Abstract
A 59-year-old woman was found unresponsive at home. Initial neurologic examination revealed aphasia and right-sided weakness. Laboratory results demonstrated a serum calcium level of 17.3 mg/dL (corrected serum calcium for albumin concentration was 16.8 mg/dL). Extensive workup for intrinsic aetiology of hypercalcemia was unrevealing. Further discussion with family members and investigation of the patient’s home for over-the-counter medications and herbal supplements revealed chronic ingestion of calcium carbonate tablets. CT angiogram of the brain revealed multifocal intracranial vascular segmental narrowing, which resolved on a follow-up cerebral angiogram done 2 days later. These findings were consistent with reversible cerebral vasoconstriction syndrome.
Appropriate blood pressure control with parenteral agents, calcium channel blockade with nimodipine and supportive care therapies resulted in significant improvement in neurologic status. By discharge, patient had near-complete resolution of neurologic symptoms.
Keywords: drugs and medicines, adult intensive care, drugs: CNS (not psychiatric), neurological injury, stroke
Background
The pathophysiology of the neurologic manifestations of hypercalcemia remains poorly understood. Severe cerebral vasoconstriction is a rare, but important complication.1 This report describes the presentation, workup and management of a woman with acute neurologic injury resulting from excess use of calcium supplements.
Reversible cerebral vasoconstriction syndrome (RCVS) represents a condition of reversible cerebral vascular injury, considered to be along the spectrum of migraine and posterior reversible encephalopathy syndrome (PRES), with cerebral infarction being an important complication. The incidence of RCVS is unknown, but diagnosis is occurring with increasing frequency for unclear reasons. Improved use of non-invasive vascular imaging modalities like CT angiography (CTA) has been postulated as a cause in the literature.1 The characteristic angiographic findings of RCVS are segmental narrowing and dilatation of the cerebral vasculature, with a ‘string of beads’ appearance of one or more arteries.2
We report a case of hypercalcemia-induced RCVS due to excessive use of over-the-counter calcium-containing supplements.
Case presentation
A 59-year-old woman presented to the emergency department via emergency medical services after being found unresponsive in her home by a family friend. Her last known normal was 1 day prior to presentation. Medical history was notable for hypothyroidism, essential hypertension and dyspepsia. Four months prior, she was hospitalised at another institution for left tibia and fibula fractures sustained in a fall from standing height. At that time, she was also hypercalcemic, with rapid correction to normal calcium levels solely with intravenous fluids. No specific aetiology was found for the hypercalcemia during that admission.
On arrival to our emergency department, she was noted to have a Glasgow Coma Scale score of 6. No verbal response or eye opening was noted to verbal and tactile stimuli. Withdrawal to pain was noted only in the left upper extremity, while she appeared to have right hemiplegia. Cranial nerve examination showed right gaze preference with intermittent, spontaneous, conjugate roving eye movements. The remainder of her physical examination was unremarkable. She was intubated for airway protection thereafter. She was also severely hypertensive on presentation with systolic blood pressures ranging from 200 to 230 mm Hg and diastolic blood pressures ranging from 105 mm Hg to 125 mm Hg.
Investigations
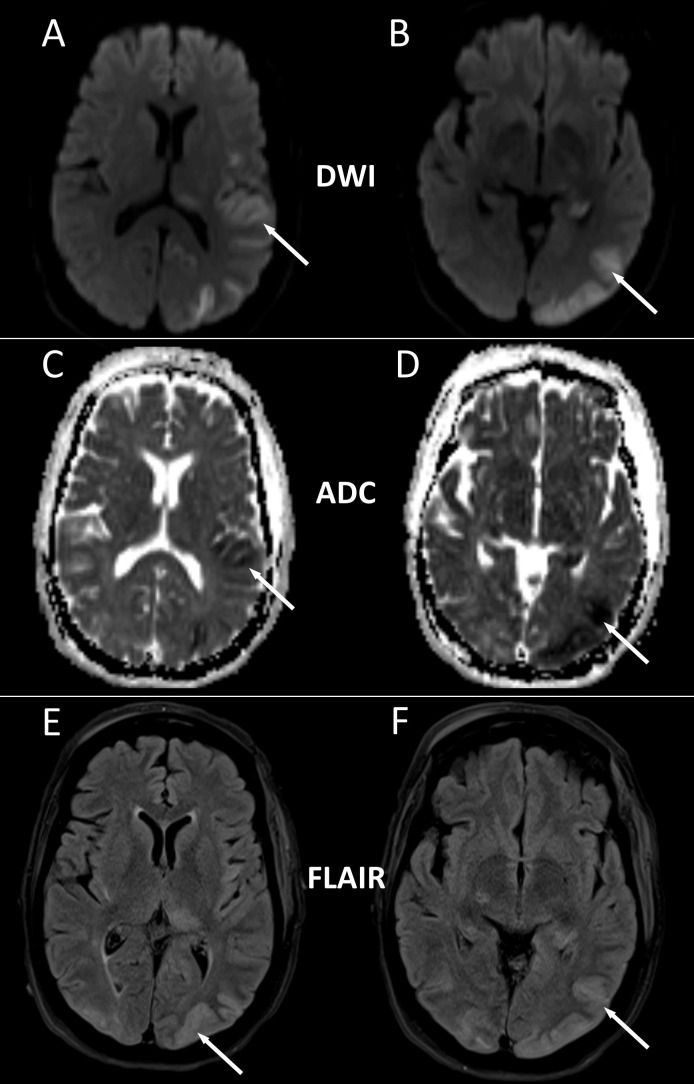
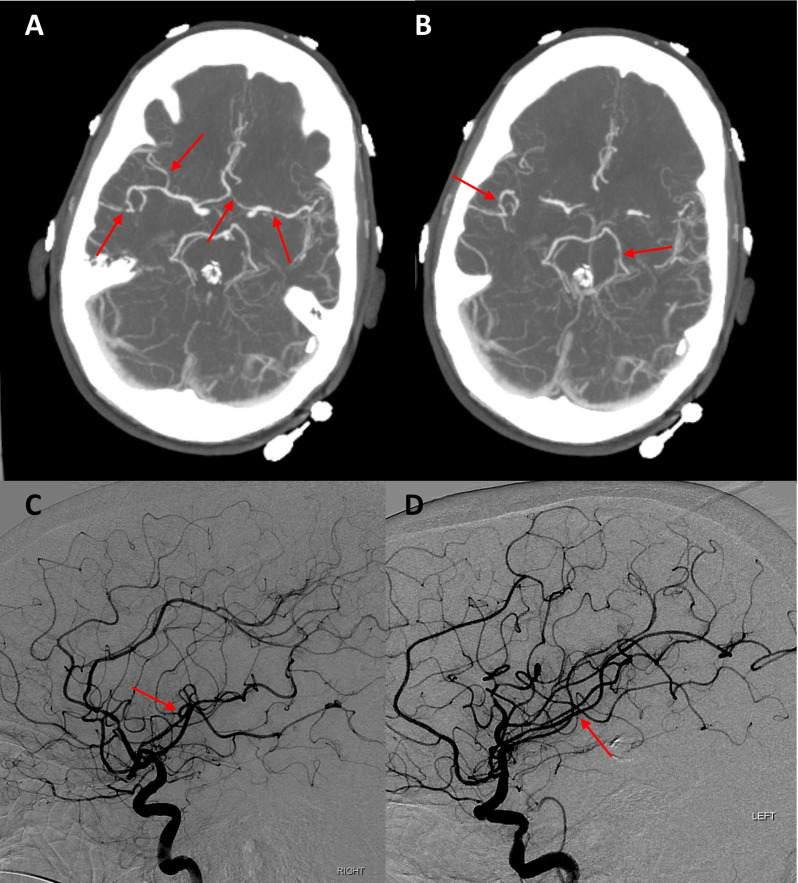
Table 1 shows the results of her blood and urine tests. Initial CT scan of the head without contrast showed no acute intracranial abnormality. Chest radiography showed no evidence for cardiopulmonary disease. Lumbar puncture showed mildly elevated protein (72 mg/dL) with normal white cell count (2 cells/mm3), unremarkable glucose and red cell count. Cerebrospinal fluid cultures and viral studies were negative. CT scan of the chest, abdomen and pelvis with contrast was obtained and did not show any evidence of malignancy. Continuous electroencephalography (EEG) revealed no evidence for subclinical seizures or epileptogenic activity. Subsequently, MRI of the brain was obtained (figure 1), with diffusion-weighted images and apparent diffusion coefficient maps that showed acute infarction of the left parieto-occipital cortex, left posterior thalamus, hippocampus, anterior temporal cortex and insula without any vasogenic oedema on T2 Fluid-attenuated inversion recovery (FLAIR). CTA of the head and neck demonstrated multifocal intracranial vascular narrowing (figure 2). Cerebral angiography was intentionally delayed by 2 days, looking for reversibility of findings on CTA expected in parallel with observed clinical improvement. Cerebral angiography showed largely normal appearing intracerebral vessels (figure 2), with almost complete resolution of the focal narrowing of vessels seen on the CTA.
Table 1.
Laboratory data
| Laboratory test | Admission | Discharge | Reference range |
| Complete blood count with differential | |||
| White cell count (×103/µL) | 32.1 | 8.5 | 4.8–10.8 |
| Haemoglobin (g/dL) | 178 | 88 | 120-160 |
| Haematocrit (%) | 52.9 | 26.4 | 37.0–47.0 |
| Platelets(x109/L) | 406 | 384 | 160–360 |
| Comprehensive metabolic panel | |||
| Blood urea nitrogen (mg/dL) | 52 | 19 | 8–24 |
| Creatinine (mg/dL) | 2.35 | 0.92 | 0.50–1.50 |
| Calcium (mg/dL) | 17.3 | 9.7 | 8.5–10.5 |
| Additional blood tests | |||
| Intact parathyroid hormone (pg/mL) | 7 | 12–72 | |
| Parathyroid hormone-related peptide (pmol/L) | 0.5 | <4.2 | |
| 25-Hydroxy vitamin D (ng/mL) | 32 | >30 | |
| 1–25 Dihydroxy vitamin D (ng/mL) | 8.7 | 19.9–79.3 | |
| Vitamin A (μg/dL) | 39 | 32.5–78.0 | |
| M spike | None | None | |
| Urine studies | |||
| Protein/creatinine (mg/g) | 2826.09 | 0.00–200.00 | |
| Calcium (mg/dL) | 7.9 | ||
| Bence Jones protein | Negative | ||
| Free kappa (mg/L) | 17.59 | 3.30–19.40 | |
| Free lambda (mg/L) | 16.81 | 5.71–26.30 | |
| Kappa/lambda ratio | 1.05 | 0.26–1.65 | |
Figure 1.
MRI brain with hyperintensity (white arrows) seen on diffusion-weighted image (DWI) (A and B), with apparent diffusion coefficient (ADC) maps (C and D) showing corresponding hypointensity (white arrows), indicating acute infarctions of the left occipital cortex, posterior thalamus, hippocampus, anterior temporal cortex, insula and posterior parietal cortex. Corresponding fluid-attenuated inversion recovery (FLAIR) image (E and F) showing expected T2 signal hyperintensity (white arrows) in areas of acute infarction, but absence of vasogenic oedema in white matter regions.
Figure 2.
Multifocal irregularities and narrowing of bilateral intracranial arteries (red arrows) (A and B), as seen on CT angiography (CTA) at the base of the brain. Cerebral angiography images acquired 48 hours after CTA showing resolution of the focal narrowing of the same vessels (red arrows) (C and D).
Differential diagnosis
There was a broad differential diagnosis at admission considering her presentation with altered mental status, focal neurologic deficit and hypercalcemia. Status epilepticus, ischaemic stroke, PRES, malignancy (intracranial or extracranial), cranial and/or extracranial infection with toxic–metabolic encephalopathy and paraneoplastic syndrome were all considered and evaluated for based on laboratory, imaging and procedural data. She was started on empiric broad-spectrum antibiotics until lumbar puncture excluded central nervous system (CNS) infection. Primary CNS vasculitis was also an important consideration given the presentation and was excluded based on lumbar puncture findings and cerebral angiography. Brain MRI did not reveal any evidence of PRES. Continuous EEG monitoring ruled out status epilepticus or subclinical seizures.
Workup for severe hypercalcemia included evaluation for hypervitaminosis D, hyperparathyroidism, multiple myeloma and sarcoidosis. Negative evaluation for alternative aetiology and rapid correction in serum calcium levels with crystalloid solution led to the conclusion that her hypercalcemia was related to accidental calcium overdose. The patient’s son brought in her medications and supplements from home, and included was a nearly empty, large bottle of calcium carbonate that had been purchased shortly prior to presentation. It was reported that the patient was taking up to 10 calcium carbonate tablets several times per day prior to admission.
Treatment
Along with general intensive care and ventilator management, the patient was initially treated with broad-spectrum, parenteral antibiotics as well as intravenous clevidipine to control the blood pressure with gradual normalisation over several days following admission. Permissive hypertension with systolic pressure up to 180 mm Hg was allowed for effective cerebral perfusion. Serum calcium corrected rapidly to normal levels with intravenous crystalloid solution alone; the patient did not need loop diuretics, calcitonin or bisphosphonate therapy, nor did she need renal replacement therapy. Following negative cultures, antibiotics were discontinued after 5 days of empiric therapy, and the patient was started on secondary prevention with aspirin and statin. The patient was started on a 14-day course of nimodipine for the treatment of cerebral vasoconstriction. Following extubation, physical, occupational and speech therapies were engaged.
Outcome and follow-up
Progressively her mental status improved and patient started following commands. She continued to have expressive aphasia along with right-sided weakness. Over the course of hospitalisation, however, her neurologic deficits improved with near-complete resolution by the time of her discharge. Patient did not require inpatient rehabilitation at hospital discharge and went directly home.
Discussion
Few case reports have described PRES or cerebral infarction associated with hypercalcemia, specifically secondary to over-the-counter supplements and medications. Most cases of hypercalcemia-induced PRES or cerebral infarction have been described in the setting of malignancy, hypervitaminosis D or hyperparathyroidism.3 4 We found two published cases of hypercalcemia-induced PRES secondary to overuse of calcium supplementation, both presenting with seizure.5 6 We describe the first case of cerebral infarction and RCVS induced by excessive use of calcium supplements.
Although the pathophysiology remains poorly understood, it has been hypothesised that PRES and RCVS represent a continuum related to cerebral vascular dysregulation and dysfunction, given the overlap of many clinicoradiographic features.7–9 Animal models have demonstrated that calcium acts to initiate actin–myosin binding within cerebrovascular smooth muscle cells to promote contractility and cause endothelial cell inflammation and injury.10 Rats with diet-induced hypercalcemia show a transformation of their endothelial cells to a predominantly proinflammatory phenotype.11
Calabrese et al proposed the following diagnostic criteria for RCVS: (1) multifocal segmental cerebral artery vasoconstriction on direct or indirect (CT or MR) angiography, (2) no evidence for aneurysmal subarachnoid haemorrhage, (3) normal or near normal cerebrospinal fluid analysis, (4) severe, acute headaches, with or without additional neurologic signs or symptoms and (5) reversibility of angiographic abnormalities within 12 weeks of onset.12 Although rare, focal neurologic deficits, which can be transient or persistent (8%–43%), and seizures (1%–17%) have been reported in RCVS.4 12–14 When cerebral infarction does occur, the infarcts are mainly in arterial watershed regions of the cerebral hemispheres. Conditions associated with RCVS include pregnancy and puerperium, exposure to drugs such as triptans or selective serotonin reuptake inhibitors among others, hypercalcemia, cannabis use and other rare disorders like porphyria.1 2 15 16 To diagnose RCVS, direct or indirect cerebral angiography is needed to show segmental narrowing and dilatation, with a ‘string of beads’ appearance of one or more arteries.2
This case demonstrates that hypercalcemia induced by excess use of over-the-counter supplements alone, without additional proinflammatory states such as malignancy and hyperparathyroidism can lead to RCVS. Even with rapid correction of hypercalcemia, cerebral vasoconstriction can result in both acute and chronic neurologic insults. A thorough workup for alternative causes should be undertaken specially to rule out underlying malignancy. Early diagnosis of RCVS is crucial as that helps direct further treatment plan and potentially limit secondary ischaemic injury. This case also highlights the importance of reviewing all over-the-counter supplements during admission, something that can be easily overlooked.
Learning points.
Ensure that a thorough medication reconciliation performed on hospital admission includes over-the-counter medications and herbal supplements.
Hypercalcemia can induce a variety of neurologic sequelae, including cerebral vasoconstriction. The serum calcium level at which cerebral vasoconstriction is seen is unknown, but is most commonly seen with an initial serum calcium greater than 13 mg/dL.
Posterior reversible encephalopathy syndrome and reversible cerebral vasoconstriction syndrome represent a spectrum of pathophysiology, related to vascular dysregulation in the central nervous system and endothelial injury promoting a proinflammatory milieu.
Footnotes
Contributors: ARS took the lead in writing the manuscript. All authors provided critical feedback and helped shape the analysis and manuscript. AKS and SD provided expertise in neurology and neurocritical care, reviewed and interpreted imaging findings and assisted in the editing process. PJM was heavily involved in the planning and design of the manuscript; he also provided expertise in critical care and assisted with the editing process.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Next of kin consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Singhal AB, Topcuoglu MA, Fok JW, et al. Reversible cerebral vasoconstriction syndromes and primary angiitis of the central nervous system: clinical, imaging, and angiographic comparison. Ann Neurol 2016;79:882–94. 10.1002/ana.24652 [DOI] [PubMed] [Google Scholar]
- 2.Ducros A. Reversible cerebral vasoconstriction syndrome. Lancet Neurol 2012;11:906–17. 10.1016/S1474-4422(12)70135-7 [DOI] [PubMed] [Google Scholar]
- 3.Kim JH, Kim MJ, Kang JK, et al. Vasogenic edema in a case of hypercalcemia-induced posterior reversible encephalopathy. Eur Neurol 2005;53:160–2. 10.1159/000086127 [DOI] [PubMed] [Google Scholar]
- 4.Chen T-H, Huang C-C, Chang Y-Y, et al. Vasoconstriction as the etiology of hypercalcemia-induced seizures. Epilepsia 2004;45:551–4. 10.1111/j.0013-9580.2004.57003.x [DOI] [PubMed] [Google Scholar]
- 5.Chan TLH, Mayich M, Budhram A, et al. Teaching images in headache: concurrent Hypercalcemia-Induced reversible cerebral vasoconstriction syndrome and posterior reversible encephalopathy syndrome. Headache 2019;59:933–5. 10.1111/head.13528 [DOI] [PubMed] [Google Scholar]
- 6.Kastrup O, Maschke M, Wanke I, et al. Posterior reversible encephalopathy syndrome due to severe hypercalcemia. J Neurol 2002;249:1563–6. 10.1007/s00415-002-0895-x [DOI] [PubMed] [Google Scholar]
- 7.Walker GL, Williamson PM, Ravich RB, et al. Hypercalcaemia associated with cerebral vasospasm causing infarction. J Neurol Neurosurg Psychiatry 1980;43:464–7. 10.1136/jnnp.43.5.464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camara-Lemarroy CR, Gonzalez-Moreno EI, Ortiz-Corona JdeJ, et al. Posterior reversible encephalopathy syndrome due to malignant hypercalcemia: physiopathological considerations. J Clin Endocrinol Metab 2014;99:1112–6. 10.1210/jc.2013-3487 [DOI] [PubMed] [Google Scholar]
- 9.Singhal AB, Hajj-Ali RA, Topcuoglu MA, et al. Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch Neurol 2011;68:1005–12. 10.1001/archneurol.2011.68 [DOI] [PubMed] [Google Scholar]
- 10.Feske SK. Posterior reversible encephalopathy syndrome: a review. Semin Neurol 2011;31:202–15. 10.1055/s-0031-1277990 [DOI] [PubMed] [Google Scholar]
- 11.Režić-Mužinić N, Cikeš-Čulić V, Božić J, et al. Hypercalcemia induces a proinflammatory phenotype in rat leukocytes and endothelial cells. J Physiol Biochem 2013;69:199–205. 10.1007/s13105-012-0202-y [DOI] [PubMed] [Google Scholar]
- 12.Calabrese LH, Dodick DW, Schwedt TJ, et al. Narrative review: reversible cerebral vasoconstriction syndromes. Ann Intern Med 2007;146:34–44. 10.7326/0003-4819-146-1-200701020-00007 [DOI] [PubMed] [Google Scholar]
- 13.Marra A, Vargas M, Striano P, et al. Posterior reversible encephalopathy syndrome: the endothelial hypotheses. Med Hypotheses 2014;82:619–22. 10.1016/j.mehy.2014.02.022 [DOI] [PubMed] [Google Scholar]
- 14.Longo DL, Witherspoon JM. Focal neurologic symptoms in hypercalcemia. Neurology 1980;30:200–1. 10.1212/WNL.30.2.200 [DOI] [PubMed] [Google Scholar]
- 15.Jensen J, Leonard J, Salottolo K, et al. The epidemiology of reversible cerebral vasoconstriction syndrome in patients at a Colorado comprehensive stroke center. J Vasc Interv Neurol 2018;10:32–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Uhegwu N, Bashir A, Hussain M, et al. Marijuana induced reversible cerebral vasoconstriction syndrome. J Vasc Interv Neurol 2015;8:36–8. [PMC free article] [PubMed] [Google Scholar]