Abstract
Background:
The incidence of primary hyperparathyroidism has increased 300% in the U.S in the past 30 years, and secondary hyperparathyroidism is almost universal in patients with end stage renal disease. We assessed the presence of environmental chemicals in human hyperplastic parathyroid tumors as possible contributing factors to this increase.
Methods:
Cryopreserved hyperplastic parathyroid tumors and normal human parathyroids were analyzed by gas chromatography and liquid chromatography coupled to ultra-high resolution mass spectrometry, bioinformatics and biostatistics.
Results:
Detected environmental chemicals included polychlorinated biphenyls (PCB), polybrominated diphenyl ethers (PBDE), dichloro-diphenyl-trichloroethane (DDT) derivatives, and other insecticides. Ninety-nine percent had p,p’-dichlorodiphenyldichloroethylene (DDE); over 50% contained other environmental chemicals, many classified as endocrine disruptors. PCB-28 and PCB-49 levels correlated positively with parathyroid tumor mass. PBDE-47 concentrations in tumors were inversely correlated with patients’ serum calcium levels. Cellular metabolites in pathways of purine and pyrimidine synthesis and mitochondrial energy production were associated with tumor growth and with p,p’-DDE in pHPT tumors. In normal parathyroids, p,p′-DDE, PCB-28, PCB-74, PCB-153, but not p,p′-DDT or PCB-49, were detected.
Conclusion:
Environmental chemicals are present in human parathyroid tumors and warrant detailed epidemiologic and mechanistic studies to test for causal links to the growth of human parathyroid tumors.
Introduction
The diagnosis of primary hyperparathyroidism (pHPT) has increased more than 3-fold over the past 30 years, faster than the increase in the aging population, suggesting possible environmental effects in addition to a combination of increased awareness, improved screening, and earlier diagnosis of subclinical disease 1. Secondary HPT (sHPT), which is caused by renal failure-related hyperphosphatemia and decreased 1,25-dihydroxy-vitamin D production leading to increased PTH production and parathyroid hyperplasia, is almost universal (>90%) in patients with chronic renal disease requiring dialysis (CKD5). Multiple endocrine disrupting environmental chemicals (including bisphenol A and plasticizers) elute from dialysis tubing and medical devices2, 3, raising concern about the potential contribution of chemical exposure to tumor growth. We studied tissues from both pHPT and sHPT because of the heightened interest in environmental factors which impact tumor development in various endocrine diseases4, 5.
Our objective was to assess a panel of persistent environmental chemicals in human hyperplastic parathyroid tumors, using mass spectrometry and metabolomics. Development of high-resolution metabolomics (HRM) has provided new technological and conceptual tools to link peripheral metabolic pathways to disease characteristics and etiology 6. Using gas chromatography-coupled ultra-high resolution mass spectrometry which provides outstanding sensitivity for measuring low abundance chemicals, we tested for the presence of environmental chemicals in human hyperplastic parathyroid tumors as possible contributing factors to the increased incidence of HPT.
Methods
Patient selection
The Institutional Review Board of Emory University approved this retrospective study using a clinical database of HPT patients (2,677 patients with parathyroid tumors treated by parathyroidectomy between 2001 and 2015 at Emory University). Forty patients with pHPT and 40 patients with renal-failure related sHPT were selected and matched for sex and age at time of parathyroidectomy. Clinical outcomes were obtained from this IRB-exempt parathyroid postoperative follow-up database. Patients with known multiple endocrine neoplasia (MEN) were excluded.
Cryopreservation of tumors
All parathyroid tissues had been collected during surgeries for hyperparathyroidism, when the clinical findings were multiple gland enlargement, necessitating sub-total or near-total parathyroidectomy. Parathyroid mass was estimated intraoperatively by diameter in three dimensions and by the calculation of the volume of an ellipsoid7. Parathyroid specimens were minced, suspended in RPMI 1640 medium with 10% human serum albumin and 10% DMSO, and stored at −80 °C.
Preparation of normal human parathyroid tissue
No cryopreserved normal human parathyroids were available at Emory University because it is not our practice to resect normal parathyroids during surgery for hyperparathyroidism. Instead, normal human thyroids resected from deceased organ donors were purchased from The National Disease Research Interchange (NDRI), Philadelphia, Pa.; and to date, four normal human parathyroids have been dissected from these thyroids for use as controls, flash frozen in sterile cryotubes in liquid nitrogen, and then stored at −80°C for 3-4 weeks before analysis.
Quantification of environmental chemicals
Thawed tumor fragments (20 mg) from each of 80 patients were homogenized in 1 mL isooctane and vacuum concentrated. Duplicate injection volumes of 4 μL were analyzed on GC-MS (Q-Exactive GC Orbitrap GC-MS/MS) using a DB-5MS column. Environmental chemicals were identified based on the spectral pattern and retention time of authentic standards (Cambridge isotopes). Within each batch of 20 samples, standard reference material (SRM) 1958 (National Institute of Standards & Technology) was used, and the levels of environmental chemicals were quantified by a previously established reference standardization using known analyte levels in SRM 1958 8. For normal parathyroid glands, 40 mg tissue was extracted and quantified for environmental chemicals. A conversion factor of 20 was used to account for the dispersion of chemicals with cryopreservation (50 mg tissue in 1 mL preservation media). This was not to provide absolute quantitation of normal glands but relative comparison of two very different preservation methods.
High-Resolution Untargeted Metabolomics
Metabolites were extracted from tissue homogenates (50 μL) in acetonitrile:water (2:1) containing internal standards following procedures as described previously 17. Each sample was analyzed with an Orbitrap Fusion Tribrid mass spectrometer (85 to 2000 m/z); in triplicate with negative electrospray ionization (ESI) on C18 chromatography. Mass spectral data were extracted with apLCMS 9 and xMSanalyzer 10. Following quantile-normalization, the unpaired limma test was used to select features that differed between pHPT and sHPT groups, and P values were adjusted for multiple comparisons using Benjamini and Hochberg false discovery rate (FDR) 11. Partial least squares discriminant analysis (PLS-DA) was used to select top discriminatory features and then the selected features were merged with limma test results. Features with Q < 0.25 (FDR adjusted) and PLS-DA VIP score > 2.0 were studied by pathway enrichment analyses using mummichog20 with a less stringent criteria of Q < 0.25 to obtain optimal coverage of metabolites (100-500 metabolites) for permutation testing (personal communication with Shuzhao Li). This approach protects against type 1 statistical error by permutation testing in pathway enrichment analysis that is additional to FDR-adjusted unpaired limma test 12. Metabolic features were annotated with xMSannotator 13; and identities of selected metabolites were further confirmed relative to authentic standards.
Statistics
Data are presented as the mean and standard error of the mean. Welch’s t test was used to determine significant differences between two groups, and Mann-Whitney U test was used as a nonparametric test when normality assumption failed by Shapiro-Wilk test (P < 0.05). Environmental chemicals were correlated with patient characteristics using SigmaPlot 14.0 (Systat Software, Inc). All other bioinformatics analyses were performed in R Studio version 1.1.447 (RStudio, Inc). The significance level was P < 0.05 for all tests 11.
Results
Patient characteristics.
Demographic information and baseline characteristics of patients with hyperplastic parathyroid tumors, 40 with pHPT and 40 with sHPT, are provided in Table 1. The groups were matched for gender composition with the majority being female, as well as for age. The sHPT patients had a 3-fold greater mass of parathyroid tumors compared to pHPT patients (3532 vs 1206 mg), and 7-fold higher serum PTH levels (1588 vs 226 pg/mL). These differences are expected between pHPT and sHPT patients requiring parathyroid surgery since pHPT and sHPT are understood to be fundamentally different diseases in causality. Compared to the normal mass of all 4 parathyroid glands (~180 mg total) and PTH levels (< 65 pg/mL), these high values underscore the disease severity in patients with tumor hyperplasia. The serum calcium levels, which are normally under tight parathyroid control, were modestly elevated above normal range (upper limit: 10.3 mg/dl). The Ca levels were relatively high for patients with sHPT because their albumin levels typically are low. Higher values for urea nitrogen, creatinine and phosphate were noted in sHPT, as expected.
Table 1.
Characteristics of patients with primary hyperplastic parathyroid tumors (pHPT) or renal-failure-related secondary parathyroid tumors (sHPT) in this study. Values shown are mean values and standard errors are shown in parentheses.
| All n = 80 (female: 69% ) | pHPT n = 40 (female: 75%) | sHPT n = 40 (female: 63%) | P value pHPT vs sHPT | |
|---|---|---|---|---|
| Age | 54 (1.4) | 56 (1.9) | 51 (1.9) | N.S. |
| Total mass of parathyroid (mg)a | 2369 (276) | 1206 (190) | 3532 (449) | <0.001 |
| PTH (intact, pg/mL) | 905 (124) | 226 (58.5) | 1588 (187) | <0.001 |
| Calcium (mg/dL) | 10.8 (0.1) | 10.9 (0.2) | 10.6 (0.1) | N.S. |
| Blood urea nitrogen ( mg/dL) | 24.4 (1.8) | 15.6 (1.1) | 33.7 (2.8) | <0.001 |
| Creatinine (mg/dL) | 4.7 (0.8) | 1.1 (0.1) | 8.6 (1.3) | <0.001 |
| Phosphate (mg/dL) | 4.2 (0.2) | 2.9 (0.2) | 5.4 (0.3) | <0.001 |
Calculated values from the volume of parathyroid.
Quantification of environmental chemicals in parathyroid tumors
The levels of environmental chemicals in plasma are typically three to five orders of magnitude lower than endogenous metabolites 14. Because a relatively small amount of tissue (20 mg) was used for analysis, the chemical detection capability was limited by the sensitivity of the instrumentation. Despite this, 66 persistent organic chemicals were identified in the 80 samples. Thirty-two of the chemicals were quantified using reference standardization with SRM1958; among them, 11 were detected at high frequency (>50% patients), but there were no significant differences in their concentrations between pHPT and sHPT tumors (Figure 1). The most frequently detected chemical (p,p′-DDE) was almost universally present: 79 out of 80 patients had a mean level of 31.4 ppb (median: 3.3 ppb) ranging from 0.02 to 510.4 ppb. Other DDE-related chemicals also were detected frequently in parathyroid tumors, including DDT, the parent compound of DDE; and DDD (dichlorodiphenyldichloroethane), a minor component of the initially manufactured technical mixture (Figure 1).
Figure 1.
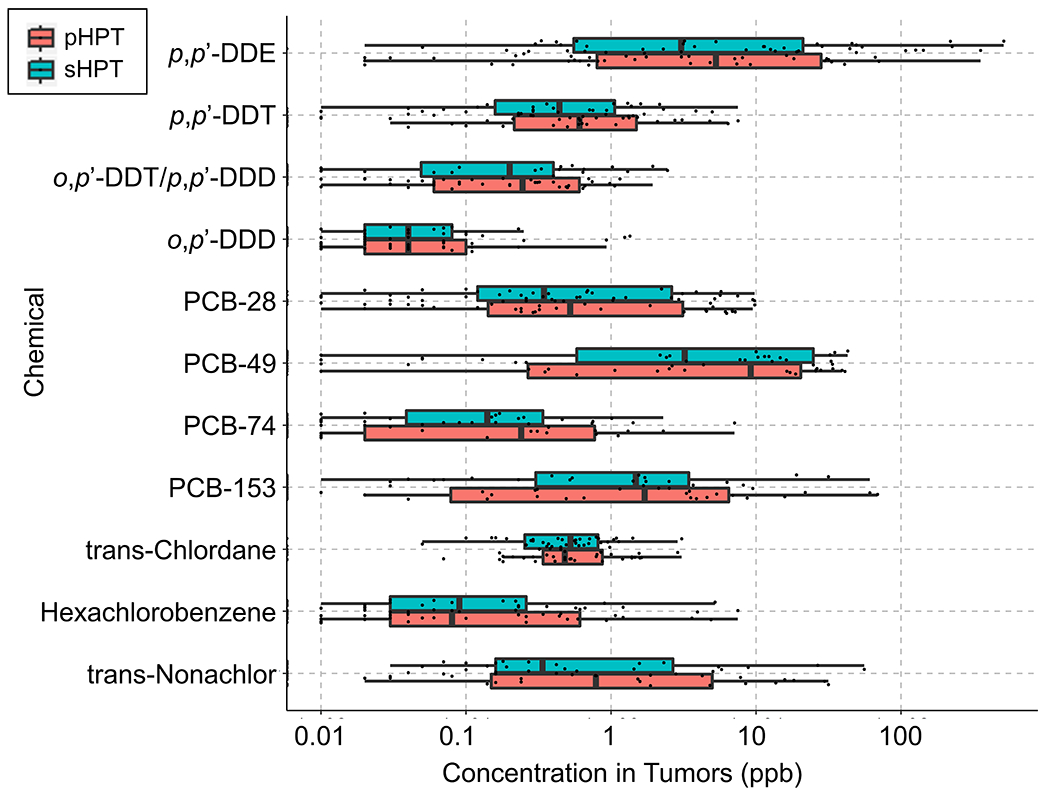
Concentration and distribution of 11 persistent environmental chemicals in parathyroid tumors detected in more than 50% of 80 patients with parathyroid hyperplasia.
A number of PCB congeners were detected frequently including PCB-28, PCB-49, PCB-74 and PCB-153. Similar to p,p’-DDE, these chemicals had a wide range of levels, spanning 3 to 4 orders of magnitude. Of note, the lower-chlorinated PCB-49, considered as a less persistent yet highly estrogenic congener15, had the highest mean level (7.5 ppb) among all PCB congeners.
Other frequently detected persistent chemicals (now globally banned) included trans-chlordane and trans-nonachlor (organic pesticides), as well as hexachlorobenzene (used as a fungicide), which were present at relatively low median levels (< 1ppb) (Figure 1).
Quantification of environmental chemicals in normal human parathyroids
A small number (n = 4) of normal parathyroid samples were obtained from NDRI post-mortem tissue repository for use as controls, as described in Methods. Of the 11 most frequently detected chemicals in parathyroid tumors, five chemicals (p,p’-DDT, p,p’-DDD/o,p’-DDT, o,p’-DDD, trans-chlordane and PCB-49) were not detectable in any normal parathyroid glands (Table 3). Three chlorinated pesticides including p,p’-DDE (4.8±2.1 ng/g), hexachlorobenzene (0.09 ± 0.06 ng/g) and trans-nonachlor (0.47 ± 0.38 ng/g) were detected in all 4 normal parathyroids at levels in the same range as in the parathyroid tumors. Three PCBs (PCB-28, PCB-74, and PCB-153) were detected in only some of the normal controls (Table 3).
Table 3.
Significant correlations (raw P < 0.05) between persistent organic pollutant levels and patient characteristics are presented with Pearson’s correlation coefficiency and false discovery rate (FDR) adjusted Q values, for overall patient samples, primary hyperplastic parathyroid tumors (first number in parentheses), or renal-failure-related secondary parathyroid tumors (second number in parentheses).
| Chemical | Patient characteristics | Pearson’s correlation coefficiency | P value | FDR-Q | Detection frequency |
|---|---|---|---|---|---|
| PCB-28 | Total massa | 0.266 (N.S., 0.364) | 0.017 (N.S., 0.021) | 0.114 (N.S., 0.221) | 75/80 |
| PCB-28 | Citrate | 0.267 (N.S., 0.357) | 0.016 (N.S., 0.024) | 0.108 (N.S., 0.228) | 75/80 |
| PCB-28 | Xanthine | N.S. (0.506, N.S.) | N.S. (0.001, N.S.) | N.S. (0.010, N.S.) | 75/80 |
| PCB-49 | Total massa | 0.229 (N.S., 0.339) | 0.041 (N.S., 0.032) | 0.199 (N.S., 0.279) | 49/80 |
| PCB-49 | Citrate | 0.256 (N.S., 0.370) | 0.022 (N.S., 0.019) | 0.131 (N.S., 0.205) | 49/80 |
| PCB-49 | Xanthine | N.S. (0.505, N.S.) | N.S. (0.001, N.S.) | N.S. (0.010, N.S.) | 49/80 |
| p,p′-DDE | Age | 0.250 (N.S., 0.429) | 0.026 (N.S., 0.006) | 0.139 (N.S., 0.079) | 79/80 |
| p,p′-DDE | Guanosine | 0.263 (N.S., 0.377) | 0.018 (N.S., 0.017) | 0.115 (N.S., 0.191) | 79/80 |
| o,p’-DDT/ p,p’-DDD | Total massa | N.S. (0.370, N.S.) | N.S. (0.019, N.S.) | N.S. (0.118, N.S.) | 58/80 |
| o,p’-DDT/p,p’-DDD | Xanthine | N.S. (0.370, N.S.) | N.S. (0.019, N.S.) | N.S. (0.118, N.S.) | 58/80 |
| o,p’-DDT/p,p’-DDD | Cytidine | N.S. (0.475, N.S.) | N.S. (0.002, N.S.) | N.S. (0.020, N.S.) | 58/80 |
| o,p’-DDT/p,p’-DDD | Fumarate | N.S. (0.370, N.S.) | N.S. (0.019, N.S.) | N.S. (0.118, N.S.) | 58/80 |
| PBDE-47 | Calcium | −0.313 (−0.439, N.S.) | 0.005 (0.005, N.S.) | 0.037 (0.038, N.S.) | 34/80 |
Calculated values from the volume of parathyroid.
Correlations of environmental chemicals to HPT disease characteristics
The levels of chemicals were correlated with tumor growth and endocrine disruption in these patients. PCB-28 and PCB-49 levels both correlated significantly with parathyroid tumor mass, mainly driven by sHPT patients (Table 2). Importantly, both PCB congeners (one tri-chlorinate and one tetra-chlorinate), frequently were detected at relatively high levels in patient tumors (Figure 1). Both PCB-28 and PCB-49 correlated with levels of citrate, a fundamental mitochondrial intermediate that regulates biosynthesis, also driven mainly by sHPT patients, but PCB-28 and PCB-49 were significantly associated with xanthine [a nucleotide base (purine) metabolite] only in pHPT patients (Table 2).
Table 2.
Environmental chemicals detected in normal human parathyroids. Intact normal parathyroid glands (40 mg) dissected from donated post-mortem thyroids were prepared, analyzed, and quantified for environmental chemicals as described in Methods.
| Chemical | Mean (ng/g) | SE (ng/g) | Detection Frequency |
|---|---|---|---|
| p,p′-DDE | 4.858 | 2.095 | 4 out of 4 |
| PCB-28 | 0.003 | 0.004 | 1 out of 4 |
| PCB-74 | 0.016 | 0.013 | 3 out of 4 |
| PCB-153 | 0.171 | 0.128 | 2 out of 4 |
| hexachlorobenzene | 0.086 | 0.064 | 4 out of 4 |
| trans-nonachlor | 0.471 | 0.380 | 4 out of 4 |
| Non-detectable in any normal tissue: | |||
| p,p′-DDT | 0 out of 4 | ||
| p,p′-DDD/o,p′-DDT | 0 out of 4 | ||
| o,p′-DDD | 0 out of 4 | ||
| PCB-49 | 0 out of 4 | ||
| trans-chlordane | 0 out of 4 | ||
Several DDT derivatives correlated with metabolic intermediates. p,p’-DDE correlated with guanosine, a purine nucleotide, and o,p’-DDT/p,p’-DDD was associated with xanthine and cytidine, fumarate (a TCA cycle intermediate generating energy) and total tumor mass in pHPT patients (Table 2). In addition, p,p’-DDE levels correlated positively with patient age. PBDE-47, detected in 43% patient tumors, had an inverse correlation with calcium levels in pHPT patients (Table 2).
High-resolution metabolomics (HRM) of parathyroid tumors in pHPT and sHPT
HRM provides a promising approach for understanding complex disease etiology by deep-phenotyping the metabolic activities in cells. A metabolic feature is defined by the accurate m/z, retention time and ion intensity. Using the limma test, 1105 out of 7655 metabolic features were different between pHPT and sHPT tumors (FDR adjusted Q < 0.25, Figure 2A). Metabolites also correlated both positively and negatively over a broad range of m/z and retention time, indicating a wide spectrum of metabolic alteration (Figure 2A).
Figure 2.
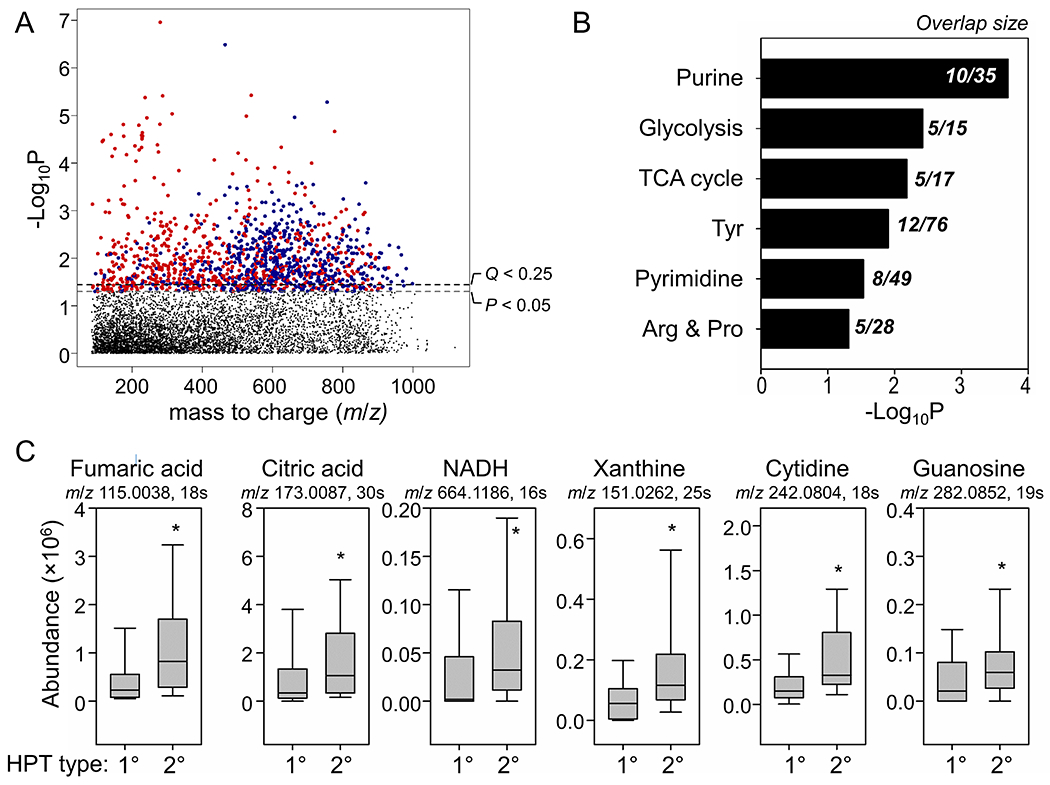
Metabolic profiles that were different between primary and secondary parathyroid hyperplasia (1° vs 2° HPT). (A) Type I Manhattan plot for −log P vs m/z features with C18 chromatography separation and negative ESI mode. Red indicates positive correlation between significant features (P < 0.05) and DDT exposure and blue indicates negative correlation. (B) Metabolic pathways enriched by significant features (FDR Q < 0.25 and VIP > 2.0) that were different between 1° vs 2° HPT. The overlap size is the number of significant metabolites in each pathway. (C) Representative metabolite abundance from the TCA cycle and purine and pyrimidine metabolic pathways. * P < 0.05 between 1° vs 2° HPT.
Among 1105 features, 318 were selected that best discriminated between pHPT and sHPT (PLS-DA test, VIP > 2.0). Pathway enrichment analysis with mummichog was performed to understand functional alterations of the metabolome. Mummichog pathway analysis uses statistical testing in permutation and enrichment to assess overall significance of the pathway and provides an additional layer of protection against type I error 16. Results showed that the metabolites which differed between pHPT and sHPT were enriched in metabolic pathways of purine metabolism, glycolysis, the tricarboxylic acid (TCA) cycle, tyrosine metabolism, pyrimidine metabolism, arginine and proline metabolism (Figure 2B).
The corresponding levels of representative metabolites in the TCA cycle pathway and the nucleotide base synthesis pathway are presented in Figure 2C. There were consistently higher levels of TCA cycle intermediates (fumaric acid, citric acid and NADH) that are critical for energy production in sHPT versus pHPT (raw P < 0.05). Similarly, higher levels of metabolites responsible for purine and pyrimidine synthesis were significantly elevated in sHPT (Table 1).
Discussion
The increasing incidence of hyperpathyroidism has been attributed to several factors, including the development of automated serum calcium measurements in the early 1970’s which led to the diagnosis of more patients 17, a delayed effect of exposure to fallout from atmospheric nuclear testing (in the 1950’s and 1960’s) 18, nuclear irradiation of the neck and upper chest for benign diseases, screening for osteoporosis, including serum calcium determination, and nutritional factors such as vitamin D deficiency 19. We have investigated the possibility of additional factors which might increase hyperparathyroidism, specifically environmental toxicants.
Our pilot study for the first time detected a wide list of environmental chemicals in human parathyroid tumors, including three categories of persistent organic pollutants that have been shown to promote tumor growth and disrupt the endocrine system: DDT, PCBs and PBDEs. Both DDT and PCBs have been banned for several decades, and PBDEs are also being phased out. However, because of their resistance to biodegradation and resulting health concerns, these chemicals have been monitored in the general population in the National Health and Nutrition Examination Survey (NHANES). Many NHANES participants during 2003–2004 had measurable levels of DDT, PCBs and PBDEs, yet the geometric mean of serum levels were in general much lower than the levels we detected in parathyroid tumors 20.
DDE persists in the body longer than DDT, so serum DDE levels may be an indicator of historic exposure and may be 10-fold higher than DDT levels in the same person 20. The relative levels of DDE-related chemicals have been shown to reflect the amount of manufactured DDT and DDD, consistent with levels detected in human and environment biomonitoring studies (p,p’-DDE > p,p’-DDT > o,p’-DDT > o,p’-DDD)25. In our study, the large range of p,p’-DDE levels in human parathyroid tumors spanning 4 orders of magnitude suggests that there is substantial difference in exposure and/or elimination of this chemical in patients with hyperparathyroidism and is consistent with previous measures of variation of DDT or DDE levels in human plasma. 20
The finding that several DDT derivatives correlated positively with metabolic intermediates (guanosine, xanthine, cytidine, and fumarate) and with tumor mass in pHPT patients suggests a potential role of these chemicals in disrupting metabolic regulation of biosynthesis and proliferation.
In our study, PCB-28 and PCB-49 were detected at over 50-fold higher levels in parathyroid tumors compared to previously reported human serum levels20, and PCB-74 and PCB-153 showed a similar trend. PBDE-47, although detected in only 43% of tumors, also showed a higher average level. On the other hand, PCB-28 was detected in only 1 of 4 normal parathyroids, and no PBC-49 was found in these controls. These comparisons suggested that parathyroid glands potentially can be susceptible to enrichment of these chemicals relative to other biological systems. This may be attributed to the high lipid content of parathyroids, or to the interaction of chemicals with specific receptors in parathyroid cells.
The parathyroid tumors were collected over a large span of time for about 14 years with no difference between pHPT and sHPT. Metabolites stored long-term at −80°C have been shown to change in concentration by 15% 21. Although the persistent chemicals reported in this study have much longer half-lives than metabolites, one caveat is that the levels of chemicals may be slightly lower than what were present in the fresh tissue. For endogenous metabolites, because of their high abundance, even a 15% change would not have major impact.
Endocrine disruption by environmental chemicals has received substantial attention in various organ systems including thyroid, mammary glands and reproductive organs9. The “thin eggshell phenomenon” has been well demonstrated as an effect of DDT interfering with calcium and vitamin D metabolism in birds 22. DDT also can inhibit the ability of calmodulin to transport Ca2+ ions for neurotransmitter release 23. Further evidence on p,p’-DDT and p,p’-DDE inhibition of Ca2+-ATPase suggests a functional perturbation caused by DDT activities 24. PCBs have been found to have similar effects on Ca2+-ATPase and are capable of sensitizing calcium channels such as ryanodine receptors 25. It is still unclear how calcium interference is related to disruption of parathyroid function, but it is possible that any activities that interfere with Ca2+ clearance, intracellular Ca2+ release or antagonize Ca2+ binding to the calcium sensing receptor can trigger the signaling of hypertrophy.
Other calcium-dependent mechanisms which stimulate proliferation also could impact parathyroid hypertrophy. We found that more severe parathyroid hypertrophy in sHPT was associated with elevation in mitochondrial energy metabolism. Mitochondrial bioenergetics are tightly controlled by calcium through at least three calcium-dependent enzymes: pyruvate dehydrogenase, α-ketoglutarate dehydrogenase and isocitrate dehydrogenase. Rewiring of mitochondrial bioenergetics has been universally found in association with the abnormal proliferation of cells, including in cancer 30
In conclusion, this is the first report of the presence of multiple environmental chemicals in human parathyroid tumors. Previous evidence of their endocrine disrupting activities suggest that these persistent pollutants might play a role in the disruption of calcium regulation in parathyroid cells and in promotion of parathyroid tumor growth. Further study will be required to determine the reason for the concentration of organic pollutants in human parathyroid tumors, while, in general, lower levels of the same chemicals were present in normal parathyroid tissue. Detailed epidemiologic and mechanistic studies will be warranted to test for causal links to the dramatic rise in primary hyperparathyroidism.
Footnotes
Meeting information:
American Association of Endocrine Surgeons, April 4-6, 2020, Birmingham, AL (Cancelled due to COVID-19 pandemic)
References
- 1.Collier A, Portelli M, Ghosh S, Nowell S, Clark D. Primary hyperparathyroidism: Increasing prevalence, social deprivation, and surgery. Endocrine research. 2017;42:31–5. [DOI] [PubMed] [Google Scholar]
- 2.Tereshchenko LG, Posnack NG. Does plastic chemical exposure contribute to sudden death of patients on dialysis? Heart Rhythm. 2019;16:312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanno Y, Okada H, Kobayashi T, Takenaka T, Suzuki H. Effects of endocrine disrupting substance on estrogen receptor gene transcription in dialysis patients. Therapeutic Apheresis and Dialysis. 2007;11:262–5. [DOI] [PubMed] [Google Scholar]
- 4.Leijs MM, Tusscher GWt, Olie K, Teunenbroek Tv, Aalderen WMCv, Voogt Pd, et al. Thyroid hormone metabolism and environmental chemical exposure. Environmental Health. 2012;11:S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeh MW, Ituarte PH, Zhou HC, Nishimoto S, Amy Liu I- L, Harari A, et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. The Journal of Clinical Endocrinology & Metabolism. 2013;98:1122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu X, Li S, Cirrilo P, Krigbaum N, Tran V, Ishikawa T, et al. Metabolome wide association study of serum DDT and DDE in pregnancy and early postpartum. Reproductive Toxicology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milas M, Weber CJ. Near-total parathyroidectomy is beneficial for patients with secondary and tertiary hyperparathyroidism. Surgery. 2004;136:1252–60. [DOI] [PubMed] [Google Scholar]
- 8.Go YM, Walker DI, Liang Y, Uppal K, Soltow QA, Tran V, et al. Reference standardization for mass spectrometry and high-resolution metabolomics applications to exposome research. Toxicological sciences. 2015;148:531–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu T, Park Y, Johnson JM, Jones DP. apLCMS--adaptive processing of high-resolution LC/MS data. Bioinformatics. 2009;25:1930–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uppal K, Soltow QA, Strobel FH, Pittard WS, Gernert KM, Yu T, et al. xMSanalyzer: automated pipeline for improved feature detection and downstream analysis of large-scale, non-targeted metabolomics data. BMC Bioinformatics. 2013;14:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological). 1995;57:289–300. [Google Scholar]
- 12.Uppal K, Walker DI, Liu K, Li S, Go YM, Jones DP. Computational metabolomics: a framework for the million metabolome. Chem Res Toxicol. 2016;29:1956–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uppal K, Walker DI, Jones DP. xMSannotator: an R package for network-based annotation of high-resolution metabolomics data. Analytical Chemistry. 2017;89:1063–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rappaport SM, Barupal DK, Wishart D, Vineis P, Scalbert A. The blood exposome and its role in discovering causes of disease. Environmental Health Perspectives. 2014;122:769–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolff MS, Camann D, Gammon M, Stellman SD. Proposed PCB congener groupings for epidemiological studies. Environ Health Perspect. 1997;105:13–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S, Park Y, Duraisingham S, Strobel FH, Khan N, Soltow QA, et al. Predicting network activity from high throughput metabolomics. PLOS Computational Biology. 2013;9:e1003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heath H 3rd, Hodgson SF, Kennedy MA. Primary hyperparathyroidism: Incidence, morbidity, and potential economic impact in a community. The New England journal of medicine. 1980;302:189–93. [DOI] [PubMed] [Google Scholar]
- 18.Hundahl SA. Perspective: National Cancer Institute summary report about estimated exposures and thyroid doses received from iodine 131 in fallout after Nevada atmospheric nuclear bomb tests. CA: A Cancer Journal for Clinicians. 1998;48:285–98. [DOI] [PubMed] [Google Scholar]
- 19.Adami S, Marcocci C, Gatti D. Epidemiology of primary hyperparathyroidism in Europe. Journal of bone and mineral research. 2002;17:N18–23. [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Fourth report on human exposure to environmental chemicals, updated tables, (January 2019). Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Accessed April 9th 2019: https://www.cdc.gov/biomonitoring/biomonitoring_summaries.html. [Google Scholar]
- 21.Haid M, Muschet C, Wahl S, Rómisch-Margl W, Prehn C, Móller G, et al. Long-term stability of human plasma metabolites during storage at− 80 C. Journal of proteome research. 2018;17:203–11. [DOI] [PubMed] [Google Scholar]
- 22.Bitman J, Cecil HC, Harris SJ, Fries GF. DDT induces a decrease in eggshell calcium. Nature. 1969;224:44–6. [DOI] [PubMed] [Google Scholar]
- 23.Costa LG. Toxic effects of pesticides. Casarett and Doull’s toxicology: the basic science of poisons. 2008;8:883–930. [Google Scholar]
- 24.Hamel A, Mergler D, Takser L, Simoneau L, Lafond J. Effects of low concentrations of organochlorine compounds in women on calcium transfer in human placental syncytiotrophoblast. Toxicological Sciences. 2003;76:182–9. [DOI] [PubMed] [Google Scholar]
- 25.Pessah IN, Cherednichenko G, Lein PJ. Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol Ther. 2010;125:260–85. [DOI] [PMC free article] [PubMed] [Google Scholar]


