Abstract
Drug-induced sarcoidosis-like reactions (DISRs) are systemic granulomatous diseases that develop in the context of a new drug onset. Ipilimumab is an immune checkpoint inhibitor (ICI) approved for the treatment of advanced melanoma which has been associated with DISR. Differential diagnosis between tumour progression and DISR by positron emission tomography/computed tomography (PET/CT) in patients treated with an ICI can be a challenge. A 31-year-old woman was diagnosed with a stage IIIB melanoma in her back. Ipilimumab 10 mg/kg was initiated. After 1 month of finishing the treatment a routine, PET/CT showed multiple enlarged mediastinal and hilar lymph nodes FDG-positive. A transbronchial biopsy showed sarcoid-like granulomatous infiltration which favoured the diagnosis of DISR related to ipilimumab. The patient remained asymptomatic and lymphadenopathy regressed progressively after 11 months. Our work highlights the importance of differentiating DISR from tumour progression, before unnecessary changes in therapeutic strategies. PET/CT is a useful diagnostic tool for its follow-up.
Keywords: dermatology, radiology (diagnostics), drugs and medicines, immunological products and vaccines, skin cancer
Background
Drug-induced sarcoidosis-like reactions (DISRs) are systemic granulomatous diseases clinically indistinguishable from sarcoidosis that develop in the context of a new drug onset. However, differentially to sarcoidosis, they usually improve or resolve after this drug withdrawal.1
The monoclonal antibody against cytotoxic T-lymphocyte antigen 4 (CTLA-4) ipilimumab is an immune checkpoint inhibitor (ICI) agent approved for the treatment of unresectable or metastatic melanoma. The Food and Drug Administration approved it also for adjuvant treatment of patients with cutaneous melanoma with pathological involvement of regional lymph nodes who have undergone complete resection, including total lymphadenectomy.2 It induces an increased activation of T-cell against tumour cells favouring tumour responses.3 Eggermont et al showed that ipilimumab adjuvant therapy prolonged survival in stage III melanoma.2 However, severe immunotherapy-related adverse events have been reported.4 Among these, DISR and lymphadenopathy have been described.4 Here, we report a patient with DISR/lymphadenopathy related to ipilimumab initially diagnosed by positron emission tomography/computed tomography (PET/CT) and confirmed by a lymph node biopsy after treatment with ipilimumab. Differential diagnosis between tumour progression and DISR was a challenge, and thereby we consider essential the correct diagnosis of this rare adverse event. Finally, we conducted a literature review of previous case reports of DISR/lymphadenopathy in patients treated with ICI and described the main findings.
Case presentation
A 31-year-old woman was diagnosed with American Joint Committee on Cancer (AJCC) (eighth edition) stage IIIB B-Raf proto-oncogene, serine/threonine kinase (BRAF) V600 melanoma of the right scapular area (Breslow 1.7 mm, ulcerated) with one clinically detected affected node on right axilla. After resection and right axillary lymphadenectomy (1 of 32 lymph nodes removed was positive for metastatic melanoma), the patient started adjuvant treatment with ipilimumab 10 mg/kg. After 3 months of treatment after which the patient had received four doses of ipilimumab, she developed a grade 3 hepatic toxicity which required corticosteroid therapy. A follow-up PET/CT performed 1 month after finishing ipilimumab showed multiple enlarged mediastinal, hilar and subdiaphragmatic lymph nodes with F18 fluorodioxyglucose (FDG)-positive (maximum standardized uptake value (SUVmax): 16.2) (figure 1). A transbronchial biopsy showed sarcoid-like granulomatous infiltration. Neither parenchymal lung disease nor pulmonary fibrosis were found in the computed tomography (CT) (stage 1, Siltzbach classification) (figure 2). At diagnosis, angiotensin converting enzyme (ACE) levels were 24.2 IU/L (normal). The results of bacterial, mycobacterial and fungus cultures were negative. Pulmonary function tests did not show obstructive nor restrictive ventilatory defects. However, there was a slight affectation of the diffusion capacity (figure 3).
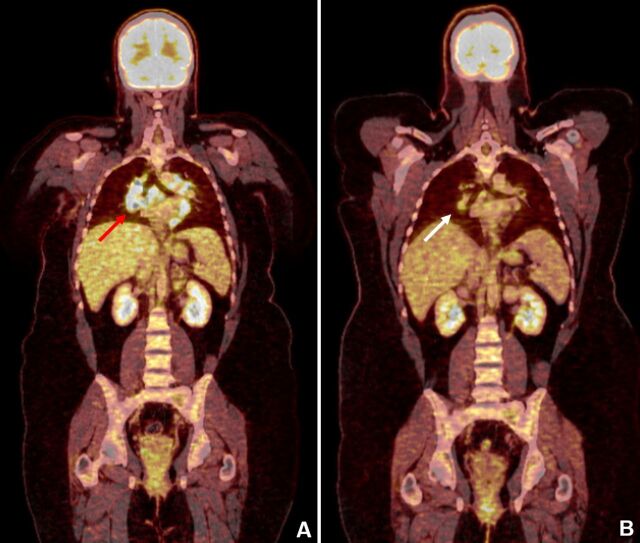
Figure 1.
(A) PET/CT done 1 month after finishing ipilimumab shows enlarged mediastinal and hilar lymph nodes with FDG-positive (red arrow). (B) PET/CT done 11 months after finishing ipilimumab shows almost complete resolution (white arrow).
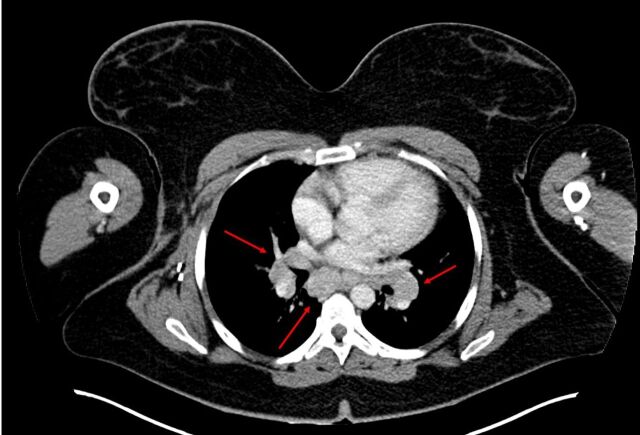
Figure 2.
Chest CT scan done 1 month after finishing ipilimumab shows enlarged mediastinal and hilar lymph nodes (red arrows).
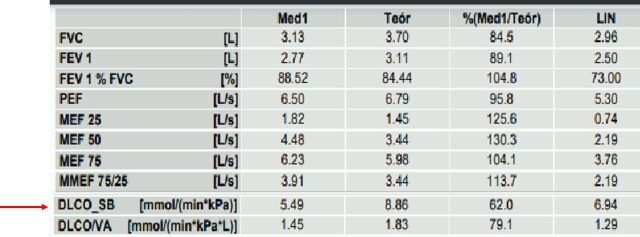
Figure 3.
Pulmonary function tests did not show obstructive or restrictive ventilatory defects. However, there was a slight affectation of the diffusion capacity (red arrow). FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; PEF, peak expiratory flow; MEF, maximal expiratory flow; MMEF, maximal (mid-)expiratory flow; DLCO_SB, single-breath diffusing capacity of the lung for CO; DLCO/VA, diffusing capacity of the lung for CO divided by the alveolar volume; LIN, lower limit of normal.
Differential diagnosis
Differential diagnosis between tumour progression, pseudoprogression and DISR should be done and it can be a challenge. Biopsy remains the gold standard for differentiating them. However, in some cases when biopsy is not possible, other clinical data should be considered. Hence, the absence of constitutional syndrome, the normality of blood tests and the reduction in the hypermetabolic activity in subsequent PET/CT after discontinuing ipilimumab would be compatible with DISR rather than with tumour progression or pseudoprogression. A systemic infection (including tuberculosis) should also be ruled out. Our patient was not septic and, furthermore, both the biopsy and its culture ruled out the infectious origin. DISR is clinically indistinguishable from sarcoidosis. However, it develops in the context of a new drug onset. Our patient did not have previous history of sarcoidosis before initiating ipilimumab. Moreover, differentially to sarcoidosis, DISR usually improves or resolves after drug withdrawal, as observed in our patient. Finally, a new different malignant neoplasm should be considered in the differential diagnosis. Biopsy is, together with other clinical data, essential to rule out this diagnosis.
Treatment
Ipilimumab had been discontinued due to hepatic toxicity. Prednisone 1 mg/kg/day (90 mg/day) was started and tapered progressively during 10 weeks for the grade 3 immune-related hepatitis. Prednisone dose was reduced by 10 mg every 7 days until dose was 10 mg/day. Finally, a dose of 5 mg/day was administered for 7 days, and then stopped. This treatment was also adequate for DISR, although in this case it was not necessary as our patient remained asymptomatic and ipilimumab had been already discontinued.
Outcome and follow-up
Lymphadenopathy and hypermetabolic activity remained stable in successive PET/TC performed 3 months after finishing ipilimumab, although the patient did not present any related symptom. However, in spite of not receiving any specific treatment, subsequent PET/CT performed at 6th, 8th and 11th month after finishing ipilimumab showed progressive reduction in lymphadenopathy and hypermetabolic activity up to near resolution (figure 1).
Unfortunately, 6 months after finishing ipilimumab, the patient developed a brain metastasis located in the left primary motor area which could be surgically resected. One month after, however, she developed a new brain metastasis in the left parieto-occipital cortex. A multidisciplinary committee decided to start stereotactic radiosurgery to treat the resection bed and the new metastasis. Moreover, dabrafenib 150 mg two times a day and trametinib 2 mg once daily were initiated. Left parieto-occipital metastasis remained stable during 26 months with this treatment. Recently, 5 years after melanoma diagnosis, a follow-up MR evidenced progression of the brain metastasis. According to the multidisciplinary committee decision, the metastasis has been surgically resected without relevant sequelae, and the patient continues in treatment with targeted therapy. No new metastasis has been detected since now.
Discussion
Immunological checkpoint inhibitors have revolutionised advanced melanoma treatment as they act increasing anti-tumour immune response.3 Ipilimumab, a monoclonal antibody to CTLA-4 was the first treatment to show an overall survival benefit in metastatic melanoma in a randomised clinical trial.5 Nevertheless, it has been associated with a considerable number of systemic adverse events, some of them life-threatening.4 DISR is a rare adverse event related to ICI therapy.6 Tumour necrosis factor alpha antagonist, interferons, highly active antiretroviral therapy, cisplatin, interleukin (IL)-2 and BRAF inhibitors are other drugs related to DISR.1 7 The differential diagnosis should be done mainly with tumour recurrence and with infections.1
After our literature review, we found 25 works describing 39 patients suffering DISR/lymphadenopathy related to ICI treatment. Of these patients, 21 (54%) were women and median age was 57 years (26–79 years). Median time when clinical appeared from ICI start was 4.5 months (0.8–24 months), consistent with our patient. Melanoma encountered for 32/39 of the tumours treated with ICI. Globally, these works showed that 27/39 (78%) patients had cutaneous manifestations of DISR syndrome. Ipilimumab (15/39), pembrolizumab (10/39) and nivolumab (7/39) encountered for most of the DISR/ lymphadenopathy related to ICI. Suspension of ICI alone (17/39) or together with corticoid therapy (17/39) were the most frequent treatment.7–10 Generally, if DISR/lymphadenopathy related to ICI treatment presents as an uncomplicated disease it may not require ICI therapy discontinuation. However, in patients with severe involvement suspension of ICI and/or corticosteroids use should be considered, although they would have a negative effect on the efficacy of ICI.7
Persistence of DISR/lymphadenopathy in the setting of checkpoint inhibitors has been described.7–10 Our review found that persistence was only described in three patients although having discontinued ICI treatment.7–10 In our patient, hypermetabolic lesions disappeared after 11 months.
Bronstein et al showed in their work that patients treated with ipilimumab who presented radiologic manifestations had better tumour response than those without (55% vs 10%, respectively).11 Tumour response was mentioned in 31/39 patients of the papers reviewed previously. Globally, 16/31 patients are referred as responders to ICI treatment (6/31 complete response) and 15/31 as no responders (8/31 progressive disease).7–10 Our patient progressed in spite of the treatment and DISR. Globally, these data are insufficient to know if DISR/lymphadenopathy is associated with a better tumour response.
Our work contributes with a new histopathological confirmed case of DISR/Lymphadenopathy related to ipilimumab and highlights the importance of knowing DISR following ICI treatment and differentiating it from tumour progression and pseudoprogression before changing therapeutic strategy. Withdrawal of ICI with or without corticosteroid treatment is effective in most cases and persistence are not common. PET/CT is a useful tool for its follow-up.
Patient’s perspective.
After all we had achieved, knowing results from this PET/CT which reported that probably I had metastasis all over my body, was very hard. My oncologist suspected that in fact it was due to an inflammatory reaction due to ipilimumab treatment. However, I thought at first moment she said it to prevent me from knowing the crude reality. But my rational thinking made me believe my oncologist in whom I have total confidence. Fortunately, a transbronchial biopsy confirmed that in fact I suffered sarcoidosis, and that I did not have disease progression. It was a great new to have sarcoidosis, although it sounds strange. I have practically no symptoms today, and probably they are related to my current treatment.
Learning points.
Sarcoidosis-like reactions should be suspected in patients with melanoma with a new onset of multiple FDG-positive enlarged lymph nodes by PET/CT during or after treatment with an immune checkpoint inhibitor.
Biopsy remains the gold standard for differentiating sarcoidosis-like reactions from tumour progression.
Differential diagnosis is essential to avoid unnecessary therapeutic strategy changes.
Persistence of sarcoidosis-like reactions after discontinuing ipilimumab is rare.
Steroid therapy can be required in some cases with good responses.
Acknowledgments
Thanks to our patients and their families who are the main reason for our studies. The authors thank Dr Juan Sebastián Blanco Cano for his technical support in PET/CT images interpretation. We would also like to thank Dr Sabina Ruiz Janer for her critical reading of our paper and Mr Leonardo González Cruz for spelling and grammar check throughout the entire document.
Footnotes
Twitter: @carlos_gocr
Contributors: We declare that this is an original and non-previously published paper, and all persons who have made substantial contributions to the work reported in the manuscript including writing and editing assistance have given me written permission. CG-C made substantial contributions to the conception and design of the work, the acquisition, analysis and interpretation of data. He drafted the work. He approved the final version published. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. DB, EM-C and VG-P made substantial contributions to the conception and design of the work, the acquisition, analysis and interpretation of data. They revised the work critically for important intellectual content. They approved the final version published. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Chopra A, Nautiyal A, Kalkanis A, et al. Drug-Induced Sarcoidosis-Like reactions. Chest 2018;154:664–77 https://doi.org/ 10.1016/j.chest.2018.03.056 [DOI] [PubMed] [Google Scholar]
- 2.Eggermont AMM, Chiarion-Sileni V, Grob J-J, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med 2016;375:1845–55 https://doi.org/ 10.1056/NEJMoa1611299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchbinder EI, Desai A. Ctla-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol 2016;39:98–106 https://doi.org/ 10.1097/COC.0000000000000239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse events associated with immune checkpoint blockade in patients with cancer: a systematic review of case reports. PLoS One 2016;11:e0160221 https://doi.org/ 10.1371/journal.pone.0160221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thumar JR, Kluger HM. Ipilimumab: a promising immunotherapy for melanoma. Oncology 2010;24:1280–8. [PubMed] [Google Scholar]
- 6.Firwana B, Ravilla R, Raval M, et al. Sarcoidosis-like syndrome and lymphadenopathy due to checkpoint inhibitors. J Oncol Pharm Pract 2017;23:620–4 https://doi.org/ 10.1177/1078155216667635 [DOI] [PubMed] [Google Scholar]
- 7.Dimitriou F, Frauchiger AL, Urosevic-Maiwald M, et al. Sarcoid-like reactions in patients receiving modern melanoma treatment. Melanoma Res 2018;28:230–6 https://doi.org/ 10.1097/CMR.0000000000000437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tetzlaff MT, Nelson KC, Diab A, et al. Granulomatous/sarcoid-like lesions associated with checkpoint inhibitors: a marker of therapy response in a subset of melanoma patients. J Immunother Cancer 2018;6:14 https://doi.org/ 10.1186/s40425-018-0323-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paolini L, Poli C, Blanchard S, et al. Thoracic and cutaneous sarcoid-like reaction associated with anti-PD-1 therapy: longitudinal monitoring of PD-1 and PD-L1 expression after stopping treatment. J Immunother Cancer 2018;6:52 https://doi.org/ 10.1186/s40425-018-0372-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fakhri G, Akel R, Salem Z, et al. Pulmonary sarcoidosis activation following neoadjuvant pembrolizumab plus chemotherapy combination therapy in a patient with non-small cell lung cancer: a case report. Case Rep Oncol 2017;10:1070–5 https://doi.org/ 10.1159/000484596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bronstein Y, Ng CS, Hwu P, et al. Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. AJR Am J Roentgenol 2011;197:W992–1000 https://doi.org/ 10.2214/AJR.10.6198 [DOI] [PubMed] [Google Scholar]