Abstract
We describe the case of a 21-year-old man with a background of sickle cell disease (SCD) who was on acute presentation in a sickle cell crisis required immediate intensive care admission with red blood cell exchange and ventilatory support. He had right frontal lobe infarcts and extensive bilateral deep white matter lesions most likely secondary to fat embolism. Inpatient investigations demonstrated a patent foramen ovale, explaining the route of spread of the fat embolus. He then had a transcatheter closure of the atrial defect. The patient needed prolonged inpatient rehabilitation. He was discharged from hospital in a wheelchair secondary to severe lower limb neurology and bilateral knee heterotopic ossification. He lives with the possibility of early onset dementia and cognitive decline, requiring constant care. The case highlights the multiple manifestations of SCD and their diverse and debilitating consequences.
Keywords: musculoskeletal and joint disorders, haematology (incl blood transfusion), stroke, sickle cell disease, disability
Background
Around 14 000 people are living with sickle cell disease (SCD) in the UK, and an increase in numbers is inevitable with immigration.1 The unpredictable nature of a sickle cell crisis can present as a debilitating state and an array of pathology, some of which may be life threatening and confusing because of multisystem involvement. Physicians need to be aware of the diverse and multiorgan involvement of SCD.
Case presentation
A 21-year-old man of Afro-Germanic descent and known background of SCD of Sickle Cell Anaemia (HbSS)-type presented to the accident and emergency department with worsening body pain. On examination, he was found tachycardic, tachypnoeic, feverish and drowsy. His haemoglobin level had dropped from a baseline of 97 to 72 g/L on presentation, then further down to 55 g/L within less than 4 hours.
The patient was admitted to the intensive treatment unit (ITU) for red blood cell (RBC) exchange transfusion and later required continuous positive airway pressure ventilatory support, as the arterial blood gasses showed rising CO2 levels. CT of the chest showed bilateral lower lobe consolidations. Despite these measures, he had a fluctuating level of consciousness that was 5–7 initially on the Glasgow Coma Scale then fell to 3. Due to a drop in his consciousness, falling respiratory rates and apnoeic spells, he was intubated and mechanically ventilated. Concerns about his level of consciousness led to neuroimaging with a CT scan of the head. The scan revealed a subacute infarct in the right frontal region (figure 1). As the patient’s reduced conscious level did not improve and seemed disproportionate to the size and area of his stroke, MRI imaging of the brain was also performed 4 days after the CT scan. The MRI confirmed the right frontal lobe infarct and revealed widespread lesions in the deep white matter in both hemispheres in keeping with cerebral fat embolism (CFE) (figures 2 and 3). A normal MR angiogram (MRA) of the cerebral vasculature ruled out moyamoya-like vasculopathy, also favouring the diagnosis of CFE. Interestingly, MRI imaging did not show any evidence of silent cerebral infarcts, an otherwise common feature in HbSS disease.2
Figure 1.
Brain imaging confirms a subacute ischaemic stroke. CT head revealed a low attenuation area in the right frontal lobe (white arrow) suggestive of an acute/subacute ischaemic event.
Figure 2.
MRI brain with FLAIR and DWI sequences shows features of cerebral fat embolisation. Fluid-attenuated inversion recovery (A–D) and diffusion-weighted (E–H) axial images showing abnormalities in splenium of corpus callosum (long white arrows), head of caudate nucleus (short white arrows), deep white matter of both hemispheres (black arrows) in addition to the infarcted right medial frontal gyrus (dotted white arrows) seen on CT. DWI, diffusion-weighted imaging FLAIR, Fluid attenuated inversion recovery.
Figure 3.
MRI brain with SWI sequence confirms widespread microhaemorrhages. Susceptibility-weighted images (A–D) showing extensive punctuate low signal areas throughout cerebellum, brain stem, cerebral white matter denoting microhaemorrhages. SWI, susceptibility-weighted imaging.
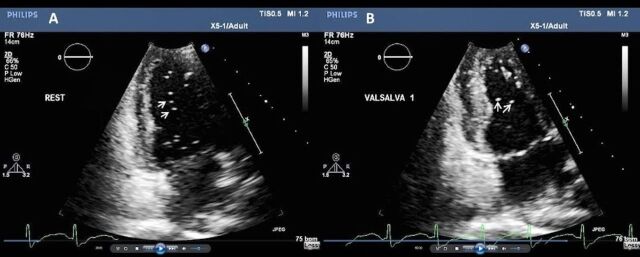
He required 10 units of RBCs initially, then two units of pooled platelets, two units of fresh frozen plasma and a further unit of RBCs. Once stable, he was stepped down from ITU to a rehabilitation unit. Clinical examination revealed mild tetraparesis, more pronounced on the left with altered sensation, proprioception and neuropathic pain in the lower limbs. Neurological investigations demonstrated critical illness neuromyopathy based on a mixed electromyography (EMG) picture. Cardiac investigations with a bubble echocardiogram disclosed a patent foramen ovale (PFO) (figure 4). He also had severe painful flexion contractures of both knees and moderate flexion contractures of both hips. Lower limb X-rays showed extensive heterotopic ossification (HO) around his knee joints (figure 5). Worthy of note, he had bilateral painful knee swellings 3 weeks beforehand, but X-rays found no bony abnormality at the time. He was managed with intravenous pamidronate, as well as naproxen, pregabalin and transdermal fentanyl patches. He continued on his established regime for SCD with hydroxycarbamide (18 mg/kg) 1 g from Monday to Friday and 1.5 g on Saturday and Sunday, along with penicillin prophylaxis.
Figure 4.
Bubble echo confirms a patent foramen ovale. As part of his stroke work-up, the patient underwent bubble echo study using intravenous injection of agitated saline and blood. This showed evidence of bubbles (white arrows) crossing the interatrial septum at rest (A) and during Valsalva manoeuvre (B), indicative of a patent foramen ovale.
Figure 5.

Pelvic and knee X-rays showing heterotopic ossification. Plain anteroposterior (AP) radiograph of the pelvis (A), right (B) and left (C) knees showed areas of soft tissue calcification adjacent to the right greater trochanter, the medial collateral ligament and to the medial aspect of the medial femoral condyle (white arrows), in keeping with heterotopic ossifications.
When better, the patient was transferred to a cardiac centre where he underwent transcatheter closure of the PFO with a 35 mm Occlutech ASD Occlude (Occlutech International AB, Helsingborg, Sweden). On his return from cardiac treatment, he was reviewed by the orthopaedic team for consideration for surgery for his severe bilateral knee HO; surgery was deemed futile at that time due to the risk of worsening recurrence and possibility of limiting the patient to a wheelchair long term.
Furthermore, on reviewing the patient, his cognitive impairment became apparent as he was unable to retain or recall information or previous conversations for reasonable lengths of time and he found decision-making challenging. This factor added further complexity to his rehabilitation.
Outcome and follow-up
After a protracted 7 months of admission, the patient was discharged from hospital in a wheelchair secondary to severe lower limb neuropathy, disuse muscle atrophy and painful bilateral knee HO. He lives with the possibility of chronic cognitive impairment requiring constant care. He is to continue with physiotherapy in the community and to maintain follow-up with the haematology department for SCD. He will have further appointments with a specialist orthopaedic team, to reconsider the suitability of surgery for his HO.
Discussion
This case report reviews the diverse manifestations and disabling consequences of an acute sickle cell crisis. At 21 years of age, this young man had experienced the full spectrum of pathology associated with SCD, from an acute crisis requiring ITU support to a debilitating chronic disease state. He went on to develop fat embolism, a frontal lobe cerebral infarct and had extensive HO, another rare manifestation of SCD.3 His clinical condition was made worse by congenital heart disease and cognitive impairment, and he required extensive rehabilitation before he could return to home and community living.
SCD is inherited as an autosomal recessive condition. Biochemically, the formation of an abnormal haemoglobin S is due to substitution of a single amino acid in the β-globin chain (valine for glutamic acid). This substitution ultimately leads to distortion of normally biconcave RBCs to acquire a crescent shape, known as sickling.4
In venous circulation, the HbS forms insoluble polymers leading to a sickled state. In arterial circulation, the HbS unpolymerises to render an unsickled state. This repetitive sickling and unsickling state eventually take its toll leading to irreversible red cell membrane damage, adherence to endothelial cells and release of inflammatory mediators. These changes are the basis for chronic anaemia and repeated episodes of ischaemic vaso-occlusion leading to a crisis that can affect multiple organs.5 The estimated median life expectancy of a patient with SCD is up to the fifth decade.6 Many of them will suffer from chronic anaemia and its vaso-occlusive complications.7 8
The course and complications of a sickle cell crisis are unpredictable and varied. Some patients may exhibit a severe acute crisis, which may lead to multiorgan damage, impairing the neurological, circulatory or musculoskeletal systems.8 9
A common complication of vaso-occlusive crises is generalised bone marrow infarction and necrosis leading to non-traumatic fat embolism syndrome, features of that include respiratory distress, altered mental status, acute kidney injury, anaemia, fever and tachycardia.10 Presence of an intracardiac or pulmonary right-to-left shunt allows fat emboli to enter the brain (CFE), with its neurological sequelae. A study by Dowling et al on 170 patients with SCD showed that the presence of right-to-left shunt was significantly higher in patients who experience a stroke than those who do not (45.6% vs 23.6%, p<0.001), with a higher prevalence of intrapulmonary shunting.11 An earlier study by the same group found that the prevalence of PFO or other intracardiac shunts was as high as 25% (10 of 40) in children with SCD who suffered either an overt or silent ischaemic stroke, compared with less than 12% (7 of 60) in children with stroke but without SCD.12 The authors proposed that intracardiac shunts such as PFO could be an independent modifiable risk factor for stroke or recurrent stroke in children with SCD. Indeed, ischaemic strokes are frequent in SCD, estimated to occur in about 11% of children with HbSS by age 199 and reported to be 220 times higher in children with HbSS than in those without SCD.13 14 Importantly, the prevalence and incidence of an ischaemic stroke are four times higher in patients with HbSS disease compared with those with Sickle Cell Disease (HbSC).9 In our patient, a bubble echo study showed a PFO. The increased pulmonary pressures caused by his chest crisis may have contributed to an increased right-to-left shunt via the interatrial septal defect, and intrapulmonary shunting may have also occurred concomitantly. As CFE tends to affect the periventricular, deep and subcortical white matter and deep grey nuclei as opposed to the cerebral cortex,15 16 it is plausible that our patient had a genuine thromboembolic event causing an ischaemic stroke in the right medial frontal gyrus in the context of a procoagulant state due to sickle cell crisis, presence of PFO and lack of vasculopathy on MRA. In contrast, the other white matter lesions were due to CFE. Razdan et al reported two cases of ischaemic stroke in young adult patients with SCD where the presence of PFO was confirmed.17 One of them, an 18-year-old boy, had HbSC disease and initially presented with transient right-sided sensorimotor symptoms involving the arm and leg, and a visual field deficit of the left lower quadrant, which later recurred. MRI of the brain revealed restricted diffusion in the left temporal region involving the left optic radiation, in keeping with a thromboembolic ischaemic event. Importantly, and similar to our case, there was no evidence of intracerebral vasculopathy on MRA. He was treated with exchange transfusion, and as PFO was demonstrated on bubble echo, commenced on anticoagulation until transcatheter PFO closure. He had no further Transient ischaemic attack (TIAs) or strokes in a 6 years of follow-up. The second case, a 21-year-old woman with HbSS, presented initially with a left-sided headache and severe expressive dysphasia. MRI demonstrated an acute ischaemic stroke in the distribution of the left middle cerebral artery, and she was treated with exchange transfusion to reduce HbS concentration below 30%. Bubble echo study demonstrated a PFO. Unlike the other cases, intracranial CT angiogram showed moyamoya disease in this patient. Chronic hypertransfusion therapy was recommended, but the patient did not comply and represented 4 months later with right-sided weakness, dysarthria and intermittent confusion. She was treated with exchange transfusion again and commenced on warfarin. A multidisciplinary decision was reached not to close the PFO given its small size, and the concomitant presence of severe intracerebral vasculopathy (moyamoya), which is generally irreversible and associated with high ischaemic stroke risk. A crucial difference between these two cases and ours is that of the absence of CFE. Both of these cases presented with acute, well-defined and localised neurological deficits, whereas our patient mainly had altered conscious level, most likely attributable to CFE, while the ischaemic event was more of an incidental finding on initial brain imaging. Nevertheless, it highlights the potential benefits of investigating for and treating PFO in patients with SCD with stroke, particularly in the absence of intracerebral vasculopathy.
Interestingly, CFE has also been reported in SCD without evidence of an intracardiac or intrapulmonary shunt,18 suggesting a systemic pathology that also affects the brain directly. While fat embolism syndrome is a rare complication of SCD with a predilection for heterozygous (HbSC) patients (only 15% of patients were homozygous (HbSS)) according to a recent systematic review,19 CFE can have devastating long-term consequences, including both sensorimotor and higher function deficits with chronic cognitive impairment, brain volume loss and demyelination.15 Therefore, early suspicion and recognition of the condition are paramount in order to avoid delay in treatment. MRI scan of the brain, and susceptibility-weighted imaging (SWI) in particular, has an invaluable role in demonstrating CFE.10 15 16 20 Diffusion-weighted imaging (DWI) typically shows innumerable bright, punctuate-restricted diffusion in a starfield pattern throughout the brain, reflecting cytotoxic oedema related to haemorrhage and infarction from cerebral vessel occlusion by fat emboli. At the same time, SWI frequently demonstrates susceptibility artefact in the corresponding foci representing microhaemorrhage.10 It is important to note that these changes, and in particular those on DWI sequence, show a dynamic change with time and become more confluent, unlike the early spot-like pattern.15 However, access to MRI imaging should not delay prompt treatment, especially if clinical suspicion of CFE is high. Fat embolism syndrome can present with a myriad of clinical signs and symptoms that occur following the release of fat emboli into the systemic circulation. Like our case, most patients have severe pain, fever, respiratory distress, altered mental status and other end-organ damage (heart, kidneys, liver) early in their presentation.19 21–25 Gendreau et al retrospectively analysed 25 patients with SCD admitted to intensive care, who underwent MRI imaging of the brain for acute non-focal neurological signs. Patients with confirmed CFE by SWI-MRI (10 of 25) had more fever and severe cardiopulmonary involvement, more consciousness alteration with a lower Glasgow Coma Scale (GCS) level, a lower platelet count and higher lactate dehydrogenase level compared with their non-CFE counterparts26, suggesting that these characteristics may be helpful to distinguish patients with CFE and recognise the condition early. Reduced haemoglobin and very high ferritin levels are also the characteristic laboratory findings.19 27
Emergency exchange transfusion is the mainstay of treatment and may improve the functional outcome, with some achieving full recovery with prolonged rehabilitation.22 According to a recent review by Tsitsikas et al, awareness of fat embolism syndrome in SCD has improved recently, with the increased use of red cell exchange resulting in reduced mortality (33% between 2014 and 2018 vs 64% before 2014), but at the expense of patients left with severe neurological impairment and only 20% making a full recovery.19 28 Therefore, as overall outcomes remain unsatisfactory, additional therapeutic measures such as plasma exchange and pre-emptive transfusion for high-risk patients have been suggested.19 24
Furthermore, our patient had another rather unusual complication of his sickle cell crisis in the form of HO affecting predominantly not only his knee joints but also the right hip to some extent. The authors of this case report are aware of only one previously reported case of extensive HO in SCD.3 The most common predisposing conditions to HO include trauma, spinal cord injury, traumatic brain injury, prolonged sedation and immobilisation and even acute respiratory distress syndrome.29–32 Interestingly, in a case series of 11 patients requiring prolonged ventilation in critical care, including one with fat embolism (but with aetiology not documented), Argyropoulou et al described HO in the area of the inner most part of the vastus medialis muscle, the same pattern was observed in our patient.33 Therefore, in our patient, HO of the knees may have been a consequence of his critical illness, rather than his SCD directly. Furthermore, the authors also found that the typical X-ray changes of HO only appeared around 23 days after the clinical diagnosis, compared with 1.5 days delay when using MRI. In our patient, also an initial X-ray for his knee pains showed no changes, whereas 20 days later, HO was evident on the repeated X-ray. This time interval delayed treatment and hence may have impacted on the severity of HO. Surgical resection of the excess bony tissue may be performed to help joint mobility. Surgery should be performed only once the HO matures and becomes less active metabolically, typically 6 months after the initiation of HO, as otherwise there are risks of recurrence.34 Non-surgical treatment options include the use of NSAIDs, bisphosphonates35 and symptomatic treatment with analgesics. Physical therapy in the form of passive range-of-movement exercise may help to prevent or mitigate HO in some but not all types of injuries.33 With most medical conditions, prevention and early treatment are preferable but necessitate early detection of HO.
Our case report emphasises the importance of early recognition of the diverse and potentially devastating pathologies associated with sickle cell crisis.
Patient’s perspective.
Despite the recent clinical life-changing events that the patient had endured, he considers himself ‘lucky to be alive’ and extremely optimistic about the future, such as love and becoming a photographer or a graphic designer. He takes inspiration from his older brother who also has sickle cell disease and has managed to complete his master’s degree in finance.
Although he never envisaged himself as a wheelchair user, he is adjusting well and hopes that this would be temporary until he is reviewed for HO surgery.
He has mentally transitioned from ‘whether he deserves this’ to life ‘is not a bed of roses’ and to having gained ‘a better angle of life’. He has learnt to turn pessimism to optimism.
He finds being in a nursing home with “old people” the most difficult and challenging part of his journey as he is unable to see his family because of the current pandemic.
The pain due to HO is severe enough to keep him awake, but he manages this by watching TV and playing games until he ‘just falls asleep’.
Learning points.
Patients in a sickle cell disease (SCD) crisis may present with varied signs and symptoms that may affect multiple organ systems.
In this patient, there were several unusual manifestations like a cerebral infarction, a congenital atrial septal defect and cognitive impairment.
The clinical presentation may rapidly change therefore influencing and affecting medical management.
There are no predictors or prognostic factors for the outcome of a crisis.
One must have a high index of suspicion for multisystemic involvement in patients with SCD.
An echocardiogram to look for a patent foramen ovale in patients with SCD with stroke is recommended, given its relatively high prevalence.
Footnotes
Twitter: @DeepakBatura
Contributors: TH—data collection, initial draft. SB—data collection, revision of draft, images and bibliography. MJF—data collection, initial draft, revision of draft. DB—drafting, revision of draft, revision of bibliography, oversight.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Dormandy E, James J, Inusa B, et al. How many people have sickle cell disease in the UK? J Public Health 2018;40:e291–5. 10.1093/pubmed/fdx172 [DOI] [PubMed] [Google Scholar]
- 2. Bernaudin F, Verlhac S, Arnaud C, et al. Chronic and acute anemia and extracranial internal carotid stenosis are risk factors for silent cerebral infarcts in sickle cell anemia. Blood 2015;125:1653–61. 10.1182/blood-2014-09-599852 [DOI] [PubMed] [Google Scholar]
- 3. Fye MA, Baumgaertner MR. Multifocal heterotopic ossification in a patient with sickle cell disease: a case report. Am J Orthop 1997;26:216–9. [PubMed] [Google Scholar]
- 4. Diez-Silva M, Dao M, Han J, et al. Shape and biomechanical characteristics of human red blood cells in health and disease. MRS Bull 2010;35:382–8. 10.1557/mrs2010.571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nagel RL PO. General pathophysiology of sickle cell anemia : Steinberg MH FB, Higgs DR, Disorders of hemoglobin. Cambridge: Cambridge University Press, 2001: 494–526. [Google Scholar]
- 6. Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. life expectancy and risk factors for early death. N Engl J Med 1994;330:1639–44. 10.1056/NEJM199406093302303 [DOI] [PubMed] [Google Scholar]
- 7. Pathare A, Kindi SA, Daar S, et al. Cytokines in sickle cell disease. Hematology 2003;8:329–37. 10.1080/10245330310001604719 [DOI] [PubMed] [Google Scholar]
- 8. Tanabe P, Spratling R, Smith D, et al. Ce: understanding the complications of sickle cell disease. Am J Nurs 2019;119:26–35. 10.1097/01.NAJ.0000559779.40570.2c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood 1998;91:288–94. [PubMed] [Google Scholar]
- 10. Kang JH, Hargett CW, Sevilis T, et al. Sickle cell disease, fat embolism syndrome, and "starfield" pattern on MRI. Neurol Clin Pract 2018;8:162–4. 10.1212/CPJ.0000000000000443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dowling MM, Quinn CT, Ramaciotti C, et al. Increased prevalence of potential right-to-left shunting in children with sickle cell anaemia and stroke. Br J Haematol 2017;176:300–8. 10.1111/bjh.14391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dowling MM, Lee N, Quinn CT, et al. Prevalence of intracardiac shunting in children with sickle cell disease and stroke. J Pediatr 2010;156:645–50. 10.1016/j.jpeds.2009.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Earley CJ, Kittner SJ, Feeser BR, et al. Stroke in children and sickle-cell disease: Baltimore-Washington cooperative young stroke study. Neurology 1998;51:169–76. 10.1212/WNL.51.1.169 [DOI] [PubMed] [Google Scholar]
- 14. Kwiatkowski JL, Zimmerman RA, Pollock AN, et al. Silent infarcts in young children with sickle cell disease. Br J Haematol 2009;146:300–5. 10.1111/j.1365-2141.2009.07753.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuo K-H, Pan Y-J, Lai Y-J, et al. Dynamic MR imaging patterns of cerebral fat embolism: a systematic review with illustrative cases. AJNR Am J Neuroradiol 2014;35:1052–7. 10.3174/ajnr.A3605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mossa-Basha M, Izbudak I, Gurda GT, et al. Cerebral fat embolism syndrome in sickle cell anaemia/β-thalassemia: importance of susceptibility-weighted MRI. Clin Radiol 2012;67:1023–6. 10.1016/j.crad.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 17. Razdan S, Strouse JJ, Naik R, et al. Patent foramen ovale in patients with sickle cell disease and stroke: case presentations and review of the literature. Case Rep Hematol 2013;2013:1–5. 10.1155/2013/516705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nathan CL, Aamodt WW, Yalamarti T, et al. Cerebral fat embolism syndrome in sickle cell disease without evidence of shunt. eNeurologicalSci 2019;14:19–20. 10.1016/j.ensci.2018.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tsitsikas DA, May JE, Gangaraju R, et al. Revisiting fat embolism in sickle syndromes: diagnostic and emergency therapeutic measures. Br J Haematol 2019;186:e112–5. 10.1111/bjh.15941 [DOI] [PubMed] [Google Scholar]
- 20. Yeap P, Kanodia AK, Main G, et al. Role of susceptibility-weighted imaging in demonstration of cerebral fat embolism. BMJ Case Rep 2015. 10.1136/bcr-2014-207581. [Epub ahead of print: 08 Jan 2015]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Greaves P, Mathew V, Peters C, et al. Successful outcome of three patients with sickle-cell disease and fat embolism syndrome treated with intensive exchange transfusion. Clin Case Rep 2017;5:39–43. 10.1002/ccr3.727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maroni A, Dauger S, Chomton M. Fat embolism syndrome in a child with sickle cell disease. J Pediatr 2019;214:236. 10.1016/j.jpeds.2019.06.029 [DOI] [PubMed] [Google Scholar]
- 23. Ositelu A, Urrutia-Argueta S, Kapoor R. Neurologic recovery in systemic nontraumatic fat embolism syndrome in an elderly patient with hemoglobin SC disease: a case report. Clin Case Rep 2020;8:1816–20. 10.1002/ccr3.3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsitsikas DA, Vize J, Abukar J. Fat embolism syndrome in sickle cell disease. J Clin Med 2020. 10.3390/jcm9113601. [Epub ahead of print: 08 11 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Viseroi MI, Patel A.;, Youssef N.;, et al. Altered mental status as initial presentation of fat embolism syndrome in sickle cell crisis American thoracic Society 2019 International Conference. Am J Respir Crit Care Med 2019:A6665. [Google Scholar]
- 26. Gendreau S, Scholer M, Cecchini J, et al. Cerebral fat embolism in sickle cell disease. Am J Hematol 2020;95:E41–5. 10.1002/ajh.25686 [DOI] [PubMed] [Google Scholar]
- 27. Gangaraju R, May JE, Williams LA, et al. Fat embolism syndrome due to bone marrow necrosis in patients with hemoglobinopathies: a life-threatening complication mimicking thrombotic thrombocytopenic purpura. Am J Hematol 2019;94:E64–6. 10.1002/ajh.25363 [DOI] [PubMed] [Google Scholar]
- 28. Tsitsikas DA, Gallinella G, Patel S, et al. Bone marrow necrosis and fat embolism syndrome in sickle cell disease: increased susceptibility of patients with non-SS genotypes and a possible association with human parvovirus B19 infection. Blood Rev 2014;28:23–30. 10.1016/j.blre.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 29. Jacobs JW, De Sonnaville PB, Hulsmans HM, et al. Polyarticular heterotopic ossification complicating critical illness. Rheumatology 1999;38:1145–9. 10.1093/rheumatology/38.11.1145 [DOI] [PubMed] [Google Scholar]
- 30. Pape HC, Lehmann U, van Griensven M, et al. Heterotopic ossifications in patients after severe blunt trauma with and without head trauma: incidence and patterns of distribution. J Orthop Trauma 2001;15:229–37. 10.1097/00005131-200105000-00001 [DOI] [PubMed] [Google Scholar]
- 31. Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J Nucl Med 2002;43:346–53. [PubMed] [Google Scholar]
- 32. Sugita A, Hashimoto J, Maeda A, et al. Heterotopic ossification in bilateral knee and hip joints after long-term sedation. J Bone Miner Metab 2005;23:329–32. 10.1007/s00774-005-0608-5 [DOI] [PubMed] [Google Scholar]
- 33. Aronen JG, Garrick JG, Chronister RD, et al. Quadriceps contusions: clinical results of immediate immobilization in 120 degrees of knee flexion. Clin J Sport Med 2006;16:383–7. 10.1097/01.jsm.0000244605.34283.94 [DOI] [PubMed] [Google Scholar]
- 34. Pavey GJ, Polfer EM, Nappo KE, et al. What risk factors predict recurrence of heterotopic ossification after excision in combat-related amputations? Clin Orthop Relat Res 2015;473:2814–24. 10.1007/s11999-015-4266-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Teasell RW, Mehta S, Aubut JL, et al. A systematic review of the therapeutic interventions for heterotopic ossification after spinal cord injury. Spinal Cord 2010;48:512–21. 10.1038/sc.2009.175 [DOI] [PMC free article] [PubMed] [Google Scholar]