Abstract
Introduction
Women comprise two-thirds of people with dementia, making female sex a significant dementia risk factor. Both type 1 diabetes (T1D) and type 2 diabetes (T2D) are known dementia risk factors with an increasing global incidence. Understanding whether subtle sex differences persist in cognitive function prior to dementia in the context of diabetes may help elucidate the magnitude of sex effects on dementia risk.
Research design and methods
We examined cross-sectional data from the Study of Longevity in Diabetes (SOLID), a prospective cohort study of members of Kaiser Permanente Northern California aged 60 years and older with T1D (n=758), T2D (n=232) and without either T1D or T2D (n=247). We used factor analysis to generate summary scores of cognitive domains and used regression analyses to examine the associations between sex and cognition adjusting for sociodemographic and cardiovascular confounders.
Results
We included 1237 participants (630 women and 607 men) with mean age 68 years. By design, the distribution of men and women in T1D, T2D and no diabetes was similar. Women had better cognitive performance than men in global cognition (β=0.21, 95% CI 0.16 to 0.26), language (β=0.08, 95% CI 0.004 to 0.15), executive function (β=0.13, 95% CI 0.05 to 0.20), episodic verbal memory (β=0.68, 95% CI 0.59 to 0.77) and attention (β=0.20, 95% CI 0.11 to 0.28) but not in episodic visual memory (β=0.006, 95% CI −0.07 to 0.09) adjusting for age and education independent of diabetes status. We did not find an interaction between sex and diabetes status for any of the cognitive outcomes.
Conclusions
Women in late mid-life have better cognitive performance than men in many cognitive domains independent of the presence of T1D or T2D. Further work is required to understand whether these differences change over time or in older cohorts and to understand their relationship to subsequent dementia.
Keywords: diabetes complications, cognition, sex characteristics
Significance of this study.
What is already known about this subject?
Both female sex and diabetes are risk factors for dementia, but the underlying mechanisms are unclear.
It is not known whether the previously described sex differences in cognitive function differ depending on whether a person has type 1, type 2 or no diabetes.
What are the new findings?
In a study of 1237 people (mean age 68 years), we found that women had better cognitive performance than men in a number of cognitive domains including global cognition, language, executive function, attention and verbal memory.
Women and men had similar visual memory scores.
The sex differences in cognition did not vary by the presence or absence of diabetes or whether a person had type 1 or type 2 diabetes.
How might these results change the focus of research or clinical practice?
Our results demonstrate the presence of sex differences in cognition across diabetes states and encourage further research to better understand whether the differences we report change over time and their relationship to subsequent dementia.
Introduction
Female sex is a risk factor for dementia.1 Women make up approximately half the world population,2 yet two-thirds of people with dementia are women. This makes the disproportionate burden of dementia in women a large public health concern. The reasons for this increased risk in women are unclear but previous work suggest that there may be sex-specific etiological factors in addition to simply increased longevity.3 The global incidence of both type 1 diabetes (T1D) and type 2 diabetes (T2D) is increasing4 and, depending on the population studied, there also appears to be sex differences in diabetes incidence.5 Recently, both T1D and T2D have been shown to be associated with an increased risk of dementia.6 7 However, whether the known sex differences in dementia risk extend to those with diabetes or is altered by diabetes is poorly understood.8 There are sex-related differences in the incidence of diabetes complications,9–11 yet, it is unknown whether there are also sex differences in cognitive function related to diabetes.
Clarifying whether sex differences persist in cognitive function in the context of diabetes helps elucidate the magnitude of sex effects on dementia risk.
Neuropsychological testing in people without dementia allows the detection of subtle cognitive changes that may precede the development of dementia. This allows the potential to identify important mechanisms and risk factors prior to the development of overt functional impairment. For example, when compared with men, women tend to perform more poorly in some cognitive tasks and better in others.12 13 The reasons underlying these sex differences are unknown but may be related to the neuronal activity of sex hormones.13–15 Greater understanding of the factors associated with increased dementia risk is essential to the targeting of interventions to high-risk groups such as women. We evaluated whether the associations of sex and cognitive function in mid-later life differs by diabetes status in a group of people without dementia. Our hypothesis was that women would perform more poorly in the cognitive tests than men and these would differ by diabetes status.
Research design and methods
Study population
The Study of Longevity in Diabetes (SOLID) is a prospective cohort study of aging and diabetes that recruited members of Kaiser Permanente Northern California (KPNC) aged 60 years and older with T1D, T2D and without either T1D or T2D. Details of participant eligibility and inclusion have been published previously and briefly presented in online supplemental figure 1.16–18 Potential participants with T1D were identified in electronic medical records using International Classification of Diseases (ICD)-9 and ICD-10 codes for T1D (250.x1, 250.x3 or E10.x) or T2D (250.x0, 250.x2, E11.x). As per previous work using the SOLID dataset,16–18 individuals with diagnostic codes related to both types of diabetes were classified as having T1D if at least 75% of diagnostic codes related to diabetes were for T1D specifically and the member was prescribed insulin to reduce the risk of misclassification. Enrolled participants with T1D were then used to guide recruitment of two comparator groups: people with T2D and people without either T1D or T2D. Individuals with T1D were population frequency matched to potential participants with T2D or without either T1D or T2D. Individuals with diagnostic codes related to both types of diabetes were classified as having T2D if at least 75% of diagnostic codes related to diabetes were for T2D. Population distribution matching was performed on the following factors: sex, age (grouped as: 60–64, 65–69, 70–74, 75–79, 80–84, 85–89, 90+ years), race/ethnicity and education.
bmjdrc-2020-001646supp001.pdf (220.9KB, pdf)
Participants completed a number of items regarding demographic, general health, diabetes complications, health literacy (Rapid Estimate of Adult Literacy in Medicine-Short Form,19 mood (Geriatric Depression Scale (GDS)) and sleep quality (a modified version of the Pittsburg Sleep Quality Index (PSQI)).20 Presence of microvascular (retinopathy, neuropathy, nephropathy) and macrovascular disease (stroke, myocardial infarction) at baseline were captured through self-report of a physician’s diagnosis at baseline. A comprehensive cognitive battery, described below, was then administered by trained interviewers.
Cognitive function
We conducted factor analysis on the cognitive assessments of all participants and identified five cognitive domains: language, executive function, episodic verbal memory, episodic visual memory and simple attention. The language domain was comprised of the phonemic fluency test (F and L), the category fluency test (animals and vegetables), list sorting (two alternative lists) and Multilingual Naming Test. The executive function domain comprised the Trail Making Test (A and B), the Digit Symbol Substitution Test and the Stroop Color and Word Tests. The episodic verbal memory domain consisted of the Word List Learning Test (immediate and delayed). The episodic visual memory domain consisted of the Word List Learning Test (immediate and delayed) and the Benson Complex Figure Copy (immediate and delayed). The simple attention domain was composed of the Diamond and TMX cancellation tests. Each test score was converted to a z-score (mean=0; SD=1). For each domain, a summary score was calculated by summing the z-scores for individuals who completed at least 50% of the relevant tests. A global cognition score was calculated as the average of the five domain-specific summary scores for individuals who competed at least 50% of all cognitive function tests.
Covariables
Age was calculated from date of baseline interview and date of birth. Diabetes duration was calculated using self-reported age of diabetes onset. Race/ethnicity (white, Hispanic, Asian, African-American and other), educational attainment, presence of microvascular and macrovascular disease (retinopathy, neuropathy, nephropathy, stroke, myocardial infarction) and diabetes complications (lifetime exposure of severe hypoglycemia requiring hospital care and lifetime exposure to diabetic ketoacidosis (DKA)) were based on self-report. We categorized educational attainment as: ‘college degree or greater’ or ‘less than a college degree’.
Analytic sample
Of the 1311 individuals enrolled in SOLID (805 individuals with T1D, 248 individuals with T2D, 258 individuals without diabetes), we excluded 4 participants with missing information on educational attainment and 70 participants who were missing the global cognition score, resulting in a final analytic sample of 1237 for the whole study.
Statistical analyses
We examined the distribution of baseline characteristics in the overall sample, then by sex and by diabetes status. Within each of the categories of no diabetes, T1D and T2D, we examined mean standardized scores on global and domain-specific cognitive measures without covariate adjustment. For our main analysis, we specified linear regression models to examine the association between sex (using men as reference) and performance on global and domain-specific measures of cognition. First, we performed these models in the whole group, including diabetes status as a covariate. We then specified linear regression models of the associations between sex and the cognitive outcomes among individuals with no diabetes, T1D and T2D separately. Literature research and knowledge of previously published associations found in this sample guided our identification of covariates. In each of these models, we initially fitted base models that adjusted for age and education. We then added diabetes status, race/ethnicity, PSQI and GDS score in a stepwise fashion to explore how these factors, that have been shown to be associated with cognition in this sample,17 18 influenced any sex-cognitive performance relationship. We then tested a final model including health literacy and cardiovascular health measures (neuropathy, nephropathy, retinopathy, stroke and myocardial infarction). We also explored whether the relationship between age (adjusting for sex and education) and the cognitive scores was linear or quadratic using an Akaike Information Criterion (AIC) measure of model fit.
In the models using whole group data, we additionally included a product term sex×diabetes status to test for a potential interaction (effect modification) between sex and diabetes status on cognitive function. We also plotted the estimates of these models to visually inspect for the possibility of an interaction. We further stratified the final model (including all potentially relevant covariables) by diabetes status to explore within-strata sex-cognition relationships. In the T1D and T2D groups (separately), we additionally examined the effect of including lifetime history of DKA and/or severe hypoglycemia on sex-cognition model associations. All analyses were performed using SAS V.9.4.
Results
Sample characteristics
Of the initial 1311 people recruited into the study, a total of 1237 participants (630 women and 607 men) had sufficient data to be included in the current study. The sample characteristics of those who completed and did not complete all cognitive tests (n=70) are presented in online supplemental table 1. Broadly, those who did not complete all cognitive tests were older, less likely to hold a college degree, had lower household income, were diagnosed with diabetes at a younger age, had longer disease duration and were more likely to report a history of severe hypoglycemia and DKA than those who completed all cognitive tests. Table 1 describes the characteristics of study participants in the whole group and stratified by sex.
Table 1.
Sample characteristics stratified by sex
| Whole group | Women | Men | P value | |
| n (%) | 1237 | 630 (51) | 607 (49) | |
| Demographics | ||||
| Age (years), mean (SD) | 67.8 (6.6) | 67.6 (6.5) | 68.0 (6.7) | 0.23 |
| Race/Ethnicity, n (%) | ||||
| White | 1052 (85) | 533 (85) | 519 (86) | |
| Hispanic | 97 (8) | 59 (9) | 38 (6) | |
| Asian | 20 (2) | 10 (2) | 10 (2) | |
| African-American | 21 (2) | 10 (2) | 11 (2) | |
| Other | 47 (4) | 18 (3) | 29 (5) | 0.22 |
| College degree, n (%) | 755 (61) | 366 (58) | 389 (64) | 0.03 |
| Annual household income (US$) | ||||
| 0–59 999 | 364 (29) | 214 (36) | 150 (26) | |
| 60 000–99 000 | 364 (29) | 192 (32) | 172 (30) | |
| 100 000–199 000 | 342 (28) | 151 (26) | 191 (34) | |
| >200 000 | 89 (7) | 35 (6) | 54 (10) | <0.0001 |
| Health literacy, n (%) | ||||
| 3rd grade or below | 1 (0) | 1 (0) | 0 (0) | |
| 4th–6th grade | 7 (1) | 2 (0) | 5 (1) | |
| 7th–8th grade | 86 (7) | 27 (4) | 59 (10) | |
| High school | 1143 (92) | 600 (95) | 543 (89) | 0.001 |
| General health | ||||
| Every smoked >100 cigarettes, n (%) | 538 (43) | 262 (42) | 276 (46) | 0.19 |
| How often drink alcohol | ||||
| Do not drink, n (%) | 322 (26) | 165 (26) | 157 (26) | |
| At least monthly, n (%) | 421 (34) | 234 (37) | 187 (31) | |
| At least weekly, n (%) | 481 (39) | 226 (36) | 255 (43) | 0.04 |
| PSQI, mean (SD) | 8.0 (2.7) | 8.5 (2.8) | 7.6 (2.6) | <0.0001 |
| GDS, mean (SD) | 2.0 (2.3) | 2.2 (2.4) | 1.8 (2.1) | 0.003 |
| No diabetes, n (%) | 247 (20) | 127 (20) | 120 (20) | |
| Type 1 diabetes, n (%) | 758 (61) | 385 (61) | 373 (61) | |
| Type 2 diabetes, n (%) | 232 (19) | 118 (19) | 114 (19) | 0.99 |
| Diabetes characteristics | ||||
| Mean age at diabetes onset (years) (SD) | 34.4 (18.4) | 35.1 (19.0) | 33.7 (17.9) | 0.23 |
| Mean diabetes duration (years) (SD) | 33.1 (17.8) | 32.2 (18.0) | 34.1 (17.5) | 0.09 |
| Retinopathy, n (%) | 341 (28) | 183 (31) | 158 (28) | 0.30 |
| Neuropathy, n (%) | 374 (30) | 191 (31) | 183 (32) | 0.98 |
| Nephropathy, n (%) | 61 (5) | 29 (5) | 32 (6) | 0.49 |
| Stroke, n (%) | 100 (8) | 45 (7) | 55 (9) | 0.19 |
| Myocardial infarction, n (%) | 126 (10) | 54 (9) | 72 (12) | 0.05 |
| Severe hypoglycemia n (%) | 376 (30) | 183 (29) | 193 (32) | 0.29 |
| Diabetic ketoacidosis, n (%) | 213 (17) | 134 (21) | 79 (13) | 0.0006 |
Bold signifies p values≤0.05.
GDS, Geriatric Depression Scale; PSQI, Pittsburg Sleep Quality Index.
The mean age of men and women in all groups were similar (~68 years). Approximately 85% of the sample were of white ethnicity. Compared with men, women were less likely to have completed a college or higher degree (58% vs 64%, p=0.03) and reported lower household income (p<0.0001), lower alcohol use (p=0.04) and worse sleep quality (mean PSQI=8.5 vs mean PSQI=7.6; p<0.0001). Women had higher GDS scores than men (2.2 vs 1.8 p=0.003). By design, the distribution of men and women was similar in those with T1D, T2D and no diabetes (~50%). Table 2 describes the characteristics of study participants stratified by sex and diabetes status. The mean age of diabetes diagnosis was approximately 28 years in those with T1D and was approximately 55 years in those with T2D. In those with T1D, men had a little longer duration of diabetes (40.4 years, SD=13.9) than women (38.0 years, SD=16.0). A similar pattern was seen in those with T2D, but this difference was not statistically significant (men: 14.5 years; women: 13.5 years). Participants with T1D reported higher rates of microvascular and macrovascular complications, severe hypoglycemia and DKA than those with T2D or with diabetes. However, these rates were similar between men and women except for lifetime exposure to DKA which was more commonly reported by women (35%) than men (21%) (p<0.0001).
Table 2.
Sample characteristics stratified by diabetes status
| T1D | T2D | No diabetes | ||||
| Women | Men | Women | Men | Women | Men | |
| n (%) | 385 (51) | 373 (49) | 118 (51) | 114 (49) | 127 (51) | 120 (49) |
| Demographics | ||||||
| Age (years), mean (SD) | 67.0 (6.2) | 67.5 (6.4) | 68.6 (7.0) | 68.8 (7.1) | 68.4 (6.8) | 69.0 (7.2) |
| Race/Ethnicity, n (%) | ||||||
| White | 330 (86) | 317 (85) | 98 (83) | 97 (85) | 105 (83) | 105 (88) |
| Hispanic | 19 (5) | 9 (2) | 19 (16) | 15 (13) | 21 (17) | 14 (12) |
| Asian | 10 (3) | 9 (2) | 0 (0) | 1 (1) | 0 (0) | 0 (0) |
| African-American | 10 (3) | 11 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other | 16 (4) | 27 (7) | 1 (1) | 1 (1) | 1 (1) | 1 (1) |
| College degree, n (%) | 228 (59) | 245 (66) | 67 (57) | 72 (63) | 71 (56) | 72 (60) |
| Annual household income (US$) | ||||||
| 0–59 999 | 134 (35) | 94 (25) | 49 (42) | 34 (30) | 31 (24) | 22 (18) |
| 60 000–99 000 | 115 (30) | 104 (28) | 36 (31) | 36 (32) | 41 (32) | 32 (27) |
| 100 000–199 000 | 88 (23) | 115 (31) | 23 (19) | 29 (25) | 40 (31) | 47 (39) |
| >200 000 | 22 (6) | 30 (8) | 3 (3) | 10 (9) | 10 (8) | 14 (12) |
| Health literacy, n (%) | ||||||
| 3rd grade or below | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| 4th–6th grade | 1 (0) | 3 (1) | 1 (1) | 1 (1) | 0 (0) | 1 (1) |
| 7th–8th grade | 14 (4) | 29 (8) | 10 (8) | 13 (11) | 3 (2) | 17 (14) |
| High school | 370 (96) | 341 (91) | 107 (91) | 100 (88) | 123 (97) | 102 (85) |
| General health | ||||||
| Ever smoked >100 cigarettes, n (%) | 157 (41) | 159 (43) | 57 (48) | 62 (54) | 48 (38) | 55 (46) |
| How often drink alcohol | ||||||
| Do not drink, n (%) | 104 (27) | 109 (30) | 41 (35) | 31 (27) | 20 (16) | 17 (14) |
| At least monthly, n (%) | 134 (35) | 96 (26) | 50 (42) | 47 (41) | 50 (39) | 44 (37) |
| At least weekly, n (%) | 143 (37) | 161 (44) | 26 (22) | 35 (31) | 57 (45) | 59 (49) |
| PSQI, mean (SD) | 8.6 (2.9) | 7.7 (2.6) | 8.6 (2.4) | 7.9 (2.7) | 8.1 (2.7) | 6.9 (2.1) |
| GDS, mean (SD) | 2.3 (2.5) | 1.9 (2.1) | 2.6 (2.7) | 2.0 (2.2) | 1.2 (1.4) | 1.2 (1.5) |
| Diabetes characteristics | ||||||
| Age at diabetes onset (years), mean (SD) | 28.9 (16.4) | 27.2 (13.7) | 55.3 (11.2) | 54.3 (13.4) | N/A | N/A |
| Diabetes duration (years), mean (SD) | 38.0 (16.0) | 40.3 (13.9) | 13.4 (9.9) | 14.5 (12.6) | N/A | N/A |
| Retinopathy, n (%) | 175 (45) | 145 (39) | 7 (6) | 12 (11) | 1 (1) | 1 (1) |
| Neuropathy, n (%) | 155 (40) | 147 (39) | 26 (22) | 30 (26) | 10 (8) | 6 (5) |
| Nephropathy, n (%) | 28 (7) | 31 (8) | 0 (0) | 1 (1) | 1 (1) | 0 (0) |
| Stroke, n (%) | 30 (8) | 34 (9) | 7 (6) | 13 (11) | 8 (6) | 8 (7) |
| Myocardial infarction, n (%) | 44 (11) | 50 (13) | 6 (5) | 16 (14) | 4 (3) | 6 (5) |
| Severe hypoglycemia n (%) | 178 (46) | 191 (51) | 5 (4) | 2 (2) | N/A | N/A |
| Diabetic ketoacidosis, n (%) | 134 (35) | 78 (21) | 0 (0) | 1 (1) | N/A | N/A |
Bold signifies p values≤0.05.
GDS, Geriatric Depression Scale; N/A, not available; PSQI, Pittsburg Sleep Quality Index; T1D, type 1 diabetes; T2D, type 2 diabetes.
Associations between sex and cognitive outcomes
Table 3 presents the associations between female sex and the different cognitive domains across the whole group and stratified by diabetes status.
Table 3.
Multiple linear regression models of sex and cognitive domains in the whole group and stratified by diabetes status*
| Model covariables | Global cognition | Language | Executive function | Episodic verbal memory | Episodic visual memory | Attention |
| β (female sex) (95% CI) |
β (female sex) (95% CI) |
β (female sex) (95% CI) |
β (female sex) (95% CI) |
β (female sex) (95% CI) |
β (female sex) (95% CI) |
|
| Age+education |
0.21 (0.16 to 0.26) |
0.08 (0.004 to 0.15) |
0.13 (0.05 to 0.20) |
0.68 (0.59 to 0.77) |
0.006 (−0.08 to 0.09) |
0.20 (0.11 to 0.28) |
| Age+education+ diabetes status |
0.21 (0.16 to 0.26) |
0.08 (0.004 to 0.15) |
0.13 (0.05 to 0.20) |
0.68 (0.59 to 0.77) |
0.006 (−0.07 to 0.09) |
0.20 (0.11 to 0.28) |
| Sex×diabetes interaction term (p) in above model |
0.34 | 0.92 | 0.83 | 0.21 | 0.88 | 0.12 |
| Age+education+race |
0.22 (0.17 to 0.26) |
0.08 (0.01 to 0.15) |
0.12 (0.05 to 0.20) |
0.67 (0.58 to 0.77) |
0.01 (−0.07 to 0.09) |
0.20 (0.12 to 0.29) |
| Age+education+race+ diabetes status |
0.22 (0.17 to 0.27) |
0.08 (0.02 to 0.15) |
0.13 (0.06 to 0.20) |
0.68 (0.58 to 0.77) |
0.007 (−0.07 to 0.09) |
0.20 (0.12 to 0.29) |
| Sex×diabetes interaction term (p) in above model |
0.35 | 0.86 | 0.80 | 0.22 | 0.88 | 0.11 |
| Age+education+race+PSQI+GDS score |
0.24 (0.18 to 0.29) |
0.10 (0.03 to 0.17) |
0.16 (0.08 to 0.23) |
0.71 (0.61 to 0.80) |
0.01 (−0.07 to 0.10) |
0.23 (0.14 to 0.31) |
| Age+education+race+ PSQI+GDS score+diabetes status |
0.24 (0.18 to 0.29) |
0.10 (0.03 to 0.17) |
0.16 (0.08 to 0.23) |
0.71 (0.61 to 0.81) |
0.007 (−0.08 to 0.09) |
0.22 (0.14 to 0.31) |
| Sex×diabetes interaction term (p) in above model |
0.64 | 0.90 | 0.92 | 0.52 | 0.79 | 0.28 |
| Age+education+race+PSQI+ GDS score+diabetes status+neuropathy+nephropathy+ retinopathy+stroke+MI+health literacy |
0.20 (0.15, 0.25) |
0.05 (−0.03 to 0.12) |
0.11 (0.04 to 0.18) |
0.67 (0.58 to 0.77) |
−0.01 (−0.10 to 0.07) |
0.21 (0.12 to 0.30) |
| Diabetes status stratification | ||||||
| Type 1 diabetes* |
0.25 (0.18 to 0.31) |
0.09 (−0.002 to 0.19) |
0.15 (0.05 to 0.25) |
0.70 (0.58 to 0.83) |
0.01 (−0.10 to 0.13) |
0.28 (0.16 to 0.39) |
| Type 2 diabetes* |
0.19 (0.07 to 0.31) |
0.13 (−0.03 to 0.30) |
0.13 (−0.03 to 0.29) |
0.65 (0.42 to 0.87) |
−0.05 (−0.21 to 0.11) |
0.15 (−0.07 to 0.37) |
| No diabetes* |
0.24 (0.13 to 0.35) |
0.08 (−0.07 to 0.24) |
0.19 (0.04 to 0.35) |
0.79 (0.59 to 1.00) |
0.05 (−0.14 to 0.24) |
0.14 (−0.05 to 0.33) |
Bold signifies p values≤0.05.
*Models adjusted for age, education, race, PSQI score and GDS score.
GDS, Geriatric Depression Scale; MI, myocardial infarction; PSQI, Pittsburg Sleep Quality Index.
We used a linear term for age as it resulted in a slightly better model fit than a quadratic term (AIC 1548.5 vs 1551.3). In the whole group, women had better cognitive performance than men in global cognition (β=0.21, 95% CI 0.16 to 0.26), language (β=0.08, 95% CI 0.004 to 0.15), executive function (β=0.13, 95% CI 0.05 to 0.20), episodic verbal memory (β=0.68, 95% CI 0.59 to 0.77) and attention (β=0.20, 95% CI 0.11 to 0.28) (all p<0.01) but not in episodic visual memory (β=0.006, 95% CI −0.07 to 0.09) adjusting for age and education. The stepwise inclusion of diabetes status, race/ethnicity, PSQI and GDS score did not meaningfully change these associations (maximum β change=25%). We did not find a statistically significant sex×diabetes status interaction for any of the cognitive outcomes in any of the adjusted models. The inclusion of measures of cardiovascular health and health literacy resulted in an attenuation of the associations between female sex and the cognitive outcomes with the previously described association between female sex and greater performance in language no longer statistically significant (β=0.05, 95% CI −0.03 to 0.12).
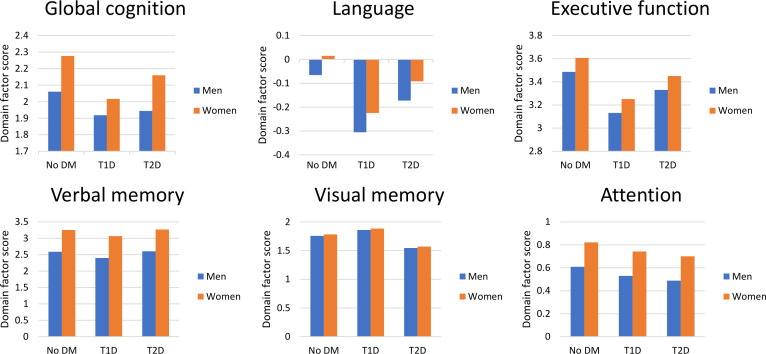
In exploratory sensitivity analysis, we examined the associations between sex and cognition adjusting for age, education, race, PSQI and GDS stratified by diabetes status (table 2). In those with T1D, women had better performance in global cognition (β=0.25, 95% CI 0.18 to 0.31), language (β=0.09, 95% CI −0.002 to 0.19), executive function (β=0.15, 95% CI 0.05 to 0.25), episodic verbal memory (β=0.70, 95% CI 0.58 to 0.83) and attention than men (β=0.28, 95% CI 0.16 to 0.39) (all p≤0.05). The addition of history of DKA to the fully adjusted models in those with T1D resulted in the weakening of the association of female sex with better language performance (β=0.09, 95% CI −0.008 to 0.18, p=0.07) but did not meaningfully change the other reported associations. The further addition of history of severe hypoglycemia requiring hospitalization did not result in any further meaningful change in the reported associations. In those with T2D, women had better cognitive performance than men in global cognition (β=0.19, 95% CI 0.07 to 0.31) and episodic verbal memory (β=0.65, 95% CI 0.42 to 0.87) (all p≤0.001) but not the other cognitive domains. The inclusion of history of DKA and/or severe hypoglycemia did not meaningfully change our results. In those without diabetes, female sex was associated with better global cognition (β=0.24, 95% CI 0.13 to 0.35), executive function (β=0.19, 95% CI 0.04 to 0.35) and episodic verbal memory (β=0.79, 95% CI 0.59 to 1.00) than men (all p<0.01). There were no associations between sex and language, episodic visual memory or attention factors among individuals without T1D or T2D. Figure 1 provides a visual representation of the associations between sex and predicted cognitive domain factor scores within diabetes status strata using the models described in table 3. The unadjusted mean cognitive factor scores by sex and diabetes status are presented in online supplemental table 2. The inclusion of measures of cardiovascular health and health literacy resulted in minimal attenuation of the associations between female sex and the cognitive outcomes (data not shown), with the only meaningful change being the previously described association between female sex and greater executive function in those without diabetes was no longer statistically significant (β=0.11, 95% CI −0.04 to 0.26).
Figure 1.
Associations between sex and predicted cognitive performance by diabetes status*. *Adjusted for age, race/ethnicity, education, Pittsburg Sleep Quality Index score and Geriatric Depression Scale score. Models based on a white person aged 70 years with a college degree. DM, diabetes mellitus; T1D, type 1 diabetes; T2D, type 2 diabetes.
Discussion
In this sample of people without dementia, we found that women performed better than men globally and in many of the individual cognitive domains regardless of diabetes status. Furthermore, the amplitude of the sex differences appeared similar across diabetes states. To our knowledge, previous studies have not examined for the presence of sex differences in cognitive function across T1D and T2D. These results suggest that the well-described better cognitive functioning of women compared with men without dementia is maintained in the presence of both T1D and T2D.
Previous work has reported that in people without diabetes, women have better cognitive functioning than men. Both the original analyses of the Health and Retirement Study21 and the follow-up of the same study performing a modified version of the Telephone Interview for Cognitive Status on 18 982 people aged 51 years or older, reported that women had better global cognition and cognitive subdomains scores than men.22 A similar pattern was observed in a study of 2503 participants in the Framingham Heart Study where women (mean age >60 years) had significantly higher performance on visual/spatial memory, verbal memory and attention/concentration than men.23 Reconciling this observation with the greater dementia risk in women has led others to suggest that better cognitive test performance of women may mask functional cognitive changes leading to delayed diagnosis and at more advanced stages.3
Fewer studies have examined the role of sex differences on cognitive outcomes in people with diabetes.8 24–30 Research on cognitive outcomes in older people with T1D have started only recently, partly due to the relatively recent ability of people with T1D to reach older ages. In those studies that have examined the associations between sex and cognition, specific sex difference data have not been presented.
Studies of sex differences in cognition in people with T2D have mostly focused on dementia as the outcome of interest. A 2016 meta-analysis of 2.3 million people reported that women with T2D had a greater risk of dementia than men with T2D.8 When examined by dementia subtype, women had a greater risk than men only for vascular dementia. The results from the Action for Health in Diabetes, a randomized controlled clinical trial of a 10-year intensive lifestyle intervention in people aged 45–76 years with T2D reported that women (n=2323) had a 30% lower prevalence of mild cognitive impairment (MCI; a precursor to dementia) and better cognitive performance at study completion than men (n=1479).31 The authors reported that these sex differences were not attributable to other risk factor profile characteristics, T2D treatment or glycemic control differences. Our observation that between-sex differences in cognition was similar in all three groups and that the better cognitive performance in women was independent of occurrence of DKA lends credence to the argument that differences in glycemic control or diabetes treatments do not seem to explain the between-sex cognitive differences we report. More detailed work is required to better understand the role of more nuanced measures of glycemic control in sex differences and cognitive outcomes, which are lacking in our study.
Previous work has reported that men tend to perform better than women in visual memory tasks.12 However, in our sample, we found that men and women had similar visual memory ability. The implications of this are unclear. Further work, including the use of sensitive neuroimaging biomarkers of function and structure would help explore the significance of this finding.
The biological or mechanistic factors underlying the generally better cognitive function in women than men remain unclear. Sex hormones are known to be neuroprotective in both sexes13 and the results of some studies suggest that women’s hormonal profile may explain some of the cognitive differences seen. Women with estrogen deficiency (induced either by surgical or hormone antagonism) show a reduction in verbal memory that is reversible with estrogen treatment or resumption of normal ovarian function.14 15 However, it is unknown whether, or how, hormonal changes might be related to function in a particular cognitive domain. Work performed in the Alzheimer’s Disease Neuroimaging Dataset suggests that these sex-cognitive domain differences are preserved even in the presence of disease, with women with amnestic MCI displaying better verbal memory than men with MCI.32 Furthermore, the authors reported that these differences remained apparent even though hippocampal volume (important in verbal memory) was similar. These results suggest that there may be important between-sex differences in brain structure that might explain difference in cognitive function. Although no consistent patterns of sex-related brain structural differences have been found,33–46 differences in number and density of neurons as well as brain size may all be important in understanding sex-related patterns in specific cognitive domains as well as sex differences in future dementia risk.47 48
We found that the associations between sex and cognitive function were independent of the known associations between diabetes and cognition. Similarly, the associations between diabetes and cognitive function did not vary by sex. Although our study suggests that the cognitive action of these two important risk factors are independent of each other, it is important to recognize the possibility that factors associated with sex and diabetes may still interact in ways we did not detect. The pathways through which both diabetes and female sex lead to increased risk of dementia remain unknown. Additional research is needed to examine the protective role of female hormones on vascular risk factors and the implications of menopause on this loss of protection and long-term brain health.
This study has a number of strengths including the large sample size of people with T1D, inclusion of well-matched T2D and without diabetes samples, and the use of detailed comprehensive cognitive tests allowing for investigation of the effects of sex on specific cognitive domains. This study also has some limitations. We lacked comprehensive detail of other factors of interest such as severity of comorbidities, biomarkers of glucose control and anthropometry such as body mass index. On visualization of the data, if any sex-diabetes class interactions are present, they appear to be very subtle and would require much larger sample sizes. Previous work in large meta-analyses has reported that in people with diabetes, women have a greater risk of stroke10 and coronary heart disease11 than men. We did not see this pattern in our cohort. It is likely that volunteer study participants are healthier than their non-volunteering counterparts. This is particularly the case when recruiting older people with T1D, who are likely to have had better glycemic control and fewer health complications throughout life than their counterparts who have since died, which may lead to survivor bias. We found a similar pattern within our sample, whereby those who completed all the cognitive tests appeared healthier with greater education and annual household income that those who did not complete all cognitive tests. It is possible that the associations we report would be different in those who had poorer glycemic control, greater burden of diabetes-related or non-diabetes-related comorbidities. The greater than expected age at diagnosis of those with T1D might be reflective of the biases of healthier people to enroll in studies or be the result of survivor-bias, whereby those with earlier age of diagnosis may not have survived to the age to be eligible to be included in our study. It is also possible that cognitive changes in our cohort were too subtle to detect with our neuropsychological tests. Future plans include obtaining objective measures of health from the medical record and following enrolled participants longitudinally for cognitive change. As we currently present cross-sectional analyses, it is important to understand whether these patterns continue over time or whether there are between-sex differences in cognitive trajectories.
In summary, women in mid-later life have better cognitive performance than men in many cognitive domains and this is independent of the presence of T1D or T2D. Further work is required to understand whether these differences change over time and their relationship to subsequent dementia.
Footnotes
Contributors: CM contributed to study design, data interpretation and wrote the manuscript. PG and MSB assisted with study design/data interpretation and reviewed/edited the manuscript. RAW obtained funding, assisted with study design/data interpretation and reviewed/edited the manuscript. MEL assisted with study design, conducted the analysis, assisted with data interpretation and reviewed/edited the manuscript.
Funding: This work was funded by the National Institute on Aging (AG047500 PI: RAW) and the Alzheimer’s Association/The Judy Fund (2019-AARGD-644788 PI: PG).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the Kaiser Permanente Northern California Institutional Review Board (Project Number: 1276423). All enrolled participants provided informed consent.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Alzheimer's Association 2019 Alzheimer’s disease facts and figures. Alzheimer's & Dementia 2019;15:321–87. 10.1016/j.jalz.2019.01.010 [DOI] [Google Scholar]
- 2.World Health Organisation Sex ratio. Available: http://www.searo.who.int/entity/health_situation_trends/data/chi/sex-ratio/en/2019
- 3.Nebel RA, Aggarwal NT, Barnes LL, et al. Understanding the impact of sex and gender in Alzheimer's disease: a call to action. Alzheimers Dement 2018;14:1171–83. 10.1016/j.jalz.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patterson CC, Dahlquist GG, Gyürüs E, et al. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet 2009;373:2027–33. 10.1016/S0140-6736(09)60568-7 [DOI] [PubMed] [Google Scholar]
- 5.Gale EA, Gillespie KM. Diabetes and gender. Diabetologia 2001;44:3–15. 10.1007/s001250051573 [DOI] [PubMed] [Google Scholar]
- 6.Smolina K, Wotton CJ, Goldacre MJ. Risk of dementia in patients hospitalised with type 1 and type 2 diabetes in England, 1998-2011: a retrospective national record linkage cohort study. Diabetologia 2015;58:942–50. 10.1007/s00125-015-3515-x [DOI] [PubMed] [Google Scholar]
- 7.Kuo C-L, Lu C-L, Chang Y-H, et al. Population-Based cohort study on dementia risk in patients with type 1 diabetes mellitus. Neuroepidemiology 2018;50:57–62. 10.1159/000486719 [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee S, Peters SAE, Woodward M, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 2016;39:300–7. 10.2337/dc15-1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev 2016;37:278–316. 10.1210/er.2015-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters SAE, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet 2014;383:1973–80. 10.1016/S0140-6736(14)60040-4 [DOI] [PubMed] [Google Scholar]
- 11.Peters SAE, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia 2014;57:1542–51. 10.1007/s00125-014-3260-6 [DOI] [PubMed] [Google Scholar]
- 12.Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychol Bull 1995;117:250–70. 10.1037/0033-2909.117.2.250 [DOI] [PubMed] [Google Scholar]
- 13.Pike CJ, Carroll JC, Rosario ER, et al. Protective actions of sex steroid hormones in Alzheimer's disease. Front Neuroendocrinol 2009;30:239–58. 10.1016/j.yfrne.2009.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henderson VW, Sherwin BB. Surgical versus natural menopause: cognitive issues. Menopause 2007;14:572–9. 10.1097/gme.0b013e31803df49c [DOI] [PubMed] [Google Scholar]
- 15.Craig MC, Fletcher PC, Daly EM, et al. Reversibility of the effects of acute ovarian hormone suppression on verbal memory and prefrontal function in pre-menopausal women. Psychoneuroendocrinology 2008;33:1426–31. 10.1016/j.psyneuen.2008.08.006 [DOI] [PubMed] [Google Scholar]
- 16.Lacy ME, Gilsanz P, Eng C, et al. Severe hypoglycemia and cognitive function in older adults with type 1 diabetes: the study of longevity in diabetes (solid). Diabetes Care 2020;43:541–8. 10.2337/dc19-0906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eng CW, Gilsanz P, Lacy ME, et al. Locus of control and cognition in older adults with type 1 diabetes: evidence for sex differences from the study of longevity in diabetes (solid). Alzheimer Dis Assoc Disord 2020;34:25–30. 10.1097/WAD.0000000000000352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilsanz P, Lacy ME, Beeri MS, et al. Sleep quality and cognitive function in type 1 diabetes: findings from the study of longevity in diabetes (solid). Alzheimer Dis Assoc Disord 2020;34:18–24. 10.1097/WAD.0000000000000351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arozullah AM, Yarnold PR, Bennett CL, et al. Development and validation of a short-form, rapid estimate of adult literacy in medicine. Med Care 2007;45:1026–33. 10.1097/MLR.0b013e3180616c1b [DOI] [PubMed] [Google Scholar]
- 20.Buysse DJ, Reynolds CF, Monk TH, et al. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh sleep quality index (PSQI). Sleep 1991;14:331–8. [PubMed] [Google Scholar]
- 21.McArdle JJ, Fisher GG, Kadlec KM. Latent variable analyses of age trends of cognition in the health and retirement study, 1992-2004. Psychol Aging 2007;22:525–45. 10.1037/0882-7974.22.3.525 [DOI] [PubMed] [Google Scholar]
- 22.Díaz-Venegas C, Downer B, Langa KM, et al. Racial and ethnic differences in cognitive function among older adults in the USA. Int J Geriatr Psychiatry 2016;31:1004–12. 10.1002/gps.4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Downer B, Fardo DW, Schmitt FA. A summary score for the Framingham heart study neuropsychological battery. J Aging Health 2015;27:1199–222. 10.1177/0898264315577590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brands AMA, Biessels GJ, de Haan EHF, et al. The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care 2005;28:726–35. 10.2337/diacare.28.3.726 [DOI] [PubMed] [Google Scholar]
- 25.Musen G, Tinsley LJ, Marcinkowski KA, et al. Cognitive function deficits associated with long-duration type 1 diabetes and vascular complications. Diabetes Care 2018;41:1749–56. 10.2337/dc17-1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Duinkerken E, Schoonheim MM, Sanz-Arigita EJ, et al. Resting-State brain networks in type 1 diabetic patients with and without microangiopathy and their relation to cognitive functions and disease variables. Diabetes 2012;61:1814–21. 10.2337/db11-1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Artero S, Ancelin M-L, Portet F, et al. Risk profiles for mild cognitive impairment and progression to dementia are gender specific. J Neurol Neurosurg Psychiatry 2008;79:979–84. 10.1136/jnnp.2007.136903 [DOI] [PubMed] [Google Scholar]
- 28.Kim Y-H, Kim NH, Jung M-H, et al. Sex differences in metabolic risk indicator of dementia in an elderly urban Korean population: a community-based cross-sectional study. Geriatr Gerontol Int 2017;17:2136–42. 10.1111/ggi.13049 [DOI] [PubMed] [Google Scholar]
- 29.Sherzai D, Sherzai A, Lui K, et al. The association between diabetes and dementia among elderly individuals: a nationwide inpatient sample analysis. J Geriatr Psychiatry Neurol 2016;29:120–5. 10.1177/0891988715627016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang K-C, Woung L-C, Tsai M-T, et al. Risk of Alzheimer's disease in relation to diabetes: a population-based cohort study. Neuroepidemiology 2012;38:237–44. 10.1159/000337428 [DOI] [PubMed] [Google Scholar]
- 31.Espeland MA, Carmichael O, Yasar S, et al. Sex-Related differences in the prevalence of cognitive impairment among overweight and obese adults with type 2 diabetes. Alzheimers Dement 2018;14:1184–92. 10.1016/j.jalz.2018.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sundermann EE, Biegon A, Rubin LH, et al. Better verbal memory in women than men in MCI despite similar levels of hippocampal atrophy. Neurology 2016;86:1368–76. 10.1212/WNL.0000000000002570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ritchie SJ, Cox SR, Shen X, et al. Sex differences in the adult human brain: evidence from 5216 UK Biobank participants. Cereb Cortex 2018;28:2959–75. 10.1093/cercor/bhy109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001;14:21–36. 10.1006/nimg.2001.0786 [DOI] [PubMed] [Google Scholar]
- 35.Riello R, Sabattoli F, Beltramello A, et al. Brain volumes in healthy adults aged 40 years and over: a voxel-based morphometry study. Aging Clin Exp Res 2005;17:329–36. 10.1007/BF03324618 [DOI] [PubMed] [Google Scholar]
- 36.Sullivan EV, Rosenbloom M, Serventi KL, et al. Effects of age and sex on volumes of the thalamus, pons, and cortex. Neurobiol Aging 2004;25:185–92. 10.1016/S0197-4580(03)00044-7 [DOI] [PubMed] [Google Scholar]
- 37.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham heart study: establishing what is normal. Neurobiol Aging 2005;26:491–510. 10.1016/j.neurobiolaging.2004.05.004 [DOI] [PubMed] [Google Scholar]
- 38.Chen X, Sachdev PS, Wen W, et al. Sex differences in regional gray matter in healthy individuals aged 44-48 years: a voxel-based morphometric study. Neuroimage 2007;36:691–9. 10.1016/j.neuroimage.2007.03.063 [DOI] [PubMed] [Google Scholar]
- 39.Greenberg DL, Messer DF, Payne ME, et al. Aging, gender, and the elderly adult brain: an examination of analytical strategies. Neurobiol Aging 2008;29:290–302. 10.1016/j.neurobiolaging.2006.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Resnick SM, Goldszal AF, Davatzikos C, et al. One-Year age changes in MRI brain volumes in older adults. Cereb Cortex 2000;10:464–72. 10.1093/cercor/10.5.464 [DOI] [PubMed] [Google Scholar]
- 41.Lemaître H, Crivello F, Grassiot B, et al. Age- and sex-related effects on the neuroanatomy of healthy elderly. Neuroimage 2005;26:900–11. 10.1016/j.neuroimage.2005.02.042 [DOI] [PubMed] [Google Scholar]
- 42.Murphy DG, DeCarli C, McIntosh AR, et al. Sex differences in human brain morphometry and metabolism: an in vivo quantitative magnetic resonance imaging and positron emission tomography study on the effect of aging. Arch Gen Psychiatry 1996;53:585–94. 10.1001/archpsyc.1996.01830070031007 [DOI] [PubMed] [Google Scholar]
- 43.Xu J, Kobayashi S, Yamaguchi S, et al. Gender effects on age-related changes in brain structure. AJNR Am J Neuroradiol 2000;21:112–8. [PMC free article] [PubMed] [Google Scholar]
- 44.Cowell PE, Turetsky BI, Gur RC, et al. Sex differences in aging of the human frontal and temporal lobes. J Neurosci 1994;14:4748–55. 10.1523/JNEUROSCI.14-08-04748.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Velsen EFS, Vernooij MW, Vrooman HA, et al. Brain cortical thickness in the general elderly population: the Rotterdam scan study. Neurosci Lett 2013;550:189–94. 10.1016/j.neulet.2013.06.063 [DOI] [PubMed] [Google Scholar]
- 46.Gur RE, Gur RC. Gender differences in aging: cognition, emotions, and neuroimaging studies. Dialogues Clin Neurosci 2002;4:197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perneczky R, Drzezga A, Diehl-Schmid J, et al. Gender differences in brain reserve : an (18)F-FDG PET study in Alzheimer's disease. J Neurol 2007;254:1395–400. 10.1007/s00415-007-0558-z [DOI] [PubMed] [Google Scholar]
- 48.Mosconi L, Berti V, Quinn C, et al. Sex differences in Alzheimer risk: brain imaging of endocrine vs chronologic aging. Neurology 2017;89:1382–90. 10.1212/WNL.0000000000004425 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2020-001646supp001.pdf (220.9KB, pdf)