Abstract
The diagnosis of Non-Hodgkin lymphoma is increasingly common among the elderly. It is well recognized that this patient population may benefit from therapy. No guidelines exist for chemotherapy dosing, and a clear assessment of potential treatment benefits has not been previously reported. In this single institution study, we report the toxicities and survival of septuagenarians and octogenarians with large cell lymphoma treated with chemo-immuno therapy with or without radiation, as primary therapy with curative intent. One hundred two patients were identified, we identified 37 patients over the age of 70 with diffuse large B-cell lymphoma (DLBCL), and sixty five patients younger DLBCL patients served as controls. Our results suggest that chemotherapy dose reductions are common among elderly and that, despite planned dose reductions; these patients are susceptible to toxicities. This age group can receive an attenuated dose when considering an anthracycline based chemo-immunotherapy without compromising overall survival.
Keywords: Chemotherapy, toxicities, elderly, Diffuse large B cell lymphoma
Introduction:
Cancer is the leading cause of death among 60–85 year-olds. With the aging population and better control of comorbid illnesses, the global burden of cancer treatment continues to rise. The incidence of most malignancies has decreased in recent decades; however, the incidence of non-Hodgkin’s lymphoma (NHL) has steadily risen [1]. Among white men >75 years of age in the United States, the incidence of NHL increased by 300–400 percent during the latter half of the 20th century [2]. Diffuse large B- cell lymphoma (DLBCL) accounts for 40% of NHL and is the most common histological subtype of NHL diagnosed in the United States.
In the early 1990s, cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) was shown to be equally efficacious and better tolerated than other multidrug regimens for the treatment of DLBCL [3, 4]. The anti-CD20 chimeric antibody, rituximab, was later added to CHOP (R-CHOP) and was found to further improve clinical outcomes without the addition of significant toxicity. R-CHOP has since become the standard therapy [5, 6].
R-CHOP chemotherapy has been shown to be curative in more than half of patients with DLBCL [7], but the adoption of doxorubicin-based chemotherapy in the treatment of elderly patients with DLBCL has not been universal. DLBCL patients with advanced age are more likely to receive dose reduced R-CHOP or non-anthracycline chemotherapy when compared with younger patients [8]. Concerns about poor performance status, coexistent illnesses, and an increased risk of treatment-related cardiotoxicity and neutropenic fever likely contribute to the under treatment of elderly patients with aggressive lymphomas [9–13]. The use of granulocyte-colony stimulating factor (G-CSF) and the improvement in supportive care may contribute to increased success in treating elderly patients with R-CHOP. Indeed, more recent studies suggest elderly patients can receive R-CHOP intensity similar to younger patients and demonstrate similar treatment outcomes [5, 14–17].
Despite promising advancements in the treatment of aggressive lymphomas, the diffusion of CHOP-based therapy for the elderly has been slow. Between 1991 and 2002, nearly half of patients aged ≥ 66 years of age diagnosed with aggressive NHL in the United States did not receive doxorubicin-based chemotherapy [18]. Furthermore, even after the advent of rituximab, 30% of all elderly patients diagnosed with large cell lymphoma through 2002 in the United States received no chemotherapy [19].
Previous studies have established chemotherapy relative dose intensity (RDI) as an important prognostic factor for survival in patients treated with CHOP-based therapy [20, 21]. Elderly patients that received CHOP intensity comparable to the younger individuals had similar long-term outcomes [17]. An RDI of CHOP-based therapy less than 70% has been associated with poorer long- term outcomes [17, 22]. In this study, we investigate the use of R-CHOP in treating elderly patients with evaluation of relative dose intensity, treatment related toxicity, and overall treatment response in patients aged 70 or greater (pts ≥70) compared with those younger than 70 (pts<70).
Patients and Methods:
Patient Selection
The study population was selected using an electronic medical record (EMR) database maintained by the Vanderbilt University Medical Center. The database contains electronic medical records (EMR) linked to chemotherapy administration data and is easily searchable using ICD-9 coding. After obtaining IRB approval, we identified patients who completed R-CHOP therapy for follicle center cell lymphoma (FCCL), grade 3 or DLBCL between January 2000 and January 2010. Patients with histologic transformation were excluded from the study. All pathology was reviewed and confirmed by the Vanderbilt Hematopathology Division. Patients treated outside our institution, and those who received RCHOP for other diagnoses were excluded. The EMR containing 4095 hematology clinic patients identified 324 individuals with ICD-9 coding corresponding to DLBCL. Subsequent chart review identified 102 individuals who completed R-CHOP for FCCL grade 3 or DLBCL between January 2000 and January 2010.
Co-morbidity Scoring:
In addition to baseline laboratory and pathology data, individual pre-treatment co-morbidities were identified and scored using the Cumulative Illness rating Scale for Geriatrics (CIRS-G) [23]. This co-morbidity scoring includes a severity rating from 0 (no problem) to 4 (extremely severe problem and/or organ failure) for organ systems using published scoring guidelines [24, 25]. Medication, laboratory data, and documented history and physical examination at the time of chemotherapy initiation were used to complete the retrospective CIRS-G. Scoring for the hematology organ system was omitted because all subjects would have been identical for that scale. The number of organ systems with a score of 3 (severe problem and/or hard to control chronic problem) or greater was calculated for each individual patient. In addition, the sum of each of the 13 individual organ systems was used as a marker of co-morbidity.
Treatment
Most patients started treatment with the plan to complete six 21-day cycles of R-CHOP. The standard doses of chemotherapy were cyclophosphamide 750mg/m2, doxorubicin 50mg/m2, vincristine 1.4 mg /m2, prednisone 100mg daily for 5 days, and rituximab 375mg/m2. Patients who underwent short course R-CHOP (3 or 4 cycles) combined with involved field radiotherapy (IFRT) due to limited stage disease were also included in the final analysis. Treatment delays and dose reductions were documented, and chemotherapy dose intensity was calculated. The average relative dose intensity (ARDI) was derived by dividing the total actual dose received (mg/m2)/total administration period (weeks) by the total planned dosage of all courses /total administration period planned for all courses). The use of G-CSF or pegylated G-CSF was recorded for each individual R-CHOP cycle to allow identification of its use as both primary and secondary prevention.
Outcomes
Treatment related toxicity, treatment response, and overall survival were endpoints. The EMR allowed the identification of chemotherapy-related toxicities and hospitalizations during chemotherapy treatment. Chemotherapy adverse events were recorded in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events [26]. Treatment response was guided by FDG-PET imaging and responses to treatment were categorized as complete remission, partial remission, stable disease, or disease progression as defined by the International Harmonization Project [27, 28]. The EMR and the Social Security Death Index [29] were used to define individual clinical outcomes through December 2010. Overall survival (OS) was calculated from the initiation of chemotherapy to the time of death, whereas event free survival (EFS) was calculated from the initiation of chemotherapy to the time of relapse or death from any cause.
Statistical Analysis
Two groups were identified: those younger than 70 years (pts <70) and those age 70 years or older (pts≥70). Baseline patient characteristics were compared between the two groups using analysis of variance (ANOVA) for numerical data and the χ2 test for categorical variables. The association of baseline characteristics and the likelihood of R-CHOP dose reduction was analyzed using multivariate logistic regression. Finally, OS, EFS, and 3 year survival rate were calculated using the Kaplan-Meier method. All retrospective data was collected and managed using REDCap electronic data capture tools [30]. Statistical analysis employed the SPSS software suite [31].
Results:
Baseline clinical characteristics are presented in Table 1. Thirteen (4%) subjects were excluded from the study because they had not completed R-CHOP therapy at the time of study closure. Two patients among those diagnosed with DLBCL did not receive anthracycline-based chemotherapy and were not included in the study: one individual was aged 90 and received rituximab alone, while the second patient was diagnosed concurrently with a systemic infection and did not survive to chemotherapy initiation.
Table 1:
Baseline Characteristics (n = 102)
| Characteristic | Age less than 70 years (n= 65) | Age 70 years or greater (n= 37) | P value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Sex (male:female) | 34 | 52 | 18 | 49 | NS |
| Age, years | |||||
| Mean | 56 | 79 | |||
| Median | 59 | 79 | |||
| Range | 20–70 | 70–90 | |||
| Baseline Data (average) | |||||
| BMI (kg/m2) | 30.3 | 24.8 | 0.001 | ||
| BSA | 2 | 1.8 | 0.003 | ||
| ANC (in thousands) | 5.1 | 5.1 | NS | ||
| ALC (in thousands) | 1.6 | 1.4 | NS | ||
| Hgb (g/dL) | 12.6 | 12.4 | NS | ||
| Hct (%) | 38.2 | 38 | NS | ||
| Blood Albumin (g/dL) | 3.8 | 3.8 | NS | ||
| Blood LDH (U/L) | 270 | 336 | NS | ||
| CIRS Co-morbidity Score* | 5.8 | 7.5 | 0.005 | ||
| Disease Stage (Ann Arbor) | |||||
| I-II | 26 | 40 | 12 | 32 | NS |
| III-IV | 39 | 60 | 25 | 68 | |
| ECOG PS | |||||
| 0–1 | 60 | 92 | 30 | 81 | NS |
| 2–4 | 5 | 8 | 7 | 9 | |
| LDH Level | |||||
| >Normal (>225) | 29 | 45 | 16 | 43 | NS |
| No data | 1 | 2 | 1 | 2 | |
| No. of extranodal sites | |||||
| 0–1 | 42 | 65 | 29 | 78 | NS |
| 2 or greater | 23 | 35 | 8 | 22 | |
| B symptoms | 9 | 14 | 8 | 22 | NS |
| Blood Albumin g/dL | |||||
| <3.5 | 9 | 14 | 7 | 19 | NS |
| No data | 1 | 2 | |||
| Anemia | |||||
| Hgb <12, g/dL | 22 | 34 | 15 | 40 | NS |
| Histology | |||||
| Diffuse large B cell lymphoma | 59 | 91 | 35 | 95 | NS |
| High grade follicular | 6 | 9 | 2 | 5 | |
| International prognostic Index | |||||
| 0–2 (low/low-intermediate) | 41 | 63 | 18 | 48.5 | NS |
| 3–5 (high-intermediate/high) | 23 | 35 | 18 | 48.5 | |
| 1 | 2 | 1 | 3 | ||
| Age-adjusted IPI | |||||
| 0–1 (low/low-intermediate) | 42 | 64 | 20 | 54 | NS |
| 2–3 (high-intermediate/high) | 22 | 33 | 16 | 43 | |
| No data | 1 | 2 | 1 | 3 | |
| CIRS Score of 3 or more | |||||
| At least 1 system | 15 | 23 | 16 | 43 | 0.03 |
LDH: Lactate dehydrogenase, BMI: body mass index, BSA: body surface area, ANC: absolute neutrophil count, Hgb: Hemoglobin, Hct: hematocrit, PS: performance status, ALC: absolute lymphocyte count, IPI: International prognostic index
Cumulative Illness Rating Scale (CIRS) was used to measure multi-morbidity. Thirteen of fourteen organ systems identified by CIRS were evaluated for each patient (hematological system not scored). Severity for each system rated as: 0 – no problem; mild or past significant,; 2-moderate, first line therapy; 3 – severe or hard to control chronic; 4 – Severe functional impairment and/or organ failure
Baseline characteristics
Of the 102 identified patients, 37 (36%) were 70 years old or older, with a median age of 79 years (range 70–90). The median age of patients younger than 70 years was 59 years (range 20–70). Eight (7.8%) patients had FCCL grade 3b. Differences in baseline BMI and body surface area (BSA) were statistically significant between age groups P<0.001 and P<0.003 respectively. Baseline laboratory data including absolute neutrophil count, absolute lymphocyte count, hemoglobin, serum albumin level, and serum LDH level were similar between age groups. In addition, disease stage, international prognostic index (IPI), and age-adjusted IPI were not statistically different between the groups. Baseline co-morbidities quantified with CIRS-G scoring showed that pts ≥70 had a higher average CIRS score (7.5 vs. 5.8, P <0.005), and a greater proportion had at least one severe or not optimally controlled chronic baseline condition (43% vs. 23 %, OR 2.5, P< 0.03).
Dose reductions:
As shown in Table 2, the two age groups received a similar number of chemotherapy cycles (mean 5.8 vs. 5.7). The aged group was more likely to have a dose reduction planned prior to R-CHOP initiation (22% vs. 5%, OR 5.7, P<0.008). Furthermore, pts≥ 70 experienced a greater frequency of any-type dose reduction during treatment (73% vs. 18%, OR 12, P<0.001). While patients ≥70 experienced more planned and mid-treatment dose reductions, the average relative dose intensity given remained greater than 70% of reference standard intensity in 32 of 37 (86%) patients. Of particular note, dose reduction of R-CHOP was planned prior to treatment initiation for 8 (22%) pts ≥70, but only 1 of these patients received less than a 70% RDI at the conclusion of therapy. Furthermore, only 4 of the aged patients (11%) received doxorubicin at RDI < 10mg/m2/week. Primary prevention of neutropenic fever with prophylactic colony stimulating factor from the onset of R-CHOP was more commonly administered among the pts≥70 (92% vs. 28%, OR 29, P<0.001). In addition, all pts≥70 received G-CSF at least once during R-CHOP treatment compared to 44% of pts<70 who received no G-CSF during their R-CHOP therapy (OR 1.86, P<0.001).
Table 2:
Treatment data
| Aged less than 70 years (n = 65) | Aged 70 or greater (n = 37) | P value | |||
|---|---|---|---|---|---|
| Number of chemotherapy cycles | |||||
| Median (range) | 6 (3–7) | 6 (3–8) | NS | ||
| Mean (standard deviation) | 5.7 (1.0) | 5.8(1.1) | |||
| Relative dose intensity (mg/m2/week) | |||||
| Doxorubicin* | 15.6 | 13.5 | 0.001 | ||
| Cyclophosphamide | 236 | 220 | 0.04 | ||
| Vincristine | 0.6 | 0.5 | 0.02 | ||
| Prednisone | 158 | 150 | NS | ||
| Rituximab | 120 | 116 | NS | ||
| No. | % | No | % | ||
| Dose delay of 7 days or greater | 12 | 23 | 11 | 30 | NS |
| Chemotherapy Dose Reduction | |||||
| At least one chemotherapy dose | 12 | 18 | 27 | 73 | 0.001 |
| Planned dose reduction at onset | 3 | 5 | 8 | 22 | 0.008 |
| Doxorubicin dose intensity* | |||||
| At least 10mg/m2/week | 63 | 98 | 33 | 89 | 0.04 |
| Less than 10mg/m2/week | 1 | 2 | 4 | 11 | |
| Average relative dose intensity^ | |||||
| Received 70% or greater of standard RDI | 60 | 94 | 32 | 86 | NS |
| Received less than 70% of standard RDI | 4 | 6 | 5 | 14 | |
| G-CSF Use | |||||
| Used as prophylaxis (any cycle) | 43 | 66 | 37 | 100 | 0.001 |
| Prophylaxis from cycle 1 | 18 | 28 | 34 | 92 | 0.001 |
One patient aged less than 70 received doxil in place of doxorubicin and is not included in the analysis
Relative dose intensity calculated using reference standard CHOP-R schedule – Doxorubicin 50mg/m2 (day 1), Cyclophosphamide 750mg/m2 (day 1), Vincristine 1.4mg/m2; max 2mg (day 1), Prednisone 100mg (day 1–5), and Rituximab 375mg/m2 (day 1); GCSF: granulocyte colony stimulating factor
Toxicity and Survival:
Treatment outcomes and chemotherapy related toxicities are presented in table 3. Frequency of neutropenia and febrile neutropenia were similar between the age groups (grade 3–4 neutropenia: 43% vs. 45%; febrile neutropenia: 22% vs. 17%). While the rates of adverse events were similar between the two age groups, the elderly group was more likely to be hospitalized during treatment (54% vs. 32%, OR 2.5, P<0.03). Here, increased co-morbidities in the aged population contributed to hospitalizations for non-neutropenic infections, falls with traumatic fractures, and overall failure to thrive. Ultimately, two treatment related deaths occurred, both in pts≥70, and were related to cardiac and pulmonary toxicity.
Table 3:
Treatment toxicity and response
| Aged less than 70 years (n= 65) | Aged 70 years or greater (n= 37) | P value | |||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Response | |||||
| Complete Remission (CR) | 59 | 91 | 35 | 95 | NS |
| Non CR | 6 | 9 | 2 | 5 | |
| Chemotherapy related toxicities | |||||
| ANC <1000 (grade 3–4) | 29 | 45 | 16 | 43 | NS |
| Emesis (grade 2–4) | 8 | 12 | 0 | 0 | 0.02 |
| Neuropathy (grade 2–4) | 5 | 8 | 8 | 22 | 0.04 |
| Cardiac toxicity (grade 3–4) | 0 | 0 | 1 | 3 | NS |
| Pulmonary Toxicity (grade 3–4) | 1 | 2 | 1 | 3 | NS |
| Febrile Neutropenia | 11 | 17 | 8 | 22 | NS |
| Hospitalized during treatment | 21 | 32 | 20 | 54 | 0.03 |
| Treatment related mortality | 0 | 0 | 2 | 5 | NS |
A complete remission (CR) was achieved in 92% of the patients and a difference was not observed between the two age groups (95% vs. 91%). Median follow up time was 32 months for pts≥70 years and 36 months for pts<70. The 3 year survival rate in pts≥70 was 73% compared to 89% in pts <70. Both OS and EFS are plotted in Figure I. At the time of outcome data collection, no statistical difference was observed between the age groups using the log rank test. Finally, a multivariate logistic regression model was used to identify baseline clinical predictors of RCHOP dose reduction. Results are presented in Table 4. Age, BSA and co-morbidity scoring were statistically significant predictors of dose reduction after controlling for sex, LDH level, disease stage, performance status, and G-CSF use.
Figures 1a and 1b:
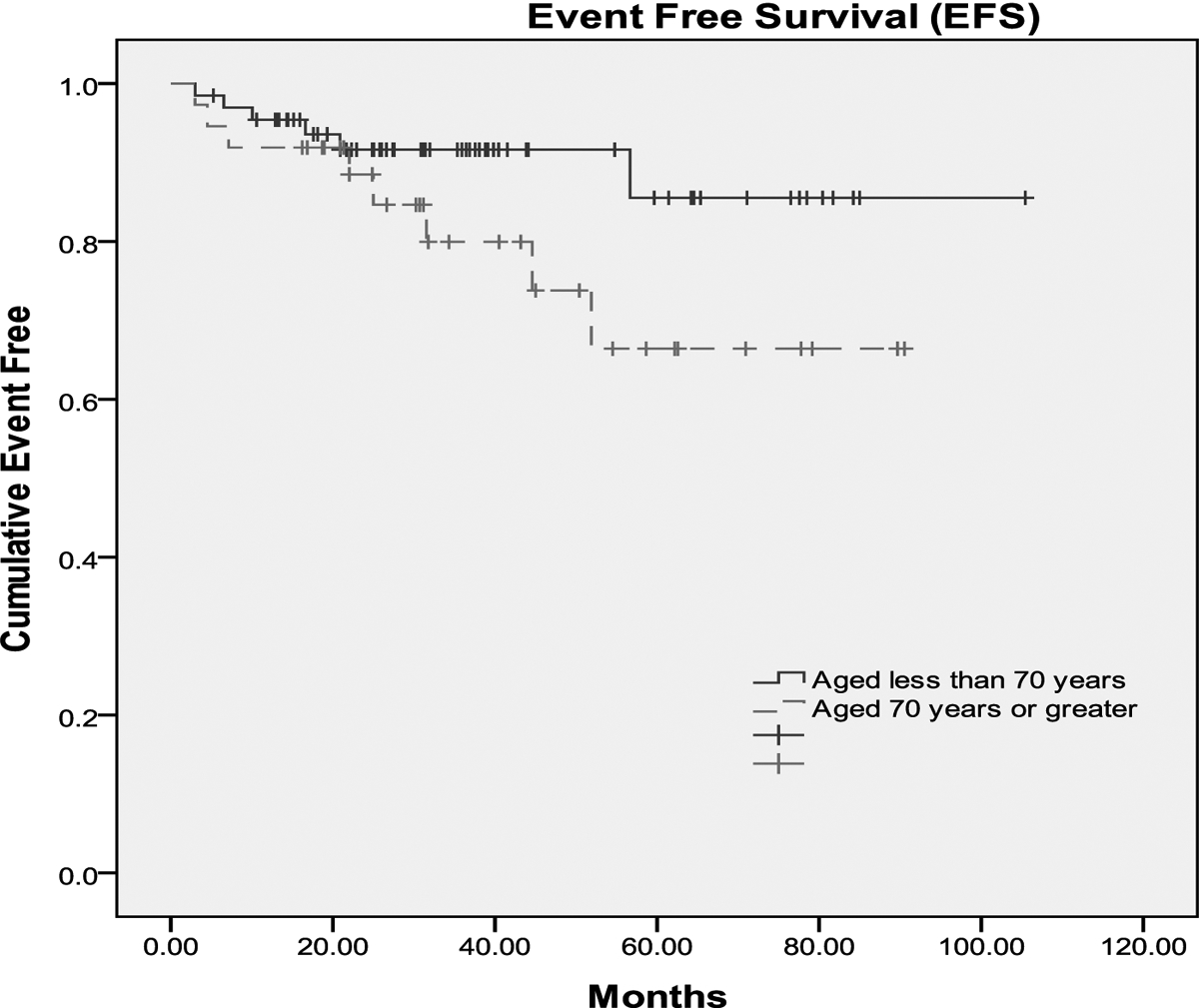
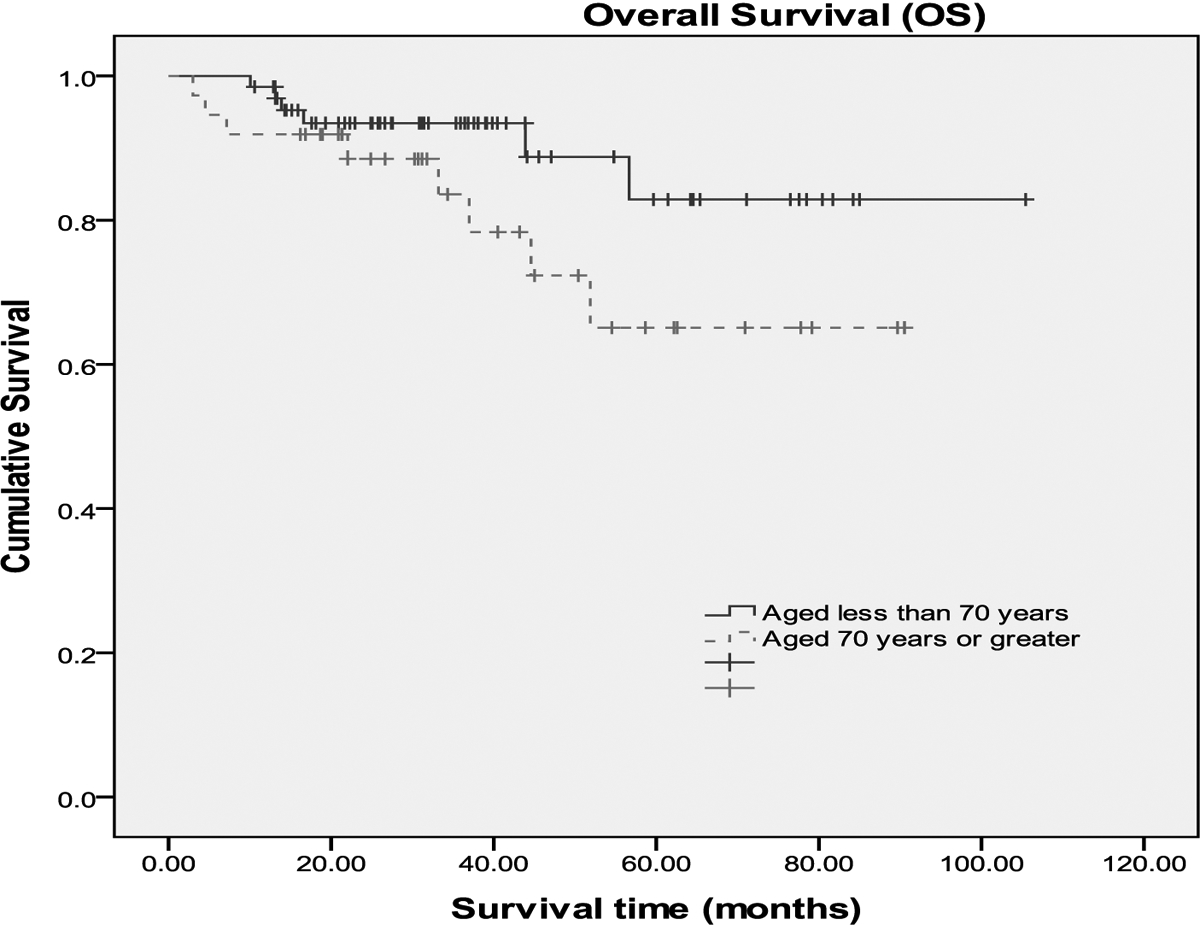
Event free survival (fig 1a) and Overall survival (fig 1b) of pts≥70 and pts<70 years of age. Median follow up time was 32 months for pts≥70 years and 36 months for pts<70
Table 4:
Multivariate Logistic Regression Analysis for Dose Reduction in Patients treated with RCHOP for Aggressive NHL (N = 100).
| Covariate | OR | P value (95% CI) |
|---|---|---|
| Age > 70 years | 11 | 0.001 (2.7–45.9) |
| Male | 0.96 | NS (0.13–3.4) |
| BSA > 2 | 0.2 | 0.03 (.05–.85) |
| LDH > normal | 0.7 | NS (0.2–2.1) |
| Disease stage ≥ III | 2.4 | NS (0.7–8.3) |
| Performance status ≥ 2 | 3.7 | NS (0.7–19.4) |
| Total CIRS comorbidity score | 1.2 | 0.04 (1.005–1.4) |
| Prophylactic CSF starting in first cycle | 0.98 | NS (0.2–3.7) |
Discussion:
R- CHOP chemotherapy is curative in over half of patients with DLBCL. Nationwide, in 1991–2002, patients continued to receive sub-optimal regimens or dose reductions that may adversely influence their outcomes. In our study of 102 patients with DLBCL or FCCL grade 3 lymphoma, the majority of patients (91%) was able to receive RCHOP with at least 70% relative dose intensity. Most (92%) patients in the older group received G-CSF as primary prophylaxis from the onset of R-CHOP therapy, and this likely contributed to the observation of comparable rates of grade 3–4 neutropenia (43% vs.45%) and neutropenic fever (22% vs. 17%) between pts≥70 and pt<70 years.
Along with comparable relative R-CHOP dose intensities and treatment toxicities, the two age groups experienced similar response rates. The overall rate of complete remission (CR) of 92% is higher than all published randomized phase III trials using RCHOP in the treatment of DLBCL, and is probably related to the fact that the majority of patients had low or low-intermediate IPI, along with the use of PET-CT in assessing treatment response. The RICOVER-60 and the LNH98–5 trials [5, 14] reported CRs in 78% and 75% of elderly patients treated with RCHOP respectively, however, these studies used CT response assessment and also included patients with other NHL subtypes that may response less-well to RCHOP. Our study employed PET-CT to assess treatment response, and PET-CT has been shown to further characterize up to 50% of partial responses based on CT as CR on PET-CT [32]. Finally, achieving a relative dose intensity of >70% in the majority of patients likely contributed to the excellent CR rate observed. In the LNH98–5 study, it was reported that 90% of patients received 90% of the ‘planned doses’ of doxorubicin and cyclophosphamide. This study had already incorporated a planned dose reduction by 50% of doxorubicin and cyclophosphamide for grade 3 or 4 cytopenia. The actual delivered dose has not been reported in any previous trials.
In the pre-rituximab era, study by Dixon et al., showed that dose reduction in CHOP chemotherapy by 50% for patients >65 years resulted in inferior complete response rates (37%)compared with those who were able to start therapy at full doses (CR=52%) [33]. Vose et al., presented the results of The Nebraska Lymphoma Study Group studying the significance of age on response rate and survival. In this study, patients over the age of 70 received a planned 1/3 dose reduction. The rate and durability of complete response was similar in patients receiving dose reductions. The survival of patients was significantly lower as compared with patients <60years. The differences in survival did not seem to be influenced by either the malignancy or therapy [34]. Therapy can be individualized for the elderly based on performance status and co-morbidities.
Our study is limited by its relatively small size and by its setting at a single institution that may suffer a potential bias of chemotherapy delivery among the elderly. A large percentage of patients initially screened were excluded from the study because they presented following relapse and had previously received RCHOP outside the institution. Thus, selection bias exists for the included patients who elected to be treated at Vanderbilt University Medical Center for frontline therapy. It was beyond the scope of our study to identify patients who were not referred to our facility, but the well documented under treatment of elderly patients with aggressive lymphoma likely affected referral of elderly patients with significant co-morbidities from the community setting. Furthermore, while our study methods were able to identify two patients with a DLBCL diagnosis who did not receive RCHOP secondary to life-limiting co-morbidities, our methods identified only individuals seen in the outpatient setting. Therefore, it is possible additional patients in whom the diagnosis was made in the inpatient setting and did not survive to discharge were not identified nor included in our study population. Co-morbidity scoring was used to help adjust for potential effects of selection bias in our aged population. Here, pts≥70 had significant medical co-morbidities with over 40% having at least one severe or not optimally controlled chronic baseline condition based on Cumulative Illness rating Scale for Geriatrics (CIRS-G).
In our study, one patient experienced grade 3 cardiac toxicity. This patient had no known cardiac history. In monitoring patients for drug induced cardiac toxicity, cardiac biomarkers such as Troponin –I (cTnI) and β-natriuretic (BNP) peptide along with echocardiogram for monitoring ejection fraction among patients who have received a cumulative anthracycline dose of >200 mg/m2 (ie., 4 cycles of standard dose CHOP therapy) are emerging as important recommendations in identifying potential anthracycline toxicity [9, 35, 36]. This is specifically true and should be implemented among the elderly patients.
The incidence of NHL in elderly patients continues to rise and presents a growing clinical challenge. Our study suggests the vast majority of patients with DLBCL can be successfully treated with RCHOP regardless of age. The advent of G-CSF and improved supportive care likely contributed to the similar rates of severe treatment toxicity observed between pts≥70 and pts<70 years. Concern over insufficient use of doxorubicin-based chemotherapy in elderly patients with DLBCL likely is justified, as recent United States population based studies show poor diffusion of these treatments in elderly patients [18, 19]. In our study, even after adjusting for baseline clinical characteristics, age remained an independent predictor of RCHOP dose reduction. Recent studies of standardized dose-reduced regimens [37, 38] are encouraging in the quest to identify optimal chemotherapy dose intensity for elderly patients with aggressive lymphoma, but additional prospective studies that include co-morbidity scoring are needed to help identify target RCHOP intensity for elderly patients.
Conclusion:
With the aging population and the increasing number of patients diagnosed with large B cell lymphoma, more definite standards need to be established for the treatment of patients ≥ 70 years. We demonstrate that patients > 70 years can receive modest dose reductions without compromising the response rates or overall survival rate. Despite dose reductions, there is an increased rate of toxicities and hospitalizations among this age group. Until prospective studies identify the recommended doses, for the elderly with good performance status and no significant co-morbidities, full dose therapy with growth factors could be initiated with close monitoring of cytopenias and cardiac status which could result in the need for dose reduction. These recommendations are based on a clinical experience form a single institution and require validation in future trials. A national consortium through collaborations among various cooperative groups need to be established to make clear recommendations regarding chemotherapy dosing in patients > 70 years with large B cell lymphoma.
Funding:
NR funding provided by NCRR/NIH, and 5K-12 CA090625-09
References:
- 1.Muller AM, et al. , Epidemiology of non-Hodgkin’s lymphoma (NHL): trends, geographic distribution, and etiology. Ann Hematol, 2005. 84(1): p. 1–12. [DOI] [PubMed] [Google Scholar]
- 2.Devesa SS and Fears T, Non-Hodgkin’s lymphoma time trends: United States and international data. Cancer Res, 1992. 52(19 Suppl): p. 5432s–5440s. [PubMed] [Google Scholar]
- 3.Fisher RI, et al. , Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med, 1993. 328(14): p. 1002–6. [DOI] [PubMed] [Google Scholar]
- 4.Gordon LI, et al. , Comparison of a second-generation combination chemotherapeutic regimen (m-BACOD) with a standard regimen (CHOP) for advanced diffuse non-Hodgkin’s lymphoma. N Engl J Med, 1992. 327(19): p. 1342–9. [DOI] [PubMed] [Google Scholar]
- 5.Coiffier B, et al. , CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med, 2002. 346(4): p. 235–42. [DOI] [PubMed] [Google Scholar]
- 6.Feugier P, et al. , Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol, 2005. 23(18): p. 4117–26. [DOI] [PubMed] [Google Scholar]
- 7.Fisher RI, Miller TP, and O’Connor OA, Diffuse aggressive lymphoma. Hematology Am Soc Hematol Educ Program, 2004: p. 221–36. [DOI] [PubMed] [Google Scholar]
- 8.Lyman GH, et al. , Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin’s lymphoma: a nationwide study. J Clin Oncol, 2004. 22(21): p. 4302–11. [DOI] [PubMed] [Google Scholar]
- 9.Dodos F, et al. , Usefulness of myocardial performance index and biochemical markers for early detection of anthracycline-induced cardiotoxicity in adults. Clin Res Cardiol, 2008. 97(5): p. 318–26. [DOI] [PubMed] [Google Scholar]
- 10.Limat S, et al. , Early cardiotoxicity of the CHOP regimen in aggressive non-Hodgkin’s lymphoma. Ann Oncol, 2003. 14(2): p. 277–81. [DOI] [PubMed] [Google Scholar]
- 11.Gomez H, et al. , Elderly patients with aggressive non-Hodgkin’s lymphoma treated with CHOP chemotherapy plus granulocyte-macrophage colony-stimulating factor: identification of two age subgroups with differing hematologic toxicity. J Clin Oncol, 1998. 16(7): p. 2352–8. [DOI] [PubMed] [Google Scholar]
- 12.Lyman GH, et al. , Risk of febrile neutropenia among patients with intermediate-grade non-Hodgkin’s lymphoma receiving CHOP chemotherapy. Leuk Lymphoma, 2003. 44(12): p. 2069–76. [DOI] [PubMed] [Google Scholar]
- 13.Bertini M, Boccomini C, and Calvi R, The Influence of advanced age on the treatment and prognosis of diffuse large-cell lymphoma (DLCL). Clin Lymphoma, 2001. 1(4): p. 278–84. [DOI] [PubMed] [Google Scholar]
- 14.Pfreundschuh M, et al. , Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol, 2008. 9(2): p. 105–16. [DOI] [PubMed] [Google Scholar]
- 15.Mey UJ, et al. , Pegfilgrastim as hematopoietic support for dose-dense chemoimmunotherapy with R-CHOP-14 as first-line therapy in elderly patients with diffuse large B cell lymphoma. Support Care Cancer, 2007. 15(7): p. 877–84. [DOI] [PubMed] [Google Scholar]
- 16.Case DC Jr., et al. , Community-based trial of R-CHOP and maintenance rituximab for intermediate- or high-grade non-Hodgkin lymphoma with first-cycle filgrastim for older patients. Clin Lymphoma Myeloma, 2007. 7(5): p. 354–60. [DOI] [PubMed] [Google Scholar]
- 17.Lee KW, et al. , Doxorubicin-based chemotherapy for diffuse large B-cell lymphoma in elderly patients: comparison of treatment outcomes between young and elderly patients and the significance of doxorubicin dosage. Cancer, 2003. 98(12): p. 2651–6. [DOI] [PubMed] [Google Scholar]
- 18.Grann VR, et al. , Outcomes and diffusion of doxorubicin-based chemotherapy among elderly patients with aggressive non-Hodgkin lymphoma. Cancer, 2006. 107(7): p. 1530–41. [DOI] [PubMed] [Google Scholar]
- 19.Link BK, et al. , Diffuse large B-cell lymphoma in the elderly: diffusion of treatment with rituximab and survival advances with and without anthracyclines. Leuk Lymphoma, 2011. 52(6): p. 994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosly A, et al. , Achievement of optimal average relative dose intensity and correlation with survival in diffuse large B-cell lymphoma patients treated with CHOP. Ann Hematol, 2008. 87(4): p. 277–83. [DOI] [PubMed] [Google Scholar]
- 21.Pettengell R, Schwenkglenks M, and Bosly A, Association of reduced relative dose intensity and survival in lymphoma patients receiving CHOP-21 chemotherapy. Ann Hematol, 2008. 87(5): p. 429–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirakawa T, et al. , Importance of maintaining the relative dose intensity of CHOP-like regimens combined with rituximab in patients with diffuse large B-cell lymphoma. Ann Hematol, 2010. 89(9): p. 897–904. [DOI] [PubMed] [Google Scholar]
- 23.Miller MD, et al. , Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res, 1992. 41(3): p. 237–48. [DOI] [PubMed] [Google Scholar]
- 24.Hudon C, Fortin M, and Soubhi H, Abbreviated guidelines for scoring the Cumulative Illness Rating Scale (CIRS) in family practice. J Clin Epidemiol, 2007. 60(2): p. 212. [DOI] [PubMed] [Google Scholar]
- 25.Salvi F, et al. , A manual of guidelines to score the modified cumulative illness rating scale and its validation in acute hospitalized elderly patients. J Am Geriatr Soc, 2008. 56(10): p. 1926–31. [DOI] [PubMed] [Google Scholar]
- 26.Common Terminology Criteria for Adverse Events (CTCAE) and Common Toxicity Criteria (CTC), V3.Available at http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 27.Pfistner B, et al. , International harmonization of trial parameters in malignant lymphoma. Eur J Haematol Suppl, 2005(66): p. 53–4. [DOI] [PubMed] [Google Scholar]
- 28.Cheson BD, et al. , Revised response criteria for malignant lymphoma. J Clin Oncol, 2007. 25(5): p. 579–86. [DOI] [PubMed] [Google Scholar]
- 29.Ancestry.com.Social Security Death Index [database on-line] Provo, UR, USA: Ancestry.com Operations Inc, 2010. [date unknown], [no volume]. [Google Scholar]
- 30.Harris PA, et al. , Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform, 2009. 42(2): p. 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.PASW Statistics 18, SPSS Inc;2009. [Google Scholar]
- 32.Juweid ME, et al. , Response assessment of aggressive non-Hodgkin’s lymphoma by integrated International Workshop Criteria and fluorine-18-fluorodeoxyglucose positron emission tomography. J Clin Oncol, 2005. 23(21): p. 4652–61. [DOI] [PubMed] [Google Scholar]
- 33.Dixon DO, et al. , Effect of age on therapeutic outcome in advanced diffuse histiocytic lymphoma: the Southwest Oncology Group experience. J Clin Oncol, 1986. 4(3): p. 295–305. [DOI] [PubMed] [Google Scholar]
- 34.Vose JM, et al. , The importance of age in survival of patients treated with chemotherapy for aggressive non-Hodgkin’s lymphoma. J Clin Oncol, 1988. 6(12): p. 1838–44. [DOI] [PubMed] [Google Scholar]
- 35.Pichon MF, et al. , Drug-induced cardiotoxicity studied by longitudinal B-type natriuretic peptide assays and radionuclide ventriculography. In Vivo, 2005. 19(3): p. 567–76. [PubMed] [Google Scholar]
- 36.Nousiainen T, et al. , Natriuretic peptides as markers of cardiotoxicity during doxorubicin treatment for non-Hodgkin’s lymphoma. Eur J Haematol, 1999. 62(2): p. 135–41. [DOI] [PubMed] [Google Scholar]
- 37.Musolino A, et al. , Activity and safety of dose-adjusted infusional cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy with rituximab in very elderly patients with poor-prognostic untreated diffuse large B-cell non-Hodgkin lymphoma. Cancer, 2011. 117(5): p. 964–73. [DOI] [PubMed] [Google Scholar]
- 38.Wilson WH, et al. , Phase II study of dose-adjusted EPOCH and rituximab in untreated diffuse large B-cell lymphoma with analysis of germinal center and post-germinal center biomarkers. J Clin Oncol, 2008. 26(16): p. 2717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]


