Abstract
Glucose homeostasis is maintained through the secretion of peptide hormones, such as insulin, somatostatin, and glucagon, from islets of Langerhans, clusters of endocrine cells found in the pancreas. This report describes an LC-MS method using multiple reaction monitoring for quantitation of insulin, C-peptide, glucagon, and somatostatin secretion from human islet populations. For rapid analysis, a 5 min separation was achieved using a 2.1 × 30 mm (i.d. × length) C18 column with 2.7 μm diameter core shell particles. A sacrificial protein hydrolysate was used with the sample and found to improve signal magnitude, repeatability, and to reduce carryover between runs. At optimized gradient conditions, the gradient run time was 4.55 min producing an average peak width of 0.3 min, a minimum resolution of 1.2, and a peak capacity of 20. As a proof of concept, the method was used to measure secretions from static incubations of human islets from 2 donors. Insulin and C-peptide were quantified and matched well with literature values of these hormones. We expect that this antibody-free quantitation of multiple hormones secreted from islets will provide insights into the temporal relationships of these peptides in the future.
Keywords: Core shell, superficially porous, UHPLC, diabetes
1. Introduction:
Islets of Langerhans are the endocrine unit in the pancreas which release peptide hormones critical for regulating proper blood glucose homeostasis. The major peptide hormones released from islets include insulin, glucagon, and somatostatin, with other peptides such as ghrelin, C-peptide, pancreatic polypeptide, and islet amyloid polypeptide, also being secreted [1]. The mechanisms that control the timing and amount of hormones released is still uncertain for many of these, but are known to involve the blood glucose level, small molecules, and paracrine interactions from the other peptides [2], [3], [4]. It is clear that the secretion patterns are markedly different between healthy and diseased states, such as type 2 diabetes [5], [6]. Developing analytical methods that can resolve multiple peptide hormones released from islets simultaneously, and in a temporally-resolved manner, is essential in understanding the control mechanisms of secretion, and how they may go awry.
In the quantitation of islet hormone secretions, antibody-based methods are most commonly employed. The “gold-standard” analytical techniques for measurement of these hormones are radioimmunoassays (RIA) and enzyme-linked immunosorbent assay (ELISA), both of which leverage antibody binding to achieve highly selective and sensitive detection. However, these assays are time intensive, and rely on separate kits for each peptide hormone analyzed. Some multiplexed immunoassays have been described [7], [8], [9], [10], but they are only suitable for 2–4 analytes. It would be ideal to quantify multiple hormones simultaneously in a rapid manner to increase measurement throughput.
For targeted quantitation of small molecules, peptides, and proteins in complex samples, LC-MS/MS methods using a triple quadrupole mass analyzer operating in multiple reaction monitoring (MRM) mode are increasingly being used [11], [12], [13], [14]. These assays have been shown to be capable of sensitive detection of many unique species and have compared favorably in detection sensitivity and selectivity to the antibody binding techniques [15], [16]. To aid in quantitation, internal standards (IS) composed of isotopic analogues of the analytes are added to correct for variations in ionization efficiencies in ESI [17]. MRM assays have been developed for glucose-regulating hormones from complex samples. For example, insulin was successfully quantified in human serum with a limit of quantitation (LOQ) of 18 pM. This method was also compared with a US Food and Drug Administration standard immunochemiluminometric evaluation for insulin levels in plasma and showed strong agreement across the range of concentrations tested [15]. A similar method for glucagon was able to achieve a lower limit of quantitation (LLOQ) of 12.5 pg mL−1 which enabled detection of endogenous levels of glucagon and showed good agreement with a standard glucagon RIA [16]. While both of these methods produced impressively low LOQ, they were not aimed at analysis of multiple hormones or rapid analysis.
To decrease analysis time in MRM assays, a rapid separation should be performed. This would increase experimental throughput and also may allow the method to be used in an online manner with the biological cells to obtain temporally resolved hormone profiles. As an example, online analysis of a cellular bioreactor with LC-MS detection was used with multiple SPE columns and produced a sampling frequency of ~9 min [18]. Another example was the use of an SPE column in-line with a microfluidic system that enabled detection of non-esterified fatty acid secretion from 3T3-L1 cells [19]. Both of these systems allowed online analysis of cellular release with LC-MS detection, but neither performed gradient elution LC which could improve limits of detection. Other methods to decrease separation time and potentially be used for online analysis are capillary LC columns, or short columns that enable high efficiency separations, such as those provided by core-shell stationary phase particles [20].
Here, an LC-MS method is presented that was developed with the goal of rapid and simultaneous quantitation of four common glucose-regulating hormones secreted from islets, somatostatin, C-peptide, insulin, and glucagon. Separation of the peptide hormones was performed within 5 min using a 2.1 × 30 mm C18 column with 2.7 μm diameter fused core particles. To improve analyte and IS` response, while also enhancing the reproducibility over multiple injections, the sample solvent was optimized with a sacrificial protein hydrolysate. Using the method, glucose-regulating hormones released from a collection of human islets was quantified after incubation in low and high glucose solutions.
2. Experimental:
2.1. Chemicals and Reagents
LC grade water (H2O) and LC grade acetonitrile (ACN) were purchased from VWR (Radnor, PA). All peptides were specific to human unless otherwise stated. Somatostatin-14, ([ring-D5] Phe6) somatostatin-14 (somatostatin IS), C-peptide trifluoroacetate salt, and ([13C6]Leu14)-glucagon (1–29) trifluoroacetate salt (glucagon IS) were purchased from BaChem (Torrance, CA). Bovine serum albumin (BSA) was purchased from EMD Chemicals (San Diego, CA). SILu™Pep C-peptide (([13C6]Leu26+30)-C-peptide) (C-peptide IS), glucagon, human insulin, SILu™Prot insulin, human (([15N7] (all amino acids excluding Thr30 in β chain)) labeled insulin) (insulin IS), and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise specified. A balanced salt solution (BSS) was used in certain experiments and was composed of 25 mM tricine, 125 mM NaCl, 5.9 mM KCl, 1.2 mM MgCl2, and 2.4 mM CaCl2 adjusted to a pH of 7.4 and contained different amounts of glucose as described in the text. All vials used in both method development and generation of calibration curves were low adsorption glass autosampler vials (Sigma-Aldrich).
2.2. Preparation and Analysis of Calibration Standards
Somatostatin, C-peptide, glucagon, insulin, and all IS were solubilized according to vendor protocols to a concentration of 1 mg mL−1. The solvents used were: H2O for somatostatin, somatostatin IS, and glucagon IS, 8.7 M acetic acid for C-peptide and C-peptide IS, 0.05 M acetic acid for glucagon, 0.01 M HCl for insulin, and 0.35 M acetic acid for insulin IS. These stock solutions were stored at −20 °C for long term storage or 4 °C for short term storage.
From this point forward, all water and ACN contained 0.1% formic acid (FA) unless otherwise stated. Hydrolyzed BSA (Hyd-BSA) was made according to a previously published protocol[21]. Briefly, intact BSA was dissolved in H2O at a concentration of 10 mg mL−1. This solution was further diluted by the addition of ACN to a total volume of 7.5 mL. Aliquots of this solution were heated for 5 min at 95 °C before cooling on ice for 30 min. The aliquots were then pooled and centrifuged for 30 min at 15,000 g. The supernatant (6 mL) was collected, and 3 mL of H2O were added. A 1 mL aliquot from this solution was removed and diluted to 200 mL with 50:50 (v:v) ACN:H2O. Of this solution, 120 mL were removed and combined with 80 mL of H2O. This solution, which had a final solvent composition of 30:70 ACN:H2O is referred to as Hyd-BSA throughout the remainder of the text.
One set of calibration curves was made to compare Hyd-BSA and 30:70 ACN:H2O as the sample solvent. All vials used for the Hyd-BSA calibration curves, were incubated for 2 h in Hyd-BSA prior to addition of any reagents. Analyte and IS stock solutions were diluted to 1 μM (with the exception of insulin IS because the stock concentration of 365 nM was sufficient) using either Hyd-BSA or 30:70 ACN:H2O. Necessary volumes of analyte (0, 1.5, 3, and 6 μL) and constant IS volumes (3 μL for all except insulin, 8.2 μL) were combined and diluted with either Hyd-BSA or 30:70 ACN:H2O to 100 μL to make calibration standards for both curves. Final concentrations were 0, 15, 30, and 60 nM for the analytes and 30 nM for all IS.
A second calibration curve was made to examine the instrument response at lower analyte concentrations and with BSS to mimic the islet experiments. Analyte and IS stock solutions were diluted to 2 μM and 200 nM, respectively (with the exception of insulin IS which was diluted to 50 nM), with Hyd-BSA. BSS containing 1 mM glucose was used to further dilute analyte solutions to concentrations of 10, 20, 100, 200, and 400 nM. 5 μL of these solutions were further diluted to 100 μL via the introduction of 35 μL of IS and 60 μL of Hyd-BSA. Final concentrations of the analytes were 0.5, 1, 5, 10, and 20 nM and IS concentration was 10 nM.
2.3. Preparation and Analysis of Samples Collected from Human Islets
Human islet equivalents (IEQ), proprietary islet media standard (PIM(S)™), proprietary islet media human AB serum (PIM(ABS)™), and proprietary islet media glutamine/glutathione mixture (PIM(G)™) were purchased from Prodo Laboratories (Aliso Viejo, CA). Human islet samples were from deidentified cadaveric organ donors and therefore exempt from Institutional Review Board approval. Islets from two donors were used in two experiments and donor characteristics are provided in the Appendix (Table A-1). Experiment 1 was performed using islets from donor 1 and was prior to the incorporation of somatostatin into the method. Experiment 2 was performed with islets from donor 2 and incorporated all four peptides.
Human islets were recovered according to the protocol established by Prodo Laboratories. Briefly, four batches of 250 islet equivalents (IEQ) were received in separate 50 mL vials. IEQ is a term used to normalize the size variability of islets. One IEQ is equal to an islet of 150 μm in diameter; islets with smaller diameters are therefore fractions of an IEQ and islets with larger diameters are larger than 1 IEQ. To the best of our ability, all 250 IEQ were removed from the vial and allowed to recover overnight in PIM(S) media at 37 °C and 5% CO2. The following day, 250 IEQ were washed and placed into 50 μL of BSS with 1 mM glucose in a microcentrifuge tube.
In experiment 1, the islets were incubated at 37 °C and 5% CO2 for 75 min. One 5 μL aliquot was then removed from the microcentrifuge tube for analysis of hormone secretions. A 5 μL spike of BSS with 151 mM glucose was added to the tube to bring the final concentration of glucose to 16 mM and placed back in the incubator for another 75 min. An additional 5 μL aliquot was removed after the incubation period was complete.
For experiment 2, the islet incubation period was extended from 75 min to 2 h and three 5 μL aliquots were removed after incubation with 1 mM glucose. The concentration of glucose was then adjusted to 16 mM by the addition of a 15 μL spike of BSS with 51 mM glucose before being placed back into the incubator for another 2h. Three additional 5 μL aliquots were collected after this incubation period.
To normalize the amount of peptide hormones secreted in both experiments, the islets were lysed, and their remaining hormone content measured. Lysis was performed as described previously [22]. Briefly, all BSS except the last 10 μL that contained islets was removed from the tube and 90 μL of cell lysing solution (75% ethanol, 23.5% H2O and 1.5% HCl) was added. This solution with the islets was sonicated for 10 min. After sonication, 70 and 90 μL of lysate from experiment 1 and 2, respectively, were transferred to a low adsorption vial and dried with nitrogen. The dried solute was reconstituted with Hyd-BSA (280 μL for experiment 1 or 360 μL for experiment 2) to make lysate stock solution. This stock solution was diluted to three different ratios with Hyd-BSA: 1:1.4, 1:10, and 1:50 for experiment 1 and1:1.5, 1:7.7, and 1:63 for experiment 2. This was done so the response of all 3 analytes examined in experiment 1 and the 4 analytes examined in experiment 2 would fit within the calibration range in at least one of the dilutions. The final sample volumes for these lysates were maintained at 100 μL although 5 μL of somatostatin IS were included in experiment 2 which made the total IS volume 30 μL for experiment 1 and 35 μL for experiment 2.
Both aliquots of islet secretions from experiment 1 (1 at 1 mM glucose, 1 at 16 mM glucose) and the six aliquots from experiment 2 (3 at 1 mM glucose, 3 at 16 mM glucose) were diluted to a final volume of 100 μL with IS and Hyd-BSA as described in Section 2.2 (somatostatin IS not included in experiment 1). Daily calibration curves for quantitation of islet secretion and lysate samples were made using the low (0.5 nM) and high (20 nM) calibrants.
2.4. LC-MRM experiments
LC-MRM experiments were performed using a Thermo Scientific™ (Waltham, MA) Vanquish™ Flex UHPLC system with a Split Sampler Module™ and a Thermo Scientific™ TSQ Quantis™ triple quadrupole mass spectrometer. The UHPLC system was a binary pump and the two mobile phases (MP) used were H2O with 0.1% FA (MPA) and ACN with 0.1% FA (MPB). Separations were performed on a 2.1 × 30 mm (internal diameter (i.d.) × length) ES-C18 column (Halo Peptide, Mac-Mod Analytical, Chadds Ford, PA, USA). Injection volume was 5 μL, MP flowrate was 0.2 mL min−1, and column temperature was held constant at 30 °C. The optimized gradient began at 21.3% MPB and held for 1 min. The MP composition underwent a linear change to 45.9% MPB over 4.55 min.
The TSQ Quantis™ mass spectrometer was operated in selected reaction monitoring mode. Electrospray settings were: spray voltage of 3500 V, sheath gas setting of 4.09 L min−1, an auxiliary gas setting of 6.4 L min−1, ion transfer tube temperature of 325 °C, and a capillary temperature of 350 °C. Monitored transitions for each analyte and IS were found by directly infusing standard and IS. The transitions used are listed in Table A-2. A dwell time of 50 ms was used for each transition.
2.5. Data analysis
Chromatograms were analyzed using Xcalibur™ (Thermo Scientific) software. Retention time precision was evaluated by the percent relative standard deviation (%RSD) for each analyte and IS over 20 runs. Calibration curves were built by plotting the average ratio of analyte peak area to IS peak area at each analyte concentration from three runs. Error bars in all plots are equal to ± 1 SD unless otherwise noted. Linear trendlines were fit to the data and utilized to interpolate concentrations of analyte from islet samples. Concentrations determined from the analyzed samples, along with the total dilution performed, were used to calculate the moles of peptides secreted from islets as well as moles in islet lysate. Limits of detection for each analyte were calculated as three times the standard deviation of the 0.5 nM analyte runs divided by the slope of the appropriate calibration curve.
To compare the measured secretion values with those reported in literature, two different normalization methods were used. The first was to calculate the percentage of each hormone release, with respect to the total content of that hormone, per minute of incubation time at each glucose condition [23]. The second method was to convert the measured values to pg mL−1 and normalized to IEQ and incubation time [24]. In both of these methods, the average of the measured values in the 5 μL aliquots were extrapolated to the total amount in the 50 μL tube.
3. Results and Discussion:
The ability to identify multiple peptide hormones released from islets of Langerhans simultaneously and in a rapid manner would allow for unprecedented examination of islet biology [25]. With this information, the interplay of the various hormones could be examined, and how these relationships change in disease state quantified. We set out to develop a robust LC-MS method that would allow antibody-free quantitation of multiple peptide hormones secreted from islets of Langerhans.
3.1. Optimization of separation conditions
Due to the complexity of the extracellular solution matrix and the necessity to quantify low amounts of released hormones, a rapid LC-MS method using MRM was developed. A main factor driving the development of the method was the ability for rapid quantitation, so a separation time < 5 min was used as a criterion.
To achieve these goals while maintaining resolution of the peptide hormones, several columns were tested, but ultimately a 2.1 × 30 mm (i.d. × length) C18 column using 2.7 μm core shell particles (2.7 μm total diameter, 1.7 μm solid core) was chosen. The development and use of core shell stationary phase particles has shown to be an effective way to reduce peak dispersion, increase mobile phase velocity, while maintaining low backpressure [20]. Using this column with a 0.2 mL min−1 flow rate kept the separation of the four peptide hormones under 5 min, maintained a backpressure between 40–70 bar, and produced highly efficient separations.
Initial separations and subsequent blank runs showed carryover of analytes. To reduce this, the %ACN in the sample solvent was increased in 10% increments. It was found that 30% ACN reduced carryover to less than 10% in blanks performed immediately after separations. Additional benefits to the increased ACN in the sample solvent were the enhanced responses of the analytes and IS, and an improvement in the %RSD of the response over multiple injections (Figure A-1). While higher %ACN in the sample reduced carryover further, it also led to low retention of the analytes and poor separations, so 30% ACN was deemed optimal.
Method development began with the implementation of scouting gradients. The initial sample solvent contained 30% ACN, but we found that somatostatin (the least retained analyte) did not elute during the initial 0.75 min hold if the MPB starting percentage was < 22%. The sample solvent must have diluted with the starting MP gradient to lessen its strength and enable retention [26]. However, if the starting MPB percentage was > 22%, somatostatin was observed during the initial hold. Starting MPB percentages lower than 20% necessitated steep gradients to ensure all analytes were eluted by 5 min. These gradients were found to be detrimental to resolution of the later-eluting species. A starting %MPB of 21.3 was found to be the best compromise between separation time and retention and was implemented into the method.
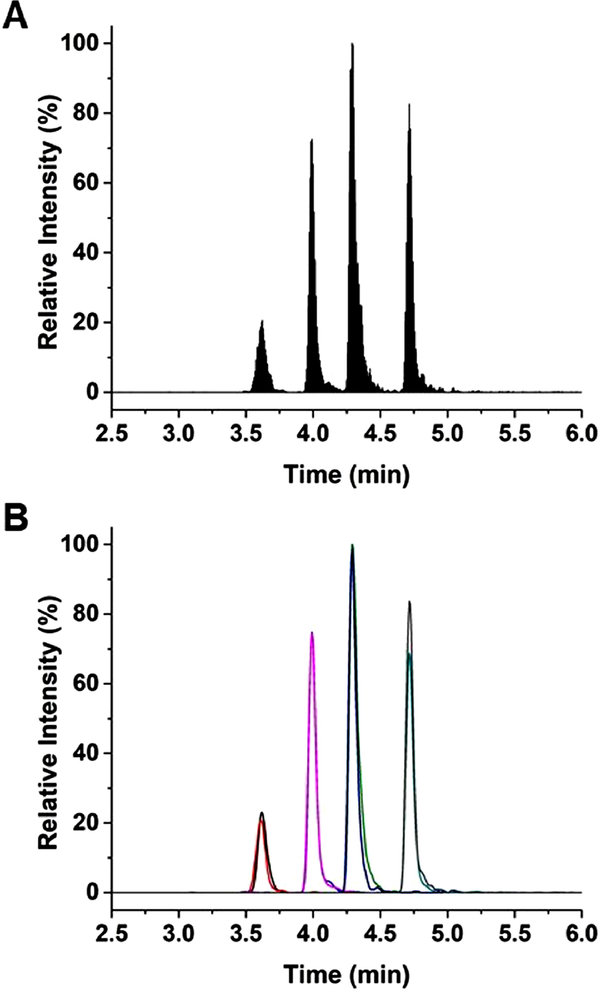
After the starting %MPB was determined, linear gradients with different slopes of %MPB vs. time were evaluated. It was often seen for the later-eluting analytes (insulin and glucagon) that they would separate from their IS when using shallow gradients. To maintain overlap, the slope of the gradient was increased, but was found to decrease resolution between the peptide hormones. The gradient that satisfied all conditions was found to be 21.3 to 45.9 %MPB over 4.55 min. The results of this separation can be seen in Figure 1A which shows the total ion chromatogram (TIC). The average peak width for all species using the optimized gradient was 0.3 min, whereas resolution between unique hormones was a minimum of 1.2 between C-peptide and insulin, while 1.4 for the other species. Retention times of all analyte and IS species had less than 0.2% RSD over the course of 20 runs. Using a gradient run time of 4.55 min and an average peak width of 0.3 min, the peak capacity of the separation was determined to be approximately 20 [27]. To help reduce carryover, MPB was cycled after each separation. This procedure included holding MPB at 45.9% after the separation for 2.20 min before reducing to 21.3% and holding for 1.50 min. MPB was increased to 65% and held for 1.50 min. The %MPB was reduced to 21.3 and held for 5 min of re-equilibration prior to the next sample injection.
Figure 1: Separation of glucose-regulating hormones.
(A) The TIC using the final separation conditions, which consisted of a gradient from 21.3 to 45.9% MPB beginning at 0.75 min over 4.55 min, is shown. Order of elution was somatostatin and somatostatin IS, C-peptide and C-peptide IS, insulin and insulin IS, and glucagon and glucagon IS. Concentrations of analytes and IS were 30 nM. (B) The extracted chromatograms of somatostatin (black), somatostatin IS (red), C-peptide (blue), C-peptide IS (pink), insulin (green), insulin IS (purple), glucagon (teal), and glucagon IS (gray) are shown using the ion transitions listed in Table A-2.
3.2. Utilization of Hyd-BSA as sample solvent
While the original sample solvent (30:70 ACN:H2O) was sufficient for the development of initial separation conditions, the calibration curves indicated that peak areas for IS increased with increasing analyte concentration, especially for later-eluting analytes. As an example, the %RSD of the insulin IS peak area across the calibration was higher than 50%, even though its concentration was constant for all runs. This was thought to be an effect of the higher concentrations of analytes in the calibration acting as adsorption competitors for the lower-concentration IS. This effect, combined with low responses of somatostatin, insulin, and glucagon below 100 nM led us to investigate non-specific adsorption in the system. Non-specific adsorption can drastically affect detection of low concentration analytes in LC-MRM analyses. Common remedies include modifications to the sample solvent, including higher percent of organic solvent, higher percent of acidic additives, and the use of low adsorption autosampler vials [28]. Trials featuring all three of these adjustments to the LC-MRM method were attempted and none improved performance to desired levels, so an alternative approach using adsorption competition with sacrificial additives was used. The additives are introduced into the sample solvent to occupy non-specific binding sites and allow more accurate responses of low concentration species [28]. BSA is a common additive for this purpose, but can be detrimental when the sample solvent contains high percentages of organic or acidic additives [29]. However, recent work has shown that adding a peptide hydrolysate of BSA (Hyd-BSA) can mimic the ability of the intact protein to adsorb to nonspecific sites while being less affected by the organic and acidic modifiers [21]. Therefore, the addition of Hyd-BSA to the sample solvent was explored.
Calibration curves were generated using the original sample solvent (30:70 ACN: H2O) and with Hyd-BSA. The protocol included the addition of Hyd-BSA to all stock solutions as well as incubation of sample vials with Hyd-BSA solution for 2 h prior to addition of sample. Concentrations for this set of calibration curves were 0, 15, 30, and 60 nM for the analytes and 30 nM for all IS.
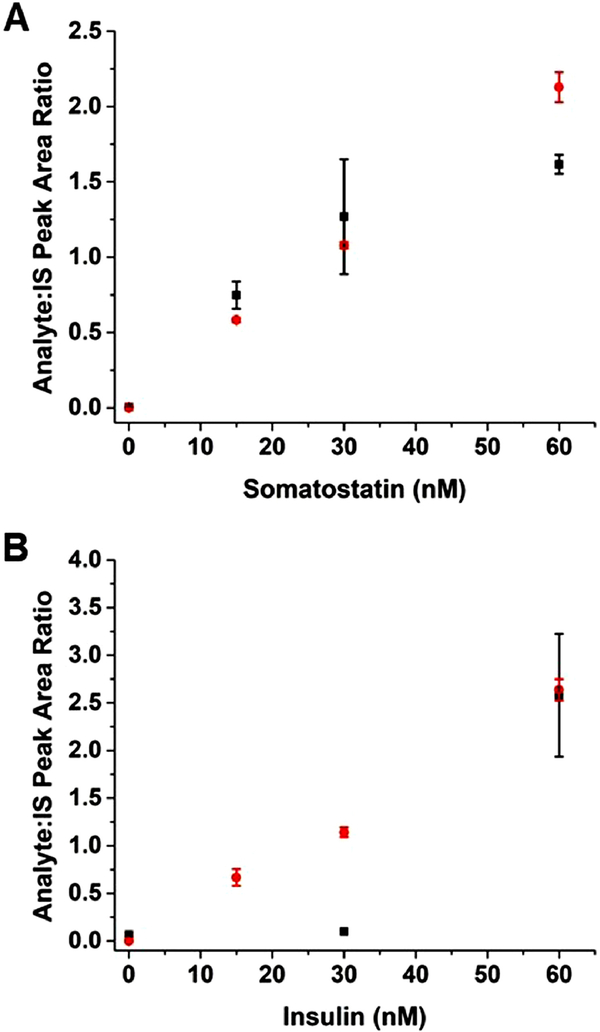
The resulting calibration curves collected for 2 analytes of interest are compared to the conditions using only 30% ACN as the sample solvent in Figure 2. The results demonstrate a clear improvement in linearity and sensitivity for somatostatin, representative of earlier-eluting species, and insulin, representative of later-eluting species with the sacrificial additive. Furthermore, a significant improvement in the absolute response for each of these species was observed. For example, 60 nM somatostatin had an average peak area response of 4500 (n = 3 runs) when diluted with 30:70 ACN:H2O which increased to an average of 20000 (n = 3 runs) using Hyd-BSA (Figure A-2). Another improvement with Hyd-BSA was seen with the IS precision over the concentration range of the calibration curve. The RSD of each IS across the calibration range when diluted with Hyd-BSA were < 15%, a drastic improvement to the > 50% RSD observed with only 30% ACN.
Figure 2: Effect of Hyd-BSA.
Calibration curves are shown when 30:70 ACN:H2O (■) and Hyd-BSA (●) were used as the sample solvent for (A) somatostatin and (B) insulin.
With these improvements, a second set of calibration curves was performed to better evaluate linearity of data at lower concentrations and to mimic the incubation conditions of islets. Analyte concentrations for this calibration curve were 0, 0.5, 1, 5, 10, and 20 nM and all IS were held at 10 nM. The sample solvent also contained 20% of the physiological salt solution, BSS, to mimic conditions for islet analysis. The resulting calibration curves generated were linear with best fits of: somatostatin, y = 0.0631x + 0.1025, r2 = 0.990; C-peptide, y = 0.1329x + 0.1950, r2 = 0.996; insulin, y = 0.1244x + 0.4275, r2 = 0.978; glucagon, y = 0.0608x + 0.1666, r2 = 0.995. All 4 curves exhibited a high degree of linearity (r2 > 0.97) over the tested range. Across all concentrations and three replicates, the range of %RSD for peak area ratios were 5–10%, 4–13%, 5–11%, and 4–18% for somatostatin, C-peptide, insulin, and glucagon, respectively. Representative traces of each of the analytes at the various concentrations tested are shown in Figure 3. These results indicated that the MRM assay maintained a high degree of linearity when using low concentrations of analytes, even in the presence of ~150 mM salt using the optimized approach. Also, as can be seen in Figure 3, with the addition of Hyd-BSA, no carryover was detected in the runs without analyte.
Figure 3: Extracted ion chromatograms of glucose-regulating hormones.
The extracted ion chromatograms of (A) somatostatin, (B) C-peptide, (C) insulin, and (D) glucagon are shown. Concentrations of analyte were those used in the calibration: 0 nM (orange), 0.5 nM (black), 1 nM (red), 5 nM (blue), 10 nM (pink), and 20 nM (green).
Responses collected at 0 nM for each analyte fell well below the linear fits in multiple experiments. As such, to calculate the LOD, the standard deviation of the 0.5 nM runs were used as described in Section 2.5 and values of 0.5, 0.2, 2, and 2 nM for somatostatin, C-peptide, insulin, and glucagon, respectively, were obtained. We expect that these are high estimates because reproducible responses of standards equal to, or lower, than these calculated LODs, with the exception of C-peptide, were obtained. For example, Figure 3 shows the difference in signal between the blank and the 0.5 nM runs for all analytes. Nevertheless, with characterization of the method complete, we then tested the ability to measure these peptide hormones from human islets of Langerhans.
3.3. Analysis of secretion from human islets of Langerhans
Two separate experiments using 250 IEQ from two donors were used on two different days. For experiment 2, three replicates were performed on each aliquot and multiple aliquots at each condition were collected to account for potential sampling error. For experiment 1, a single aliquot was collected for each glucose concentration, but three replicates were still performed. For ease in presentation, we will only discuss experiment 2, but have provided the data from experiment 1 in the Appendix.
The three low and high glucose aliquots, as well as the lysate samples at the different dilutions, underwent an addition of Hyd-BSA and IS (overall 1:20 dilution), similar to what was done for the standards in the calibration curve. Table 1 summarizes the results at the various glucose conditions and the amount in the islet lysate. The first set of values in Table 1 correspond to the amount of peptide (pmoles) measured in the 5 μL fraction during the 2-hour incubation time. These values are the average of the replicates (n = 9) for that glucose condition. The uncertainty for these values corresponds to ± 1 SD of the nine replicates. To help normalize secretion amounts by accounting for biological heterogeneity and to compare with literature values, two normalizations are also presented, the % of total content secreted min−1 and the amount of each hormone secreted in pg mL−1 IEQ−1 min−1 and were calculated as described in Section 2.5.
Table 1.
Secretion amount and rates for C-peptide and insulin in experiment 2.
| C-peptide |
Insulin |
|||||
|---|---|---|---|---|---|---|
| Secreted (pmol) | a% content secreted min−1 | pg mL−1 IEQ−1 min−1 | Secreted (pmol) | % content secreted min−1 | pg mL−1 IEQ−1 min−1 | |
| Low glucose (1 mM) | 0.23 ±0.04 | 0.015 ± 0.003 | 4.7 ± 0.8 | 0.67 ±0.11 | 0.025 ± 0.004 | 26 ± 4 |
| High glucose (16 mM) | 0.81 ±0.10 | 0.053 ± 0.007 | 16 ± 2 | 1.4 ±0.2 | 0.051 ± 0.007 | 54 ± 7 |
Measured lysate amounts (in pmol): somatostatin, 4.6 ± 0.3; C-peptide, 116 ± 8; insulin, 206 ± 12.
To summarize the results from the islet experiments, only insulin and C-peptide were detected in the secretion samples. Measurement of these peptides are unsurprising as insulin and its precursor fragment, C-peptide, are produced in β-cells within islets which make up the largest percentage of cells in the islets [1]. Somatostatin and glucagon, produced in δ- and α-cells, respectively, are found in lower percentages and have correspondingly lower secretion amounts. Although these peptides were not observed in the secretion samples, somatostatin was detected in the islet lysate. Precision of the assay, as measured by the %RSD of the replicates, ranged from 12–17% for C-peptide and 13–16% for insulin. This precision range is similar to the range of the standards in the calibration, so the majority of the uncertainty is likely from the method. Similar results were seen for Experiment 1 (Table A-3) with insulin and C-peptide being detected in the majority of samples, and glucagon being detected in the lysate. One difference between the two experiments was that C-peptide was not detected in the low glucose incubation in experiment 1. To remedy this, a longer incubation time was used for experiment 2. In both cases, the values for secretion rates calculated for both C-peptide and insulin are similar to previously published values [23], [24], [30], [31]. Considering differences between incubation conditions, methods of analyses used, and biological variability of donor, the agreement show great promise for the LC-MS method.
A potential source of experimental error may be from a previously unobserved salt adduct(s) formed with co-eluting components affecting peptide responses [32], but because MRM transitions were optimized before addition of BSS to the calibration standards, this was not diagnosed. For somatostatin and glucagon, human islets are typically composed of significantly more glucagon-containing alpha cells than somatostatin-containing delta cells. This did not appear to be the case in experiment 2. The response of somatostatin in experiment 2 was higher than all calibration ranges, while the response of glucagon was lower than all calibrations. This suggests that the glucagon content of these islets from this particular donor was abnormally low. This type of biological variability is difficult to account for.
4. Conclusions:
Understanding the mechanisms that control the secretion profiles of hormones from islets of Langerhans would lead to a better understanding of their biology and what may change during diseases like diabetes. The LC-MS/MS method developed combines a rapid separation using a short column with core shell particles and an islet-friendly high salt sample matrix. Repeatable results were observed with low carryover and low limits of detection. The ability to measure lower amounts of peptide hormones could be achieved by reductions in the dilution steps during sample preparation and the use of smaller i.d. columns with nanoflow ESI. The applicability of the method was shown by the quantitation of insulin and C-peptide release from human islets that are similar in magnitude to other reports using antibody-based methods. Continued improvement of the system is expected to lead to the development of a platform for time-resolved analysis of multiple hormones released from human islets of Langerhans.
Supplementary Material
Highlights:
30 × 2.1 mm C18 column with core-shell particles for separation of peptide hormones
0.2% RSD retention time of analytes and internal standards
Multiple reaction monitoring produced 0.5 nM detection limits in salt-filled matrix
Values of C-peptide and insulin secretion from human islets agreed with literature
Acknowledgements
We would like to thank Dr. Xinsong Lin at Florida State University for his help with instrument maintenance, as well as Professor James Edwards at St. Louis University for helpful conversations. This work was supported in part by grants from the National Institutes of Health, R01 DK080714, and using resources and/or funding provided by the NIDDK-supported Human Islet Research Network (HIRN, RRID:SCR_014393; https://hirnetwork.org; UC4 DK116283 to MGR).
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Da Silva Xavier G, The Cells of the Islets of Langerhans, J. Clin. Med 7 (2018) 54 10.3390/jcm7030054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rorsman P, Huising MO, The somatostatin-secreting pancreatic δ-cell in health and disease, Nat. Rev. Endocrinol 14 (2018) 404–414. 10.1038/s41574-018-0020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fu Z, Gilbert ER, Liu D, Regulation of Insulin Synthesis and Secretion and Pancreatic Beta-Cell Dysfunction in Diabetes, Curr. Diabetes Rev 9 (2012) 25–53. 10.2174/1573399811309010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Walker JN, Ramracheya R, Zhang Q, Johnson PRV, Braun M, Rorsman P, Regulation of glucagon secretion by glucose: Paracrine, intrinsic or both?, Diabetes, Obes. Metab 13 (2011) 95–105. 10.1111/j.1463-1326.2011.01450.x. [DOI] [PubMed] [Google Scholar]
- [5].Menge BA, Grüber L, Jørgensen SM, Deacon CF, Schmidt WE, Veldhuis JD, Holst JJ, Meier JJ, Loss of inverse relationship between pulsatile insulin and glucagon secretion in patients with type 2 diabetes, Diabetes. 60 (2011) 2160–2168. 10.2337/db11-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gumbiner B, Van Cauter E, Beltz WF, Ditzler TM, Griver K, Polonsky KS, Henry RR, Abnormalities of insulin pulsatility and glucose oscillations during meals in obese noninsulin-dependent diabetic patients: effects of weight reduction, Endocrinol. Metab 81 (1996) 2061–2068. [DOI] [PubMed] [Google Scholar]
- [7].Guillo C, Roper MG, Two-color electrophoretic immunoassay for simultaneous measurement of insulin and glucagon content in islets of Langerhans, Electrophoresis. 29 (2008) 410–416. 10.1002/elps.200700399. [DOI] [PubMed] [Google Scholar]
- [8].Guillo C, Roper MG, Simultaneous capillary electrophoresis competitive immunoassay for insulin, glucagon, and islet amyloid polypeptide secretion from mouse islets of Langerhans, J. Chromatogr. A 1218 (2011) 4059–4064. 10.1016/j.chroma.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Caslavska J, Allemann D, Thormann W, Analysis of urinary drugs of abuse by a multianalyte capillary electrophoretic immunoassay, J. Chromatogr. A. 838 (1999) 197–211. 10.1016/S0021-9673(99)00115-6. [DOI] [PubMed] [Google Scholar]
- [10].Lomasney AR, Yi L, Roper MG, Simultaneous monitoring of insulin and islet amyloid polypeptide secretion from islets of Langerhans on a microfluidic device, Anal. Chem 85 (2013) 7919–7925. 10.1021/ac401625g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hermes N, Jewell KS, Wick A, Ternes TA, Quantification of more than 150 micropollutants including transformation products in aqueous samples by liquid chromatography-tandem mass spectrometry using scheduled multiple reaction monitoring, J. Chromatogr. A 1531 (2018) 64–73. 10.1016/j.chroma.2017.11.020. [DOI] [PubMed] [Google Scholar]
- [12].Wong JMT, Malec PA, Mabrouk OS, Ro J, Dus M, Kennedy RT, Benzoyl chloride derivatization with liquid chromatography-mass spectrometry for targeted metabolomics of neurochemicals in biological samples, J. Chromatogr. A 1446 (2016) 78–90. 10.1016/j.chroma.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wei R, Li G, Seymour AB, High-throughput and multiplexed LC/MS/MRM method for targeted metabolomics, Anal. Chem 82 (2010) 5527–5533. 10.1021/ac100331b. [DOI] [PubMed] [Google Scholar]
- [14].Vidova V, Spacil Z, A review on mass spectrometry-based quantitative proteomics: Targeted and data independent acquisition, Anal. Chim. Acta 964 (2017) 7–23. 10.1016/j.aca.2017.01.059. [DOI] [PubMed] [Google Scholar]
- [15].Chen Z, Caulfield MP, McPhaul MJ, Reitz RE, Taylor SW, Clarke NJ, Quantitative insulin analysis using liquid chromatography-tandem mass spectrometry in a high-throughput clinical laboratory, Clin. Chem 59 (2013) 1349–1356. 10.1373/clinchem.2012.199794. [DOI] [PubMed] [Google Scholar]
- [16].Howard JW, Kay RG, Tan T, Minnion J, Ghatei M, Bloom S, Creaser CS, Development of a high-throughput UHPLC-MS/MS (SRM) method for the quantitation of endogenous glucagon from human plasma, Bioanalysis. 6 (2014) 3295–3309. 10.4155/bio.14.226. [DOI] [PubMed] [Google Scholar]
- [17].Bronsema KJ, Bischoff R, Van De Merbel NC, Internal standards in the quantitative determination of protein biopharmaceuticals using liquid chromatography coupled to mass spectrometry, J. Chromatogr. B 893–894 (2012) 1–14. 10.1016/j.jchromb.2012.02.021. [DOI] [PubMed] [Google Scholar]
- [18].Marasco CC, Enders JR, Seale KT, McLean JA, Wikswo JP, Real-time cellular exometabolome analysis with a microfluidic-mass spectrometry platform, PLoS One. 10 (2015) 1–19. 10.1371/journal.pone.0117685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dugan CE, Grinias JP, Parlee SD, El-Azzouny M, Evans CR, Kennedy RT, Monitoring cell secretions on microfluidic chips using solid-phase extraction with mass spectrometry, Anal. Bioanal. Chem 409 (2017) 169–178. 10.1007/s00216-016-9983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hayes R, Ahmed A, Edge T, Zhang H, Core-shell particles: Preparation, fundamentals and applications in high performance liquid chromatography, J. Chromatogr. A 1357 (2014) 36–52. 10.1016/j.chroma.2014.05.010. [DOI] [PubMed] [Google Scholar]
- [21].Verbeke F, Bracke N, Debunne N, Wynendaele E, De Spiegeleer B, LC-MS Compatible Antiadsorption Diluent for Peptide Analysis, Anal. Chem 92 (2020) 1712–1719. 10.1021/acs.analchem.9b01840. [DOI] [PubMed] [Google Scholar]
- [22].Detimary P, Jonas JC, Henquin JC, Stable and diffusible pools of nucleotides in pancreatic islet cells, Endocrinology 137 (1996) 4671–4676. [DOI] [PubMed] [Google Scholar]
- [23].Henquin JC, The challenge of correctly reporting hormones content and secretion in isolated human islets, Mol. Metab 30 (2019) 230–239. 10.1016/j.molmet.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gomez Y, Navarro-Tableros V, Tetta C, Camussi G, Brizzi MF, A versatile model of microfluidic perifusion system for the evaluation of C-peptide secretion profiles: Comparison between human pancreatic islets and HLSC-derived islet-like structures, Biomedicines. 8 (2020) 1–13. 10.3390/biomedicines8020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hellman B, Salehi A, Gylfe E, Dansk H, Grapengiesser E, Glucose generates coincident insulin and somatostatin pulses and antisynchronous glucagon pulses from human pancreatic islets, Endocrinology. 150 (2009) 5334–5340. 10.1210/en.2009-0600. [DOI] [PubMed] [Google Scholar]
- [26].Alsehli B, Dolan JW, The role of the injection solvent, LC GC Asia Pacific. 25 (2012) 19–21. [Google Scholar]
- [27].Neue UD, Theory of peak capacity in gradient elution, J. Chromatogr. A 1079 (2005) 153–161. 10.1016/j.chroma.2005.03.008. [DOI] [PubMed] [Google Scholar]
- [28].Maes K, Smolders I, Michotte Y, Van Eeckhaut A, Strategies to reduce aspecific adsorption of peptides and proteins in liquid chromatography-mass spectrometry based bioanalyses: An overview, J. Chromatogr. A 1358 (2014) 1–13. 10.1016/j.chroma.2014.06.072. [DOI] [PubMed] [Google Scholar]
- [29].Taevernier L, Wynendaele E, D’Hondt M, De Spiegeleer B, Analytical quality-by-design approach for sample treatment of BSA-containing solutions, J. Pharm. Anal 5 (2015) 27–32. 10.1016/j.jpha.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Henquin JC, Dufrane D, Kerr-Conte J, Nenquin M, Dynamics of glucose-induced insulin secretion in normal human islets, Am. J. Physiol. Endocrinol. Metab 309 (2015) E640–E650. 10.1152/ajpendo.00251.2015. [DOI] [PubMed] [Google Scholar]
- [31].Henquin JC, Dufrane D, Gmyr V, Kerr-Conte J, Nenquin M, Pharmacological approach to understanding the control of insulin secretion in human islets, Diabetes Obes. Metab 19 (2017) 1061–1070. 10.1111/dom.12887. [DOI] [PubMed] [Google Scholar]
- [32].Kruve A, Kaupmees K, Adduct Formation in ESI/MS by Mobile Phase Additives, J. Am. Soc. Mass Spectrom 28 (2017) 887–894. 10.1007/s13361-017-1626-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.