Pibouin-Fragner L, Zhang J, Genet G, Tong R, Dubrac A, Eichmann A. The Robo4 cytoplasmic domain is dispensable for vascular permeability and neovascularization. Nat Commun. 2016;7:13517. doi: 10.1038/ncomms13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okada Y, Funahashi N, Tanaka T, Nishiyama Y, Yuan L, Shirakura K, Turjman AS, Kano Y, Naruse H, Suzuki A, et al. Endothelial cell-specific expression of roundabout 4 is regulated by differential DNA methylation of the proximal promoter. Arterioscler Thromb Vasc Biol. 2014;34:1531–1538. doi: 10.1161/ATVBAHA.114.303818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian R, Liu Z, Zhang H, Fang X, Wang C, Qi S, Cheng Y, Su G. Investigation of the regulation of roundabout4 by hypoxia-inducible factor-1α in microvascular endothelial cells. Invest Ophthalmol Vis Sci. 2015;56:2586–2594. doi: 10.1167/iovs.14-14409. [DOI] [PubMed] [Google Scholar]
- 30.Dai C, Gong Q, Cheng Y, Su G. Regulatory mechanisms of Robo4 and their effects on angiogenesis. Biosci Rep. 2019;39:BSR20190513. doi: 10.1042/BSR20190513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. eLife. 2015;4:e05005. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao S, Wassler M, Zhang L, Li Y, Wang J, Zhang Y, Shelat H, Williams J, Geng YJ. MicroRNA-133a regulates insulin-like growth factor-1 receptor expression and vascular smooth muscle cell proliferation in murine atherosclerosis. Atherosclerosis. 2014;232:171–179. doi: 10.1016/j.atherosclerosis.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Z, He F, OuYang S, Li Y, Ma F, Chang H, Cao D, Wu J. miR-140-5p could suppress tumor proliferation and progression by targeting TGFBRI/SMAD2/3 and IGF-1R/AKT signaling pathways in Wilms' tumor. BMC Cancer. 2019;19:405. doi: 10.1186/s12885-019-5609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grigelioniene G, Suzuki HI, Taylan F, Mirzamohammadi F, Borochowitz ZU, Ayturk UM, Tzur S, Horemuzova E, Lindstrand A, Weis MA, et al. Gain-of-function mutation of microRNA-140 in human skeletal dysplasia. Nat Med. 2019;25:583–590. doi: 10.1038/s41591-019-0353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y, Liu D, Xi Y, Li J, Liu B, Li J. Upregulation of miRNA-140-5p inhibits inflammatory cytokines in acute lung injury through the MyD88/NF-κB signaling pathway by targeting TLR4. Exp Ther Med. 2018;16:3913–3920. doi: 10.3892/etm.2018.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu TT, Zhang WF, Yin YL, Liu YH, Song P, Xu J, Zhang MX, Li P. MicroRNA-140-5p targeting tumor necrosis factor-α prevents pulmonary arterial hypertension. J Cell Physiol. 2019;234:9535–9550. doi: 10.1002/jcp.27642. [DOI] [PubMed] [Google Scholar]
- 38.Li H, Ouyang XP, Jiang T, Zheng XL, He PP, Zhao GJ. MicroRNA-296: A promising target in the pathogenesis of atherosclerosis? Mol Med. 2018;24:12. doi: 10.1186/s10020-018-0012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meiler S, Baumer Y, Toulmin E, Seng K, Boisvert WA. MicroRNA 302a is a novel modulator of cholesterol homeostasis and atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:323–331. doi: 10.1161/ATVBAHA.114.304878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nazari-Jahantigh M, Wei Y, Noels H, Akhtar S, Zhou Z, Koenen RR, Heyll K, Gremse F, Kiessling F, Grommes J, et al. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J Clin Invest. 2012;122:4190–4202. doi: 10.1172/JCI61716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rotllan N, Ramírez CM, Aryal B, Esau CC, Fernández-Hernando C. Therapeutic silencing of microRNA-33 inhibits the progression of atherosclerosis in Ldlr−/− mice - brief report. Arterioscler Thromb Vasc Biol. 2013;33:1973–1977. doi: 10.1161/ATVBAHA.113.301732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baumer Y, McCurdy S, Alcala M, Mehta N, Lee BH, Ginsberg MH, Boisvert WA. CD98 regulates vascular smooth muscle cell proliferation in atherosclerosis. Atherosclerosis. 2017;256:105–114. doi: 10.1016/j.atherosclerosis.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang T, Zhou H, Chen Y, Zhang P, Wang T. The biphasic effects of the oxLDL/β2GPI/anti-β2GPI complex on VSMC proliferation and apoptosis. Cell Signal. 2019;57:29–44. doi: 10.1016/j.cellsig.2019.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.