Abstract
Purpose
AZD1775 is a WEE1 kinase inhibitor targeting G2 checkpoint control, preferentially sensitizing TP53-deficient tumor cells to DNA damage. This phase I study evaluated safety, tolerability, pharmacokinetics, and pharmacodynamics of oral AZD1775 as monotherapy or in combination with chemotherapy in patients with refractory solid tumors.
Patients and Methods
In part 1, patients received a single dose of AZD1775 followed by 14 days of observation. In part 2, patients received AZD1775 as a single dose (part 2A) or as five twice per day doses or two once per day doses (part 2B) in combination with one of the following chemotherapy agents: gemcitabine (1,000 mg/m2), cisplatin (75 mg/m2), or carboplatin (area under the curve, 5 mg/mL⋅min). Skin biopsies were collected for pharmacodynamic assessments. TP53 status was determined retrospectively in archival tumor tissue.
Results
Two hundred two patients were enrolled onto the study, including nine patients in part 1, 43 in part 2A (including eight rollover patients from part 1), and 158 in part 2B. AZD1775 monotherapy given as single dose was well tolerated, and the maximum-tolerated dose was not reached. In the combination regimens, the most common adverse events consisted of fatigue, nausea and vomiting, diarrhea, and hematologic toxicity. The maximum-tolerated doses and biologically effective doses were established for each combination. Target engagement, as a predefined 50% pCDK1 reduction in surrogate tissue, was observed in combination with cisplatin and carboplatin. Of 176 patients evaluable for efficacy, 94 (53%) had stable disease as best response, and 17 (10%) achieved a partial response. The response rate in TP53-mutated patients (n = 19) was 21% compared with 12% in TP53 wild-type patients (n = 33).
Conclusion
AZD1775 was safe and tolerable as a single agent and in combination with chemotherapy at doses associated with target engagement.
INTRODUCTION
DNA damage–induced checkpoint control is essential for the maintenance of genomic stability. One of the key proteins regulating the G2 checkpoint is the tyrosine kinase WEE1,1-3 which inhibits the action of its direct substrate cyclin-dependent kinase (CDK) 1 by phosphorylation of the Tyr15 residue,3-6 resulting in cell cycle arrest and allowing time for DNA repair. After DNA damage, TP53-mediated induction of p21Waf1/Cip1 also contributes to cell cycle arrest by activating the G1 checkpoint and strengthening the G2 checkpoint. Mutations in TP53 occur commonly in cancer, causing defective and weakened G1 and G2 checkpoints, respectively, and rendering cells highly dependent on activated WEE1 to achieve cell cycle arrest in response to DNA damage. Consequently, WEE1 inhibition abrogates the G2 checkpoint and selectively sensitizes TP53-deficient cells to DNA-damaging chemotherapy7 via premature mitotic entry and mitotic catastrophe.8,9
In addition to these events at the G2/M boundary, WEE1 also phosphorylates and inhibits the activity of CDK2 during S phase, allowing regulation of DNA synthesis and maintenance of replication forks.9,10 Therefore, WEE1 inhibition may result in increased DNA synthesis and nucleotide insufficiency that reduces replication fork speed, leading to replication fork stalling and double-strand breaks.11 Such events may be lethal to cancer cells with baseline replicative stress or compromised DNA repair proficiency or may exacerbate the effects of DNA-damaging agents irrespective of TP53 status. The small-molecule inhibitor AZD1775 (formerly MK-1775), a pyrazolopyrimidine derivative, is a potent and specific inhibitor of WEE1.12,13 Preclinically, AZD1775 induced cell death in combination with chemotherapy and preferentially sensitized TP53-deficient tumor cell lines to various anticancer agents, including gemcitabine, cisplatin, carboplatin, and radiation.12-16 The enhancement of antitumor activity by AZD1775 correlated with inhibition of CDK1 Y15 phosphorylation in tumor tissue and skin hair follicles in a dose-dependent manner, suggesting pCDK1 to be a useful pharmacodynamic biomarker.12 Additionally, in WiDr colorectal xenografts treated with gemcitabine plus AZD1775, reduced pCDK1 in tumor correlated with expression changes in genes associated with the G2 checkpoint comprising a WEE1 signature.13 This gene signature was also observed in animal skin samples, suggesting that pharmacodynamic markers can also be quantitatively assessed in surrogate tissues.
Other studies have demonstrated that AZD1775-mediated potentiation of antimetabolite chemotherapeutics can occur independent of TP53 status in both hematologic and solid tumor models.17 Additionally, AZD1775 has demonstrated single-agent activity in subsets of cell lines with either wild-type or mutant TP53. Sensitivity has been correlated with induction of DNA damage (assayed by phosphorylated histone H2AX [γH2AX]), without evidence of premature mitosis (assayed by phosphorylated histone H3),18,19 and may occur in cells under oncogene-addicted20 or epigenetically mediated replication stress21 or in cells with homologous recombination repair deficiency.22
The objectives of this phase I study were to determine the maximum-tolerated doses (MTDs), dose-limiting toxicities (DLTs), and biologically effective dose and to characterize safety and tolerability, the pharmacokinetic and pharmacodynamic profile, biomarkers of biologic activity, and the preliminary antitumor activity of oral AZD1775, both as monotherapy and in combination with gemcitabine, cisplatin, or carboplatin. On the basis of the variety of mechanisms by which AZD1775 may induce cytotoxicity, patients with tumors harboring both mutant and wild-type TP53 were enrolled.
PATIENTS AND METHODS
Patient Selection
Patients were ≥ 18 years old, with locally advanced or metastatic solid tumors, for which no standard therapy was available. All patients had an Eastern Cooperative Oncology Group performance status of ≤ 1, adequate organ function, and evaluable and/or measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST, version 1.0).23 See the Appendix (online only) for additional inclusion and exclusion criteria.
Study Design and Drug Treatment
This phase I, open-label, nonrandomized three-arm dose-escalation study was conducted in eight centers in the United States, Canada, and Europe (ClinicalTrials.gov identifier: NCT00648648). Cohorts of patients were treated at sequentially increasing dose levels of oral AZD1775 (Fig 1). Dose escalation in combination with chemotherapy was performed according to a modified toxicity probability interval scheme that used a 30% DLT rate. AZD1775 was titrated using a modified Fibonacci design allowing for 50%, 40%, and 30% dose increments in subsequent dose levels24 (Appendix). The MTD of AZD1775 was evaluated for each of the three chemotherapy treatment arms separately.
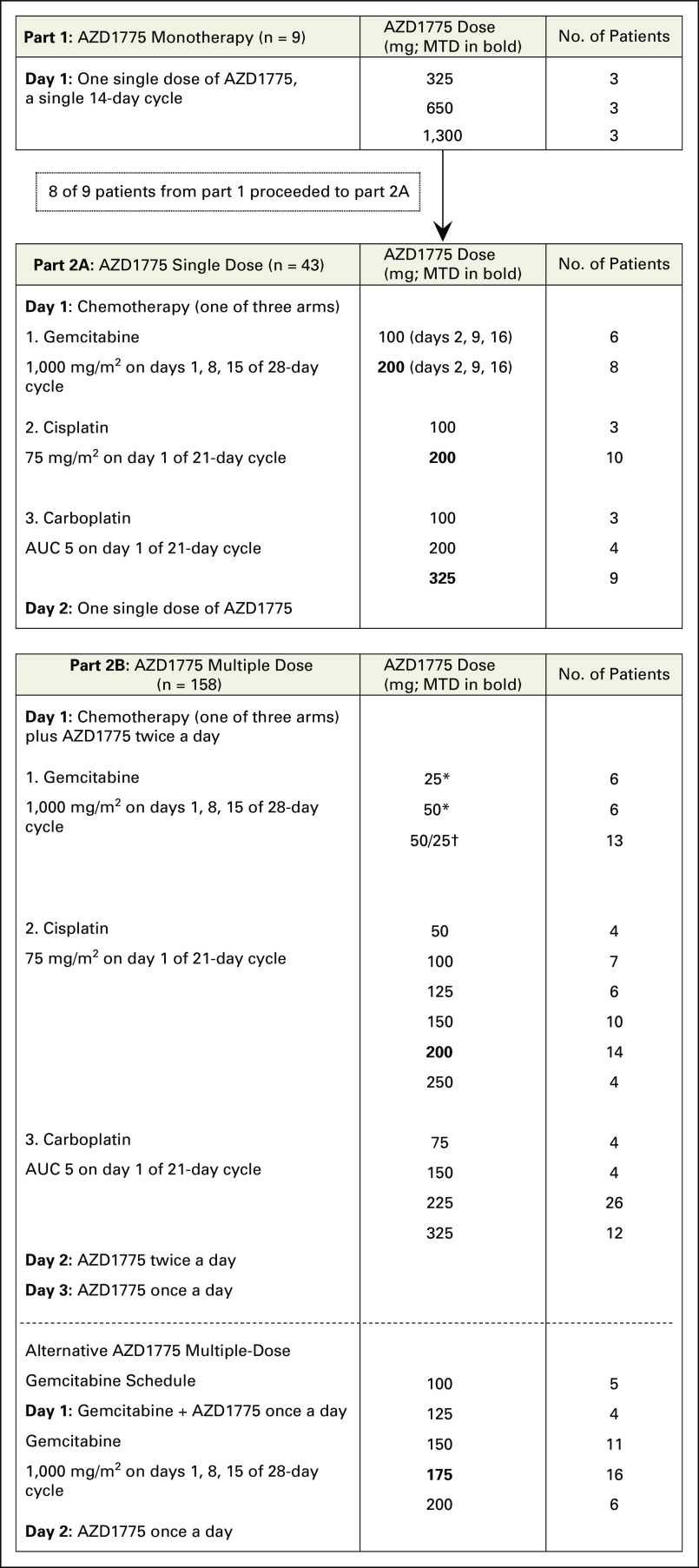
Fig 1.
Study setup. The first part of the study consisted of monotherapy with AZD1775 given as one single dose. Parts 2A and 2B consist of three different treatment arms, with gemcitabine, carboplatin, or cisplatin, in the following two different schedules: one single dose of AZD1775 administered the day after the chemotherapy (part 2A) or five doses of AZD1775 given twice a day, with the first dose always starting concomitantly with chemotherapy. (*) AZD1775 administered on days 1 to 3, 8 to 10, and 15 to 17. (†) AZD1775 50 mg twice a day on days 1, 8, and 15; AZD1775 25 mg twice a day on days 2, 9, and 16; and 25 mg once a day on days 3, 10, and 16. AUC, area under the curve; MTD, maximum-tolerated dose.
AZD1775 monotherapy consisted of a single dose followed by 14 days of observation (part 1), after which patients moved to part 2A, in which a single dose of AZD1775 was given 24 hours after standard chemotherapy with gemcitabine (1,000 mg/m2), cisplatin (75 mg/m2), or carboplatin (area under the curve [AUC], 5 mg/mL⋅min). Part 2B consisted of a multiple-dose regimen of AZD1775 (administered twice a day for 2 days and once a day for 1 day) starting concomitantly with chemotherapy. Patients were assigned to a chemotherapy arm according to the judgment of the investigator.
Alternate schedules of AZD1775 in combination with gemcitabine were explored. The first schedule involved AZD1775 50 mg twice a day on day 1, 25 mg twice a day on day 2, and 25 mg once a day on day 3, with gemcitabine given simultaneously with administration of the first dose of AZD1775. The second schedule involved a once-daily dose of AZD1775 (varying from 100 to 200 mg) for 2 days, with the first dose given simultaneously with administration of gemcitabine.
Safety and Assessments
Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0).25 DLTs were defined as any grade 4 or 5 hematologic toxicity (with the exception of grade 4 anemia and leukopenia, grade 4 neutropenia lasting for < 7 days, and grade 4 thrombocytopenia lasting for < 4 days [except if a platelet transfusion was required]) and any grade 3, 4, or 5 nonhematologic toxicity (with specific exceptions; Appendix) during the first treatment cycle. Tumor assessments were performed at screening, every two cycles, and whenever there was suspicion of disease progression.
Pharmacokinetic Assessments
In all parts of the study, blood samples for pharmacokinetic analysis were collected during cycle 1 at selected time points up to 48 hours after administration of the last dose of AZD1775 and analyzed by hydrophilic interaction liquid chromatography coupled with tandem mass spectrometry, as previously described (Appendix).26
Exploratory Biomarker and Pharmacodynamic Assessments
Baseline tumor samples were collected to correlate TP53 mutation with pharmacodynamic and clinical response. Analysis of TP53 status was performed by polymerase chain reaction (PCR) and sequencing of exons 4 to 9.
Target inhibition of AZD1775 was assessed as a decrease of pCDK1 (Tyr15) relative to total CDK1 measured in skin biopsies using quantitative multiplex immunohistochemistry. On the basis of preclinical data linking this pharmacodynamic marker with in vitro and in vivo efficacy,12 a 50% decrease of pCDK1 after AZD1775, compared with after chemotherapy and before AZD1775, with a one-sided P < .05, was defined as evidence of target engagement.
Hair follicles were analyzed by quantitative PCR for the WEE1 signature,13 a gene expression–based pharmacodynamic biomarker that consists of a composite score calculated from the average fold change of up- and downregulated genes relative to before dose. Gene expression was measured at pre- and postdose time points for the following eight genes identified as potential candidates by microarray: CLSPN, FBXO5, MCM10, CCNE1, CCNE2, EGR1, HIST12BD, and MYB. These genes are closely associated with the G2 checkpoint and commonly modulated by AZD1775 in both TP53-mutant and wild-type cell lines, as well as in skin samples derived from subcutaneous xenograft tumors in rats treated with gemcitabine and AZD1775.13
Statistical Analyses
Safety assessments, tumor response, pharmacokinetic parameters, and pharmacodynamic biomarkers were analyzed by descriptive statistics. An analysis of variance was conducted for each quantitative PCR gene on the log fold-change (after dose to before dose) scale. The various treatment and dose combinations were included as distinct categorical factors so that all observations were used to estimate a common residual variance; hence, tests were not dependent on variance estimates derived from only a few patients. A Hochberg multiplicity adjustment was applied over the three monotherapy doses tested (adjusting for multiple tests within the gene).
RESULTS
Patient Characteristics
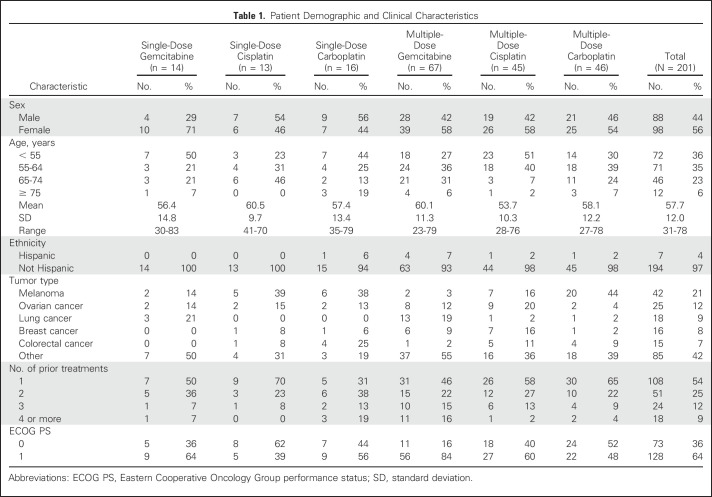
In total, 202 patients were treated, of whom 176 were evaluable for response (Table 1). Eight of nine patients completed part 1 of the study and continued in part 2A. The most common tumor types were melanoma (n = 4, 44%) and lung cancer (n = 2, 22%) in part 1, and melanoma (n = 42, 21%), ovarian cancer (n = 25, 12%), breast cancer (n = 17, 8%), colorectal cancer (n = 16, 8%), and lung cancer (n = 15, 7%) in part 2.
Table 1.
Patient Demographic and Clinical Characteristics
Safety and Tolerability
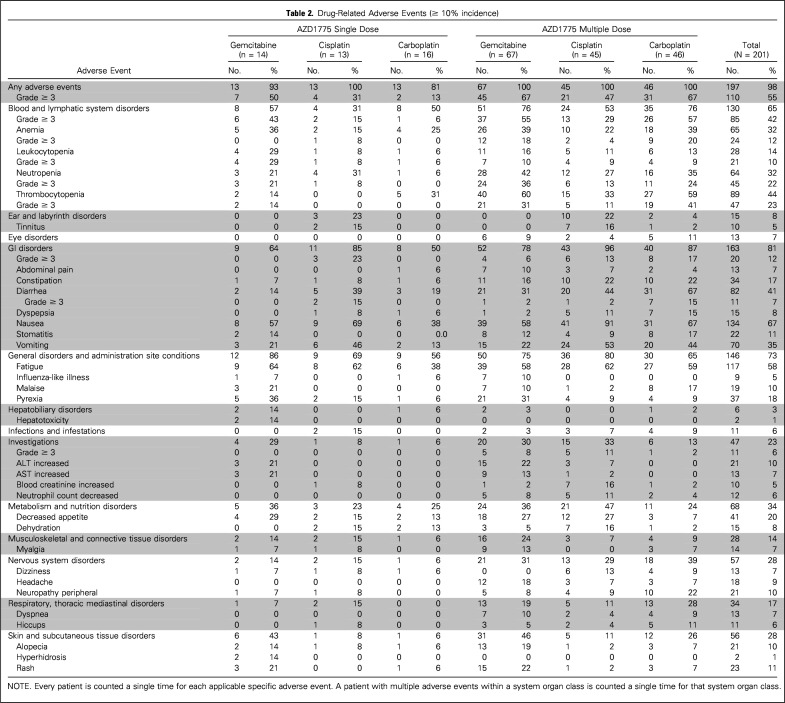
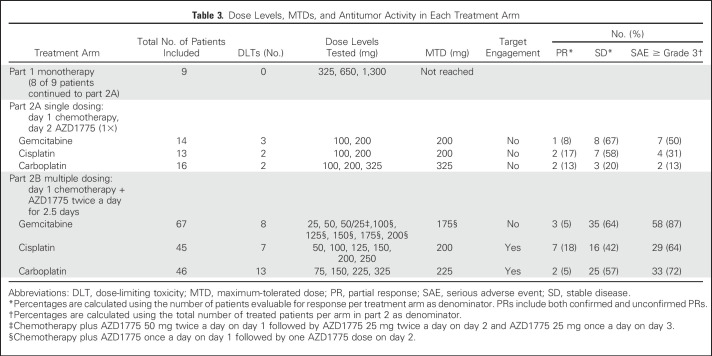
Five (56%) of nine patients treated in part 1 with a single dose of AZD1775 experienced a drug-related adverse event (AE), with the most frequently reported events being diarrhea (22%) and fatigue (22%). In part 2, 38 patients (19%) had a serious treatment-related AE. The most common treatment-related AEs were GI disorders (nausea [67%], vomiting [35%], and diarrhea [41%]), fatigue (58%), and hematologic toxicity [thrombocytopenia [44%], neutropenia [32%], and anemia [32%]; Table 2). DLT criteria were not observed with AZD1775 monotherapy so the MTD was not formally defined. For combination therapy, MTDs were defined in all treatment arms and consisted of AZD1775 225 mg twice a day for 2.5 days every 21 days, 200 mg twice a day for 2.5 days every 21 days, and 175 mg once a day for 2 days weekly for 3 consecutive weeks out of every 4-week cycle, combined with carboplatin (AUC 5), cisplatin (75 mg/m2), and gemcitabine (1,000 mg/m2 weekly for 3 consecutive weeks out of every 4-week cycle), respectively, for the multiple dosing regimens (Fig 1).
Table 2.
Drug-Related Adverse Events (≥ 10% incidence)
Antitumor Activity
Seventeen (10%) of 176 evaluable patients achieved a partial response (PR; of whom seven patients [4%] had confirmed PR) and 94 patients (53%) had stable disease lasting at least 6 weeks as best overall response (Table 3). Responses have been observed in patients with ovarian cancer (n = 7), melanoma (n = 3), breast cancer (n = 2), head and neck cancer (n = 3), colorectal cancer (n = 1), and squamous cell carcinoma of the skin (n = 1). Baseline tumor samples were evaluable from 52 patients. Among 19 patients with tumors harboring TP53 mutation, four patients (21%) achieved a PR. Thirty-three patients had TP53 wild-type tumors, of whom four patients (12%) achieved a PR.
Table 3.
Dose Levels, MTDs, and Antitumor Activity in Each Treatment Arm
Pharmacokinetics
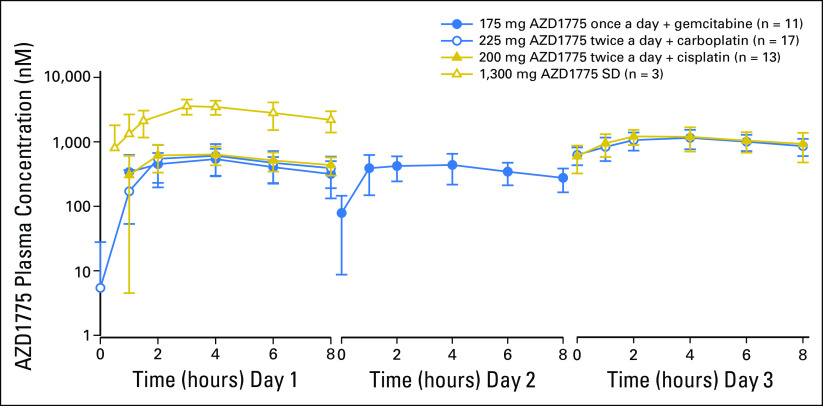
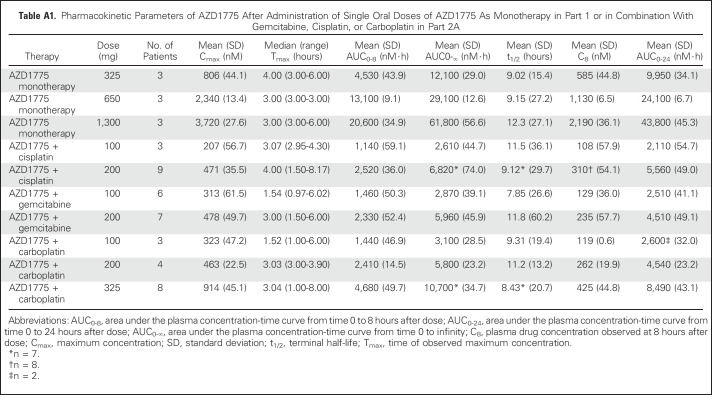
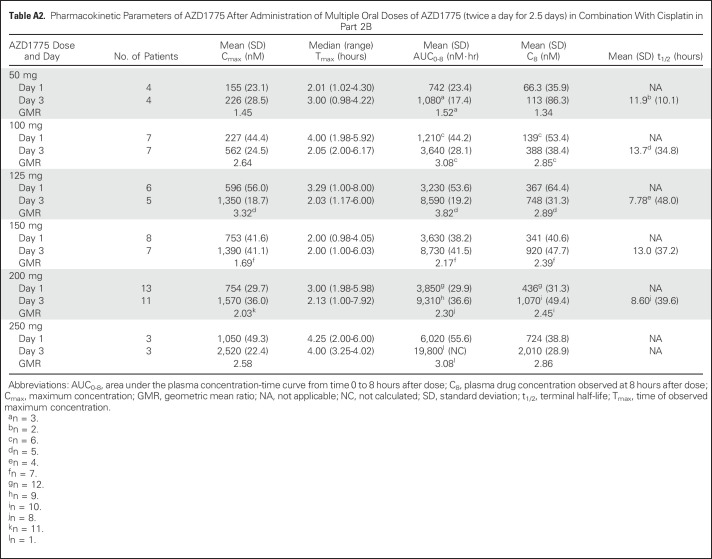
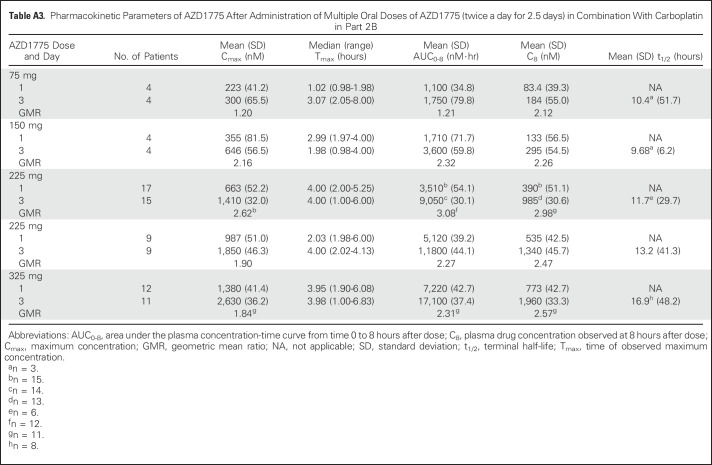
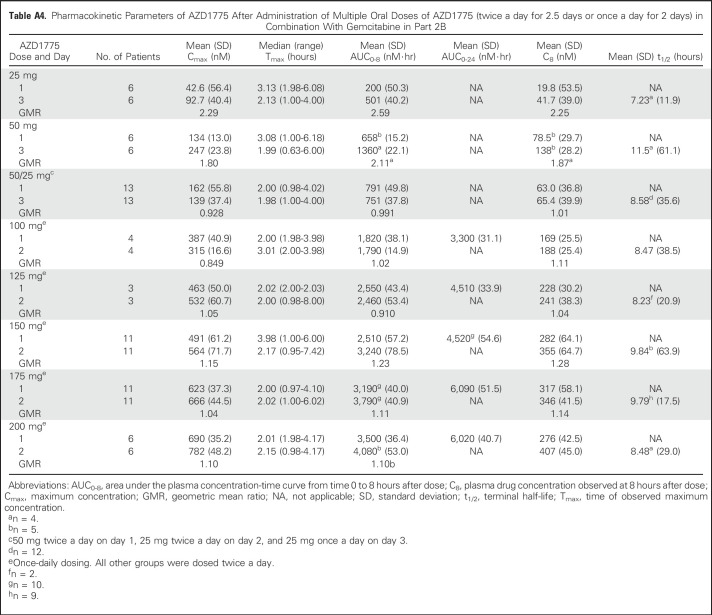
Plasma exposure increased approximately dose proportionally in both monotherapy and combination therapy arms, with moderate to high variability (Fig 2 and Appendix Tables A1 to A4, online only). Accumulation ratios (geometric mean ratio = day 3/day 1) for the area under the plasma concentration-time curve from time 0 to 8 hours after dose, maximum concentration, and plasma drug concentration observed at 8 hours after dose for twice-daily dosing averaged from 0.991 to 3.82, 0.928 to 3.32, and 1.01 to 2.98, respectively, across tested AZD1775 doses in combination with chemotherapy. The pharmacokinetic target of plasma drug concentration at 8 hours after dose of 240 nM, which was associated with maximal efficacy in rat tumor xenograft studies, was achieved at AZD1775 100 mg in combination with cisplatin and AZD1775 150 mg in combination with carboplatin on day 3 of the multiple AZD1775 dosing regimen (twice a day for 2.5 days), but not at the MTD of AZD1775 in the multiple-dose regimen in combination with gemcitabine. The alternate dosing regimen of AZD1775 125 mg once a day for 2 days in combination with gemcitabine achieved the pharmacokinetic target on day 2. Pharmacokinetic parameters of AZD1775 were not significantly different between the three chemotherapy groups.
Fig 2.
Mean concentration-time profiles for single-dose (SD) AZD1775 and multiple doses of AZD1775 alone and in combination with gemcitabine, carboplatin, or cisplatin (semi-log plot).
The antiemetic aprepitant is a substrate and a weak to moderate inhibitor of CYP3A4. Although the use of strong CYP3A4 inhibitors was prohibited, administration of aprepitant was permitted as supportive care according to institutional guidelines. Comparing the pharmacokinetic parameters of AZD1775 in patients with and without concomitant administration of aprepitant showed an approximate 40% increase in exposure (P < .001 for AUC from time 0 to 8 hours after dose on days 1 and 3).
Exploratory Biomarker and Pharmacodynamic Analyses
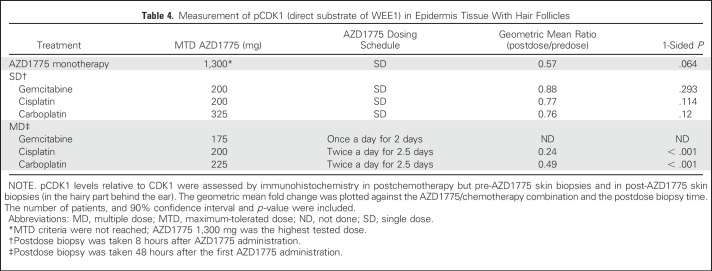
pCDK1 levels relative to total CDK1 were assessed by immunohistochemistry in pre- and postdose hair follicles in skin biopsies taken from behind the ear. In the combination arms, the predose biopsy was taken after chemotherapy but before AZD1775. Target engagement was demonstrated in the multidose regimen in combination with cisplatin or carboplatin (Table 4). With the gemcitabine multiple-dose regimen, target engagement was not achieved at the MTD of AZD1775 25 mg (twice a day for 2.5 days) or with a regimen of AZD1775 50 mg twice a day on day 1, 25 mg twice a day on day 2, and 25 mg once a day on day 3. In an alternate schedule, the MTD of AZD1775 175 mg once a day for 2 days surpassed the dose needed to achieve target engagement in the other arms, but skin biopsies of patients treated at this dose level were not available for pCDK1 analysis.
Table 4.
Measurement of pCDK1 (direct substrate of WEE1) in Epidermis Tissue With Hair Follicles
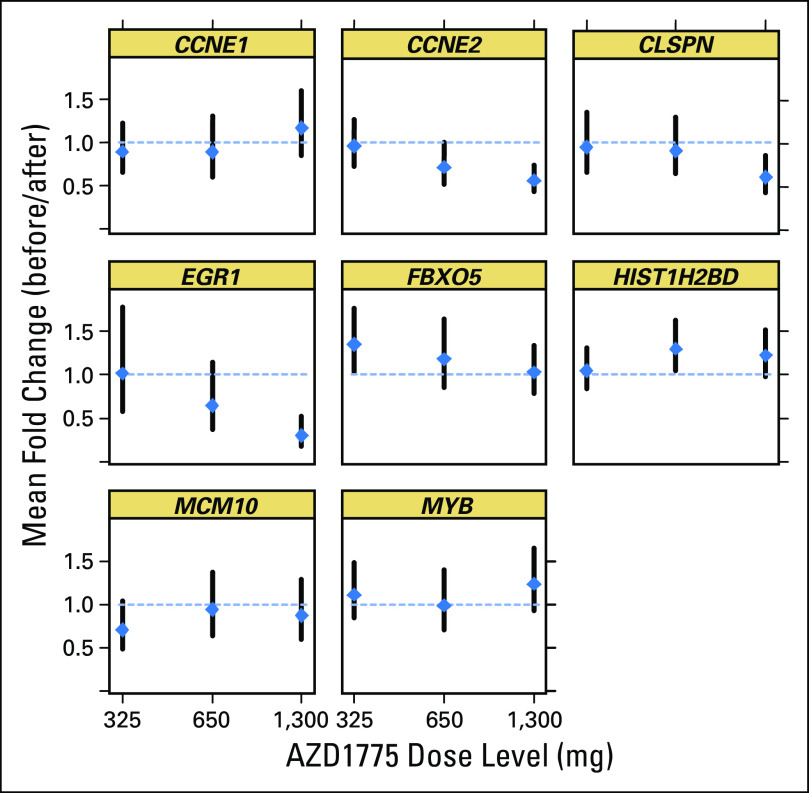
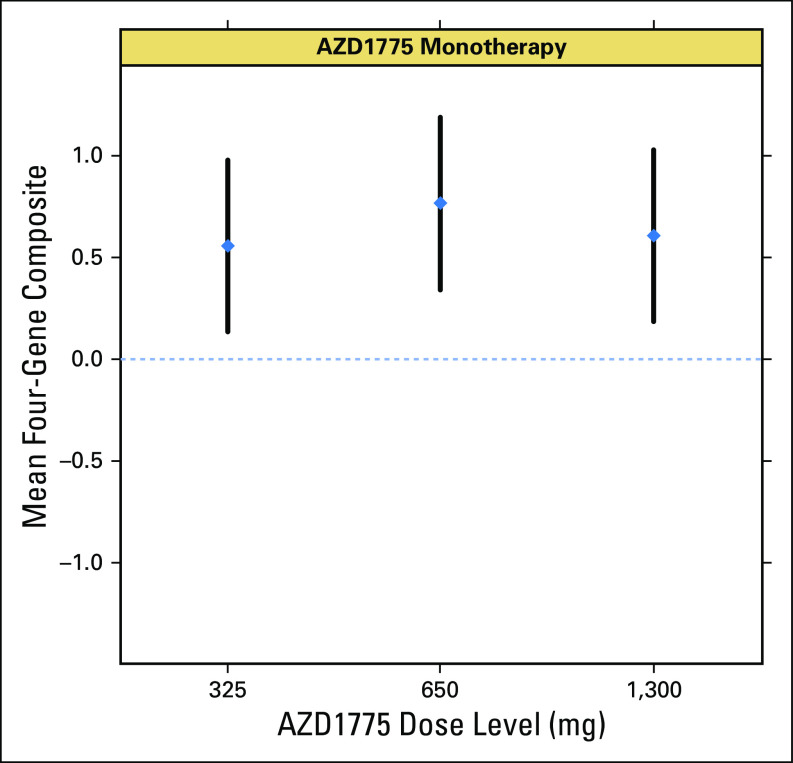
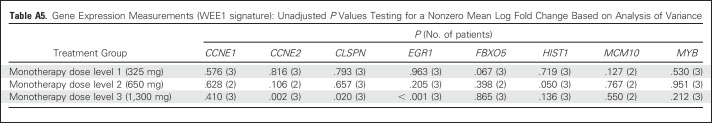
Gene expression measurements (WEE1 signature13) demonstrated that four of the eight selected genes (CCNE2, EGR1, CLSPN, and HIST12BD) showed significant changes in expression after monotherapy, consistent with preclinical expectations (P < .05, unadjusted for multiplicity). Most notable were the effects on expression of EGR1 and CCNE2 (P < .003 and P = .005, respectively, after adjustment for multiplicity) at the highest dose levels, suggesting a dose-response correlation. A composite score derived from the four genes that showed expression changes in the direction consistent with that expected on the basis of preclinical data (specifically, CCNE2, CLSPN, and MCM10 as downregulated set and HIST1H2BD as upregulated gene) showed a consistent trend indicating target engagement at all monotherapy doses, although a strong dose-response trend is not evident using the limited data available (Appendix).
DISCUSSION
In this study, we explored the tolerability, safety, and antitumor activity of the WEE1 inhibitor AZD1775 in combination with cisplatin, carboplatin, and gemcitabine, on the basis of the potentiation by AZD1775 of their antitumor activity in vitro and in vivo.12 In general, AZD1775 was well tolerated. In combination with chemotherapy, toxicities observed in the AZD1775 single-dose regimen were consistent with those expected for the individual chemotherapeutic agents. However, in the AZD1775 multiple-dose regimen, toxicities likely related to AZD1775 were observed, including bone marrow suppression, nausea, vomiting, diarrhea, fatigue, and hiccups. Episodes of nausea, vomiting, and diarrhea occurred primarily at days 2 to 3, suggesting a correlation with exposure.
The pharmacokinetic parameters of AZD1775 were approximately linear and increased in a dose-proportional manner, and were not significantly changed in combination with chemotherapy (Appendix Tables A1 to A4). However, we found a significant difference in AZD1775 exposure between patients treated with and without aprepitant, likely the result of CYP3A4 inhibition by aprepitant. In vitro data suggested that the major pathway of AZD1775 metabolism in humans involves CYP3A4, although FMO3 and FMO5 may be involved as well. Given the 40% increase in AZD1775 exposure upon concomitant use of aprepitant, this drug-drug interaction was considered clinically relevant, and the use of aprepitant has been prohibited in subsequent studies until further crossover drug-drug interaction studies are conducted.
Since early in vitro experiments examining the sequence of gemcitabine and AZD1775 administration demonstrated greatest antitumor activity when AZD1775 was given approximately 24 hours after exposure to DNA-damaging agents,12 patients in part 2A received the chemotherapy infusion on day 1 and one dose of AZD1775 24 hours (± 2 hours) after chemotherapy on day 2. The relatively short half-life of AZD1775 in vivo, as well as preclinical data that emerged while the study was ongoing, suggested that multiple doses of AZD1775 administered with chemotherapy would increase the combinatorial efficacy without affecting tolerability.16 To maximize checkpoint escape in cancer cells that transition through S phase during the time of treatment with chemotherapy, the protocol was amended, and AZD1775 was given twice a day for five doses in all three treatment arms, composing part 2B of the study. However, this schedule did not allow us to achieve doses in combination with gemcitabine that met predicted pharmacokinetic levels for efficacy or the minimum threshold required for target engagement, prompting us to investigate an attenuated once-daily schedule. After this adjustment, doses in combination with gemcitabine were achieved consistent with proof of mechanism that was demonstrated in the other arms, with reduced pCDK1 relative to total CDK1 in post-treatment skin biopsies compared with postchemotherapy and pre-AZD1775 skin biopsies. Together with changes in gene expression in hair follicles observed after monotherapy that reflected a previously defined WEE1 signature, evidence of WEE1 inhibition in surrogate tissue was established in this study. Using the defined doses and schedules, further confirmatory pharmacodynamic assessments in optimally timed tumor biopsies after chemotherapy and after chemotherapy and AZD1775 will be required to confirm proof of mechanism in tumor tissue.
Although the patient population was heavily pretreated, PRs and instances of prolonged stable disease were achieved. Mechanistically, tumors harboring TP53 mutation or p53 pathway alteration are expected to benefit most from the addition of AZD1775 to cytotoxic chemotherapy. Indeed, our data suggested that tumors from responding patients were mildly enriched for TP53 mutations, given the response rates of 21% and 12% in TP53-mutated and TP53 wild-type patients, respectively. However, larger patient sample sizes, better knowledge of the underlying biology, and a more detailed characterization of p53 pathway components in resistant and sensitive tumors will be necessary to optimize the identification of patients most likely to derive benefit from chemotherapy and AZD1775 combinations.
Notably, AZD1775 is being actively developed in high-grade serous ovarian cancer, a tumor type where TP53 mutation is ubiquitous, in combination with carboplatin (ClinicalTrials.gov identifiers: NCT01164995, NCT01357161) or gemcitabine (NCT02101775). Preliminary results have demonstrated promising antitumor activity with AZD1775 plus carboplatin in patients with platinum-resistant ovarian cancer,27 as well as a significant increase in progression-free survival with AZD1775 added to paclitaxel and carboplatin when compared with paclitaxel plus carboplatin alone in patients with platinum-sensitive ovarian cancer.28
Further development of AZD1775 may also occur in combination with radiation therapy, particularly in glioblastoma, where WEE1 is overexpressed and radiosensitizing effects have been demonstrated in preclinical models.29-33 Additionally, preclinical synergism has been observed with CHK1 inhibitors34-37; combined WEE1/CHK1 inhibition, if tolerable, may achieve even more potent G2 checkpoint abrogation in concert with DNA-damaging agents. Interestingly, the activation of CDK1 afforded by WEE1 inhibition may also predispose to immunotherapy responses in tumors that have undergone epithelial-mesenchymal transition, prompting interest in combinations with immune checkpoint blockade.38
On the basis of the multiple-dose regimen (twice a day for 2.5 days) established in this study, a monotherapy study was launched with a similar schedule administered for up to 2 weeks of every 21-day cycle. The MTD was 225 mg, with biopsies after the fifth dose demonstrating reduced CDK1-Y15 phosphorylation and induction of γH2AX. Responses were observed among patients carrying BRCA mutations.39 Such work may also inform the optimal populations to study in combination trials. In summary, we have established tolerable doses of oral AZD1775 in combination with cisplatin, carboplatin, and gemcitabine that exceed threshold pharmacokinetic levels for efficacy and preliminary pharmacodynamic evidence of WEE1 inhibition in concert with these DNA-damaging agents.
Appendix
Patient Inclusion and Exclusion Criteria
Eligible patients had adequate bone marrow (absolute neutrophil count ≥ 1,500/μL; platelet count 100,000/μL; hemoglobin ≥ 9 g/dL), liver function (serum total bilirubin ≤ 1.5× upper limit of normal [ULN] or direct bilirubin ≤ ULN for patients with serum total bilirubin > 1.5× ULN; ALT and AST ≤ 2.5× ULN or ≤ 5× ULN for patients with liver metastases; if alkaline phosphatase ≥ 2.5× ULN, the liver fraction had to be ≤ 2.5× ULN), renal function (serum creatinine ≤ 1.5× ULN or ≥ 60 mL/min for patients with creatinine levels > 1.5× ULN), and adequate coagulation status (international normalized ratio or prothrombin time ≤ 1.5× ULN; activated partial thromboplastin time ≤ 1.5× ULN). Previous anticancer treatment had to be completed at least 4 weeks before study entry. Up to four prior cytotoxic chemotherapy regimens were permitted. Drugs or other products known to be metabolized by CYP3A4 or to inhibit or induce CYP3A4 were not allowed. Patients with CNS metastases were also excluded unless they were clinically stable for 1 month before study entry (ie, no evidence of new enlarging CNS metastasis and off corticosteroids or on a stable dose of corticosteroids for ≥ 2 weeks). Other exclusion criteria included ongoing systemic infections, symptomatic ascites or pleural effusion, pregnancy, and hypersensitivity to the chemotherapy.
Study Design and Treatment
The study received approval of the institutional medical ethical review boards and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. Written informed consent was given by all patients before inclusion onto the study.
Part 1 of the study (one single dose of AZD1775) used a dose-escalation scheme with 100% dose increments and dose level 1 of AZD1775 325 mg. Dose level 1 was calculated on the basis of a dose of 180 mg/m2 (average body-surface area of 1.8 m2) and rounded to the closest multiple of 25. The dose of 180 mg/m2 of AZD1775 was established as the maximum no-effect level in a single-dose oral toxicity study in dogs.
Combination therapy with AZD1775 and chemotherapy (parts 2A and 2B) used a modified Fibonacci scheme. The modified Fibonacci scheme used 50%, 40%, and 30% dose increments in subsequent dose levels. The toxicity probability interval targets a dose-limiting toxicity (DLT) rate of 30% and allows escalation or de-escalation on the basis of the number of DLTs observed at a given dose level. Upon definition of a preliminary maximum-tolerated dose in six patients, a cohort expansion for a total of 13 evaluable patients was triggered. During cohort expansion, dose assignment actions continued based on continuous assessment of tolerability information. In case of DLT or toxicity after cycle 1, dose modification to a lower dose level was permitted in individual patients.
Safety Assessments
Demographic data and medical history were collected during screening. Physical examination, vital signs, and other safety assessments (Eastern Cooperative Oncology Group performance status, 12-lead ECG, hematology and biochemistry, and relevant tumor markers) were performed before dose and throughout treatment.
DLTs were defined as any grade 4 or 5 hematologic toxicity (with the exception of grade 4 anemia and leukopenia, grade 4 neutropenia lasting for < 7 days, and grade 4 thrombocytopenia lasting for < 4 days [except if a platelet transfusion was required]) and any grade 3, 4, or 5 nonhematologic toxicity (with the specific exception of grade 3 nausea, vomiting, diarrhea, or dehydration occurring in the setting of inadequate compliance with supportive care measures and lasting for < 48 hours, alopecia [of any grade], and inadequately treated hypersensitivity reactions).
Pharmacokinetic Assessments
Whole-blood samples of 4 mL each, for determination of AZD1775 plasma concentrations, were collected at the following time points: part 1 (monotherapy): before dose (time 0) and then 0.5, 1, 1.5, 3, 4, 6, 8, 24, and 48 hours after the administration of AZD1775; part 2A (AZD1775 single-dose combination therapy): cycle 1, day 1: before dose (time 0) and then 0.5, 1, 1.5, 3, 4, 6, 8, 24, and 48 hours after the administration of AZD1775; parts 2B and 3 (AZD1775 multiple-dose combination therapy): cycle 1, days 1 and 3: before dose and then 1, 2, 4, 6, and 8 hours after the first administration of AZD1775 (plus chemotherapy on day 1); cycle 1, day 2: before dose (before the third administration of AZD1775). Twenty-four and 48 hours after the fifth administration of AZD1775 were optional time points for blood sample collection. In the gemcitabine plus once-daily for 2 days AZD1775 dosing regimen, the time points for plasma collection on days 1 and 2 were similar to days 1 and 3 of the AZD1775 multiple-dose schedule (part 2B). For pharmacokinetic parameters, see Appendix Tables A1 to A4.
Pharmacodynamic Assessments
pCDK1.
Preclinical data indicated that the hair bulb is the preferable tissue for pCDK1 analysis. However, this study demonstrated that bulbs are only present in a minority of patient specimens. Therefore, epidermis of the scalp behind the ears (containing hair follicles) was used for pCDK1 analysis because it is also an actively proliferating tissue and present in all punch biopsies.
AZD1775, by inhibition of WEE1, reduces pCDK1 levels relative to total CDK1. Phosphorylation of CDK1 is induced by chemotherapy, especially gemcitabine. Therefore, correction for the chemotherapy effect was applied in the analysis of the postdose skin biopsy samples.
WEE1 signature.
Plucked hair follicles were obtained before and after AZD1775 dose (8 ± 2 hours after [last] oral administration of AZD1775 in cycle 1). Skin biopsies were obtained before and after AZD1775 dose (8 ± 2 hours [parts 1 and 2A] and within 2 hours [part 2B] after last oral administration of AZD1775 in cycle 1).
Quantitative polymerase chain reaction assays were performed for all clinical hair follicle samples from the single-dose regimen to analyze gene expression of a selected group of genes, also referred to as the WEE1 signature. A signature responsive to AZD1775 was derived from preclinical experiments13 and assessed in hair follicles collected at baseline and 8 hours after dose from patients participating in the monotherapy part of this study. Briefly, a composite score was calculated as the average fold change of genes downregulated relative to predose levels subtracted from the average fold change of genes upregulated relative to predose levels. The initial eight-gene signature (HIST1H2BD, EGR1, CCNE1, CCNE2, CLSPN, MCM10, FBOX5, and MYB) was refined based on an interim analysis that pooled all the treatment groups (not just monotherapy) and determined which genes showed significant effects in a direction consistent with preclinical experiments. This led to a reduced four-gene signature (upregulated: HISTH1HSBD; and downregulated: CCNE2, CLSPN, and MCM10).
Individual measurements that decreased below the limit of quantification established via an assay validation process (Ct > 34.06) were not used in the analysis. Fold-change (postdose v predose) values were calculated using the comparative Ct method (ΔΔCt). Statistical tests providing P values were conducted using the log (base 2) of fold change. As a quality control check, trends in fold change versus RNA yield were checked, and in general, there did not seem to be any strong trends for the genes examined. An analysis of the predose/postdose changes in the house keeping genes was conducted to confirm that significant results were not being driven by effects on those genes.
An analysis of variance was conducted for each gene to estimate the mean fold change at each of the combinations of treatment and dose level. All treatments and dose levels were included in a single analysis of variance model for each gene as distinct categorical factors so that all observations were used to estimate a common residual variance. However, findings conducted in a separate study of standard of care therapies suggested that the natural course of gene expression changes over the time period of interest (24 to 32 hours) after receipt of standard of care is a confounding factor in interpreting the effects of AZD1775 in the combination setting. Hence, statistical inference was restricted to just the monotherapy results. The Hochberg step-up procedure was used to report P values adjusted for the multiple tests (for different monotherapy dose combinations) within each gene (Appendix Figs A1 and A2; Appendix Tables A5 and A6, online only).
Fig A1.
Gene signature: fold-change means and 90% CIs for AZD1775 monotherapy doses (back-transformed from statistics on log2 scale).
Fig A2.
Gene signature: quantitative polymerase chain reaction four-gene signature score means and 90% CIs for monotherapy doses.
Table A1.
Pharmacokinetic Parameters of AZD1775 After Administration of Single Oral Doses of AZD1775 As Monotherapy in Part 1 or in Combination With Gemcitabine, Cisplatin, or Carboplatin in Part 2A
Table A2.
Pharmacokinetic Parameters of AZD1775 After Administration of Multiple Oral Doses of AZD1775 (twice a day for 2.5 days) in Combination With Cisplatin in Part 2B
Table A3.
Pharmacokinetic Parameters of AZD1775 After Administration of Multiple Oral Doses of AZD1775 (twice a day for 2.5 days) in Combination With Carboplatin in Part 2B
Table A4.
Pharmacokinetic Parameters of AZD1775 After Administration of Multiple Oral Doses of AZD1775 (twice a day for 2.5 days or once a day for 2 days) in Combination With Gemcitabine in Part 2B
Table A5.
Gene Expression Measurements (WEE1 signature): Unadjusted P Values Testing for a Nonzero Mean Log Fold Change Based on Analysis of Variance
Table A6.
Gene Signature: Adjusted P Values Testing for a Nonzero Mean Log Fold Change Based on Analysis of Variance (Hochberg adjustment applied to all tests within a given gene)
Footnotes
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00648648.
AUTHOR CONTRIBUTIONS
Conception and design: Suzanne Leijen, Tim Demuth, Shelonitda Rose, Amit M. Oza, Jan H.M. Schellens, Geoffrey I. Shapiro
Provision of study materials or patients: Anna C. Pavlick, Raoul Tibes, Lee Rosen, Jan H.M. Schellens, Geoffrey I. Shapiro
Collection and assembly of data: Suzanne Leijen, Robin M.J.M. van Geel, Anna C. Pavlick, Raoul Tibes, Lee Rosen, Shelonitda Rose, Mark A. Lee, Amit M. Oza, Jan H.M. Schellens, Geoffrey I. Shapiro
Data analysis and interpretation: Suzanne Leijen, Robin M.J.M. van Geel, Anna C. Pavlick, Raoul Tibes, Lee Rosen, Albiruni R. Abdul Razak, Raymond Lam, Mark A. Lee, Tomoko Freshwater, Stuart Shumway, Li Wen Liang, Amit M. Oza, Jan H.M. Schellens, Geoffrey I. Shapiro
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase I Study Evaluating WEE1 Inhibitor AZD1775 As Monotherapy and in Combination with Gemcitabine, Cisplatin, or Carboplatin in Patients With Advanced Solid Tumors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Suzanne Leijen
No relationship to disclose
Robin M.J.M. van Geel
No relationship to disclose
Anna C. Pavlick
Consulting or Advisory Role: Bristol-Myers Squibb, Novartis, Merck
Research Funding: Bristol-Myers Squibb (Inst), Millennium (Inst), Novartis (Inst), Merck (Inst), Celldex (Inst)
Raoul Tibes
Consulting or Advisory Role: Merck, Karyopharm Therapeutics
Lee Rosen
Research Funding: Merck
Albiruni R. Abdul Razak
Research Funding: Merck, Novartis, EntreMed, Boehringer Ingelheim, Bristol-Myers Squibb, Cayo Pharmaceutical, Eli Lilly, Genentech, Roche
Raymond Lam
Employment: Merck
Stock or Other Ownership: Merck
Research Funding: Merck
Tim Demuth
Employment: Novartis, Sandoz
Stock or Other Ownership: Novartis
Shelonitda Rose
Employment: Merck, Advaxis
Stock or Other Ownership: Merck, Advaxis
Travel, Accommodations, Expenses: Merck, Advaxis
Mark A. Lee
Employment: Merck
Stock or Other Ownership: Merck
Tomoko Freshwater
Employment: Merck
Stuart Shumway
Employment: Merck
Li Wen Liang
Employment: Merck Sharp & Dohme
Amit M. Oza
Honoraria: WebRx
Research Funding: AstraZeneca (Inst), Roche (Inst), Merck (Inst), Clovis Oncology (Inst)
Jan H.M. Schellens
No relationship to disclose
Geoffrey I. Shapiro
Consulting or Advisory Role: Vertex Pharmaceuticals, G1 Therapeutics, Eli Lilly, EMD Serono, Pfizer
Research Funding: Pfizer (Inst), Genentech (Inst), Bayer (Inst), Immune Design (Inst), Vertex (Inst), Millennium (Inst), Puma Biotechnology (Inst), Tensha Therapeutics (Inst), Covidien (Inst), Novartis (Inst), Cellceutix (Inst), Sanofi (Inst), Cyclacel (Inst), Mirati Therapeutics (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), Eli Lilly, Aileron Therapeutics (Inst), PharmaMar (Inst), PTC Therapeutics (Inst), Merck (Inst)
REFERENCES
- 1.Coleman TR, Dunphy WG.Cdc2 regulatory factors Curr Opin Cell Biol 6877–8821994 [DOI] [PubMed] [Google Scholar]
- 2.Morgan DO.Cyclin-dependent kinases: Engines, clocks, and microprocessors Annu Rev Cell Dev Biol 13261–2911997 [DOI] [PubMed] [Google Scholar]
- 3.Parker LL, Piwnica-Worms H.Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase Science 2571955–19571992 [DOI] [PubMed] [Google Scholar]
- 4.Featherstone C, Russell P.Fission yeast p107wee1 mitotic inhibitor is a tyrosine/serine kinase Nature 349808–8111991 [DOI] [PubMed] [Google Scholar]
- 5.Lundgren K, Walworth N, Booher R, et al. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2 Cell 641111–11221991 [DOI] [PubMed] [Google Scholar]
- 6.Rowley R, Hudson J, Young PG.The wee1 protein kinase is required for radiation-induced mitotic delay Nature 356353–3551992 [DOI] [PubMed] [Google Scholar]
- 7.Leijen S, Beijnen JH, Schellens JH.Abrogation of the G2 checkpoint by inhibition of Wee-1 kinase results in sensitization of p53-deficient tumor cells to DNA-damaging agents Curr Clin Pharmacol 5186–1912010 [DOI] [PubMed] [Google Scholar]
- 8.Aarts M, Sharpe R, Garcia-Murillas I, et al. Forced mitotic entry of S-phase cells as a therapeutic strategy induced by inhibition of WEE1 Cancer Discov 2524–5392012 [DOI] [PubMed] [Google Scholar]
- 9. Do K, Doroshow JH, Kummar S: Wee1 kinase as a target for cancer therapy. Cell Cycle 12:3159-3164, 2013. [DOI] [PMC free article] [PubMed]
- 10.Vriend LE, De Witt Hamer PC, Van Noorden CJ, et al. WEE1 inhibition and genomic instability in cancer Biochim Biophys Acta 1836227–2352013 [DOI] [PubMed] [Google Scholar]
- 11. Beck H, Nähse-Kumpf V, Larsen MS, et al: Cyclin-dependent kinase suppression by WEE1 kinase protects the genome through control of replication initiation and nucleotide consumption. Mol Cell Biol 32:4226-4236, 2012. [DOI] [PMC free article] [PubMed]
- 12.Hirai H, Iwasawa Y, Okada M, et al. Small-molecule inhibition of Wee1 kinase by MK-1775 selectively sensitizes p53-deficient tumor cells to DNA-damaging agents Mol Cancer Ther 82992–30002009 [DOI] [PubMed] [Google Scholar]
- 13.Mizuarai S, Yamanaka K, Itadani H, et al. Discovery of gene expression-based pharmacodynamic biomarker for a p53 context-specific anti-tumor drug Wee1 inhibitor. Mol Cancer. 2009;8:34. doi: 10.1186/1476-4598-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bridges KA, Hirai H, Buser CA, et al. MK-1775, a novel Wee1 kinase inhibitor, radiosensitizes p53-defective human tumor cells Clin Cancer Res 175638–56482011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawabe T.G2 checkpoint abrogators as anticancer drugs Mol Cancer Ther 3513–5192004 [PubMed] [Google Scholar]
- 16.Hirai H, Arai T, Okada M, et al. MK-1775, a small molecule Wee1 inhibitor, enhances anti-tumor efficacy of various DNA-damaging agents, including 5-fluorouracil Cancer Biol Ther 9514–5222010 [DOI] [PubMed] [Google Scholar]
- 17.Van Linden AA, Baturin D, Ford JB, et al. Inhibition of Wee1 sensitizes cancer cells to antimetabolite chemotherapeutics in vitro and in vivo, independent of p53 functionality Mol Cancer Ther 122675–26842013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guertin AD, Li J, Liu Y, et al. Preclinical evaluation of the WEE1 inhibitor MK-1775 as single-agent anticancer therapy Mol Cancer Ther 121442–14522013 [DOI] [PubMed] [Google Scholar]
- 19.Kreahling JM, Gemmer JY, Reed D, et al. MK1775, a selective Wee1 inhibitor, shows single-agent antitumor activity against sarcoma cells Mol Cancer Ther 11174–1822012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weisberg E, Nonami A, Chen Z, et al. Identification of Wee1 as a novel therapeutic target for mutant RAS-driven acute leukemia and other malignancies Leukemia 2927–372015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pfister SX, Markkanen E, Jiang Y, et al: Inhibiting WEE1 selectively kills histone H3K36me3-deficient cancers by dNTP starvation. Cancer Cell 28:557-568, 2015. [DOI] [PMC free article] [PubMed]
- 22.Aarts M, Bajrami I, Herrera-Abreu MT, et al. Functional genetic screen identifies increased sensitivity to WEE1 inhibition in cells with defects in Fanconi anemia and HR pathways Mol Cancer Ther 14865–8762015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada J Natl Cancer Inst 92205–2162000 [DOI] [PubMed] [Google Scholar]
- 24.Ji Y, Li Y, Nebiyou Bekele B.Dose-finding in phase I clinical trials based on toxicity probability intervals Clin Trials 4235–2442007 [DOI] [PubMed] [Google Scholar]
- 25. National Cancer Institute: National Cancer Institute Cancer Therapy Evaluation Program Common Terminology Criteria for Adverse Events, version 3.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 26. doi: 10.1007/s00216-012-6440-6. Xu Y, Fang W, Zeng W, et al: Evaluation of dried blot spot (DBS) technology versus plasma analysis for determination of MK-1775 by HILIC-MS/MS in support of clinical studies. Anal Bioanal Chem 404:3037-3048, 2012. [DOI] [PubMed] [Google Scholar]
- 27. doi: 10.1200/JCO.2016.67.5942. Leijen S, Van Geel RMJM, Sonke GS, et al: Phase II study with Wee1 inhibitor AZD1775 plus carboplatin in patients with p53 mutated ovarian cancer refractory or resistant (<3 months) to standard first line therapy. J Clin Oncol 33, 2015 (suppl; abstr 2507) [DOI] [PubMed] [Google Scholar]
- 28. doi: 10.1158/1078-0432.CCR-20-0219. Oza AM, Weberpals JI, Provencher DM, et al: An international, biomarker-directed, randomized, phase II trial of AZD1775 plus paclitaxel and carboplatin (P/C) for the treatment of women with platinum-sensitive, TP53-mutant ovarian cancer. J Clin Oncol 33, 2015 (suppl; abstr 5507) [DOI] [PubMed] [Google Scholar]
- 29.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response Nature 444756–7602006 [DOI] [PubMed] [Google Scholar]
- 30.Mir SE, De Witt Hamer PC, Krawczyk PM, et al. In silico analysis of kinase expression identifies WEE1 as a gatekeeper against mitotic catastrophe in glioblastoma Cancer Cell 18244–2572010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarcar B, Kahali S, Prabhu AH, et al. Targeting radiation-induced G(2) checkpoint activation with the Wee-1 inhibitor MK-1775 in glioblastoma cell lines Mol Cancer Ther 102405–24142011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.PosthumaDeBoer J, Würdinger T, Graat HC, et al. WEE1 inhibition sensitizes osteosarcoma to radiotherapy. BMC Cancer. 2011;11:156. doi: 10.1186/1471-2407-11-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Witt Hamer PC, Mir SE, Noske D, et al. WEE1 kinase targeting combined with DNA-damaging cancer therapy catalyzes mitotic catastrophe Clin Cancer Res 174200–42072011 [DOI] [PubMed] [Google Scholar]
- 34.Davies KD, Cable PL, Garrus JE, et al. Chk1 inhibition and Wee1 inhibition combine synergistically to impede cellular proliferation Cancer Biol Ther 12788–7962011 [DOI] [PubMed] [Google Scholar]
- 35.Carrassa L, Chilà R, Lupi M, et al. Combined inhibition of Chk1 and Wee1: In vitro synergistic effect translates to tumor growth inhibition in vivo Cell Cycle 112507–25172012 [DOI] [PubMed] [Google Scholar]
- 36.Guertin AD, Martin MM, Roberts B, et al. Unique functions of CHK1 and WEE1 underlie synergistic anti-tumor activity upon pharmacologic inhibition. Cancer Cell Int. 2012;12:45. doi: 10.1186/1475-2867-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaudhuri L, Vincelette ND, Koh BD, et al. CHK1 and WEE1 inhibition combine synergistically to enhance therapeutic efficacy in acute myeloid leukemia ex vivo Haematologica 99688–6962014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamilton DH, Huang B, Fernando RI, et al. WEE1 inhibition alleviates resistance to immune attack of tumor cells undergoing epithelial-mesenchymal transition Cancer Res 742510–25192014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Do K, Wilsker D, Ji J, et al. Phase I study of single-agent AZD1775 (MK-1775), a Wee1 kinase inhibitor, in patients with refractory solid tumors J Clin Oncol 333409–34152015 [DOI] [PMC free article] [PubMed] [Google Scholar]