The bacterial pathogen Mycobacterium tuberculosis can enter into a persistent state in which M. tuberculosis can evade host immunity, thereby reducing the effectiveness of current tuberculosis vaccines. Understanding the factors that contribute to persistence would enable the rational design of vaccines effective against persisters.
KEYWORDS: conditional persistence, Mycobacterium tuberculosis, sterilizing immunity
ABSTRACT
Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis, can enter into a persistent state that confers resistance to antibacterial agents. Many observations suggest that persistent M. tuberculosis cells also evade the antimycobacterial immune mechanisms, thereby reducing the effectiveness of the current tuberculosis vaccine. Understanding the factors that contribute to persistence may enable the rational design of vaccines that stimulate effective immune killing mechanisms against persister cells. Independent mutations targeting the methionine and arginine biosynthetic pathways are bactericidal for M. tuberculosis in mice. However, in this study, we discovered that the addition of leucine and pantothenate auxotrophy altered the bactericidality of methionine auxotrophy. Whereas the leucine/pantothenate/methionine auxotrophic M. tuberculosis strain H37Rv ΔleuCD ΔpanCD ΔmetA was eliminated in immunocompetent mice, this strain persisted in multiple organs of immunodeficient Rag1−/− mice for at least a year. In contrast, the leucine/pantothenate/arginine auxotroph H37Rv ΔleuCD ΔpanCD ΔargB was eliminated in both immunocompetent and immunodeficient Rag1−/− mice. Our results showed that leucine and pantothenate starvation metabolically blocked the sterilization mechanisms of methionine starvation but not those of arginine starvation. These triple-auxotrophic strains should be invaluable tools for unravelling the bacterial and host factors that enable persistence and for vaccine development studies to assess the efficacy of vaccines that boost immune recognition of M. tuberculosis in the persistent state. The sterilization of the ΔleuCD ΔpanCD ΔmetA auxotroph in immunocompetent mice, but not in mice lacking an adaptive immune response, could provide a new system for studying the antimycobacterial killing mechanisms of adaptive immunity.
OBSERVATION
Tuberculosis (TB) remains a significant global health problem despite the availability and widespread use of chemotherapy and of bacille Calmette-Guerin (BCG) vaccination. Our previous research has demonstrated that the effectiveness of chemotherapy is hampered by the ability of Mycobacterium tuberculosis (Mtb) to enter into a persistent state (1, 2). We hypothesize that persistence may also be a factor in the limited effectiveness of BCG or most other approaches to TB vaccination. As part of our goal to develop better TB vaccines, we have been exploring how various host and bacterial factors modulate the immune response and bacterial survival. Understanding such factors will aid in developing improved TB vaccines and in establishing the correlates of protection in humans that will aid vaccine design and selection. BCG, because of its relative attenuation in virulence, has been used as the challenge strain in humans for testing the efficacy of novel vaccine candidates (3, 4). However, the ability of BCG to cause disseminated infections in humans (5–7) and its lack of several important immune targets, such as those contained in the esx-1 region of difference (RD1), limit its usefulness (8–11). The ideal strain for TB vaccine challenge studies in humans would be safe, susceptible to immune killing, and contain the appropriate immunological targets.
We propose that auxotrophic mutants of M. tuberculosis represent alternative human in vivo challenge organisms as they are safer than BCG, while retaining the RD1 region and other genetic loci deleted in BCG (10–12). In auxotrophic mutants, a specific biosynthetic gene or pathway is mutated, resulting in a conditional mutation in which the mutant will grow only when the product of that biosynthetic pathway is supplied. We have observed that infection with different auxotrophic mutants of M. tuberculosis can have different outcomes in vivo, with some mutants inducing bacteriostatic host responses and persisting in host tissues, whereas others induce bactericidal responses in which the bacteria are eliminated, resulting in sterilization of infected tissues. For example, M. tuberculosis leucine/pantothenate double auxotrophs persist in immunocompetent and immunocompromised mice (13, 14), whereas the M. tuberculosis methionine H37Rv ΔmetA (15) and arginine H37Rv ΔargB (16) auxotrophs are sterilized. These observations led us to hypothesize that the induction of a persister phenotype or a sterilization phenotype by nutrient limitations is governed by the nature of the targeted biosynthetic pathway.
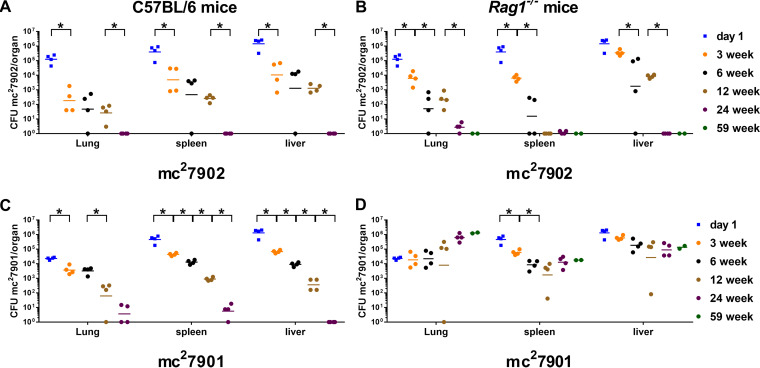
Previously, we mutated the leucine/pantothenate auxotroph H37Rv ΔleuCD ΔpanCD (mc26206) by introducing a third auxotrophy, i.e., methionine or arginine auxotrophy (17). We hypothesized that deleting either metA or argB from mc26206, which undergoes bacteriostasis in mice (13), would render the resulting strains susceptible to rapid sterilization in mice, given that when made independently the metA and argB mutations were bactericidal during mouse infections. To test this hypothesis, immunocompetent C57BL/6 mice and immunodeficient Rag1−/− mice, which lack adaptive immunity, were infected intravenously with the leucine/pantothenate/methionine auxotroph H37Rv ΔleuCD ΔpanCD ΔmetA (mc27901) or the leucine/pantothenate/arginine auxotroph H37Rv ΔleuCD ΔpanCD ΔargB (mc27902). Lungs, livers, and spleens were harvested at 3, 6, 12, 24, and 59 weeks postinjection, and bacterial burdens were determined. As expected for a triple auxotroph, the leucine/pantothenate/arginine auxotroph mc27902 was eliminated by 24 weeks in the livers, lungs, and spleens of both immunocompetent and immunodeficient mice (Fig. 1A and B). Similarly, the leucine/pantothenate/methionine auxotroph mc27901 was eliminated by 24 weeks in immunocompetent mice (Fig. 1C). However, mc27901 persisted in all three organs in immunodeficient mice during the entire 59 weeks of the experiment even though initial dissemination to the lungs (day 1) was nearly a log unit lower for mc27901 than for mc27902 (Fig. 1C and D). A small increase in the bacterial burden in the lungs of mc27901-infected immunodeficient mice was observed over time, but this was not statistically significant. In livers and spleens, titers in mc27901-infected immunodeficient mice decreased for the first 6 to12 weeks of infection, although at a lower rate than observed for mc27902, before plateauing. The ability of mc27901 to persist in immunodeficient mice but not in immunocompetent mice suggests that some component of the adaptive immune response is able to kill mc27901. This unexpected observation revealed a conditional system of persistence that renders M. tuberculosis susceptible to killing by an adaptive immune response.
FIG 1.
Strain mc27901 persists in immunocompromised Rag1−/− mice but not in immunocompetent mice. C57BL/6 mice (A and C) and Rag1−/− mice (B and D) were infected via intravenous injection with 3.8 × 106 CFU of mc27902 (H37Rv ΔleuCD ΔpanCD ΔargB) (A and B) or 3.0 × 106 CFU of mc27901 (H37Rv ΔleuCD ΔpanCD ΔmetA) (C and D). At the indicated times, four mice per group were euthanized, and lungs, livers, and spleens were homogenized to determine burden of M. tuberculosis. The 59-week time point is composed of only two Rag1−/− mice per group. Data that are statistically significant are marked with an asterisk (P < 0.05).
The profound difference in the persistence phenotypes between the two strains in the immunocompromised host was surprising. Persistence is the capacity of a subpopulation of bacterial cells to survive a sterilizing process (18–20). Persisters do not acquire a mutation but rather turn on a genetic program to survive killing by a drug or other assault. The study of the survival of these triple-auxotrophic M. tuberculosis strains in vivo highlights a few important observations. First, while the sterilization mediated by methionine starvation of our ΔmetA mutant can be reversed by combining the metA metabolic block with blocks in the leucine and pantothenate biosynthetic pathways, the arginine starvation sterilization pathway is not affected by the leucine and pantothenate auxotrophy. Second, the persistent leucine/pantothenate/methionine auxotroph mc27901 can remain protected from the innate immune system for over 1 year and cause no clinically recognizable disease in mice lacking adaptive immunity. Last, the sterilization of mc27901 in immunocompetent mice reveals the presence of an M. tuberculosis-killing mechanism that is dependent on a functional adaptive immune response.
As of today, BCG remains the only licensed TB vaccine. While numerous new TB vaccines have been developed (21), an animal model that reliably delineates the correlates of protection in humans remains to be established. We propose that the triple auxotroph mc27901, a biosafety level 2-safe laboratory strain (13, 17), which is markedly attenuated in vivo, could provide a safe test strain for assessing the efficacies of TB vaccines in humans. Since M. tuberculosis is not a recipient of conjugation in nature and the ΔleuCD, ΔpanCD, and ΔmetA deletions were shown not to be reversible or suppressible (14, 16, 22), these sets of auxotrophies are unlikely to be repaired in vivo. Indeed, only two independent mutations in M. tuberculosis are required by the World Health Organization to qualify a strain as a vaccine (23). A suitable human infection model of TB must be safe, with limited replication in vivo and easily and reproducibly quantifiable in vivo or ex vivo. Various markers for monitoring strain elimination, such as the NanoLuc gene or its optimized version Antares (24, 25), could be incorporated into mc27901 to enable expeditious evaluation of vaccine efficacy after intradermal challenges as described for BCG in the human vaccine model (3, 4). Moreover, the use of rag1−/− mice and mc27901 provides an attractive model for dissecting the components of the immune response that can elicit protection. Last, recently isolated clinical strains, which might be more relevant for vaccine assessment, can be used as substrates to generate safe triple auxotrophs as challenge strains for human vaccination models, through targeting the leucine, pantothenate, and methionine/arginine metabolic pathways by specialized transduction (13). The utility of these safe, multiple-auxotrophic strains is multifold. They provide a platform that enables direct, cost-saving, and expeditious evaluation of novel anti-TB vaccines in humans and can define correlates of protection in TB. Such strains also constitute a set of invaluable tools for unraveling the mechanisms underlying M. tuberculosis persistence, as well as for the discovery of drugs that can eliminate persisters and deliver sterilizing immunity in humans.
Experimental procedures. (i) Bacterial strains and reagents.
The M. tuberculosis strains mc27901and mc27902 were obtained from laboratory stocks. The strains were grown in 30-ml square bottles containing 5 ml Middlebrook 7H9 (Difco, Sparks, MD) supplemented with 10% (vol/vol) oleic acid-albumin-dextrose-catalase (OADC; Difco), 0.2% (vol/vol) glycerol, d-pantothenate (24 mg/liter), l-leucine (50 mg/liter), l-methionine (50 mg/liter), l-arginine (200 mg/liter), and 0.05% (vol/vol) tyloxapol at 37°C with shaking. Plating was done using Middlebrook 7H10 (Difco) supplemented with 10% (vol/vol) OADC, 0.2% (vol/vol) glycerol, d-pantothenate (24 mg/liter), l-leucine (50 mg/liter), l-methionine (50 mg/liter), and l-arginine (200 mg/liter), and the plates were incubated at 37°C for 4 to 8 weeks. Media and chemicals were obtained from Sigma (St. Louis, MO) or ThermoFisher Scientific (Waltham, MA).
(ii) Mouse infection.
Female C57BL/6 mice were obtained from Envigo (Somerset, NJ). Female Rag1−/− mice (6 to 8 weeks old) were bred in-house. Animal protocol AUP20171114 was approved by the Einstein Animal Institute, which is accredited by the American Association for the Use of Laboratory Animals (DHEW publication no. [NIH] 78-23, revised 1978), and accepts as mandatory the NIH “Principles for the Use of Animals.” The mc27901 and mc27902 strains were grown to mid-log phase (optical density at 600 nm [OD600] of ∼0.6 to 0.8), pelleted by centrifugation, and washed twice with Dulbecco’s phosphate-buffered saline (DPBS) containing 0.05% tyloxapol (DPBS-tyloxapol). Cells were resuspended in DPBS-tyloxapol, sonicated twice for 10 s, and diluted in DPBS-tyloxapol to a concentration of 1.5 × 107 CFU/ml. Mice were infected via tail vein injection (0.2 ml injected). At day 1 and at weeks 3, 6, 12, 24, and 59, mice were euthanized. The lungs, spleens, and livers were homogenized in DPBS to determine CFU per organ. Serial dilutions of the lysates in DPBS were plated on fully supplemented Middlebrook 7H10 plates (see above), and the lowest dilutions were also plated on Middlebrook 7H10 plates without amino acid supplements. The plates were incubated at 37°C for 4 to 8 weeks to obtain CFU counts.
(iii) Statistics.
Differences between groups were analyzed by an unpaired, nonparametric Mann-Whitney test using GraphPad Prism 7.05 (San Diego, CA).
ACKNOWLEDGMENTS
W.R.J. acknowledges the support from the National Institutes of Health grants AI026170, AI111276, and AI132940 for this work. J.C. and S.A.P. acknowledge R01AI137344. J.C. acknowledges R01AI139297.
We thank Bing Chen, Mei Chen, and John Kim for technical assistance with the mouse experiments.
Footnotes
Citation Vilchèze C, Porcelli SA, Chan J, Jacobs WR, Jr. 2021. Sterilization by adaptive immunity of a conditionally persistent mutant of Mycobacterium tuberculosis. mBio 12:e02391-20. https://doi.org/10.1128/mBio.02391-20.
REFERENCES
- 1.Jain P, Weinrick BC, Kalivoda EJ, Yang H, Munsamy V, Vilcheze C, Weisbrod TR, Larsen MH, O’Donnell MR, Pym A, Jacobs WR, Jr. 2016. Dual-reporter mycobacteriophages (Phi2DRMs) reveal preexisting Mycobacterium tuberculosis persistent cells in human sputum. mBio 7:e01023-16. doi: 10.1128/mBio.01023-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vilcheze C, Hartman T, Weinrick B, Jain P, Weisbrod TR, Leung LW, Freundlich JS, Jacobs WR, Jr. 2017. Enhanced respiration prevents drug tolerance and drug resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 114:4495–4500. doi: 10.1073/pnas.1704376114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blazevic A, Xia M, Turan A, Tennant J, Hoft DF. 2017. Pilot studies of a human BCG challenge model. Tuberculosis (Edinb) 105:108–112. doi: 10.1016/j.tube.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Harris SA, White A, Stockdale L, Tanner R, Sibley L, Sarfas C, Meyer J, Peter J, O’Shea MK, Manjaly Thomas Z-R, Hamidi A, Satti I, Dennis MJ, McShane H, Sharpe S. 2018. Development of a non-human primate BCG infection model for the evaluation of candidate tuberculosis vaccines. Tuberculosis (Edinb) 108:99–105. doi: 10.1016/j.tube.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aznar Martinez L, Lopez Cubillana P, Lopez Abad A, Vidal Crespo N, Gomez Gomez GA. 2019. Bacillus Calmette-Guerin in immunosuppressed patient with high-grade nonmuscle invasive bladder carcinoma. Urol Int 103:242–244. doi: 10.1159/000501108. [DOI] [PubMed] [Google Scholar]
- 6.Bonarriba CR, de la Cruz-Ruiz M, Gomez-Marques G. 2013. Intravesical bacillus Calmette-Guerin in immunosuppressed patients with carcinoma in situ. Nefrologia 33:429–431. doi: 10.3265/Nefrologia.pre2012.Oct.11759. [DOI] [PubMed] [Google Scholar]
- 7.Croce E, Hatz C, Jonker EF, Visser LG, Jaeger VK, Bühler S. 2017. Safety of live vaccinations on immunosuppressive therapy in patients with immune-mediated inflammatory diseases, solid organ transplantation or after bone-marrow transplantation − a systematic review of randomized trials, observational studies and case reports. Vaccine 35:1216–1226. doi: 10.1016/j.vaccine.2017.01.048. [DOI] [PubMed] [Google Scholar]
- 8.Groschel MI, Sayes F, Shin SJ, Frigui W, Pawlik A, Orgeur M, Canetti R, Honore N, Simeone R, van der Werf TS, Bitter W, Cho S-N, Majlessi L, Brosch R. 2017. Recombinant BCG expressing ESX-1 of Mycobacterium marinum combines low virulence with cytosolic immune signaling and improved TB protection. Cell Rep 18:2752–2765. doi: 10.1016/j.celrep.2017.02.057. [DOI] [PubMed] [Google Scholar]
- 9.Hsu T, Hingley-Wilson SM, Chen B, Chen M, Dai AZ, Morin PM, Marks CB, Padiyar J, Goulding C, Gingery M, Eisenberg D, Russell RG, Derrick SC, Collins FM, Morris SL, King CH, Jacobs WR, Jr. 2003. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc Natl Acad Sci U S A 100:12420–12425. doi: 10.1073/pnas.1635213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahairas GG, Sabo PJ, Hickey MJ, Singh DC, Stover CK. 1996. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol 178:1274–1282. doi: 10.1128/jb.178.5.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pym AS, Brodin P, Brosch R, Huerre M, Cole ST. 2002. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol 46:709–717. doi: 10.1046/j.1365-2958.2002.03237.x. [DOI] [PubMed] [Google Scholar]
- 12.Behr MA, Wilson MA, Gill WP, Salamon H, Schoolnik GK, Rane S, Small PM. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520–1523. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- 13.Jain P, Hsu T, Arai M, Biermann K, Thaler DS, Nguyen A, González PA, Tufariello JM, Kriakov J, Chen B, Larsen MH, Jacobs WR, Jr. 2014. Specialized transduction designed for precise high-throughput unmarked deletions in Mycobacterium tuberculosis. mBio 5:e01245-14. doi: 10.1128/mBio.01245-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sampson SL, Dascher CC, Sambandamurthy VK, Russell RG, Jacobs WR, Jr, Bloom BR, Hondalus MK. 2004. Protection elicited by a double leucine and pantothenate auxotroph of Mycobacterium tuberculosis in guinea pigs. Infect Immun 72:3031–3037. doi: 10.1128/iai.72.5.3031-3037.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berney M, Berney-Meyer L, Wong K-W, Chen B, Chen M, Kim J, Wang J, Harris D, Parkhill J, Chan J, Wang F, Jacobs WR, Jr. 2015. Essential roles of methionine and S-adenosylmethionine in the autarkic lifestyle of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 112:10008–10013. doi: 10.1073/pnas.1513033112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiwari S, van Tonder AJ, Vilchèze C, Mendes V, Thomas SE, Malek A, Chen B, Chen M, Kim J, Blundell TL, Parkhill J, Weinrick B, Berney M, Jacobs WR, Jr. 2018. Arginine-deprivation-induced oxidative damage sterilizes Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 115:9779–9784. doi: 10.1073/pnas.1808874115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vilcheze C, Copeland J, Keiser TL, Weisbrod T, Washington J, Jain P, Malek A, Weinrick B, Jacobs WR, Jr. 2018. Rational design of biosafety level 2-approved, multidrug-resistant strains of Mycobacterium tuberculosis through nutrient auxotrophy. mBio 9:e00938-18. doi: 10.1128/mBio.00938-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bigger JW 1944. Treatment of staphylococcal infections with penicillin. Lancet 244:497–500. doi: 10.1016/S0140-6736(00)74210-3. [DOI] [Google Scholar]
- 19.Hobby GL, Meyer K, Chaffee E. 1942. Observations on the mechanism of action of penicillin. Proc Soc Exp Biol Med 50:281–285. doi: 10.3181/00379727-50-13773. [DOI] [Google Scholar]
- 20.McCune RM, Feldmann FM, Lambert HP, McDermott W. 1966. Microbial persistence. I. The capacity of tubercle bacilli to survive sterilization in mouse tissues. J Exp Med 123:445–468. doi: 10.1084/jem.123.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porcelli SA, and, Jacobs WR, Jr 2019. Exacting Edward Jenner’s revenge: the quest for a new tuberculosis vaccine. Sci Transl Med 11:eaax4219. doi: 10.1126/scitranslmed.aax4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy E, De Silva AD, Sambandamurthy VK, Clark SO, Stavropoulos E, Jacobs WR, Brennan J, Chan J, Williams A, Colston MJ, Tascon RE. 2006. Induction of high levels of protective immunity in mice after vaccination using dendritic cells infected with auxotrophic mutants of Mycobacterium tuberculosis. Immunol Lett 103:196–199. doi: 10.1016/j.imlet.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Kamath AT, Fruth U, Brennan MJ, Dobbelaer R, Hubrechts P, Ho MM, Mayner RE, Thole J, Walker KB, Liu M, Lambert P-H, AERAS Global TB Vaccine Foundation, World Health Organization . 2005. New live mycobacterial vaccines: the Geneva consensus on essential steps towards clinical development. Vaccine 23:3753–3761. doi: 10.1016/j.vaccine.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Yeh H-W, Karmach O, Ji A, Carter D, Martins-Green MM, Ai H-W. 2017. Red-shifted luciferase-luciferin pairs for enhanced bioluminescence imaging. Nat Methods 14:971–974. doi: 10.1038/nmeth.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki K, Kimura T, Shinoda H, Bai G, Daniels MJ, Arai Y, Nakano M, Nagai T. 2016. Five colour variants of bright luminescent protein for real-time multicolour bioimaging. Nat Commun 7:13718. doi: 10.1038/ncomms13718. [DOI] [PMC free article] [PubMed] [Google Scholar]