Abstract
Purpose
Alectinib has shown activity in the CNS in phase I and II studies. To further evaluate this activity, we pooled efficacy and safety data from two single-arm phase II studies (NP28761 and NP28673; ClinicalTrials.gov identifiers: NCT01871805 and NCT01801111, respectively) in patients with ALK-positive non–small-cell lung cancer (NSCLC).
Patients and Methods
Both studies included patients with ALK-positive NSCLC who had previously received crizotinib; all patients received alectinib 600 mg twice per day. The primary end point in both studies was independent review committee (IRC)–assessed objective response rate (ORR; by Response Evaluation Criteria in Solid Tumors [RECIST] version 1.1). Additional end points (all by IRC) included CNS ORR (CORR), CNS disease control rate (CDCR), and CNS duration of response (CDOR).
Results
One hundred thirty-six patients had baseline CNS metastases (60% of the overall study populations); 50 patients (37%) had measurable CNS disease at baseline. Ninety-five patients (70%) had prior CNS radiotherapy; 55 patients completed the CNS radiotherapy more than 6 months before starting alectinib. Median follow-up time was 12.4 months (range, 0.9 to 19.7 months). For patients with baseline measurable CNS disease, IRC CORR was 64.0% (95% CI, 49.2% to 77.1%), CDCR was 90.0% (95% CI, 78.2% to 96.7%), and median CDOR was 10.8 months (95% CI, 7.6 to 14.1 months). For patients with measurable and/or nonmeasurable baseline CNS disease, IRC CORR was 42.6% (95% CI, 34.2% to 51.4%), CDCR was 85.3% (95% CI, 78.2% to 90.8%), and median CDOR was 11.1 months (95% CI, 10.3 months to not evaluable). CORR was 35.8% (95% CI, 26.2% to 46.3%) for patients with prior radiotherapy (n = 95) and 58.5% (95% CI, 42.1% to 73.7%) for patients without prior radiotherapy (n = 41). As previously reported, alectinib was well tolerated, regardless of baseline CNS disease.
Conclusion
Alectinib showed good efficacy against CNS metastases, in addition to systemic activity, in crizotinib-refractory ALK-positive NSCLC.
INTRODUCTION
Crizotinib was the first anaplastic lymphoma kinase (ALK) inhibitor to receive US Food and Drug Administration (FDA) approval for the treatment of advanced ALK-positive non–small-cell lung cancer (NSCLC) in 2011.1,2 However, although patients initially respond well to crizotinib, the majority eventually experience progressive disease (PD).3,4
Approximately 15% to 30% of all patients with lung cancer develop brain metastases, and the incidence is increasing, possibly as a result of better diagnostic methods5 or longer systemic progression-free survival (PFS) on targeted therapies. In addition, the CNS is a common site of progression for patients with ALK-positive NSCLC treated with crizotinib; the CNS was the first site of PD in 70% of patients with known brain metastases (30 of 43 patients) during crizotinib treatment.6 Further, the overall incidence of CNS metastases in patients with ALK-positive NSCLC can increase to approximately 60% after first-line crizotinib.7,8 The CNS was also a common site of PD in studies of ceritinib (approved for patients with ALK-positive NSCLC previously treated with crizotinib).7 Together, these findings highlight a need for new treatments that can offer both systemic efficacy and CNS disease control for patients with ALK-positive NSCLC.
Alectinib, a potent and highly selective ALK inhibitor, was granted approval by the Japanese Ministry of Health, Labor, and Welfare for ALK inhibitor–naïve patients with ALK-positive NSCLC in 2014 on the basis of the phase I/II AF-001JP study, which demonstrated an objective response rate (ORR) of 93.5% (95% CI, 82% to 99%), including two complete responses (CRs) and 41 partial responses (PRs).9 Patient follow-up for this study is ongoing, and to date, 19.6% of patients have achieved a CR, and the 2-year PFS rate is 76% (95% CI, 60% to 87%).10,11
Phase II studies have confirmed the efficacy of alectinib in crizotinib-refractory patients with ALK-positive NSCLC. The North American phase II NP28761 (ClinicalTrials.gov identifier: NCT01871805) study demonstrated an ORR by central independent review committee (IRC) of 52.2% (95% CI, 39.7% to 64.6%) and a disease control rate (DCR) of 79.1% (95% CI, 67.4% to 88.1%).12 The global phase II NP28673 (ClinicalTrials.gov identifier: NCT01801111) trial reported similar rates; IRC ORR was 50% (95% CI, 41% to 59%), whereas DCR was 79% (95% CI, 70% to 86%).8 Data from these studies supported the accelerated FDA approval of alectinib in December 2015 for the treatment of patients with ALK-positive NSCLC who have experienced PD on or are intolerant to crizotinib.
Alectinib has also demonstrated activity in the CNS in phase I and II studies.8,12 To further evaluate the efficacy of alectinib in the CNS, we pooled efficacy and safety data for patients with CNS disease from the phase II NP28761 and NP28673 studies.
PATIENTS AND METHODS
Study Design
Full study details are provided in the primary publications for the NP286738 and NP2876112 studies. NP28761 and NP28673 were single-arm, phase II, open-label, multicenter studies. NP28761 was conducted at 27 sites in North America, and NP28673 was a global study conducted at 56 sites in 16 countries. Both studies were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Protocols were approved by local institutional review boards or ethics committees at each participating site. Patients provided written informed consent.
Enrollment periods differed between the two studies. In NP28761, patients were enrolled between May 3, 2012, and August 4, 2014. This duration included the phase I dose-finding stage; for the phase II part, the first patient received treatment on September 4, 2013. In NP28673, patients were enrolled between June 20, 2013, and April 23, 2014.
Inclusion and Exclusion Criteria
Eligible patients had advanced or metastatic NSCLC that was previously confirmed as ALK-positive by an FDA-approved test (Vysis LSI break-apart fluorescence in situ hybridization; Abbott, Chicago, IL; retesting was not required). Patients were age ≥ 18 years, had an Eastern Cooperative Oncology Group performance status of ≤ 2, had adequate organ function, and had measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. In addition, patients were required to have PD on crizotinib according to RECIST version 1.1. Patients previously irradiated (whole-brain radiotherapy [WBRT] or stereotactic radiosurgery permitted; data on radiation type not captured) in the CNS had to have stable brain disease (no obvious clinical symptoms and no clinical deterioration) at study entry and be off corticosteroid treatment; both conditions had to be met for at least 2 weeks before the first dose of alectinib.
In both studies, patients were excluded if they had brain or leptomeningeal metastases that were symptomatic in nature and/or required treatment. Patients were also excluded from both studies if they had received prior therapy with an ALK inhibitor other than crizotinib. Patients could be chemotherapy naïve or have received prior platinum-based chemotherapy.
Procedures
All patients received oral alectinib 600 mg twice daily with a meal. Patients continued treatment with alectinib until PD, unacceptable toxicity, or withdrawal of consent. A minimum washout period of 7 days was required between the last dose of crizotinib and the first dose of alectinib.
Study End Points
The primary end point in both studies was ORR by IRC using RECIST version 1.1 in the response-evaluable population, with a coprimary end point in NP28673 of ORR by IRC in patients previously treated with chemotherapy. The key protocol-defined secondary end point was CNS ORR (CORR) by IRC; other secondary end points included duration of response (DOR), PFS, CNS DOR (CDOR), and safety.
Assessments
Tumor response and progression, including CNS response and progression, were assessed according to RECIST version 1.1 by IRC. A separate IRC assessing CNS disease consisted of specialist neuroradiologists who were blinded to systemic response. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). All patients underwent baseline tumor imaging, including computed tomography of the chest and abdomen, as well as brain imaging. CNS assessment was performed prospectively by regular brain imaging; magnetic resonance imaging was the most commonly used method; in the pooled population, 85 patients (62.5%) were assessed with magnetic resonance imaging, 38 (27.9%) with computed tomography, and 13 (9.6%) with both methods. The frequency of response or progression assessments, including brain scans, was the same regardless of baseline CNS metastases status. However, scans were taken every 6 weeks in the NP28761 study and every 8 weeks in NP28673.
Statistical Analysis
CNS end points were assessed in the following two populations: patients with measurable CNS disease at baseline and patients with measurable and/or nonmeasurable CNS disease at baseline, on the basis of RECIST version 1.1 by IRC. CORR was defined as objective tumor response rate (CR and PR) of CNS lesions in patients who had baseline CNS disease, on the basis of RECIST version 1.1 by IRC. CNS DCR (CDCR) was defined as the percentage of patients who had a best overall CNS response of PR, CR, or stable disease (SD) on the basis of RECIST version 1.1 by IRC. CDOR was defined as the time from the first observation of a CNS response until the first observation of CNS progression or death from any cause on the basis of RECIST version 1.1 by IRC. For patients with only nonmeasurable disease, response could be classed as CR, SD, or PD, but not PR.
The Clopper-Pearson method was used to construct 95% CIs for response rates. Kaplan-Meier analysis of time-to-event data (DOR) was used to estimate median event times, and the Brookmeyer-Crowley method was used to calculate two-sided 95% CIs. All analyses were performed with the use of SAS statistical software, version 9.2 (SAS Institute, Cary, NC).
RESULTS
A total of 225 patients were enrolled onto the two studies (n = 87 in NP28761 and n = 138 in NP28673; Appendix Table A1, online only). Here, we report data for the pooled CNS population only, on the basis of a data cutoff of April 27, 2015, for both studies.
Patients
This pooled CNS analysis included 136 patients (60% of the overall pooled population of the two studies) with baseline CNS disease; patient characteristics are listed in Table 1. Briefly, the median age was 51 years (range, 22 to 75 years); most patients had an Eastern Cooperative Oncology Group performance status of 1 (54%) and were younger than 65 years of age (90%). Fifty patients (37%) had measurable CNS disease at baseline, whereas 86 patients (63%) had only nonmeasurable CNS disease.
Table 1.
Pooled Baseline Characteristics for Patients Who Had Measurable and/or Nonmeasurable CNS Disease at Baseline
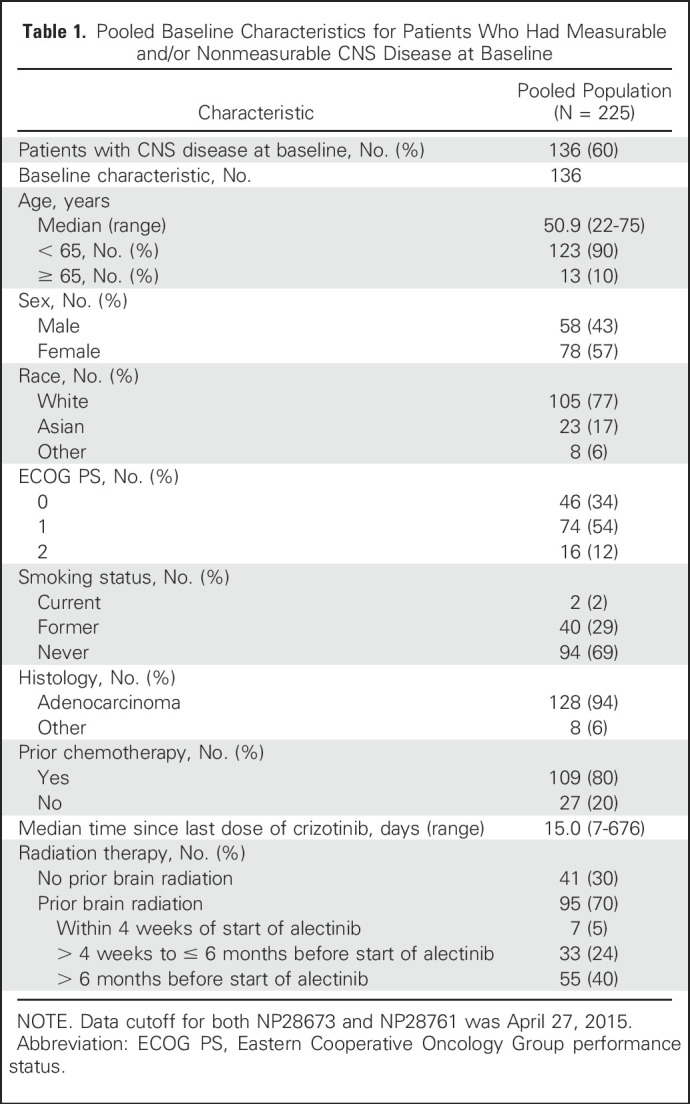
In total, 95 patients (70%) had received prior CNS radiotherapy, with 55 completing this radiotherapy greater than 6 months before starting alectinib. In patients with measurable CNS disease, 34 (68%) had received prior CNS radiotherapy, but the majority of these patients (25 of 34 patients) had completed the radiotherapy greater than 6 months before starting alectinib.
Prior Crizotinib Treatment
Median time on prior treatment with crizotinib was 350 days (interquartile range, 250 to 607 days), and the median time from last dose of crizotinib to first dose of alectinib was 15 days (range, 7 to 676 days). The best response on crizotinib was PR in 49% of patients, SD in 27% of patients, and PD in 19% of patients.
Efficacy
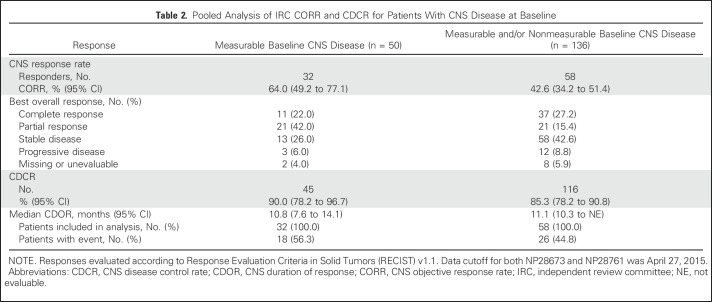
At data cutoff (April 27, 2015), the median duration of follow-up was 12.4 months (range, 0.9 to 19.7 months). In the group with measurable CNS disease at baseline, 32 of 50 patients had a CNS response, resulting in an IRC CORR of 64.0% (95% CI, 49.2% to 77.1%), with 11 CRs (22.0%) observed (Table 2 and Fig 1). The CDCR was 90.0% (95 CI, 78.2% to 96.7%) and the median CDOR was 10.8 months (95% CI, 7.6 to 14.1 months) after 56% of events.
Table 2.
Pooled Analysis of IRC CORR and CDCR for Patients With CNS Disease at Baseline
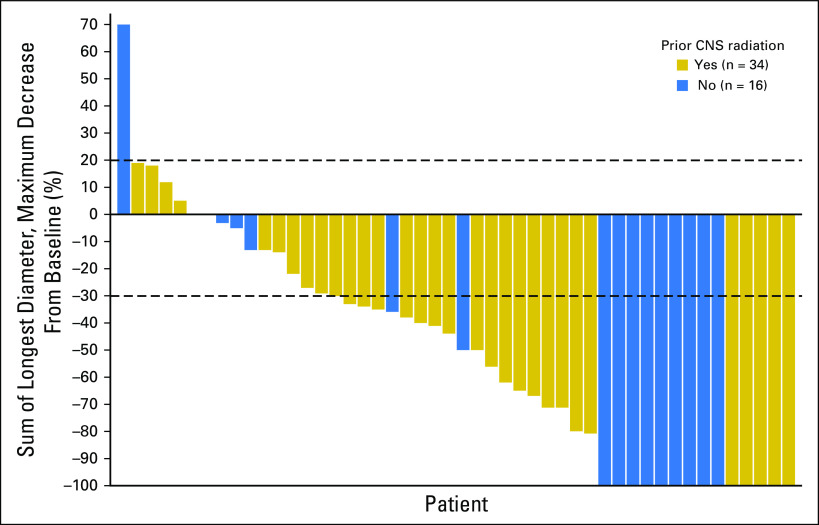
Fig 1.
Waterfall plot of best CNS response in patients with baseline measurable CNS disease by prior CNS radiation.
For patients with measurable and/or nonmeasurable CNS disease at baseline, the IRC CORR was 42.6% (95% CI, 34.2% to 51.4%), with 37 CRs (27%) observed (Table 2). The CDCR was 85.3% (95% CI, 78.2% to 90.8%) and the median CDOR was 11.1 months (95% CI, 10.3 months to not evaluable) after 45% of events (Table 2).
In patients with measurable CNS disease at baseline, CORR was 64.7% in white patients (n = 34) and 62.5% in Asian/other patients (n = 16). In patients with measurable and/or nonmeasurable CNS disease at baseline, CORRs were 43.8% and 38.7% in white (n = 105) and Asian/other patients (n = 31), respectively.
CORR by Prior Brain Radiation Therapy
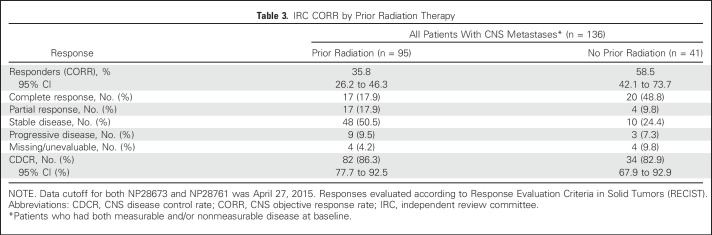
The CORR was 35.8% (95% CI, 26.2% to 46.3%) in patients who had received prior radiation (n = 95) and 58.5% (95% CI, 42.1% to 73.7%) in patients who had not received prior radiation (n = 41; Table 3 and Fig 1). In patients who had received radiation ≤ 6 months (n = 40) versus greater than 6 months prior (n = 55), the CORRs were 27.5% (95% CI, 14.6% to 43.9%) and 41.8% (95% CI, 28.7% to 55.9%), respectively. The CDCR in both patient groups was greater than 85%. CRs were seen in 18% of patients with and 49% of patients without prior radiotherapy. For the 50 patients with measurable CNS disease at baseline, the CORR was 61.8% (95% CI, 43.6% to 77.8%; CR rate, 12%) in patients who had received prior radiation (n = 34) and 68.8% (95% CI, 41.3% to 89.0%; CR rate, 44%) in patients who did not receive prior radiation (n = 16; Appendix Table A2, online only).
Table 3.
IRC CORR by Prior Radiation Therapy
Time-to-Event Summary for PFS
Overall, 83 (61%) of 136 patients with baseline CNS metastases had a PFS event at the time of data cutoff. The median time to event was 8.3 months (95% CI, 5.9 to 11.2 months), and the 6-month event-free rate was 58.0% (95% CI, 49.6% to 66.4%). For patients with measurable CNS disease only, 31 patients (62%) had an event, and the median time to event was 9.2 months (95% CI, 7.4 to 15.9 months); the 6-month event-free rate was 67.9% (95% CI, 54.9% to 80.9%). For patients without baseline CNS disease, 67 patients (59.8%) had an event, and the median time to event was 7.6 months (95% CI, 5.6 to 13.0 months); the 6-month event-free rate was 58.0% (95% CI, 48.7% to 67.3%). These results are similar to those for the overall populations of the two phase II studies.
Site of Progression
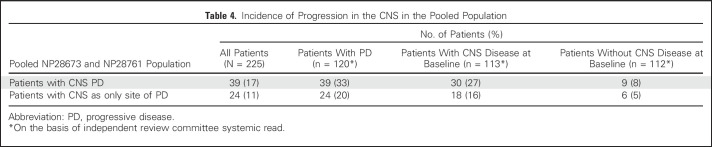
At the data cutoff (April 27, 2015), with a median follow-up time of 12.4 months (range, 0.9 to 19.7 months), only 17% of patients in the overall pooled population (n = 225) had PD in the CNS, as assessed by the IRC systemic read, and 11% had the CNS as the sole site of PD (Table 4). Of the 112 patients identified by the IRC systemic read as not having CNS disease at baseline, only 8% developed CNS metastases.
Table 4.
Incidence of Progression in the CNS in the Pooled Population
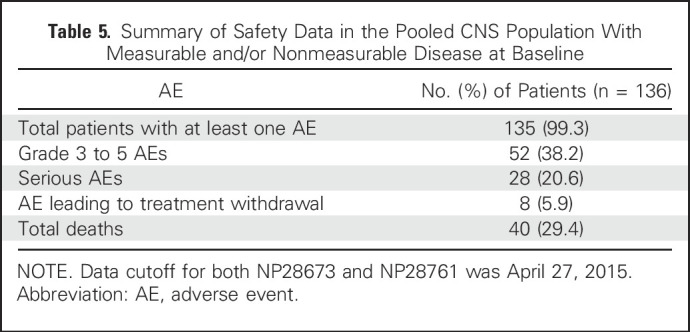
Safety
The safety profile of alectinib was consistent with previous studies9,13 and similar to the overall populations of the phase II studies8,12 (Table 5). There were low rates of dose reductions (14.7%) and withdrawals (5.9%) as a result of adverse events, confirming that alectinib was well tolerated in patients with measurable and/or nonmeasurable CNS lesions.
Table 5.
Summary of Safety Data in the Pooled CNS Population With Measurable and/or Nonmeasurable Disease at Baseline
DISCUSSION
Although patients with ALK-positive NSCLC initially respond well to the current standard treatment of crizotinib (National Comprehensive Cancer Network guidelines V6.2015),2 the majority will eventually experience PD within 1 year.3,4,14 The CNS is a common site of progression for crizotinib-treated patients, with 70% of patients experiencing CNS progression as a new or nontarget lesion during treatment,6 possibly as a result of poor CNS exposure to crizotinib.15 New treatments are needed, not only to overcome acquired resistance to crizotinib, but also to achieve better clinical efficacy in the CNS.
The phase II NP28673 and NP28761 studies have demonstrated that alectinib has both systemic and CNS efficacy in crizotinib-refractory ALK-positive NSCLC.8,12,13 Combining data from these two studies helps to characterize the role of alectinib in the CNS, as the larger patient numbers increase the precision of the pooled results and broaden the population profile. In this pooled analysis of patients with baseline CNS disease from these two studies, alectinib demonstrated good CNS efficacy, with IRC CORRs of 64.0% and 42.6% for patients with measurable and patients with measurable and/or nonmeasurable CNS disease at baseline, respectively. For patients with measurable and/or nonmeasurable CNS disease at baseline, 37 CRs (27%) were observed, demonstrating that alectinib can provide CNS benefit regardless of whether the disease is measurable according to RECIST. Importantly, the responses observed in the CNS were durable, with a CDOR of approximately 11 months, and were comparable to those observed for the overall systemic disease assessment.12,16
The promising CNS efficacy profile of alectinib in this pooled analysis is supported by previous clinical and preclinical results, which have suggested that alectinib is effective at controlling brain metastases. Preclinical studies showed that alectinib induced brain tumor regression in mouse models.17 In addition, the phase I/II AF-001JP study of alectinib in ALK-positive NSCLC reported no progression of brain metastases during alectinib therapy.9 In the Japanese bioequivalence study (JP28927; JapicCTI-132186), alectinib demonstrated efficacy in patients with ALK-positive NSCLC who had baseline brain metastases, with a reported response rate of 65%.18 Results from the phase I dose-finding part of NP28761 reported an ORR of 52% in patients with baseline brain metastases, including 29% with a CR.13 Although the current pooled analysis did not include any patients with leptomeningeal metastases, alectinib has been shown to be active in patients with ALK-positive NSCLC with leptomeningeal metastases.19,20
The ALK inhibitor ceritinib has also shown some efficacy in the CNS, although patient numbers are still relatively low. Overall, 10 of 29 patients with ALK-positive NSCLC and measurable brain disease at baseline had an objective CNS response with ceritinib, giving a CORR of 34.5% (95% CI, 17.9% to 54.3%).21 The different CORRs reported with ceritinib and alectinib may potentially be a result of different rates of efflux from the brain, which could affect the therapeutic concentrations of these agents in the CNS.22 In NP28761, paired CSF and systemic plasma samples showed alectinib penetration of the CNS, supporting a linear relationship between CSF and free alectinib concentrations in plasma.13 Furthermore, in preclinical models of intracranial metastases, alectinib demonstrated a high brain-to-plasma ratio and was transported independently of the efflux transporter, P-glycoprotein.17 Conversely, preclinical models of ceritinib reported restricted brain accumulation in the presence of P-glycoprotein or breast cancer resistance protein.23
In this pooled analysis, only 29% of patients with baseline CNS disease had received prior CNS radiation within the last 6 months, and only 5% had received CNS radiation within 1 month of starting alectinib. CORRs of 35.8% and 58.5% were observed for patients with CNS metastases with and without prior radiotherapy, increasing to 61.8% and 68.8%, respectively, for patients with measurable CNS disease only, suggesting that alectinib has clinical efficacy irrespective of radiation history. The observed CR rates (12% to 49%, depending on subgroup) are much higher than the 5% to 6% CR rates historically noted with WBRT,24,25 with the higher CR rates in patients without prior radiation likely a result of a lower disease burden in these patients. These data suggest that alectinib has CNS efficacy that is at least equivalent to the systemic efficacy observed. However, the results of this retrospective pooled analysis must be evaluated in the context of the small sample numbers for some subgroups and the single-arm design of the two studies.
The safety profile of alectinib in this combined analysis was consistent with previous studies,8,9,12,13 confirming that alectinib is well tolerated in patients with or without baseline CNS metastases. Standard WBRT can have physiologic and neurologic adverse effects, with many patients experiencing a decline in cognitive function.26 Stereotactic radiosurgery, increasingly used as an alternative to WBRT, can reduce these adverse effects but may decrease effectiveness when targeting large numbers of metastases.27 In addition, both types of radiation can result in areas of radionecrosis, which may require corticosteroids. A systemic drug treatment able to target all brain lesions with minimal toxicity could therefore offer clear advantages versus current standards of care.
In conclusion, this pooled analysis supports the results of previous alectinib studies, suggesting that alectinib has good CNS efficacy in ALK-positive NSCLC. Two ongoing phase III, head-to-head studies are comparing the systemic and CNS efficacy of alectinib versus crizotinib for patients with ALK-positive NSCLC who are ALK inhibitor naïve (global ALEX study, NCT02075840; Japanese J-ALEX study, JapicCTI-132316). Preliminary results from J-ALEX were recently presented; the PFS hazard ratio for alectinib versus crizotinib was 0.34 (99.6826% CI, 0.17 to 0.70; P < .001). Median PFS was not reached with alectinib (95% CI, 20.3 months to not reached) and was 10.2 months with crizotinib (95% CI, 8.2 to 12.0 months). Patients were not stratified according to baseline CNS metastases, but subgroup analyses found that the PFS hazard ratio for alectinib versus crizotinib in patients with baseline brain metastases was 0.08 (95% CI, 0.01 to 0.61).28
ACKNOWLEDGMENT
We thank all the NP28761 and NP28673 investigators, their patients, and their families. Third-party medical writing support, under the direction of the authors, was provided by Rhiannon Owen of Gardiner-Caldwell Communications and was funded by F. Hoffmann-La Roche.
Appendix
Table A1.
Baseline Characteristics for the Overall Study Populations
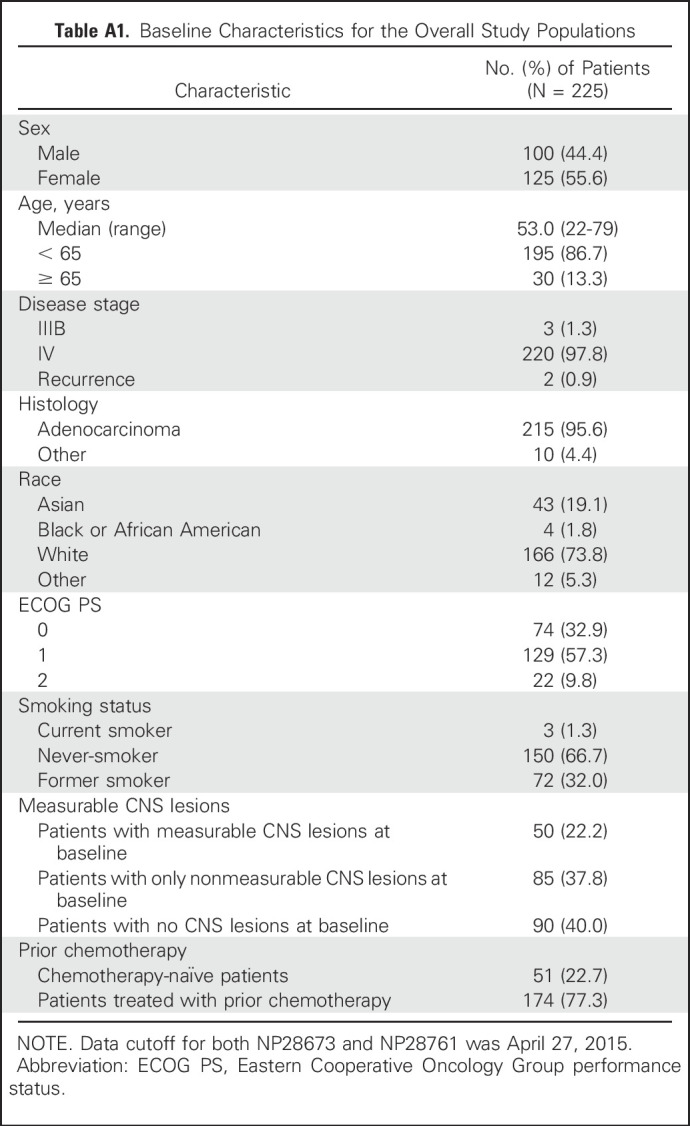
Table A2.
CORR by Prior Radiation in Patients With Measurable CNS Metastases at Baseline
Footnotes
Presented in part at the 16th International Association for the Study of Lung Cancer World Conference on Lung Cancer, Denver, CO, September 6-9, 2015.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT01871805, NCT01801111.
See accompanying editorial on page 4064
AUTHOR CONTRIBUTIONS
Conception and design: Shirish M. Gadgeel, Mark A. Socinski, D. Ross Camidge, Walter Bordogna, Sophie Golding, Ali Zeaiter, Sai-Hong Ignatius Ou
Provision of study materials or patients: Shirish M. Gadgeel, Mark A. Socinski, D. Ross Camidge, Eric Dansin, Antje Tessmer, James Chih-Hsin Yang, Ji-Youn Han, Sai-Hong Ignatius Ou
Collection and assembly of data: Alice T. Shaw, Leena Gandhi, Mark A. Socinski, D. Ross Camidge, Luigi De Petris, Dong-Wan Kim, Alberto Chiappori, Denis L. Moro-Sibilot, Michael Duruisseaux, Lucio Crino, Tommaso De Pas, Eric Dansin, Antje Tessmer, James Chih-Hsin Yang, Ji-Youn Han, Walter Bordogna, Sophie Golding, Sai-Hong Ignatius Ou
Data analysis and interpretation: Shirish M. Gadgeel, Alice T. Shaw, Ramaswamy Govindan, Leena Gandhi, Mark A. Socinski, D. Ross Camidge, Dong-Wan Kim, Alberto Chiappori, Michael Duruisseaux, Tommaso De Pas, James Chih-Hsin Yang, Walter Bordogna, Sophie Golding, Ali Zeaiter, Sai-Hong Ignatius Ou
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Pooled Analysis of CNS Response to Alectinib in Two Studies of Pretreated Patients With ALK-Positive Non–Small-Cell Lung Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Shirish M. Gadgeel
Consulting or Advisory Role: Pfizer, Novartis, Boehringer Ingelheim, Genentech, ARIAD, AstraZeneca, Bristol-Myers Squibb
Speakers' Bureau: Genentech, AstraZeneca
Research Funding: Pfizer (Inst), Clovis Oncology (Inst), Merck (Inst), Genentech (Inst), Incyte (Inst), Millennium (Inst), AstraZeneca/MedImmune (Inst), Bristol-Myers Squibb (Inst), Halozyme Therapeutics (Inst), Acerta Pharma (Inst), ACEA Biosciences (Inst), Janssen Oncology (Inst), Novartis (Inst), Five Prime Therapeutics (Inst), OncoMed (Inst)
Alice T. Shaw
Honoraria: Pfizer, Roche, Novartis
Consulting or Advisory Role: Pfizer, Novartis, Genentech, ARIAD, Roche, Daiichi Sankyo, EMD Serono, Taiho Pharmaceutical, Ignyta
Research Funding: Pfizer, Novartis, Genentech, ARIAD
Ramaswamy Govindan
Honoraria: Boehringer Ingelheim
Consulting or Advisory Role: GlaxoSmithKline, Boehringer Ingelheim, Clovis Oncology, Helsinn Therapeutics, Genentech, Abbvie, Celgene, Bayer, Novartis
Research Funding: Bayer (Inst), GlaxoSmithKline (Inst), MethylGene (Inst), Abbvie (Inst)
Travel, Accommodations, Expenses: Boehringer Ingelheim, Celgene, Merck, Amgen, Genentech, GlaxoSmithKline
Leena Gandhi
Honoraria: Merck
Consulting or Advisory Role: Genentech, Merck, Abbvie, AstraZeneca, Pfizer
Research Funding: Bristol-Myers Squibb
Mark A. Socinski
Honoraria: Genentech
Consulting or Advisory Role: Genentech
Speakers' Bureau: Genentech
Research Funding: Genentech (Inst)
D. Ross Camidge
Honoraria: Genentech
Consulting or Advisory Role: Genentech
Luigi De Petris
Honoraria: F. Hoffman-La Roche, AstraZeneca, Novartis, Quiagen
Consulting or Advisory Role: F. Hoffman-La Roche (Inst), Bristol Meyer-Squibb (Inst), Boehringer Ingelheim (Inst)
Dong-Wan Kim
No relationship to disclose
Alberto Chiappori
Consulting or Advisory Role: Clovis Oncology, Novartis, Genentech, ARIAD
Speakers' Bureau: Pfizer, Novartis, Genentech, Celgene, Boehringer Ingelheim, Merck
Research Funding: Novartis, Pfizer
Denis L. Moro-Sibilot
Honoraria: Roche, Novartis, Pfizer, ARIAD
Consulting or Advisory Role: Roche, Novartis, Pfizer
Travel, Accommodations, Expenses: Roche, Novartis, Pfizer
Michael Duruisseaux
Honoraria: Novartis
Travel, Accommodations, Expenses: Novartis, Roche, Boehringer Ingelheim, Pfizer
Lucio Crino
Honoraria: Pfizer, Boehringer Ingelheim, Bristol-Myers Squibb
Consulting or Advisory Role: Pfizer, Bristol-Myers Squibb
Travel, Accommodations, Expenses: Pfizer, Bristol-Myers Squibb
Tommaso De Pas
No relationship to disclose
Eric Dansin
Honoraria: Roche
Consulting or Advisory Role: AstraZeneca, Eli Lilly, Boehringer Ingelheim, Novartis
Research Funding: Roche (Inst)
Travel, Accommodations, Expenses: Eli Lilly, Novartis, Ipsen
Antje Tessmer
Honoraria: Novartis/Pfizer, Bristol-Myers Squibb
Consulting or Advisory Role: Chugai Pharma
Research Funding: PPD Germany (Inst)
Travel, Accommodations, Expenses: Boehringer Ingelheim
James Chih-Hsin Yang
Honoraria: Boehringer Ingelheim, Roche, Chugai Pharma, MSD, AstraZeneca
Consulting or Advisory Role: Boehringer Ingelheim, Novartis, AstraZeneca, Genentech, Clovis Oncology, Eli Lilly, MSD Oncology, Merck Serono, Celgene, Astellas Pharma, Bayer, Pfizer, Ono Pharmaceutical, Bristol-Myers Squibb, Boehringer Ingelheim (Inst), AstraZeneca (Inst)
Ji-Youn Han
Honoraria: Roche, Pfizer, AstraZeneca
Consulting or Advisory Role: MSO, Bristol-Myers Squibb, Novartis
Research Funding: Roche, Boehringer Ingelheim
Walter Bordogna
Employment: F. Hoffmann-La Roche
Stock or Other Ownership: F. Hoffmann-La Roche
Sophie Golding
Employment: F. Hoffmann-La Roche, F. Hoffmann-La Roche (I)
Stock or Other Ownership: F. Hoffman-La Roche
Ali Zeaiter
Employment: F. Hoffman-La Roche
Stock or other Ownership: F. Hoffman-La Roche
Sai-Hong Ignatius Ou
Honoraria: Genentech, Pfizer, AstraZeneca, Novartis, Boehringer Ingelheim
Consulting or Advisory Role: Genentech, Pfizer, ARIAD, Novartis
Speakers' Bureau: Genentech, AstraZeneca, Boehringer Ingelheim, Novartis
Research Funding: Pfizer (Inst), Genentech (Inst), ARIAD (Inst), AstraZeneca/MedImmune (Inst), Clovis Oncology (Inst), Daiichi Sankyo (Inst), Boehringer Ingelheim (Inst), Ignyta (Inst)
REFERENCES
- 1.Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: Updated results from a phase 1 study Lancet Oncol 131011–10192012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. doi: 10.1016/j.thorsurg.2014.12.003. National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines for non-small-cell lung cancer. V6.2015. http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. [DOI] [PubMed]
- 3.Lovly CM, Shaw AT.Molecular pathways: Resistance to kinase inhibitors and implications for therapeutic strategies Clin Cancer Res 202249–22562014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ou SH, Jänne PA, Bartlett CH, et al. Clinical benefit of continuing ALK inhibition with crizotinib beyond initial disease progression in patients with advanced ALK-positive NSCLC Ann Oncol 25415–4222014 [DOI] [PubMed] [Google Scholar]
- 5.Niemiec M, Głogowski M, Tyc-Szczepaniak D, et al. Characteristics of long-term survivors of brain metastases from lung cancer Rep Pract Oncol Radiother 1649–532011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa DB, Shaw AT, Ou SH, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases J Clin Oncol 331881–18882015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khozin S, Blumenthal GM, Zhang L, et al. FDA approval: Ceritinib for the treatment of metastatic anaplastic lymphoma kinase-positive non-small cell lung cancer Clin Cancer Res 212436–24392015 [DOI] [PubMed] [Google Scholar]
- 8.Ou SH, Ahn JS, De Petris L, et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: A phase II global study J Clin Oncol 34661–6682016 [DOI] [PubMed] [Google Scholar]
- 9.Seto T, Kiura K, Nishio M, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): A single-arm, open-label, phase 1-2 study Lancet Oncol 14590–5982013 [DOI] [PubMed] [Google Scholar]
- 10. Ohe Y, Nishio M, Kiura K, et al: A phase I/II study with a CNS-penetrant, selective ALK inhibitor alectinib in ALK-rearranged non-small cell lung cancer (ALK+ NSCLC) patients (pts): Updates on progression free survival (PFS) and safety results from AF-001JP. J Clin Oncol 33, 2015 (suppl; abstr 8061)
- 11.McKeage K.Alectinib: A review of its use in advanced ALK-rearranged non-small cell lung cancer Drugs 7575–822015 [DOI] [PubMed] [Google Scholar]
- 12.Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: A single-group, multicentre, phase 2 trial Lancet Oncol 17234–2422016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): Results from the dose-finding portion of a phase 1/2 study Lancet Oncol 151119–11282014 [DOI] [PubMed] [Google Scholar]
- 14.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer N Engl J Med 3712167–21772014 [DOI] [PubMed] [Google Scholar]
- 15.Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib J Clin Oncol 29e443–e4452011 [DOI] [PubMed] [Google Scholar]
- 16. Barlesi F, Dingemans A-MC, Ou S-HI, et al: Updated efficacy and safety results from a global phase 2, open-label, single-arm study (NP28673) of alectinib in crizotinib-refractory ALK+ non-small-cell lung cancer (NSCLC). Eur J Cancer 51, 2015 (suppl 3; abstr 3101)
- 17.Kodama T, Hasegawa M, Takanashi K, et al. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases Cancer Chemother Pharmacol 741023–10282014 [DOI] [PubMed] [Google Scholar]
- 18. Horiike A, Hida T, Nakagawa K, et al: Anti-tumor activity of alectinib against brain metastases from ALK rearranged NSCLC: Subset analysis of JP28927. Jpn J Lung Cancer 54:O-14, 2014 (abstr)
- 19.Gainor JF, Sherman CA, Willoughby K, et al. Alectinib salvages CNS relapses in ALK-positive lung cancer patients previously treated with crizotinib and ceritinib J Thorac Oncol 10232–2362015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ou SH, Sommers KR, Azada MC, et al. Alectinib induces a durable (>15 months) complete response in an ALK-positive non-small cell lung cancer patient who progressed on crizotinib with diffuse leptomeningeal carcinomatosis Oncologist 20224–2262015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shaw AT, Mehra R, Tan DSW, et al: Evaluation of ceritinib-treated patients with anaplastic lymphoma kinase rearranged (ALK+) non-small cell lung cancer (NSCLC) and brain metastases in the ASCEND-1 study. Ann Oncol 25:iv426-iv470, 2014 (suppl 4)
- 22.Misra A, Ganesh S, Shahiwala A, et al. Drug delivery to the central nervous system: A review J Pharm Pharm Sci 6252–2732003 [PubMed] [Google Scholar]
- 23.Kort A, Sparidans RW, Wagenaar E, et al. Brain accumulation of the EML4-ALK inhibitor ceritinib is restricted by P-glycoprotein (P-GP/ABCB1) and breast cancer resistance protein (BCRP/ABCG2) Pharmacol Res 102200–2072015 [DOI] [PubMed] [Google Scholar]
- 24.Bergqvist M, Brattström D, Bennmarker H, et al. Irradiation of brain metastases from lung cancer: A retrospective study Lung Cancer 2057–631998 [DOI] [PubMed] [Google Scholar]
- 25.Komatsu T, Kunieda E, Oizumi Y, et al. Clinical characteristics of brain metastases from lung cancer according to histological type: Pretreatment evaluation and survival following whole-brain radiotherapy Mol Clin Oncol 1692–6982013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brown PD, Asher AL, Ballman KV, et al: NCCTG N0574 (Alliance): A phase III randomized trial of whole brain radiation therapy (WBRT) in addition to radiosurgery (SRS) in patients with 1 to 3 brain metastases. J Clin Oncol 33, 2015 (suppl; abstr LBA4)
- 27.Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study Lancet Oncol 15387–3952014 [DOI] [PubMed] [Google Scholar]
- 28. Nokihara H, Hida T, Kondo M, et al: Alectinib (ALC) versus crizotinib (CRZ) in ALK-inhibitor naive ALK-positive non-small cell lung cancer (ALK+ NSCLC): Primary results from the J-ALEX study. J Clin Oncol 34, 2016 (suppl; abstr 9008)