Abstract
Myeloid-derived suppressor cells (MDSC) encompass a subset of myeloid cells, which suppress both innate and adaptive immune functions. Since Mycobacterium tuberculosis (M. tuberculosis) can infect these cells, interest has emerged to study the antimicrobial response of MDSC to mycobacteria causing tuberculosis. Reactive oxygen species (ROS) are critical mediators to control intracellular replication of M. tuberculosis and MDSC express high levels of these effector molecules. Here we describe the flow cytometric assessment of total cellular ROS produced by MDSC in response to infection with M. tuberculosis and compare it with the ROS activity of non-MDSC myeloid cells. To further understand the dynamics of host–pathogen interactions, we provide details on methods for measurement of the intracellular replication of M. tuberculosis within MDSC. Of note, these procedures were adopted for primary MDSC and non-MDSC subsets isolated from human immunodeficiency virus (HIV)-uninfected or HIV-infected individuals, in vitro infected with M. tuberculosis to mimic M. tuberculosis mono- or HIV-M. tuberculosis coinfection, respectively.
Keywords: Monocytic MDSC, HIV-M. tuberculosis coinfection, Cell ROS, Mitochondrial ROS, Antimicrobial activity, Flow cytometry, MDSC sorting
1. Introduction
Myeloid-derived suppressor cells (MDSC) are a heterogeneous population of myeloid origin that contributes to the negative regulation of immune function. Human MDSC are considered as lineage negative (Lin−) cells that express common myeloid markers (CD11b, CD33) and low to zero levels of HLA-DR (HLA-DR−/lo), and thus have Lin−CD11b+CD33+HLA-DR−/lo phenotype [1, 2]. Depending on the presence of CD15/CD66b or CD14, MDSC have been classified as granulocytic (CD15+ or/and CD66b+) or monocytic (CD14+) subsets, respectively [1, 3–5]. These cells suppress innate and adaptive immunity either by arginine depletion, reactive oxygen (ROS) and nitrogen (NOS) species generation, VEGF expression, and/or mediation of CD4+CD25+FoxP3+ Treg cell expansion [3, 6–8]. Considerable research, predominantly performed in animal models, has demonstrated the inhibition of antitumor and antimicrobial activity by MDSC [9–12]. Additionally, recent evidence suggests that this inhibitory activity is present in patients with certain malignancies. Genesis of MDSC is regulated by various pro-inflammatory cytokines including IL-6, IL-10, prostaglandins, stem-cell factor, GM-CSF, TGF-β, VEGF, and TNFα [7, 8].
Infection with Mycobacterium tuberculosis (M. tuberculosis) and human immunodeficiency virus type-1 (HIV) is associated with production of cytokines, which includes factors such as, IL-6, IL-10, prostaglandins, TGF-β, and TNF-α that could expand MDSC. Of note, both infections constitute the main burden of infectious disease worldwide [13, 14], potentiate one another, and accelerate disease development through mechanisms, which still remain undetermined [15–17]. Importantly, immunological suppression in individuals infected with M. tuberculosis and/or HIV persist post-treatment with respective drug regimens, preserving the risk of reactivation of M. tuberculosis both in mono- and HIV coinfection settings. We and others have demonstrated that CD14+HLA-DR−/lo monocytic MDSC numbers decline in HIV patients on anti-retroviral therapy, but remain high as compared to healthy individuals [10, 11, 18–22]. We found that compared to CD14+HLA-DRhi non-MDSC, the expression of ROS with anti-antimicrobial effect is higher in MDSC isolated from peripheral blood. Its expression declines in response to M. tuberculosis infection.
We provide a protocol for comparative analysis of cellular ROS in MDSC and non-MDSC subsets infected with M. tuberculosis using flow cytometry. We also provide a detailed methodology to determine the intracellular replication of M. tuberculosis in these cells isolated from peripheral blood, which has also been employed for in vitro-generated MDSC [23].
2. Materials
All the procedures must be carried out following the appropriate Biosafety Level (BSL) practices. M. tuberculosis should be handled in BSL-3 laboratory and flow cytometric analysis performed either in BSL-3 or outside BSL-3 laboratories after sample fixation and following the Institutional Biosafety Guidelines.
2.1. Isolation of Peripheral Blood Mononuclear Cells (PBMC)
Ficoll-Paque Premium (GE Healthcare).
Dulbecco’s phosphate-buffered saline (D-PBS) (Life Technologies).
Hemocytometer.
0.4% Trypan Blue in D-PBS (v/v) (see Note 1).
2.2. Flow Cytometry and Isolation of M-MDSC from PBMC
Anti-human-CD3-PerCP-Cy5.5 (clone SK7) antibody (dilution 1:100).
Anti-human-CD19-PerCP-Cy5.5 (clone SJ25C1) antibody (dilution 1:100).
Anti-human-CD66b-PerCP-Cy5.5 (clone G10F5) antibody (dilution 1:100).
Anti-human-CD11b-APC-eFlour780 (clone ICRF44) antibody (dilution 1:100).
Anti-human-CD33-Alexa Fluor 700 (clone WM55) antibody (dilution 1:100).
Anti-human-CD14-PE/Cy7 (clone ME5) antibody (dilution 1:100).
Anti-human-HLA DR- PE/Dazzel-594 (clone L243) antibody (dilution 1:100).
Aqua fluorescent LIVE/DEAD® Fixable Dead Cell Stain (Life Technologies) Excitation/Emission 367/526 nm (dilution 1:1000) (see Notes 2 and 3).
2.5 mM CellROX® Deep Red Oxidative Stress Reagent (C10422; Life Technologies) Excitation/Emission 640/665 nm.
Human CD3 microbeads (Miltenyi Biotec).
LS magnetic columns.
MACS Magnetic Separators.
RBC lysis buffer (BD Biosciences).
Staining buffer: 1% bovine serum albumin in D-PBS.
Cell sorting buffer (Miltenyi Biotec).
Cell collection media: fetal bovine serum with 1% Penicillin-Streptomycin.
2.3. Cell Culture
2.3.1. M-MDSC Culture
RPMI1640 media.
2.5 M HEPES.
200 mM Glutamine.
100 mM Sodium pyruvate.
10,000 units/mL Penicillin-Streptomycin.
10 mg/mL Gentamicin.
10% Heat-inactivated autologous serum (see Note 4).
Culture medium: RPMI Medium 1640 supplemented with 10% fetal calf serum, 25 mM HEPES, 2 mM glutamine, 1 mM sodium pyruvate, and 100 units/mL Penicillin-Streptomycin. Store at 4 °C.
Culture medium for infection with M. tuberculosis: RPMI Medium 1640 supplemented with 10% fetal calf serum, 25 mM HEPES, 2 mM glutamine, and 1 mM sodium pyruvate. Store at 4 °C.
Culture medium to kill extracellular M. tuberculosis: RPMI Medium 1640 supplemented with 10% fetal calf serum, 25 mM HEPES, 2 mM glutamine, 1 mM sodium pyruvate, and 50 μg/mL gentamicin, prepare fresh.
2.3.2. M. tuberculosis Culture
Green Fluorescent Protein (GFP) expressing M. tuberculosis Erdman (gift from Dr. Larry Schlesinger, Texas Biomedical Research Institute, United States).
M. tuberculosis Erdman (BEI Resources, United States).
Middlebrook 7H9 broth.
Middlebrook 7H10 agar.
10% Oleic Acid, Albumin, Dextrose, Catalase (OADC) enrichment.
20% Tween 20 in sterile water.
50% Glycerol in sterile water (see Note 5).
10% Sodium dodecyl sulfate (SDS) in sterile water.
100 mm Petri dishes.
250-mL polypropylene non-vented screw-capped conical flasks.
Middlebrook 7H9 liquid medium: Dissolve 4.7 g Middlebrook 7H9 powder in 900 mL deionized water then add 10 mL 50% glycerol and 0.25 mL 20% Tween 20. Sterilize by autoclaving at 121 °C for 20 min and cool to room temperature. Supplement the media with 100 mL OADC and store at 4 °C.
Middlebrook 7H10 agar plates: Dissolve 19 g Middlebrook 7H10 powder in 900 mL deionized water, then add 10 mL glycerol. Sterilize by autoclaving at 121 °C for 20 min and cool for 20 min. Supplement the media with 100 mL OADC and pour approximately 20 mL to each Petri plate. Once solidified, store the plates at 4 °C.
3. Methods
3.1. Flow Cytometric Sorting of M-MDSC
3.1.1. Peripheral Blood Mononuclear Cell Isolation
Mix heparinized blood with 2–4 times volume of D-PBS.
Carefully layer 30 mL of diluted blood over 20 mL of Ficoll-Paque in a 50 mL conical tube and centrifuge at 400 × g for 30 min at 25 °C in a swinging bucket rotor without brakes. The acceleration and deceleration rates need to be optimized depending on the centrifuge being used. With Thermo Scientific Sorvall ST 16R, acceleration and deceleration set at six gives an intact buffy coat layer.
Aspirate the top layer consisting of diluted plasma gently, without disturbing the white mononuclear blood cell layer.
Transfer the mononuclear cell layer to a new 50 mL conical tube. Fill the tube with D-PBS and centrifuge at 300 × g for 10 min at 25 °C. Remove the supernatant completely.
Resuspend the pellet in 50 mL D-PBS and centrifuge at 200 × g for 10 min at 25 °C. Remove the supernatant completely and proceed for CD3 depletion.
3.1.2. Depletion of CD3+ Cells
Resuspend cells in D-PBS and determine cell number of PBMC using hemocytometer (see Note 1).
Centrifuge cell suspension at 300 × g for 10 min. Remove the supernatants completely.
Resuspend the cell pellet in 80 μL of MACS sorting buffer per 107 cells.
Add 20 μL of CD3 microbeads to 107 cells, mix, and incubate for 15 min at 4 °C.
Wash the cells with 5 mL buffer and centrifuge at 300 × g for 10 min. Remove the supernatant completely.
Resuspend cells in 600 μL of MACS buffer and proceed to magnetic separation using LS column.
Place the column in a MACS Separator and prepare by rinsing with 3 mL of MACS buffer.
Load the cell suspension onto the column. Collect the unlabeled cells which pass through the magnetic column. Wash the column by adding 3 mL buffer twice, each time once the column reservoir is empty.
The CD3+ cells are trapped in column and unlabeled CD3 depleted cell fraction enriched for myeloid cells is collected in the tube.
3.1.3. Sample Preparation for Cell Sorting
Centrifuge CD3+ cells depleted cell fraction at 300 × g for 10 min and resuspend in FACS staining buffer.
Count the cells and add pre-titrated CD11b, CD33, CD14, HLA-DR, and CD3/19/66b antibody cocktail (see Note 6).
Incubate cells for 30 min at 4 °C in dark.
Wash the cells twice with 3 mL FACS staining buffer by centrifuging at 300 × g for 10 min.
Resuspend the cells in cell sorting buffer and pass through 40 μM filter to get rid of cellular debris, which may clog the cell sorter.
Using the appropriate Cell Sorter capable of Biohazard cell sorting, gate on CD3/CD19/CD66b-cell population. Collect CD11b+CD33+CD14+HLA-DRhi (non-MDSC) and CD11b+CD33+CD14+HLA-DRlo (MDSC) cell fractions in separate collection tubes in appropriate collection media (see Notes 7 and 8).
3.2. Culture of Sorted Cells and Infection with M. tuberculosis
Centrifuge sorted cell fraction, resuspend in antibiotic-free complete RPMI1640 media, and count cells by Trypan Blue exclusion method (see Note 1).
Plate the cells at a minimum cell density of 0.5 × 106 cell/mL and minimum 8 × 104 cells/well in 24-well plate.
Keep the cells for resting for 2 h by incubating at 37 °C and 5% CO2. We have not found any change in ROS expression if cells are rested overnight (ON). Subsequent methodology is shown for ON rested cells.
Gently aspirate the cell culture media and replace with fresh antibiotic-free complete RPMI1640 medium.
Infect cells with GFP-Erdman at a multiplicity of infection (MOI) 1:5 (1 cell: 5 bacteria). The total volume per well should not exceed 300 μL (see Note 9).
Incubate the cells for 1–2 h at 37 °C and 5% CO2 for infection to happen.
3.3. Flow Cytometric Determination of ROS Production Following M. tuberculosis Infection
During the last 30 min of infection, add 1 μM of CellROX diluted in prewarmed complete RPMI1640 (see Note 10).
Wrap the plate in aluminum foil and transfer to incubator, 37 °C and 5% CO2.
Gently scrape the cells and transfer to labeled FACS staining tubes.
Wash wells once with 2 mL D-PBS and add to respective staining tubes.
Centrifuge at 300 × g for 10 min at 25 °C. Carefully aspirate the buffer.
Resuspend cells in 1 mL D-PBS and add 0.5 μL Aqua Live/Dead reagent. Incubate for 15 min at room temperature in dark.
Wash the cells with 2 mL FACS staining buffer by centrifuging at 300 × g for 10 min.
Proceed with Fixation protocol approved by the Institutional Biosafety Committee to move samples out of BSL-3 for flow cytometry.
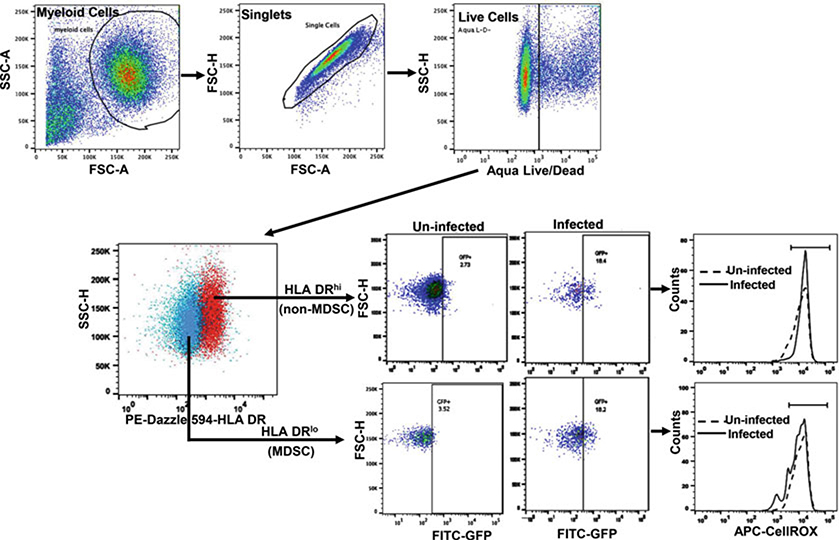
Samples are acquired on a flow cytometer analyzer with compatible lasers/filters combination and data analyzed using appropriate software or FlowJo. Dead cells are excluded and the expression of CellROX is analyzed in M. tuberculosis GFP+ cell gate (Fig. 1); and calculate Net ROS expression = [Mean fluorescence intensity of ROS by M. tuberculosis GFP+ non-MDSC—Mean fluorescence intensity of ROS by uninfected non-MDSC] and [Mean fluorescence intensity of ROS by M. tuberculosis GFP+ MDSC—Mean fluorescence intensity of ROS by uninfected MDSC]. The flow cytometry-based method can also be utilized to measure the rate of infection by measuring the percentage of GFP+ cells.
Fig. 1.
Flow cytometric determination of reactive oxygen species by MDSC in response to M. tuberculosis. Sorted MDSC and non-MDSC (HLA-DRhi) are infected with GFP-M. tuberculosis Erdman and incubated with CellROX for 30 min. Cells are stained with Live/Dead stain, formaldehyde fixed, and analyzed using flow cytometer. The gating strategy is shown
3.4. Measurement of Intracellular M. tuberculosis
Remove media after 2 h of infection in step 6 of Subheading 3.2 and wash wells three times with 1 mL D-PBS to remove non-phagocytosed bacteria and loosely adherent cells.
Add 0.5 mL/well fresh media containing gentamicin (50 μg/mL) (see Note 11).
Incubate the cells for 1–2 h at 37 °C and 5% CO2 to kill extracellular bacteria.
Remove the medium from wells and add to separate 15 mL centrifuge tubes containing 21 μL of 10% SDS. Add 3 mL additional sterile water to have the final 0.07% SDS concentration (see Note 12).
Add 1 mL of 0.07% SDS to each of the wells to lyse adherent MDSC.
Remove the lysed MDSC to separate 15 mL centrifuge tubes.
Wash the wells twice with 0.07% SDS and add to previous tubes (total volume of 3 mL).
Centrifuge the tubes (see steps 3 and 7) at 2000 × g for 20 min to pellet the mycobacteria.
Discard supernatant and resuspend the pellet in 1 mL Middlebrook 7H9 broth by gently tapping the sealed tube.
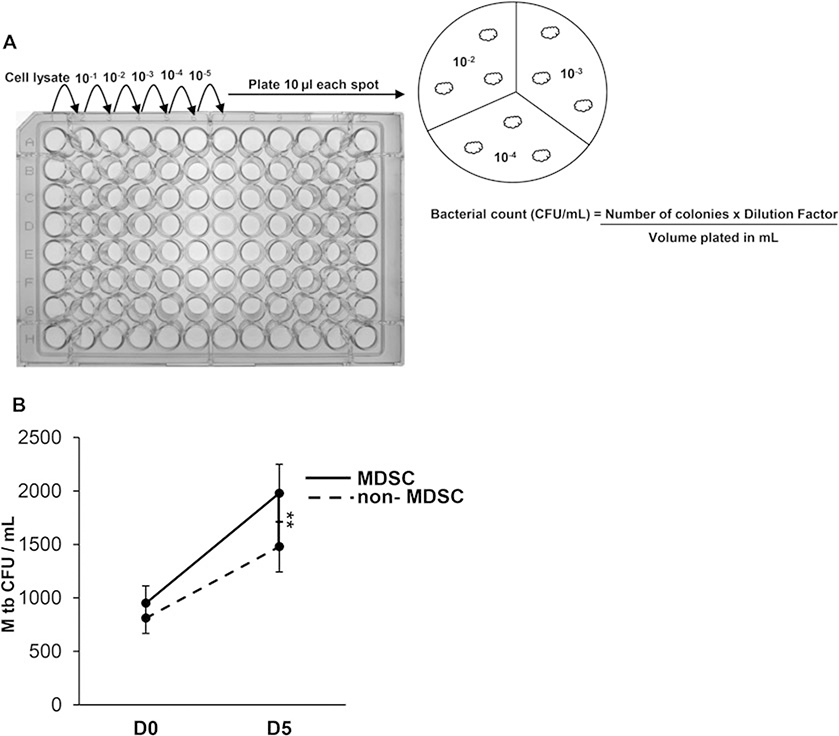
Prepare the serial dilutions in microtiter plate by adding infected cell lysate (100 μL) to the first well of a row and 90 μL of MiddleBrook 7H9 broth or D-PBS to each subsequent well. Transfer 10 μL from first well to next well and mix well by pipetting up and down to prepare 1:10 dilution. Further dilutions are made in the same manner. In triplicate plate, 10 μL of the dilution on to MiddleBrook 7H10 agar supplemented with 10% OADC and wait till dry. Once the spots are air-dried, incubate the plates at 37 °C following the Institutional Biosafety Guidelines for 3-weeks and count the colonies (Fig. 2).
Calculate colony forming units (CFUs) as: Number of colonies × Dilution factors/volume plated in mL (see Note 13).
Remaining infected MDSC are placed back to the incubator.
The above steps may be repeated at day-3 and −5 postinfection.
Fig. 2.
Intracellular replication of M. tuberculosis in MDSC isolated from peripheral blood mononuclear cells. (a) Sorted MDSC and non-MDSC are infected with M. tuberculosis Erdman at multiplicity of infection of 5 for 3 h, washed with D-PBS to remove non-phagocytosed bacteria and loosely adherent cells. Cells are treated with gentamicin (50 μg/mL) for 1–2 h at 37 °C and 5% CO2 to kill extracellular bacteria. Cells are lysed with 0.07% SDS and cellular lysates are serially diluted and plated in triplicate on Middlebrook 7H10 agar supplemented with OADC enrichment. The number of colonies are counted after 3 weeks and colony forming units (CFU)/ml determined. (b) Intracellular growth of M. tuberculosis shown at days-0 and −5 postinfection of MDSC and non-MDSC isolated from HIV-infected individuals. Data show mean values ±SEM; N = 4 donors. **p < 0.005
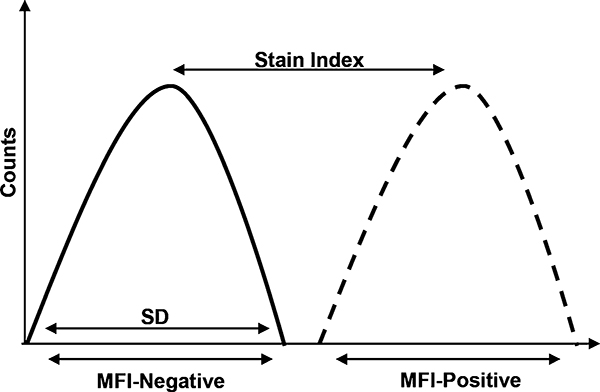
Fig. 3.
Stain index. The stain index is the ratio of mean fluorescence intensity (MFI) positive population and the MFI of negative population, divided by two times the standard deviation of the negative population
Acknowledgments
This work was supported by NIH grant AI127132 and The University of Georgia Research Foundation. A part of this work was performed with the support of the Flow Cytometry Core at the San Diego Center for AIDS Research (P30 AI036214), the VA San Diego Health Care System, and the San Diego Veterans Medical Research Foundation. We thank Ms. Tara Rambaldo and Mr. Neal Sekia, CFAR, University of California, San Diego, and Ms. Julie Nelson, Center for Tropical and Emerging Global Diseases Flow Cytometry Core, University of Georgia, Athens GA for their assistance with flow cytometry.
Footnotes
Trypan blue is used to count live cells using hemocytometer. For this, place the coverslip on the hemocytometer and apply 10 μL of cells (PBMC or sorted cells) diluted with 10 μL Trypan blue (Dilution factor 2). Grids of the hemocytometer are seen under the microscope, set of 4 × 4 squares are at each corner of the hemocytometer, count the number of cells in the 16-squares at each corner. Determine the average and calculate the cell number/mL as: Average cell count × Dilution Factor × 104/mL.
The antibody panel can be designed based on the lasers-filters combination of the flow cytometer accessible to the user. We have found this panel works best with a range of flow cytometers tested: MoFlo (Beckman Coulter), Canto II, Aria, and LSR II (all from Becton Dickinson) with very minimal to no spillover of fluorescence; this is particularly important to avoid spillover of CD14+HLA-DRhi into CD14+HLA-DR−/lo population. The controls include unstained cells and fluorescence minus one (FMO). Due to the donor-to-donor variation in the expression of various markers, we recommend to use stained compensation beads (Comp Control) to calculate compensation.
Since monocytic MDSC are CD3−CD19−CD66b−, using antibodies conjugated to same fluorophore place the cells expressing them in dump channel. This is helpful in removing contaminating cells and clean up for downstream analysis.
To avoid high level of variability between commercial serum lots and determine individual donor response, we recommend using autologous serum. We have found commercial human serum also gives similar readout. It is important to heat inactivate serum at 56 °C for 30 min, to inactivate complement and prevent cell lysis.
M. tuberculosis Erdman was cultured as previously described [24], with shaking at 70 rpm to avoid bacterial clumping. Infection stocks are prepared in Middlebrook 7H9 broth with OADC and 10% glycerol, CFU are determined after one freeze-thaw cycle, and MOI calculated for infection based on these values.
In order to avoid high background fluorescence, it is recommended to titrate antibodies and fluorescent probes for flow cytometry. For titration experiments use the cell number and experimental conditions identical to the actual assay and incorporate viability dye. A defined number of cells are stained in a total of 100 μL volume with antibodies diluted in staining buffer at 1:25, 1:50, 1:100, 1:200, and 1:400 dilution. To determine the optimal concentration, stain index is calculated: Stain index = Mean Fluorescence Intensity of (MFI) +ve population – Mean Fluorescence Intensity (MFI) of −ve population/2 Standard Deviation of −ve population. The dilution that gives the highest Stain index is the dilution to use (Fig. 3).
The selection of collection media depends on each individual laboratory, we have found collecting myeloid cells in fetal bovine serum (FBS) with 1% Penicillin-Streptomycin maintains good cell viability. It is important to wash FBS completely before proceeding for infection assays.
Since the cells are cultured post-sort, staining cells with viability dye before sorting can be avoided. Most of the currently available viability stains are dissolved in DMSO which can cause cell death of primary cells. If the cells are to be cultured post-sort, we do not prefer to include viability dye for sorting. However, it is highly recommended to include Live/Dead stain if collecting cells for gene expression or other molecular studies.
We used M. tuberculosis stocks frozen at −80 °C and actively growing cultures for infection; we found that actively growing culture gives a better readout of immediate effector molecules such as ROS.
CellROX kill the cells if used in excess. It is highly recommended that the optimal concentration to use is determined. The stained cells should be analyzed as soon as possible, latest within 24 h of fixation.
This is to ensure that only intracellular bacteria are remaining in the wells. Amikacin at 100 μg/mL can also be used in place of gentamicin, as this antibiotic is impermeable to cells.
This step is very important at day 0 postinfection to determine the optimal antibiotic concentration that kills the extracellular bacteria.
With 0.1 × 105 cell infection at MOI 1:5, we observe 10−2 or 10−3 as the highest dilution that gives countable bacterial colonies.
References
- 1.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, Rodriguez PC, Sica A, Umansky V, Vonderheide RH, Gabrilovich DI (2016) Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 7:12150 10.1038/ncomms12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talmadge JE, Gabrilovich DI (2013) History of myeloid-derived suppressor cells. Nat Rev Cancer 13(10):739–752. 10.1038/nrc3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC (2009) Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res 69(4):1553–1560. 10.1158/0008-5472.CAN-08-1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Youn JI, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI (2012) Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol 91(1):167–181. 10.1189/jlb.0311177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L (2007) Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol 25(18):2546–2553. 10.1200/JCO.2006.08.5829 [DOI] [PubMed] [Google Scholar]
- 6.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI (2009) Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol 182(9):5693–5701. 10.4049/jimmunol.0900092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lechner MG, Liebertz DJ, Epstein AL (2010) Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol 185(4):2273–2284. 10.4049/jimmunol.1000901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lechner MG, Megiel C, Russell SM, Bingham B, Arger N, Woo T, Epstein AL (2011) Functional characterization of human Cd33+ and Cd11b+ myeloid-derived suppressor cell subsets induced from peripheral blood mononuclear cells co-cultured with a diverse set of human tumor cell lines. J Transl Med 9:90 10.1186/1479-5876-9-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O’Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL (2007) MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med 204(6):1463–1474. 10.1084/jem.20062602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garg A, Spector SA (2014) HIV type 1 gp120-induced expansion of myeloid derived suppressor cells is dependent on interleukin 6 and suppresses immunity. J Infect Dis 209 (3):441–451. 10.1093/infdis/jit469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garg A, Trout R, Spector SA (2017) Human immunodeficiency virus Type-1 myeloid derived suppressor cells inhibit cytomegalovirus inflammation through Interleukin-27 and B7-H4. Sci Rep 7:44485 10.1038/srep44485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tacke RS, Lee HC, Goh C, Courtney J, Polyak SJ, Rosen HR, Hahn YS (2012) Myeloid suppressor cells induced by hepatitis C virus suppress T-cell responses through the production of reactive oxygen species. Hepatology 55 (2):343–353. 10.1002/hep.24700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Organization WH (2010) Global tuberculosis control 2010. http://www.who.int/tb/publications/global_report/2010/en/index.html
- 14.UNAIDS (2010) Chapter 2: Epidemic update. UNAIDS report on the global AIDS epidemic 2010. http://www.unaids.org/documents/20101123_GlobalReport_Chap2_em.pdf
- 15.Diedrich CR, Flynn JL (2011) HIV-1/Mycobacterium tuberculosis coinfection immunology: how does HIV-1 exacerbate tuberculosis? Infect Immun 79(4):1407–1417. 10.1128/IAI.01126-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwan CK, Ernst JD (2011) HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev 24(2):351–376. 10.1128/CMR.00042-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pawlowski A, Jansson M, Skold M, Rottenberg ME, Kallenius G (2012) Tuberculosis and HIV co-infection. PLoS Pathog 8(2):e1002464 10.1371/journal.ppat.1002464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gama L, Shirk EN, Russell JN, Carvalho KI, Li M, Queen SE, Kalil J, Zink MC, Clements JE, Kallas EG (2012) Expansion of a subset of CD14highCD16negCCR2low/neg monocytes functionally similar to myeloid-derived suppressor cells during SIV and HIV infection. J Leukoc Biol 91(5):803–816. 10.1189/jlb.1111579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knaul JK, Jorg S, Oberbeck-Mueller D, Heinemann E, Scheuermann L, Brinkmann V, Mollenkopf HJ, Yeremeev V, Kaufmann SH, Dorhoi A (2014) Lung-residing myeloid-derived suppressors display dual functionality in murine pulmonary tuberculosis. Am J Respir Crit Care Med 190(9):1053–1066. 10.1164/rccm.201405-0828OC [DOI] [PubMed] [Google Scholar]
- 20.Magcwebeba T, Dorhoi A, du Plessis N (2019) The emerging role of myeloid-derived suppressor cells in tuberculosis. Front Immunol 10:917 10.3389/fimmu.2019.00917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin A, Cai W, Pan T, Wu K, Yang Q, Wang N,Liu Y, Yan D, Hu F, Guo P, Chen X, Chen L, Zhang H, Tang X, Zhou J (2013) Expansion of monocytic myeloid-derived suppressor cells dampens T cell function in HIV-1-seropositive individuals. J Virol 87(3):1477–1490. 10.1128/JVI.01759-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vollbrecht T, Stirner R, Tufman A, Roider J, Huber RM, Bogner JR, Lechner A, Bourquin C, Draenert R (2012) Chronic progressive HIV-1 infection is associated with elevated levels of myeloid-derived suppressor cells. AIDS 26(12):F31–F37. 10.1097/QAD.0b013e328354b43f [DOI] [PubMed] [Google Scholar]
- 23.Agrawal N, Streata I, Pei G, Weiner J, Kotze L, Bandermann S, Lozza L, Walzl G, du Plessis N, Ioana M, Kaufmann SHE, Dorhoi A (2018) Human monocytic suppressive cells promote replication of Mycobacterium tuberculosis and alter stability of in vitro generated granulomas. Front Immunol 9:2417 10.3389/fimmu.2018.02417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moliva JI, Duncan MA, Olmo-Fontanez A, Akhter A, Arnett E, Scordo JM, Ault R, Sasindran SJ, Azad AK, Montoya MJ, Reinhold-Larsson N, Rajaram MVS, Merrit RE, Lafuse WP, Zhang L, Wang SH, Beamer G, Wang Y, Proud K, Maselli DJ, Peters J, Weintraub ST, Turner J, Schlesinger LS, Torrelles JB (2019) The lung mucosa environment in the elderly increases host susceptibility to Mycobacterium tuberculosis infection. J Infect Dis 220 (3):514–523. 10.1093/infdis/jiz138 [DOI] [PMC free article] [PubMed] [Google Scholar]