Abstract
This work reports the synthesis and characterization of a new C^N-based cycloplatinated(II) fluoride complex [Pt(ppy)(PPh3)F] (2, ppy = 2-phenylpyridinate), involving a Pt‒F bond. The new complex is highly luminescent in green area with a high quantum yield of 94.6% at 77K. A comparison study of the heavier of halogen derivatives reveals a descending emission quantum yield order of F > Cl > Br > I. Time-dependent density functional theory (TD-DFT) calculations ascribe the decreased emission efficiency to the decreasing trend of intra ligand (IL) transition from F to I, which accounts for the major radiative pathway. In addition, 2 is capable of the fluorinating alkyl halides leading to Csp3‒F bond formation at room temperature.
Introduction
Halide ions are among the most common ligands used for stabilizing transition metals. The size, σ-bond donation ability, trans effect, and bond strengths to low oxidation state metals increase down the halide group, while the π-bond donation and bond strength to high oxidation state metals decrease from F to I.1 As a result, transition metal complexes usually show interesting halide effect in reactivity and properties such as luminescence. While transition metal complexes with Cl, Br, and I ligands are very common, the F containing analogues are much less available, especially for late transition metals cations in low oxidation states,2–5 thus preventing a complete study across the group. For example, halide ligands (Cl, Br, I) have been shown to largely influence the emission properties of Pt(II)6–9, Pt(IV)10, 11, and Cu(I)12–15 complexes. The scarcity of transition metal fluoride complexes is largely due to their high reactivity and the limited synthetic methods, as predicted by the hard/soft acid‒base theory that the fluoride ion (hard base) is mismatched with soft acid such as Pt(II), Pd(II), Au(I).16
Our groups have been actively studying cycloplatinated complexes due to their intriguing photophysical and biological properties.17–22 Some Pt(II)23–28 and Pt(IV)29–37 fluoride complexes are known in literature, however, cycloplatinated complexes bearing a Pt–F bond are unprecendented.38–40 To the best of our knowledge, only one report discussed the halide effect on the photophysical properties of Pt(IV) complexes involving all the F, Cl, Br, and I ligands.10 A systematic investigation of the halide effect on the photophysical properties of Pt(II) is not available. Herein, we report the synthesis, photophysical properties and reactivity of a C^N-based cycloplatinated(II) complex with a fluoride ligand.
Results and Discussion
Synthesis and Structural Characterization
The Pt fluoride complex [Pt(ppy)(PPh3)F] (2, ppy = 2-phenylpyridinate) was efficiently synthesized through F/X exchange from its heavier halogen counterparts [Pt(ppy)(PPh3)X] (1, X = Cl, 1a; Br, 1b; I, 1c)41, 42 using AgF as the fluoride source (Scheme 1, yield > 85%). The halide metathesis reaction was performed in CH2Cl2 at room temperature with 1.4 equiv. of AgF (see the Experimental Section for details).
Scheme 1.
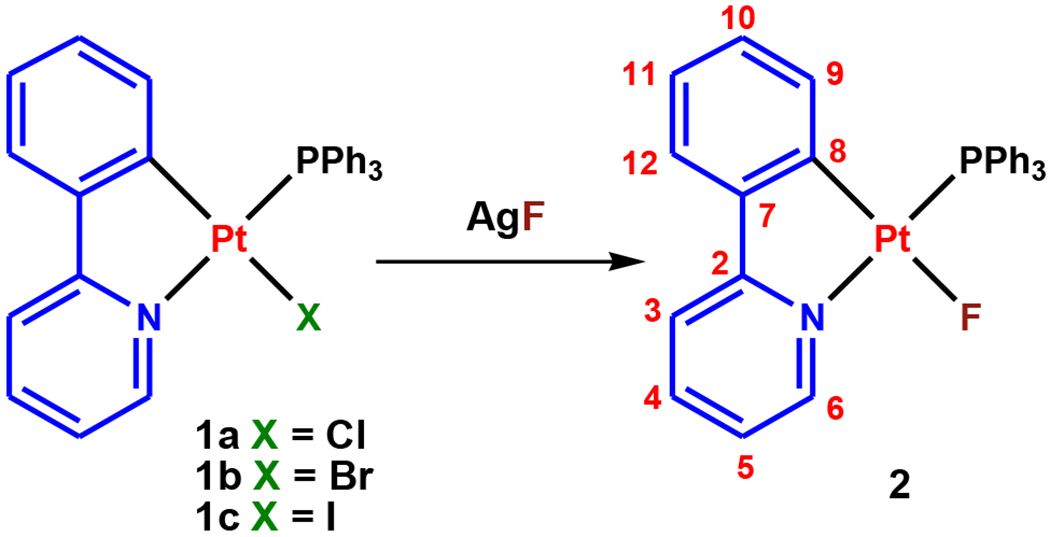
Synthetic route and ligand numbering system for 2.
The successful formation of 2 with Pt‒F bond was first identified using the multinuclear 1D (Figures 1 and S1–S5) and 2D (Figures S6–S8) NMR spectroscopy. The 19F NMR spectrum of 2 shows a sharp doublet signal (2JPF = 17 Hz) flanked by Pt satellites (1JPtF = 340 Hz) at δ = ‒241.4 ppm providing direct evidence of Pt‒F bonding. Correspondingly, a doublet signal appears in the 31P{1H} NMR spectrum of 2 (δ = 24.0 ppm) which has a 2JPF coupling constant of 17 Hz consistent with that observed from 19F NMR spectroscopy (Figure 1). This doublet signal has Pt satellites with 1JPtP = 4406 Hz which is normal for direct Pt‒P bonds in Pt(II) complexes.17, 21, 42 Expectedly, the corresponding 195Pt{1H} NMR spectrum includes a sharp doublet of doublet signal at δ = ‒3917 ppm (1JPtP = 4411 Hz, 1JPtF = 342 Hz) due to the coupling with both the coordinated P and F atoms, respectively.
Figure 1.
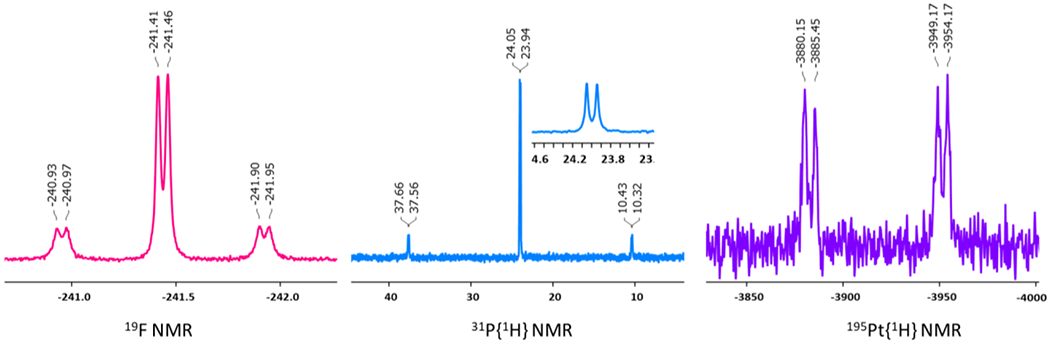
Heteronuclear NMR spectra of 2 in CD2Cl2.
The HR-ESI-Mass spectrum of 2 (Figure S9) shows an ion with an isotope pattern diagnostic for [M‒F]+ moiety (m/z = 611.1206). Finally, the molecular structure of 2 was unambiguously characterized by single crystal X-ray crystallography (Figure 2). The crystallographic data (Table S1) and the selected bond lengths and angels are collected in Table S2. The crystal structure of 2 clearly indicates that the fluoride ligand is directly bound to the Pt center and located trans to the ligating C atom of the ppy ligand. The bond length of Pt‒F is 2.042 Å, in the typical range of tetracoordinated platinum fluoride complexes.23–28 The platinum adopts a distorted-square-planar coordination geometry with angles ranging from 81.38 to 98.88° and the maximum deviation from the mean plane through PtCNPF is 0.018 Å. The fluoride ligand involves hydrogen bonding interactions with co-crystallized CH2Cl2 solvent43 (1.950 Å, Figure 2), H6 of ppy ligand42 (2.306 Å), and one hydrogen from PPh3 (2.209 Å) of the vicinal molecule. These interactions are much shorter than the sum of the van der Waals radii of H and F (2.68 Å)44–47 (Figure S10).
Figure 2.
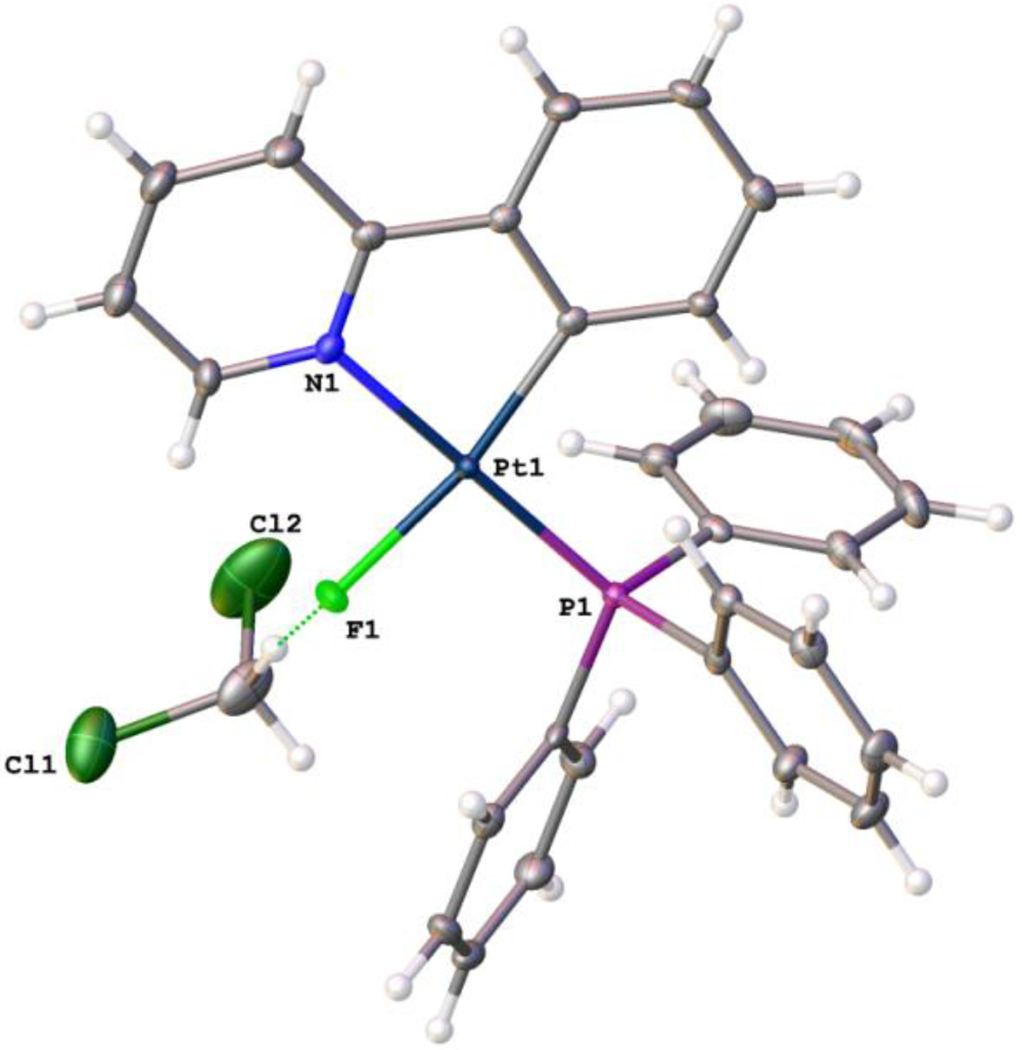
Molecular structure of 2·CH2Cl2. Ellipsoids are drawn at a 50% probability level. Selected bond lengths (Å) and angles (deg): Pt(1)-P(1) 2.2288(7), Pt(1)-F(1) 2.0417(18), Pt(1)-N(1) 2.057(2), Pt(1)-C(11) 1.994(3); F(1)-Pt(1)-P(1) 91.87(5), F(1)-Pt(1)-N(1) 87.88(9), C(11)-Pt(1)-P(1) 98.88(8), C(11)-Pt(1)-N(1) 81.38(11).
Photophysical Properties
The fluoride complex 2 is highly emissive in green area in different states and temperature conditions. In solid state at 298 K, 2 gives a completely structured emission band centered at 490 nm with a vibronic progression at 523 nm and a shoulder at around 560 nm, indicating a large composition of 3IL with small contribution of 3MLCT in the emissive state (Figure 3). The lifetime of 2 was measured to be 11.6 µs, confirming the phosphorescence character of the emission. By lowering the temperature down to 77 K, 2 exhibits a much brighter emission with a significant increase in quantum yield (Φ) from 53.1 to 94.6%. There is no tangible change in the position and shape of the emission band at 77 K compared to that of at room temperature (Figure 3). In CH2Cl2 solution state at 298 K, 2 keeps its emission in green region (Φ = 8.4%), yielding a structured emission band with a slight blue shift compared with that of the solid state (Figure 3). The emission band of the frozen solution (77 K) appears at 480 nm with a slight blue shift and more structured shape compared with that of at 298 K (Figure 3).
Figure 3.
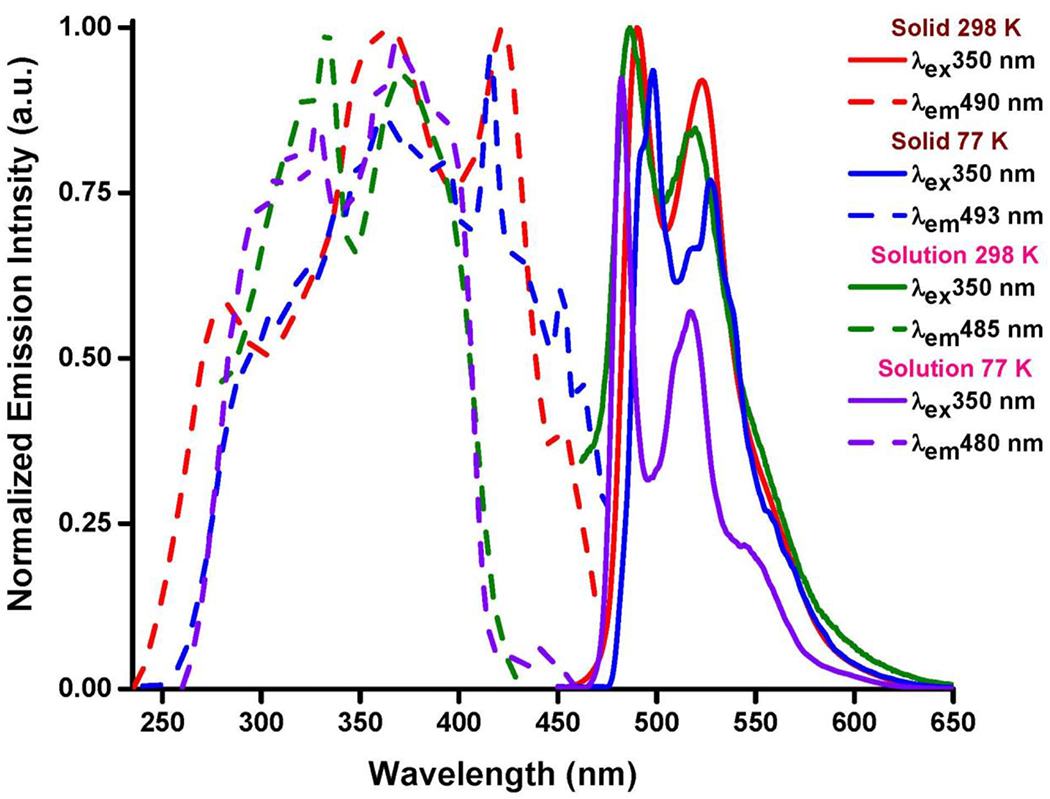
Normalized emission (solid lines) and excitation (dashed lines) spectra of 2 in the solid state and CH2Cl2 solution (10−3 M) at 298 and 77 K.
Interestingly, in solid state the similar emission bands but with decreased intensities were observed from 2 to 1c (F to I), while 1c is practically non-emissive (Figure 4 and Table 1). The descending trend of Φ values for 2 (53.1%), 1a (47.0%) and 1b (31.0%) certifies this observation. This was supported by the obtained non-radiative rate constants (knr) of the complexes (2 [4.06×104], 1a [7.36×104] and 1b [10.0×104]) which are on the increase from 2 to 1b.
Figure 4.
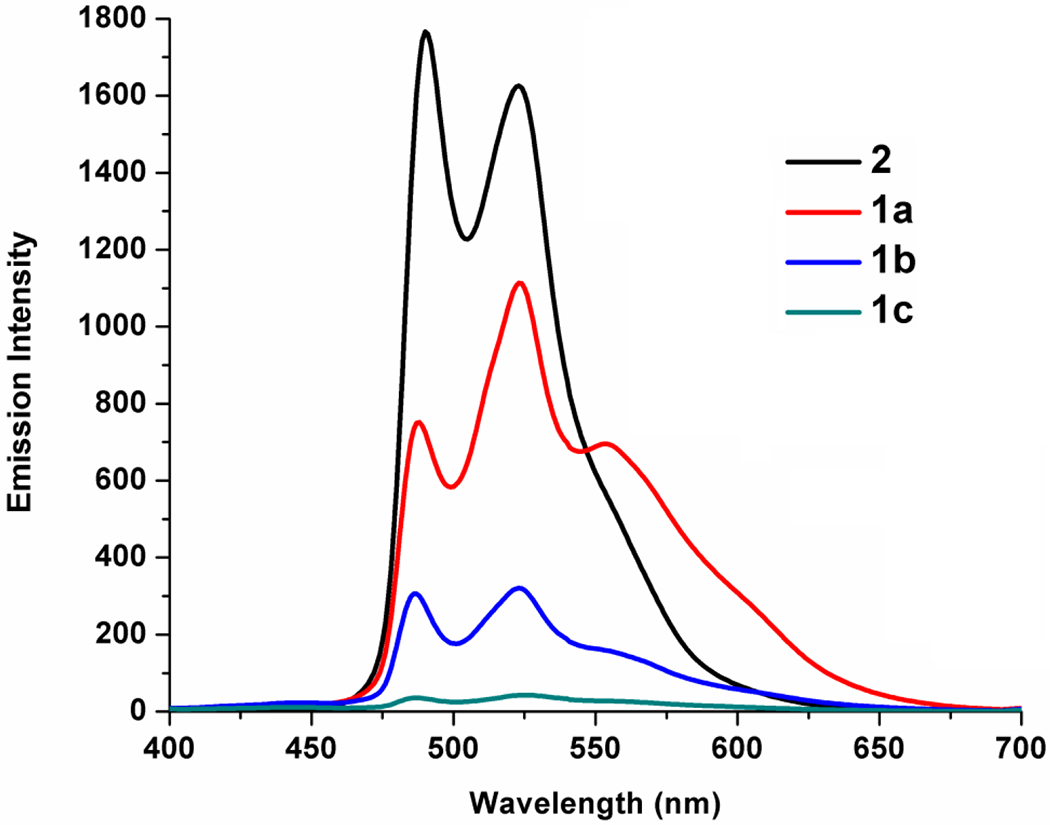
The emission spectra of 1a–c and 2 in solid state at 298 K (λex = 350 nm).
Table 1.
Numerical data of the emission properties of 1a, 1b and 2.
| Complex | λem/nm (λex/nm) | τ/μs | Φ(%) | kra | knra |
|---|---|---|---|---|---|
| 2 | 490max, 522, 560sh
(350), Solid (298 K) 493max, 529, 560sh (350), Solid (77 K) 485max, 518, 555sh (350), 10−3 M (298 K) 480max, 516, 551sh (350), 10−3 M (77 K) |
11.6 21.5 2.3 16.5 |
53.1 94.6 8.4 88.1 |
4.56×104 4.40×104 3.65×104 4.67×104 |
4.06×104 0.25×104 39.8×104 1.39×104 |
| 1a | 487, 522max, 554sh (350), Solid (298 K) | 7.2 | 47.0 | 6.52×104 | 7.36×104 |
| 1b | 487, 522max, 554sh (350), Solid (298 K) | 6.9 | 31.0 | 4.49×104 | 10.0×104 |
kr and knr were calculated according to the equations kr = Φ/τ and knr = (1/τ)-kr respectively.
To investigate the nature of electronic transitions, the UV-vis spectra were obtained for 2 and 1a–c in CH2Cl2 (Figures 5 and S11, Table S3). Density functional theory (DFT) and time-dependent DFT (TD-DFT) calculation methods were carried out for better understanding of the ground and excited states. All complexes were optimized in CH2Cl2 solution and gas phase (considered as solid state). Besides, their DFT-optimized structures (Figure S12) and the selected geometrical parameters (Table S4) are given in the Supporting Information. Then, the frontier molecular orbitals (MOs) involving “HOMO to HOMO-5” and “LUMO to LUMO+5” were calculated for all the complexes (Tables S5–S8 and visual plots in Figures S13–S16). In all the cases, HOMO is mostly localized on the Pt, ppy and halogen moieties, of which the contribution of halogen ligand increases from F to I. However, LUMO is remarkably centered on the ppy ligand (around 88%). The contribution of PPh3 is negligible in HOMO and LUMO levels, but it considerably increases in lower HOMOs and higher LUMOs.
Figure 5.
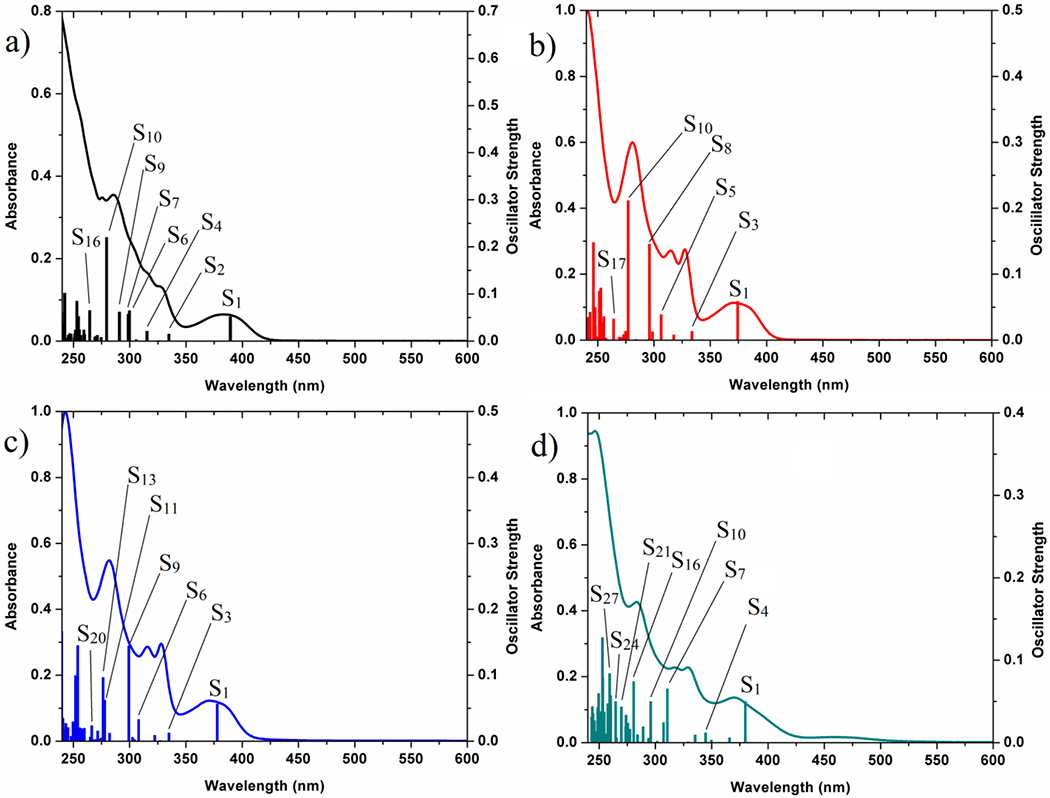
Overlaid experimental UV-vis spectra and theoretical TD-DFT bars for (a) 2, (b) 1a, (c) 1b and (d) 1c.
TD-DFT calculated electronic transitions are in good agreement with the experimental UV-vis absorption spectra for all the complexes (Figure 5). The low energy region 350–425 nm is related to the S0→S1 transition which is mainly contributed by the HOMO→LUMO transition (> 90%). This region is assigned as the mixed 1IL/1MLCT and 1XLCT (L = ppy, X = halides) characters. In compliance with the experimental data, the low energy band in 2 is red shifted compared to those of the other derivatives (Figure S17). For the more intense absorbing high energy bands (250–350 nm), in addition to IL, MLCT, and XLCT, some other characters like MLʹCT, LLʹCT, LʹLCT and XLʹCT (Lʹ = PPh3) can be observed, indicating the important role of PPh3 as the beginning or destination of the electronic transitions (Tables S9–S12).
The theoretical emission wavelengths were calculated for 2, 1a and 1b, using the energy gap between the optimized structures of S0 and T1 states in the gas phase. The calculated energy gaps between S0 and T1 are 2.516 (2), 2.595 (1a) and 2.579 (1b) eV, corresponding to the emission wavelengths of 493, 478, and 481 nm, respectively. These theoretical emissions are very close to the corresponding experimental values at 298 K (Table 1). To obtain further insight into the nature of the emissions, the frontier molecular orbitals were calculated for S0 (HOMO and LUMO) and T1 (LSOMO and HSOMO) states in the gas phase. Figure 6 depicts the HOMO-LUMO and LSOMO-HSOMO energy levels diagram for 2 while those of 1a and 1b are shown in Figures S18 and S19, respectively, and summarized in Table 2. For 2, LSOMO is close to HOMO in terms of the composition and energy. HSOMO also resembles LUMO (both localized on ppy moiety), but with a significant stabilization in energy. By looking at the LSOMO and HSOMO plots, the mixed 3IL/3MLCT/3XLCT emission character can be concluded for the complexes.
Figure 6.
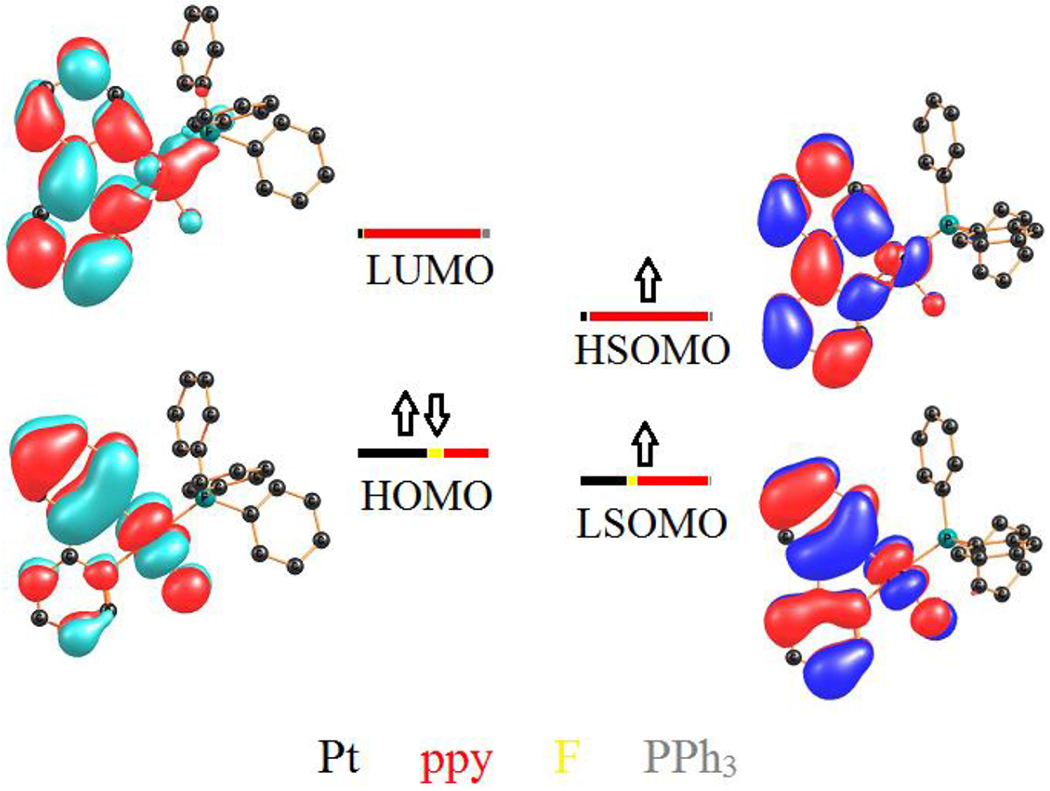
Frontier molecular orbital plots of the calculated S0 (left) and T1 (right) states of 2.
Table 2.
Composition (%) of the selected MOs in S0 and T1 states in gas phase for 2 (X = F) and 1a-c (X = Cl, Br, I).
| Complex | MO | Energy (eV) | Components (%) |
|||
|---|---|---|---|---|---|---|
| Pt | X | ppy | PPh3 | |||
| 2 | LUMO+4 | −0.495 | 20 | 1 | 9 | 69 |
| LUMO | −1.459 | 5 | 1 | 88 | 7 | |
| HOMO | −5.285 | 52 | 11 | 35 | 1 | |
| HOMO-1 | −5.986 | 89 | 1 | 2 | 8 | |
| HSOMO | −2.926 | 6 | 1 | 90 | 3 | |
| LSOMO | −5.768 | 36 | 7 | 54 | 2 | |
| 1a | LUMO+3 | −0.784 | 28 | 0 | 14 | 58 |
| LUMO | −1.547 | 5 | 1 | 89 | 6 | |
| HOMO | −5.375 | 43 | 30 | 27 | 1 | |
| HOMO-2 | −6.099 | 85 | 0 | 6 | 9 | |
| HSOMO | −3.080 | 6 | 1 | 91 | 3 | |
| LSOMO | −5.809 | 34 | 29 | 35 | 1 | |
| 1b | LUMO+3 | −0.862 | 28 | 4 | 19 | 49 |
| LUMO | −1.546 | 5 | 1 | 89 | 5 | |
| HOMO | −5.264 | 35 | 45 | 18 | 1 | |
| HOMO-2 | −6.081 | 78 | 4 | 11 | 7 | |
| HSOMO | −3.015 | 7 | 1 | 87 | 4 | |
| LSOMO | −5.699 | 30 | 37 | 30 | 3 | |
| 1c | LUMO+1 | −0.976 | 31 | 5 | 18 | 46 |
| LUMO | −1.624 | 5 | 1 | 89 | 5 | |
| HOMO | −5.136 | 25 | 62 | 10 | 3 | |
| HOMO-3 | −6.010 | 49 | 15 | 31 | 5 | |
In order to understand the halide effect on the emission of these Pt complexes, TD-DFT calculations were performed in the gas phase. Figure 7 demonstrates the comparative diagram for the energy levels of the calculated MOs of 1a‒c and 2. Similar to the solution states, the lowest electronic transitions are attributed to the HOMO→LUMO transition for all the complexes wherein the HOMO-LUMO energy gap is almost on the decrease from F to I (2 [3.826 eV], 1a [3.828 eV], 1b [3.718 eV] and 1c [3.512 eV]). In all the cases, based on the contribution percentages shown in Table 2, the HOMO→LUMO transition is assigned as mixed IL/MLCT/XLCT characters. The contribution of halide ligands in HOMO increases from F to I (Table 2, 2 [11 %], 1a [30 %], 1b [45 %] and 1c [62 %]), while the contribution of ppy ligand decreases (Table 2, 2 [35 %], 1a [27 %], 1b [18 %] and 1c [10 %]). As a result, the XLCT character grows and the IL character concedes (intra ligand transition in ppy) from F to I. Additionally, as can be observed in Figure 7, the energies of the dσ* orbitals of 2 are higher than those of the other complexes 1a-c. It suggests the higher energies of the MC states for 2 which qualitatively explain the trend of emission strength for all the complexes.
Figure 7.
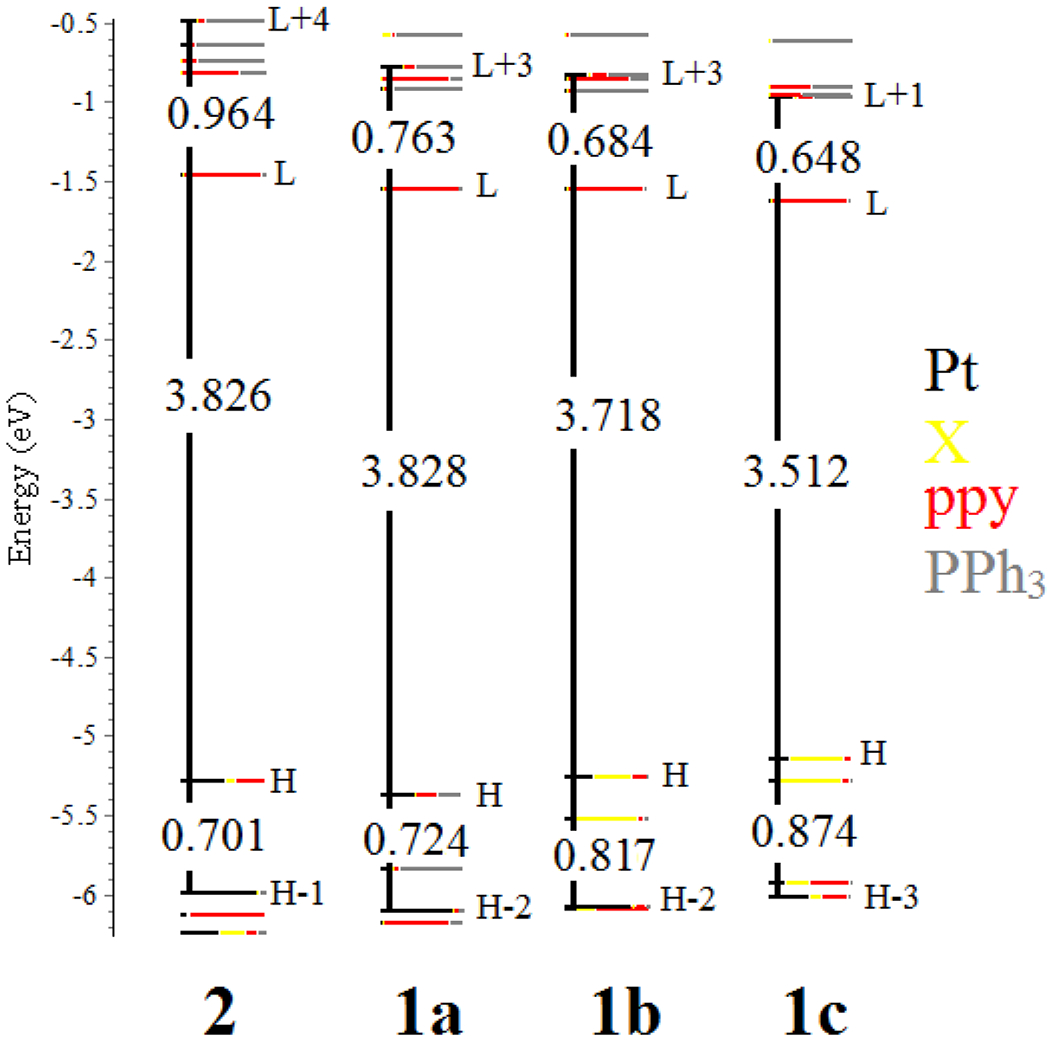
Comparative energy level diagram for the calculated MOs of 1a-c and 2 in the gas phase. The energy values are shown in eV.
Csp3–F Bond Formation
Transition metal fluoride complexes are important intermediates in C–F bond activation/formation reactions.38, 48–52 To test its reactivity for fluorination reaction, 2 was reacted with methyl iodide, ethyl iodide and allyl bromide. In situ 19F NMR monitoring of the reactions (Figures 8, S20 and S21) showed the consumption of 2 and the concomitant formation of CH3–F, C2H5–F, and allyl fluoride respectively at room temperature. The formation of the corresponding Pt complexes 1b and 1c, were observed in their 1H and 31P{1H} NMR spectra.42 The reaction was much faster for allyl bromide (completed in 10 min at room temperature) compared with that for methyl iodide and ethyl iodide. The higher reactivity of allyl bromide might result from its more electron-deficient allylic carbon and/or its strong interaction with the metal center via coordination.53
Figure 8.
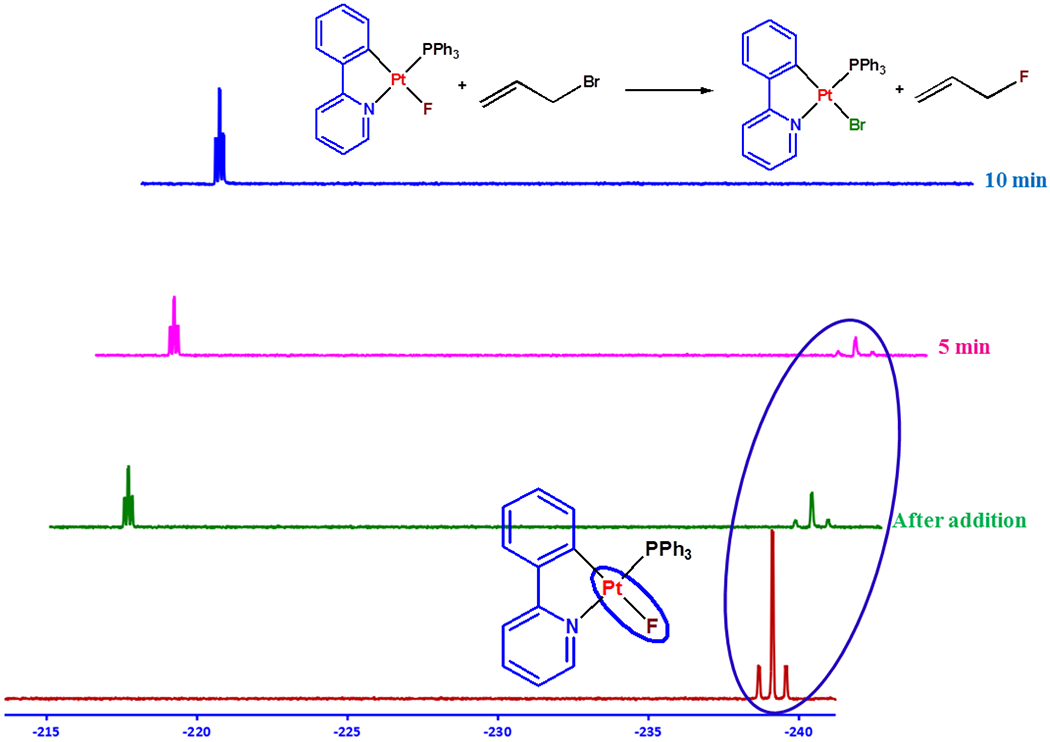
Monitoring the reaction of 2 with allyl bromide by 19F NMR spectroscopy in acetone-d6 at room temperature.
Conclusions
In summary, we report here the first example of a C^N-type cycloplatinated(II) fluoride complex. The complex exhibits an emission centered on the cyclometalated ligand in green region, being stronger (solid and solution) than its heavier halide derivatives (F ˃ Cl ˃ Br ˃ I). The theoretical calculations indicated that the nature of halogen ligand significantly affects the emissions. TD-DFT calculations exhibited that, from F to I, the contribution of XLCT character in HOMO→LUMO transition is intensified, lowering the contribution of IL character as the center of emission. Besides, the new Pt‒F complex is able to participate in Csp3‒F bond formation reactions with alkyl halides. We are currently investigating the ligand electronic effect on the reactivity, the scope of the substrates and the mechanism of C–F bond formation.
Experimental Section
General Remarks
1H (700 MHz), 13C{1H} (176 MHz), 19F (376 MHz), 31P{1H} (162 MHz) and 195Pt{1H} (64 MHz) NMR spectra were recorded on Bruker Avance 700, 400 or 300 MHz instruments at room temperature. All chemical shifts (δ) are reported in ppm relative to their corresponding external standards (SiMe4 for 1H and 13C{1H}, CFCl3 for 19F, 85% H3PO4 for 31P{1H}, Na2PtCl6 for 195Pt{1H}). The instrument for HR ESI-Mass measurement was a Shimadzu IT-TOF with an electrospray ionization source, which is part of the Arkansas Statewide Mass Spectrometry Facility. UV-vis absorption spectra were recorded on a JASCO V-770 UV-visible/NIR spectrophotometer. Emission spectra were measured on a JASCO FP-8500 spectrofluorometer. The lifetimes were measured in the phosphorimeter mode and the quantum yields of the complexes were measured using an integrating sphere. The 2-phenylpyridine (ppy), triphenylphosphine (PPh3), silver fluoride (AgF) and all the other chemicals were purchased from commercial resources. All the reactions were carried out under Argon atmosphere and in the common solvents and all solvents were purified and dried according to standard procedures before using.54 The complexes [Pt(ppy)(PPh3)Cl], 1a, [Pt(ppy)(PPh3)Br], 1b, [Pt(ppy)(PPh3)I], 1c, were prepared as published methods.42 The NMR labeling is shown in Scheme 1 for clarifying the chemical shift assignments.
Synthesis of [Pt(ppy)(PPh3)F], 2
To a solution of 1a (200 mg, 0.31 mmol, 1 eq.) in CH2Cl2 (20 mL) was added AgF (55 mg, 0.43 mmol, 1.4 eq.). The reaction mixture was stirred for 3 days in dark at room temperature, and then filtered through cotton/Celite in a glass Pasteur pipette to remove AgCl. The resulting greenish solution was concentrated to a small volume (~ 1 mL) and n-hexane (5 mL) was added to precipitate 2 as a green solid. Yield: 87% (169 mg, 0.27 mmol). HR ESI-MS(+) m/z Cacld. for C29H23NPPt [M‒F]+ 611.1215; Found 611.1206. Elem. Anal. Calcd for C29H23FNPPt (630.55): C, 55.24; H, 3.68; N, 2.22; Found: C, 55.41; H, 3.74; N, 2.19. 1H NMR (700 MHz, CD2Cl2, 295 K): δ 9.07 (ddd, 3JPtH = 28.3 Hz, 3JHH = 4.8 Hz, 4JFH = 8.7 Hz, 4JPH = 4.1 Hz, 1H, H6), 7.94 (t, 3JHH = 7.9 Hz, 1H, H4), 7.81 (d, 3JHH = 7.9 Hz, 1H, H3), 7.74 (dd, 3JHH = 7.8 Hz, 3JPH = 11.2 Hz, 6H, Ho of PPh3), 7.51 (d, 3JHH = 7.6 Hz, 1H, H12), 7.48 (t, 3JHH = 7.2 Hz, 3H, Hp of PPh3), 7.41 (t, 3JHH = 7.2 Hz, 6H, Hm of PPh3), 7.36 (t, 3JHH = 6.3 Hz, 1H, H5), 6.93 (t, 3JHH = 7.4 Hz, 1H, H11), 6.52 (ddd, 3JPtH = 49.1 Hz, 3JHH = 7.6 Hz, 4JPH = 3.9 Hz, 1H, H9), 6.50 (t, 3JHH = 7.3 Hz, 1H, H10); 13C{1H} NMR (376 MHz, CD2Cl2, 295 K): δ 164.9 (d, 3JPc = 3 Hz, C2), 146.8 (d, 2JPtc = 21 Hz, 3JFc = 13 Hz, C6), 146.1 (s, C7), 140.6 (s, C4), 139.6 (dd, 2JPtc = 83 Hz, 3JFc = 7 Hz, 3JPc = 5 Hz, C9), 135.6 (d, 3JPtc = 38 Hz, 2JPc = 13 Hz, Co of PPh3), 131.3 (d, 4JPc = 2 Hz, Cp of PPh3), 129.8 (dd, 3JPtc = 57 Hz, 4JPc = 2 Hz, 4JFc = 4 Hz, C10), 129.7 (d, 1JPc = 61 Hz, Cipso of PPh3), 128.6 (d, 3JPc = 10 Hz, Cm of PPh3), 124.0 (s, 3JPtc = 30 Hz, C12), 123.4 (s, C11), 122.3 (d, 3JPtc = 16 Hz, 4JPc = 2 Hz, C5), 118.7 (d, 3JPtc = 17 Hz, 4JPc = 2 Hz, C3); 19F NMR (376 MHz, CD2Cl2, 295 K): δ −241.4 (d, 1JPtF = 340 Hz, 2JPF = 17 Hz, 1F); 31P{1H} NMR (162 MHz, CD2Cl2, 295 K): δ 24.0 (d, 1JPtP = 4406 Hz, 2JPF = 17 Hz, 1P); 195Pt{1H} NMR (64 MHz, CD2Cl2, 295 K): δ −3917 (dd, 1JPtP = 4411 Hz 1JPtF = 342 Hz, 1Pt).
Monitoring the Reaction of 2 with Alkyl Halides (MeI, EtI and AllylBr) by NMR Spectroscopy
To a solution of 2 (10 mg, 0.016 mmol) in acetone-d6 (0.75 mL) in an NMR tube was added the appropriate alkyl halides (10 μL, 0.16 mmol for Me‒I; 26 μL, 0.32 mmol for Et‒I; 2 μL, 0.025 mmol for allyl bromide) at 298 K. The tube was then placed in the probe of the NMR spectrometer and NMR spectra were obtained at appropriate time intervals (Figures 8, S20 and S21).
X-ray Structure Determination
The appropriate crystals of 2 were obtained by slow evaporation of its saturated solution in CH2Cl2/diisopropyl ether at room temperature. Single crystal X-ray diffraction intensity data of 2 was collected at 100(2) K using a Bruker APEX-II CCD diffractometer equipped with graphite monochromated MoKα radiation (λ = 0.71073 Å). Data reduction was carried out using the program Bruker SAINT55 and an empirical absorption correction was applied based on multi-scan method.56 The structure of 2 was solved by direct method and refined by the full-matrix least-square technique on |F|2 with anisotropic thermal parameters to describe the thermal motions of all non-hydrogen atoms using the programs (SHELXS-14)57 and (SHELXL-18),58 respectively. All hydrogen atoms were located from difference Fourier map and refined isotropically. The summary of crystal data and relevant structure refinement parameters for 2 (CCDC 2008721) are given in Table S1.
Computational Details
Density functional calculations were performed with the program suite Gaussian 0959 using the B3LYP level of theory.60–62 The LANL2DZ basis set was chosen to describe Pt63, 64 and the 6–31G(d) basis set was chosen for other atoms. The geometries of complexes were fully optimized by employing the density functional theory without imposing any symmetry constraints. In order to ensure the optimized geometries, frequency calculations were performed employing analytical second derivatives. Time-dependent DFT (TD-DFT) calculations were carried out at the same level of theory and basis sets. Solvent effects have been considered by the conductor-like polarizable continuum model (CPCM)65, 66. The calculations for the electronic absorption spectra by time-dependent DFT (TD-DFT) were performed at the same level of theory.
Supplementary Material
SYNOPSIS.
A cycloplatinated(II) fluoride complex was prepared and it revealed an interesting photophysical properties and reactivity for Csp3‒F bond formation.
ACKNOWLEDGMENT
H.B. gratefully acknowledges the financial support through the startup funds from the University of Arkansas and the NIH-NIGMS (GM132906). The Institute for Advanced Studies in Basic Sciences (IASBS) Research Council (G2020IASBS32629) and the Iran National Science Foundation (Grant no. 97007977) are also gratefully acknowledged. H.R.S. wishes to acknowledge IASBS for their sabbatical leave at University of Arkansas.
Footnotes
NMR and HR ESI-MS spectra of 2, excitation and emission spectra, computational data of complexes (PDF). Crystallographic data (CIF). Cartesian coordinates (XYZ).
Accession Codes
CCDC 2008721 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
The authors declare no competing financial interest.
Contributor Information
Jiyun Hu, Department of Chemistry and Biochemistry, University of Arkansas, Fayetteville, Arkansas, 72701, United States.
Mahshid Nikravesh, Department of Chemistry, Institute for Advanced Studies in Basic Sciences (IASBS), Zanjan, 45137-66731, Iran.
Hamid R. Shahsavari, Department of Chemistry, Institute for Advanced Studies in Basic Sciences (IASBS), Zanjan, 45137-66731, Iran; Department of Chemistry and Biochemistry, University of Arkansas, Fayetteville, Arkansas, 72701, United States.
Reza Babadi Aghakhanpour, Department of Chemistry, Institute for Advanced Studies in Basic Sciences (IASBS), Zanjan, 45137-66731, Iran.
Arnold L. Rheingold, Department of Chemistry, University of California, San Diego, La Jolla, California 92093, United States.
Mia Alshami, Department of Chemistry and Biochemistry, University of Arkansas, Fayetteville, Arkansas, 72701, United States.
Yoshie Sakamaki, Department of Chemistry and Biochemistry, University of Arkansas, Fayetteville, Arkansas, 72701, United States.
Hudson Beyzavi, Department of Chemistry and Biochemistry, University of Arkansas, Fayetteville, Arkansas, 72701, United States.
References
- 1.Fagnou K; Lautens M, Halide Effects in Transition Metal Catalysis. Angew. Chem. Int. Ed 2002, 41, 26–47. [DOI] [PubMed] [Google Scholar]
- 2.Doherty NM; Hoffmann NW, Transition-Metal Fluoro Compounds Containing Carbonyl, Phosphine, Arsine, and Stibine Ligands. Chem. Rev 1991, 91, 553–573. [Google Scholar]
- 3.Murphy EF; Murugavel R; Roesky HW, Organometallic Fluorides: Compounds Containing Carbon−Metal−Fluorine Fragments of d-Block Metals. Chem. Rev 1997, 97, 3425–3468. [DOI] [PubMed] [Google Scholar]
- 4.Nahra F; Brill M; Gómez-Herrera A; Cazin CS; Nolan SP, Transition Metal Bifluorides. Coord. Chem. Rev 2016, 307, 65–80. [Google Scholar]
- 5.Pagenkopf BL; Carreira EM, Transition Metal Fluoride Complexes in Asymmetric Catalysis. Chem. Eur. J 1999, 5, 3437–3442. [Google Scholar]
- 6.Irmler P; Winter RF, Complexes trans-Pt (BODIPY)X(PEt3)2: Excitation Energy-dependent Fluorescence and Phosphorescence Emissions, Oxygen Sensing and Photocatalysis. Dalton Trans. 2016, 45, 10420–10434. [DOI] [PubMed] [Google Scholar]
- 7.Paziresh S; Aghakhanpour RB; Rashidi M; Nabavizadeh SM, Simple Tuning of the Luminescence Properties of the Double Rollover Cycloplatinated(II) Structure by Halide Ligands. New J. Chem 2018, 42, 1337–1346. [Google Scholar]
- 8.Sangari MS; Haghighi MG; Nabavizadeh SM; Pfitzner A; Rashidi M, Influence of Ancillary Ligands on the Photophysical Properties of Cyclometalated Organoplatinum(II) Complexes. New J. Chem 2018, 42, 8661–8671. [Google Scholar]
- 9.Lázaro A; Serra O; Rodríguez L; Crespo M; Font-Bardia M, Luminescence Studies of New [C,N,N′] Cyclometallated Platinum(II) and Platinum(IV) Compounds. New J. Chem 2019, 43, 1247–1256. [Google Scholar]
- 10.Juliá F; García-Legaz M-D; Bautista D; González-Herrero P, Influence of Ancillary Ligands and Isomerism on the Luminescence of Bis-cyclometalated Platinum(IV) Complexes. Inorg. Chem 2016, 55, 7647–7660. [DOI] [PubMed] [Google Scholar]
- 11.Lázaro A; Balcells C; Quirante J; Badia J; Baldomà L; Ward JS; Rissanen K; Font-Bardia M; Rodríguez L; Crespo M, Luminescent PtII and PtIV Platinacycles with Anticancer Activity Against Multiplatinum-Resistant Metastatic CRC and CRPC Cell Models. Chem. Eur. J 2020, 26, 1947–1952. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J; Duan C; Han C; Yang H; Wei Y; Xu H, Balanced Dual Emissions from Tridentate Phosphine-Coordinate Copper(I) Complexes toward Highly Efficient Yellow OLEDs. Adv. Mater 2016, 28, 5975–5979. [DOI] [PubMed] [Google Scholar]
- 13.Jia J-H; Chen X-L; Liao J-Z; Liang D; Yang M-X; Yu R; Lu C-Z, Highly Luminescent Copper(I) Halide Complexes Chelated with a Tetradentate Ligand (PNNP): Synthesis, Structure, Photophysical Properties and Theoretical Studies. Dalton Trans 2019, 48, 1418–1426. [DOI] [PubMed] [Google Scholar]
- 14.Baranov AY; Berezin AS; Samsonenko DG; Mazur AS; Tolstoy PM; Plyusnin VF; Kolesnikov IE; Artem’Ev AV, New Cu(I) Halide Complexes Showing TADF Combined with Room Temperature Phosphorescence: The Balance Tuned by Halogens. Dalton Trans 2020, 49, 3155–3163. [DOI] [PubMed] [Google Scholar]
- 15.Zink DM; Bächle M; Baumann T; Nieger M; Kühn M; Wang C; Klopper W; Monkowius U; Hofbeck T; Yersin H, Synthesis, Structure, and Characterization of Dinuclear Copper(I) Halide Complexes with P^N Ligands Featuring Exciting Photoluminescence Properties. Inorg. Chem 2013, 52, 2292–2305. [DOI] [PubMed] [Google Scholar]
- 16.Pearson RG, Hard and Soft Acids and Bases. J. Am. Chem. Soc 1963, 85, 3533–3539. [Google Scholar]
- 17.Shahsavari HR; Babadi Aghakhanpour R; Nikravesh M; Ozdemir J; Golbon Haghighi M; Notash B; Beyzavi MH, Highly Emissive Cycloplatinated(II) Complexes Obtained by the Chloride Abstraction from the Complex [Pt(ppy)(PPh3)(Cl)]: Employing Various Silver Salts. Organometallics 2018, 37, 2890–2900. [Google Scholar]
- 18.Fereidoonnezhad M; Shahsavari HR; Abedanzadeh S; Behchenari B; Hossein-Abadi M; Faghih Z; Beyzavi MH, Cycloplatinated(II) Complexes Bearing 1,1’-Bis(diphenylphosphino)ferrocene Ligand: Biological Evaluation and Molecular Docking Studies. New J. Chem 2018, 42, 2385–2392. [Google Scholar]
- 19.Fereidoonnezhad M; Kaboudin B; Mirzaee T; Babadi Aghakhanpour R; Golbon Haghighi M; Faghih Z; Faghih Z; Ahmadipour Z; Notash B; Shahsavari HR, Cyclometalated Platinum(II) Complexes Bearing Bidentate O,O′-Di(alkyl)dithiophosphate Ligands: Photoluminescence and Cytotoxic Properties. Organometallics 2017, 36, 1707–1717. [Google Scholar]
- 20.Shahsavari HR; Giménez N; Lalinde E; Moreno MT; Fereidoonnezhad M; Babadi Aghakhanpour R; Khatami M; Kalantari F; Jamshidi Z; Mohammadpour M, Heterobimetallic PtII-AuI Complexes Comprising Unsymmetrical 1,1-Bis(diphenylphosphanyl)methane Bridges: Synthesis, Photophysical, and Cytotoxic Studies. Eur. J. Inorg. Chem 2019, 2019, 1360–1373. [Google Scholar]
- 21.Jamshidi M; Babaghasabha M; Shahsavari HR; Nabavizadeh SM, The Influence of Thiolate Ligands on the Luminescence Properties of Cycloplatinated(II) Complexes. Dalton Trans 2017, 46, 15919–15927. [DOI] [PubMed] [Google Scholar]
- 22.Nazari M; Shahsavari HR, Strong Red Emissions Induced by Pt–Pt Interactions in Binuclear Cycloplatinated(II) Complexes Containing Bridging Diphosphines. Appl. Organomet. Chem 2019, 33, e5020. [Google Scholar]
- 23.Joksch M; Agarwala H; Ferro M; Michalik D; Spannenberg A; Beweries T, A Comparative Study on the Thermodynamics of Halogen Bonding of Group 10 Pincer Fluoride Complexes. Chem. Eur. J 2019, 26, 3571–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nova A; Erhardt S; Jasim NA; Perutz RN; Macgregor SA; McGrady JE; Whitwood AC, Competing C−F Activation Pathways in the Reaction of Pt(0) with Fluoropyridines: Phosphine-Assistance versus Oxidative Addition. J. Am. Chem. Soc 2008, 130, 15499–15511. [DOI] [PubMed] [Google Scholar]
- 25.Yahav A; Goldberg I; Vigalok A, Difluoro Complexes of Platinum(II) and-(IV) with Monodentate Phosphine Ligands: An Exceptional Stability of d6 Octahedral Organometallic Fluorides. Inorg. Chem 2005, 44, 1547–1553. [DOI] [PubMed] [Google Scholar]
- 26.Zhao S-B; Wang R-Y; Nguyen H; Becker JJ; Gagné MR, Electrophilic Fluorination of Cationic Pt-Aryl Complexes. Chem. Commun 2012, 48, 443–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilsson P; Plamper F; Wendt OF, Synthesis, Structure, and Reactivity of Arylfluoro Platinum(II) Complexes. Organometallics 2003, 22, 5235–5242. [Google Scholar]
- 28.Berger J; Braun T; Ahrens T; Klaering P; Laubenstein R; Braun-Cula B, The Versatile Behavior of Platinum Alkyne Complexes towards XeF2: Formation of Fluorovinyl and Fluorido Complexes. Chem. Eur. J 2017, 23, 8886–8900. [DOI] [PubMed] [Google Scholar]
- 29.Anderson CM; Crespo M; Ferguson G; Lough AJ; Puddephatt RJ, Activation of Aromatic Carbon-Fluorine Bonds by Organoplatinum Complexes. Organometallics 1992, 11, 1177–1181. [Google Scholar]
- 30.López O; Crespo M; Font-Bardía M; Solans X, Activation of C−F and C−H Bonds by Platinum in Trifluorinated [C,N,N’] Ligands. Crystal Structures of [PtFMe2{Me2NCH2CH2NHCH(CH2COMe)(2,4-C6H2F2)}] and [PtMe{Me2NCH2CH2NCH(2,3,4-C6HF3)}]. Organometallics 1997, 16, 1233–1240. [Google Scholar]
- 31.Abo-Amer A; Boyle PD; Puddephatt RJ, Oxidation of a Dimethyl Platinum(II) Complex with Xenon Difluoride: The Important Role of Solvent. Inorg. Chem. Commun 2015, 61, 193–196. [Google Scholar]
- 32.Anderson CM; Puddephatt RJ; Ferguson G; Lough AJ, Oxidative Addition of Aryl–Halogen Bonds to Platinum(II) and the Structure of a Complex Formed by Aryl–Fluoride Oxidative Addition. J. Chem. Soc., Chem. Commun 1989, 1297–1298. [Google Scholar]
- 33.Berger J; Braun T; Herrmann R; Braun B, Reactivity of Platinum Alkyne Complexes Towards N-Fluorobenzenesulfonimide: Formation of Platinum Compounds Bearing a β-Fluorovinyl Ligand. Dalton Trans 2015, 44, 19553–19565. [DOI] [PubMed] [Google Scholar]
- 34.Kaspi AW; Goldberg I; Vigalok A, Reagent-Dependent Formation of C–C and C–F Bonds in Pt Complexes: An Unexpected Twist in the Electrophilic Fluorination Chemistry. J. Am. Chem. Soc 2010, 132, 10626–10627. [DOI] [PubMed] [Google Scholar]
- 35.Dubinsky-Davidchik I; Goldberg I; Vigalok A; Vedernikov AN, Selective Aryl–Fluoride Reductive Elimination from a Platinum(IV) Complex. Angew. Chem. Int. Ed 2015, 54, 12447–12451. [DOI] [PubMed] [Google Scholar]
- 36.Yahav-Levi A; Goldberg I; Vigalok A, Synthesis and Reactivity of Unsymmetrical Difluoro Pt(IV) Complexes. J. Fluor. Chem 2010, 131, 1100–1102. [Google Scholar]
- 37.Dubinsky-Davidchik IS; Goldberg I; Vigalok A; Vedernikov AN, Unprecedented 1,3-Migration of the Aryl Ligand in Metallacyclic Aryl a-Naphthyl Pt(IV) Difluorides to Produce β-Arylnaphthyl Pt(II) Complexes. Chem. Commun 2013, 49, 3446–3448. [DOI] [PubMed] [Google Scholar]
- 38.Vigalok A, Electrophilic Halogenation–Reductive Elimination Chemistry of Organopalladium and -Platinum Complexes. Acc. Chem. Res 2015, 48, 238–247. [DOI] [PubMed] [Google Scholar]
- 39.Crespo M, Fluorine in Cyclometalated Platinum Compounds. Organometallics 2012, 31, 1216–1234. [Google Scholar]
- 40.Crespo M; Martínez M; Nabavizadeh SM; Rashidi M, Kinetico-Mechanistic Studies on C-X (X = H, F, Cl, Br, I) Bond Activation Reactions on Organoplatinum(II) Complexes. Coord. Chem. Rev 2014, 279, 115–140. [Google Scholar]
- 41.Niazi M; Shahsavari HR; Golbon Haghighi M; Halvagar MR; Hatami S; Notash B, Reactivity of a Half-Lantern Pt2(II,II) Complex with Triphenylphosphine: Selectivity in Protonation Reaction. RSC Adv 2016, 6, 76463–76472. [Google Scholar]
- 42.Niazi M; Shahsavari HR; Golbon Haghighi M; Halvagar MR; Hatami S; Notash B, Carbon-Sulfur Bond Reductive Coupling from a Platinum(II) Thiolate Complex. RSC Adv 2016, 6, 95073–95084. [Google Scholar]
- 43.Allen FH; Wood PA; Galek PTA, Role of Chloroform and Dichloromethane Solvent Molecules in Crystal Packing: An Interaction Propensity Study. Acta Cryst. 2013, B69, 379–388. [DOI] [PubMed] [Google Scholar]
- 44.Dalvit C; Vulpetti A, Weak Intermolecular Hydrogen Bonds with Fluorine: Detection and Implications for Enzymatic/Chemical Reactions, Chemical Properties, and Ligand/Protein Fluorine NMR Screening. Chem. Eur. J 2016, 22, 7592–7601. [DOI] [PubMed] [Google Scholar]
- 45.Champagne PA; Desroches J; Paquin J-F, Organic Fluorine as a Hydrogen-Bond Acceptor: Recent Examples and Applications. Synthesis 2015, 47, 306–322. [Google Scholar]
- 46.Schneider H-J, Hydrogen Bonds with Fluorine. Studies in Solution, in Gas Phase and by Computations, Conflicting Conclusions from Crystallographic Analyses. Chemical Science 2012, 3, 1381–1394. [Google Scholar]
- 47.Shahsavari HR; Babadi Aghakhanpour R; Biglari A; Niazi M; Mastrorilli P; Todisco S; Gallo V; Lalinde E; Moreno MT; Giménez N; Halvagar MR, C(sp2)–C(sp2) Reductive Elimination from a Diarylplatinum(II) Complex Induced by a S–S Bond Oxidative Addition at Room Temperature. Organometallics 2020, 39, 417–424. [Google Scholar]
- 48.Lin X; Weng Z, Transition Metal Complex Assisted Csp3–F Bond Formation. Dalton Trans 2015, 44, 2021–2037. [DOI] [PubMed] [Google Scholar]
- 49.Campbell MG; Hoover AJ; Ritter T, Transition Metal-Mediated and Metal-Catalyzed Carbon–Fluorine Bond Formation In Organometallic Fluorine Chemistry, Braun T; Hughes RP, Eds. Springer International Publishing: Cham, 2015; pp 1–53. [Google Scholar]
- 50.Hu J-Y; Zhang J-L, Hydrodefluorination Reactions Catalyzed by Transition-Metal Complexes. Top. Organomet. Chem 2015, 52, 143–196. [Google Scholar]
- 51.Kuehnel MF; Lentz D; Braun T, Synthesis of Fluorinated Building Blocks by Transition-Metal-Mediated Hydrodefluorination Reactions. Angew. Chem. Int. Ed 2013, 52, 3328–3348. [DOI] [PubMed] [Google Scholar]
- 52.Ahrens T; Kohlmann J; Ahrens M; Braun T, Functionalization of Fluorinated Molecules by Transition-Metal-Mediated C–F Bond Activation to Access Fluorinated Building Blocks. Chem. Rev 2015, 115, 931–972. [DOI] [PubMed] [Google Scholar]
- 53.Hintermann L; Läng F; Maire P; Togni A, Interactions of Cationic Palladium(II)- and Platinum(II)-η3-Allyl Complexes with Fluoride: Is Asymmetric Allylic Fluorination a Viable Reaction? Eur. J. Inorg. Chem 2006, 2006, 1397–1412. [Google Scholar]
- 54.Armarego WLF, Purification of Laboratory Chemicals. 8th ed.; Butterworth-Heinemann; 2017. [Google Scholar]
- 55.Bruker SAINT, Version 6.36 a. Bruker-AXS Inc.: Madison, WI, USA: 2002. [Google Scholar]
- 56.Bruker SMART, Version 5.625 and SADABS, Version 2.03 a. Bruker AXS Inc., Madison, Wisconsin: 2001. [Google Scholar]
- 57.Sheldrick GM, A Short History of SHELX. Acta Cryst. 2008, A64, 112–122. [DOI] [PubMed] [Google Scholar]
- 58.Sheldrick GM, Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C27, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frisch MJ; Trucks GW; Schlegel HB; Scuseria GE; Robb MA; Cheeseman JR; Scalmani G; Barone V; Mennucci B; Petersson GA; Nakatsuji H; Caricato M; Li X; Hratchian HP; Izmaylov AF; Bloino J; Zheng G; Sonnenberg JL; Hada M; Ehara M; Toyota K; Fukuda R; Hasegawa J; Ishida M; Nakajima T; Honda Y; Kitao O; Nakai H; Vreven T; Montgomery JJA; Peralta JE; Ogliaro F; Bearpark M; Heyd JJ; Brothers E; Kudin KN; Staroverov VN; Keith T; Kobayashi R; Normand J; Raghavachari K; Rendell A; Burant JC; Iyengar SS; Tomasi J; Cossi M; Rega N; Millam JM; Klene M; Knox JE; Cross JB; Bakken V; Adamo C; Jaramillo J; Gomperts R; Stratmann RE; Yazyev O; Austin AJ; Cammi R; Pomelli C; Ochterski JW; Martin RL; Morokuma K; Zakrzewski VG; Voth GA; Salvador P; Dannenberg JJ; Dapprich S; Daniels AD; Farkas O; Foresman JB; Ortiz JV; Cioslowski J; Fox DJ, Gaussian 09, Revision A.02. 2016; p Gaussian, Inc., Wallingford CT. [Google Scholar]
- 60.Becke AD, Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys 1993, 98, 5648–5652. [Google Scholar]
- 61.Miehlich B; Savin A; Stoll H; Preuss H, Results Obtained with the Correlation Energy Density Functionals of Becke and Lee, Yang and Parr. Chem. Phys. Lett 1989, 157, 200–206. [Google Scholar]
- 62.Lee C; Yang W; Parr RG, Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785. [DOI] [PubMed] [Google Scholar]
- 63.Wadt WR; Hay PJ, Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for Main Group Elements Na to Bi. J. Chem. Phys 1985, 82, 284–298. [Google Scholar]
- 64.Roy LE; Hay PJ; Martin RL, Revised Basis Sets for the LANL Effective Core Potentials. J. Chem. Theory Comput 2008, 4, 1029–1031. [DOI] [PubMed] [Google Scholar]
- 65.Cossi M; Scalmani G; Rega N; Barone V, New Developments in the Polarizable Continuum Model for Quantum Mechanical and Classical Calculations on Molecules in Solution. J. Chem. Phys 2002, 117, 43–54. [Google Scholar]
- 66.Barone V; Cossi M; Tomasi J, A New Definition of Cavities for the Computation of Solvation Free Energies by the Polarizable Continuum Model. J. Chem. Phys 1997, 107, 3210–3221. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


