Abstract
Transcription activator-like effectors (TALEs) are virulence factors of Xanthomonas that induce the expression of host susceptibility (S) genes by specifically binding to effector binding elements (EBEs) in their promoter regions. The DNA binding specificity of TALEs is dictated by their tandem repeat regions, which are highly variable between different TALEs. Mutation of the EBEs of S genes is being utilized as a key strategy to generate resistant crops against TALE-dependent pathogens. However, TALE adaptations through rearrangement of their repeat regions is a potential obstacle for successful implementation of this strategy. We investigated the consequences of TALE adaptations in the citrus pathogen Xanthomonas citri subsp. citri (Xcc), in which PthA4 is the TALE required for pathogenicity, whereas CsLOB1 is the corresponding susceptibility gene, on host resistance. Seven TALEs, containing two-to-nine mismatching-repeats to the EBEPthA4 that were unable to induce CsLOB1 expression, were introduced into Xcc pthA4:Tn5 and adaptation was simulated by repeated inoculations into and isolations from sweet orange for a duration of 30 cycles. While initially all strains failed to promote disease, symptoms started to appear between 9–28 passages in four TALEs, which originally harbored two-to-five mismatches. Sequence analysis of adapted TALEs identified deletions and mutations within the TALE repeat regions which enhanced putative affinity to the CsLOB1 promoter. Sequence analyses suggest that TALEs adaptations result from recombinations between repeats of the TALEs. Reintroduction of these adapted TALEs into Xcc pthA4:Tn5 restored the ability to induce the expression of CsLOB1, promote disease symptoms and colonize host plants. TALEs harboring seven-to-nine mismatches were unable to adapt to overcome the incompatible interaction. Our study experimentally documented TALE adaptations to incompatible EBE and provided strategic guidance for generation of disease resistant crops against TALE-dependent pathogens.
Author summary
Mutation of the EBEs of susceptibility (S) genes via genome editing and utilization of naturally occurring EBE variants have been used to generate disease resistant plants. However, TALE adaptations may lead to resistance loss, limiting the long-term efficacy of the strategy.
We utilized an experimental evolution approach to test TALEs adaptations in the Xanthomonas citri-citrus pathosystem using designer TALEs that cannot recognize the EBE of host targets. We identified adaptive TALE mutations and deletions that occurred during less than 30 cycles of repeated infections, which reconstituted the virulence on the host. Adaptive variants originated from TALEs that harbored a small number of mismatches (≤5) to the EBE, whereas designer TALEs that harbored larger number of mismatches (≥7) to the EBE failed to adapt in the duration of this study. Our study experimentally demonstrates adaptive rearrangements of TALEs during host adaptation and suggests that the potential durability in the resistance of modified crops should be a significant factor to be considered prior to their introduction into the field.
Introduction
Transcription activator-like effectors (TALEs) are bacteria-encoded eukaryotic transcriptional activators delivered into host cells through the type III secretion system (T3SS) [1]. TALE protein architecture contains N-terminal T3SS secretion and translocation signal, central DNA binding domain and C-terminal eukaryotic acidic transcriptional activation domain and nuclear localization signals (NLS) [1]. The DNA binding domain is composed of an array of 1.5–33.5 nearly identical tandem repeats of 33–34 AA [1, 2]. The 12th and 13th amino acids of each repeat, known as the “repeat-variable diresidue” (RVD), vary between repeats and dictate the affinity of each repeat to an individual nucleotide [3]. Through this recognition mechanism, the TALE repeat array determines the binding specificity of each TALE to a DNA sequence located in the promoter of host target genes that serves as an effector-binding element (EBE) [4].
Xanthomonas is one of the most economically important plant pathogens infecting most plant species [5]. TALEs are key virulence factors in numerous Xanthomonas spp. [6]. Xanthomonas TALEs induce the expression of host susceptibility (S) genes to cause disease [7]. The number of TALEs in different Xanthomonas bacteria varies from 0 (the majority of pepper and tomato infecting strains) to close to 30 (as found in X. oryzae pv. oryzicola) [7, 8]. While most non-TALE effectors of Xanthomonas are usually associated with disruption and manipulation of host defense signaling [9–11], TALEs were reported to target more diverse cellular functions. For example, multiple X. oryzae pv. oryzae (Xoo) TALEs induce the expression of rice SWEET sugar transporter genes to facilitate sucrose and glucose efflux [12–14], Tal2g of X. oryzae pv. oryzicola promotes lesion expansion and bacteria exudation by inducing the expression of sulfate transporter gene [15], AvrHah1of X. gardneri indirectly stimulates the expression of a pectate lyase gene to promote the accumulation of apoplectic fluid [16], AvrBs3 of X. euvesicatoria causes cell hypertrophy through increasing expression of pepper UPA20 [17], Tal8 of X. translucens promotes accumulation of ABA through induction of NCED in wheat [18], and PthA4 of X. citri ssp. citri (Xcc) induces hypertrophy and hyperplasia through induction of citrus CsLOB1 [19–22].
During the host-pathogen arms race, plants have evolved several strategies to combat Xanthomonas TALEs through altering or deleting the S gene promoter regions containing the EBE, utilization of executor R genes that harbor the EBE in their promoter to initiate immune response upon their induction, and recognition through NB-LRR resistance genes [23–31]. In return, Xanthomonas bacteria avoid these strategies by evolving different TALEs that target different EBEs in the S gene promoter, to target a different or functionally similar S gene, and employing interference TALEs to suppress the TALE recognition by NB-LRR [4, 27, 32]. Employment of alternative TALEs directed to the same target was reported in at least two pathosystems. Xoo strains utilize multiple TALEs (TalC, PthXo3, Tal5, and AvrXa7) to target at least three independent EBEs in the promoter of OsSWEET14 and use two other TALEs (PthXo1, and PthXo2) to induce the expression of OsSWEET11 and OsSWEET13 [12, 13, 33–35]. In addition, different EBEs in the promoter region of citrus CsLOB1 were identified to be targeted by TALEs from Xcc (PthA4/PthA*/PthAw2) [36] and X. citri ssp. aurantifolii (Xca) (PthB/PthC) [19].
Mutation of the EBE of S genes via TALEN and CRISPR mediated genome editing and utilization of naturally occurring EBE variants have been used to generate disease resistant crops, e.g., rice and citrus [37–42]. However, the tandem repeat nature of TALEs subjects them to high frequency of mutations and rearrangements [43], thus undermining the durability of resistant crops generated via mutating EBEs. It is pivotal to investigate how TALEs of pathogens adapt to the EBEs of S genes to develop successful strategies to breed or design durable disease resistance in crops.
Xanthomonas bacteria are highly specialized with narrow host range [5]. Like many other specialist pathogens, the mechanisms that dictate host specificity and adaptation are not fully understood. Investigations of host adaptation have been conducted by analyzing bacterial population genetics, reverse genetics studies or simulating host adaptation using experimental evolution. Evolutionary events, such as acquisition of novel pathogenicity associated gene clusters by horizontal genet transfer, altered regulation of metabolic genes, alteration or loss of genes associated with immune recognition by the host, and modification of existing virulence genes, were reported in host adaptation studies. For instance, acquisition of genes associated with detoxification of plant antimicrobial compounds was found to expand the host range of Enterobacteria plant pathogens Pectobacterium and Panotea to Brassicales and Allium, respectively [44, 45]. Alterations in the flg22-elicitor region in the flagella of Ralstonia solanacearum and Xanthomonas oryzae prevent the recognition by respective hosts [46, 47]. Field introduction of pepper and tomato lines bred with R genes against specific T3SS effectors of Xanthomonas euvesicartoria was followed by bacterial adaptation through disruption or modification of the targeted effectors and introduction of pathogen races that lack the corresponding effectors [48]. Experimental evolution approaches have been utilized as a tool to study host adaptation in animal and plant pathogens. Numerous studies have identified specific adaptive mutations that were involved in pathogenicity. For incidence, Pseudomonas aeruginosa experimentally evolved in mice exhibited missense mutations in the two-component sensor pmrB that regulates attachment, LPS and resistance to amicrobial compounds [49, 50]. Ralstonia solanacearum strains that experimentally evolved on bean plants harbored a mutation in the transcriptional regulator efpR, which regulates EPS production, motility and numerous metabolic processes [51, 52]. Xcc strains that evolved in resistant Meiwa kumquat via repeated inoculation and isolation harbored point mutations in the pthA4 TALE that was later verified to be associated with elicitation of immune responses [53, 54].
Experimental evolution studies of host-pathogen interactions usually focus on utilizing the experimental system as a tool for gene discovery and less on the mutational events of specific virulence factors that occur during adaptations. It remains unknown whether Xanthomonas can overcome the resistance or loss-of-susceptibility owing to the incompatible interactions between TALEs and the EBE of the corresponding susceptibility genes. We hypothesized that TALEs have the potential to overcome the mismatches in the EBE of susceptibility genes and the adaptation capacity inversely correlates with the number of mismatches. To test this hypothesis, we utilized the Xcc–citrus pathosystem [55] as a model to investigate TALE adaptations in overcoming incompatible interactions by using an experimental evolution approach. Indeed, our data provide strategic guidance for development of durable EBE-based resistance against TALE-dependent pathogens.
Results
Natural variations of citrus LOB1 and TALEs in Xanthomonas citri suggest host adaptation
We investigated the variations among TALEs (PthA4 and homologs) that target LOB1 by analyzing all available Xcc and Xca deposits in the NCBI database. We identified TALEs that display moderate to high binding affinity to the sweet orange LOB1 promoter according to target finder feature of “TAL Effector Nucleotide Targeter 2.0” [56]. The analysis identified 20 LOB1-targeting TALEs (Table 1) that contain 13 unique repeat array variants (named RVDV1-RVDV13, Table 1 and Fig 1A). The majority of the TALEs were represented by two dominant repeat array variants, RVDV1 and RVDV5. RVDV5 was identified in multiple Xcc genomes and represented by a single allelic variant. In addition, all Xcc strains containing RVDV5 were isolated from key lime or lemon trees in Florida (Table 1). On the other hand, RVDV1 was identified in six allelic variants and found in Xcc strains isolated from multiple hosts in numerous geographic regions (Table 1).
Table 1. Natural variations among TALEs targeting CsLOB1.
| RVD variant | RVD | Allelic variant | Bacteria | NCBI GenBank | Host | Geographic origin |
|---|---|---|---|---|---|---|
| 1 | NI N* NI NI NI HD HD NG HD NG NG NG NG NS HD HD NG NG | 1A | Xcc strains: 306, 306A, 5208, BL18, FB19, gd3, jx4, jx5, mf20, MN10, MN11, MN12, NT17, UI6, UI7, 03-1638-1-1 | AAM39311, AJD66579, AJZ37799, AJZ33330, AJZ28866, AJZ24451, AJZ20025, AJZ15601, AJZ11172, AJZ06700, AJZ02279, AJY97855, AJY93431, AJY88957, AJY84537, AJY80115, AUZ53767 | Citrus sinensis (Sweet Orange), C. aurantifolia (Key lime), C. paradisi (Grapefruit) | Brazil: São Paulo, USA: Florida, China: Guangdong, China: Jiangxi, Argentina |
| 1B | X. citri ɑ strain NI-1 | BAA37119 | C. natsudaidai (Amanatsu) | Japan | ||
| 1C | X. citri ɑ,b | WP_082243722 | C. sinensis (Sweet Orange) | China: Jiangxi | ||
| 1D | Xcc strains: LL074-4, LM180 | APR13430, OLR69148 | C. paradise (Grapefruit) | Martinique, Argentina | ||
| 1E | Xcc strain LH201 | APR27435 | C. hystrix (Kaffir lime) | Reunion | ||
| 1F | Xcc strain KC21 | BAF46271 | C. grandis (Pomelo) | Japan | ||
| 2 | NI NG NI HD NI HD HD NG HD NG NG NG NG NS HD NS NG NG NG | 2A | Xcc strain TX160149 | ARR15471 | C. aurantifolia (Key lime) | USA: Texas |
| 3 | NI NG NI NI NI HD HD NG HD NG NG NG NG NG HD HD NG NG | 3A | X. citri ɑ strain XW47 | ACZ62652 | C. paradise (Grapefruit) | Republic of China: Taiwan |
| 4 | NI NI NI HD HD NG HD NG NG NG NG NS HD HD HD NG | 4A | Xcc strain Xcc049 | AHB33738 | C. sinensis (Sweet Orange) | China: Chong Qing |
| 5 | NI NG NG NG NS HD HD NS HD NG NG NG NG NS HD HD NG NG | 5A | Xcc strains: Aw12879, AW13, AW14, AW15, AW16 | AGI10546, AJZ64238, AJZ51443, AJZ46823, AJZ42208 | C. aurantifolia (Key lime), C. limon (Lemon) | USA: Florida |
| 6 | NI NG NG NG NS HD HD NS HD NG NC NG NG NS HD HD NG NG |
6A | Xcc strain X0053 | ABO77779 | C. aurantifolia (Key lime) | USA: Florida |
| 7 | NI NG NG NG NS HD HD NS HD NG NG NG NG NS HD HD NG NG NG | 7A | Xcc strains: TX160042, TX160197 | ARR19110, ARR20875 | C. aurantifolia (Key lime), C. hystrix (Kaffir lime) | USA: Texas |
| 8 | NI N* NI NI NI HD HD NG HD NG NG NG NG NS HD HD HD NG NG |
8A | Xcc strain Xcc29-1 | AYL23296 | CitrusC | China: Jiangxi |
| 8B | Xcc strain Xcc29-1 | AGH79796 | CitrusC | China | ||
| 9 | NI N* NI NI NI NG HD NG HD NG NG NG NG NS HD HD NG NG |
9A | X. citri ɑ strain 3213 | AAC43587 | C. paradise (Grapefruit) | USA: Florida |
| 10 | HD N* NI NI NI HD HD NG HD NG NG NG NG NS HD HD NG NG | 10A | Xcc strain LM180 | OLR69303 | C. paradise (Grapefruit) | Argentina |
| 11 | NI N* NI NI NI HD ND NG HD NG NG NG NG NS HD HD HD ND NG | 11A | Xcc strain Xcc49 | AYL27693 | CitrusC | China: Chongqing |
| 12 | HD NG HD NG NI NG HD NG HD NI NI HD HD HD HD NG NG NG | 12A | X. citri ssp. aurantifolii strain B69, X. citri ɑ, b | WP_011153905, NP_942641, AAO72098 | CitrusC | South America |
| 13 | HD NG HD HD NI NG NI NG NI NI HD NG HD HD HD NG NG NG | 13A | X. citri ssp. aurantifolii strain ICPB 10535 | WP_088370900, EFF47385 | C. aurantifolia (Key lime) | Brazil: São Paulo |
| 13B | X. citri ssp. aurantifolii strain C340 | ABO77782 | C. aurantifolia (Key lime) | Brazil: São Paulo |
ɑ Xanthomonas ssp. is not specified in deposit or the corresponding publication.
b strain is not specified in deposit or the corresponding publication.
C Citrus species is not specified in deposit.
Allelic variant means that the backbone is not identical, but the repeat array is identical.
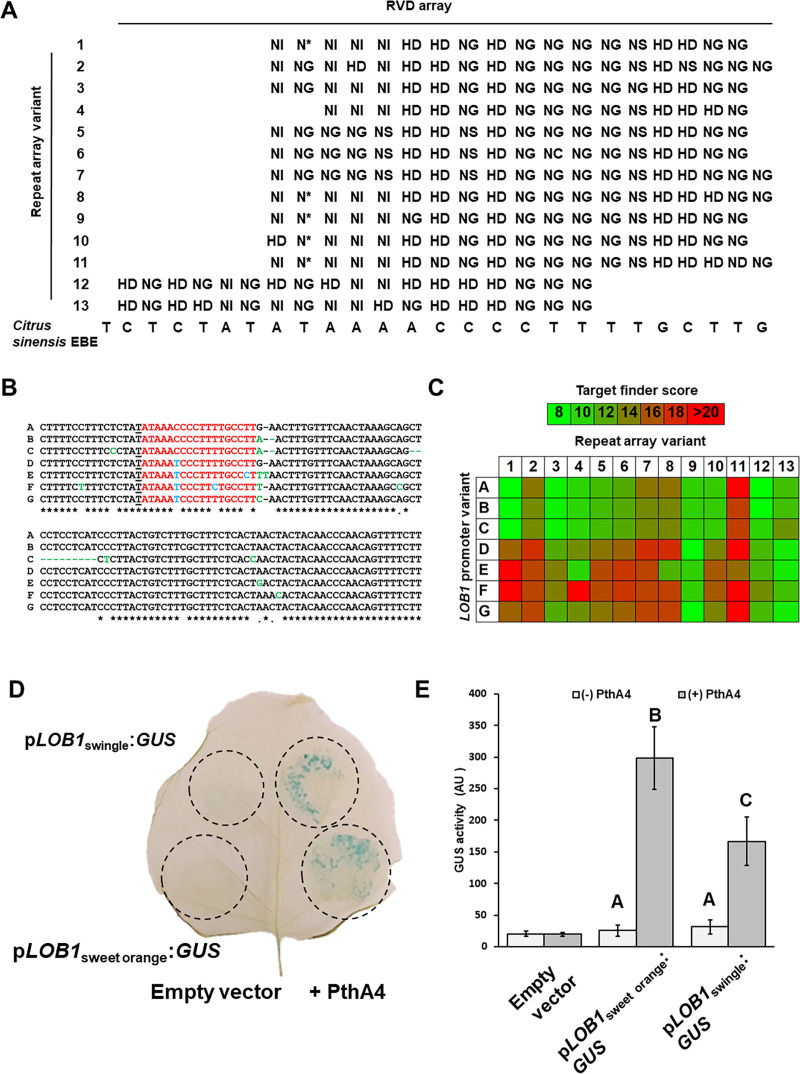
Fig 1. Variability of X. citri LOB1 targeting TALEs and the LOB1 EBE region in Rutaceae species.
A. RVD repeat arrays of LOB1 targeting TALEs from X. citri species (Sources are elaborated in Table 1). B. Sequence alignment of allelic variants (Sources are elaborated in Table 2) of the surrounding region of the TALE effector-binding elements (EBEs) from Rutaceae plants. Sequence alignment was conducted with Clustal Omega Multiple Sequence Alignment feature (https://www.ebi.ac.uk/Tools/msa/clustalo/) using default settings. Conserved residues in EBE region are marked in red. Variations in the EBE compared to allelic variant A are marked in blue. Variations in the area outside of the EBE compared to allelic variant A are marked in green. Thymidine residues proceeding EBEs are underlined. C. Target finding scores (lower scores indicate higher predicted binding affinity) of LOB1 targeting TALEs against allelic variants of Rutaceae LOB1 promoter according to TAL Effector Nucleotide Targeter 2.0 using Target Finder tool (https://tale-nt.cac.cornell.edu/). Scores are depicted in colored heat maps correlating to the ruler placed on the top of the table. D and E. Induced expression of sweet orange and Swingle citrumelo LOB1 by PthA4. Nicotiana benthamiana leaves were inoculated with Agrobacterium to co-express His-PthA4 or an empty vector with GUS reporter under the control of the LOB1 promoter from sweet orange (Citrus sinensis) or Swingle citrumelo (Poncirus trifoliata x Citrus paradisi). Expression of His-PthA4 was driven by an estradiol-inducible system and 17β-estradiol was applied at 24 h after agro-infiltration. D. Histochemical GUS staining of inoculated leave at 72 h after 17β-estradiol treatment. Experiment was repeated three times with similar results. E. GUS activity (arbitrary units [AU]) in inoculated areas was determined at 72 h after 17β-estradiol treatment. Values are means ± SE of nine biological replicates. The experiment was conducted three times and each experiment was composed of three biological replicates. Letters denote significant differences based on analysis of variance (Anova) and comparisons for all pairs using Student’s t-test (P-value < 0.05).
We assessed the phylogenetic and functional lineage of the LOB1 targeting TALEs using the QueTAL tool [57] (S1 Fig). The analysis identified at least two independent subgroups within the LOB1 targeting TALEs (S1A Fig). The first group, composed of RVDV12 and RVDV13, represented TALEs isolated from Xca strains in South America [58] (Table 1 and S1A Fig). In addition to harboring a different repeat array composition, these two TALEs also potentially target a different EBE in the LOB1 promoter, which only partially overlap with the EBE targeted by the other TALEs (Fig 1A). The second group, composed of RVDV5, RVDV6 and RVDV7, represented isolates of the lime-restricted XccAW found in North America [59] (Table 1 and S1A Fig). While functional lineage analysis based on predicted EBE binding forecast different affinities from the rest of the TALEs (S1B Fig), genome based analysis found that these three TALEs target an identical EBE in the LOB1 promoter to that of the other Xcc TALEs (Fig 1A) by utilizing different repeat arrays to target the same DNA sequence (Fig 1A). Even though the remaining TALEs share repeat stretches and high functional lineage between them (Figs 1A and S1B), distance analysis did not identify clear phylogenetic lineage (S1A Fig). It is unclear whether these TALEs were acquired or evolved independently of each other.
To investigate the relationship between the LOB1 EBEs and LOB1 targeting TALEs, we analyzed the sequences surrounding the EBEs in the LOB1 promoter regions (pLOB1) of multiple Rutaceae plants including both citrus and non-citrus (Table 2). LOB1 promoters were derived from available sequence deposits (https://www.citrusgenomedb.org/) or newly sequenced here (Table 2). We identified seven allelic variants in the LOB1 promoter (named A to G, Table 2). The majority of commercial citrus genotypes contained at least one A allele, which presumably originated from the ancestral species mandarin orange (C. reticulate) [60] (Table 2).
Table 2. Variants in the LOB1 promoter among Rutaceae species.
| Common name | Species/Genotype | LOB1 promoter variant | Comments | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | |||
| Mandarin orange* | Citrus reticulata | √ | Ancestral species | ||||||
| Pomelo* | C. maxima [(Burm.) Merr], C. grandis Swingle, Tanaka | √ | |||||||
| Citron* | C. medica | √ | |||||||
| Sweet orange+,* | C. sinensis (C. maxima × C. reticulata) | √ | √ | Commercial hybrid species | |||||
| Grapefruit+ | C. paradisi (C. maxima × sweet orange) | √ | √ | ||||||
| Lemon+ | C. limon (sour orange × citron) | √ | √ | ||||||
| Mexican lime+ | C. aurantiifolia (micrantha x citron) | √ | √ | ||||||
| Clementine* | C. clementina (Willowleaf mandarin × sweet orange) | √ | |||||||
| Sugar belle mandarin+ | “Clementine” mandarin × “Minneola” tangelo | √ | |||||||
| Alemow+ | C. macrophylla [citron × biasong (C. micrantha)] | √ | Rootstock species | ||||||
| Sour orange+ | C. aurantium (C. maxima x C. reticulata) | √ | √ | ||||||
| Swingle citrumelo+ | C. paradisi × Poncirus trifoliata | √ | √ | ||||||
| Carrizo+ | C. sinensis × Poncirus trifoliata | √ | √ | ||||||
| Hong Kong kumquat* | Fortunella hindsii | √ | Wild species | ||||||
| Meiwa kumquat+ | Fortunella crassifolia | √ | |||||||
| Trifoliate orange+,* | Poncirus trifoliata | √ | |||||||
| Chinese box orange* | Severinia buxifolia | √ | |||||||
| Papeda* | Ichang papeda | √ | |||||||
*Information is based on sequence from www.citrusgenomedb or http://citrus.hzau.edu.cn/orange.
+information is based on amplification from genomic DNA and sequencing.
Sequence analyses revealed that the 18 bp EBEPthA4 [19] of pLOB1 is 100% conserved in all commercial citrus cultivars (variants A, B and C, Fig 1B). However, we identified some sequence variations in the pLOB1 of wild Rutaceae species (variants E, F and G, Fig 1B) and in the rootstock species Carrizo, Swingle citrumelo and Sour orange (variants D and E, Fig 1B).
The affinity of each of the Xcc TALE repeat array variants to the pLOB1 variants was estimated using target finder feature of “TAL Effector Nucleotide Targeter 2.0”[56]. The analysis identified different specificity of the TALEs to specific promoter variants (Fig 1C). For instance, RVDV1 displayed high affinity to pLOB1 variants A, B and C that are present in all commercial citrus varieties but only showed moderate affinity to pLOB1 variants D, E, F and G, that are present in non-citrus Rutaceae species and rootstock varieties (Fig 1C). On the other hand, RVDV8, RVDV9, RVDV11 and RVD13 displayed only moderate affinity to the pLOB1 variants found in most commercial citrus varieties but higher affinity to the EBE found in Carrizo citrange, Swingle citrumelo, Poncirus trifoliate, C. aurantium or Ichang papeda (Fig 1C). Our analyses suggest that pLOB1-targeting TALEs of X. citri evolved different specificity to Rutaceae hosts during host adaptation. The prevalence of RVDV1 in the Xcc populations is probably due to its high affinity to the widely presented EBEs (A, B and C) in the commercial varieties.
We validated the predicated promoter binding affinity in vivo by fusing pLOB1 from sweet orange and Swingle citrumelo (variants A and E, respectively) to a GUS reporter (Fig 1D and 1E). The promoter activity was tested in the presence of pLOB1-targeting TALE PthA4 (RVDV1) using Agrobacterium mediated transient expression in Nicotiana benthamiana leaves. Consistent with the in silico prediction, PthA4 promoted significantly higher induction of sweet orange pLOB1 than that of Swingle citrumelo (Fig 1E).
Adaptation of pLOB1-targeting TALEs
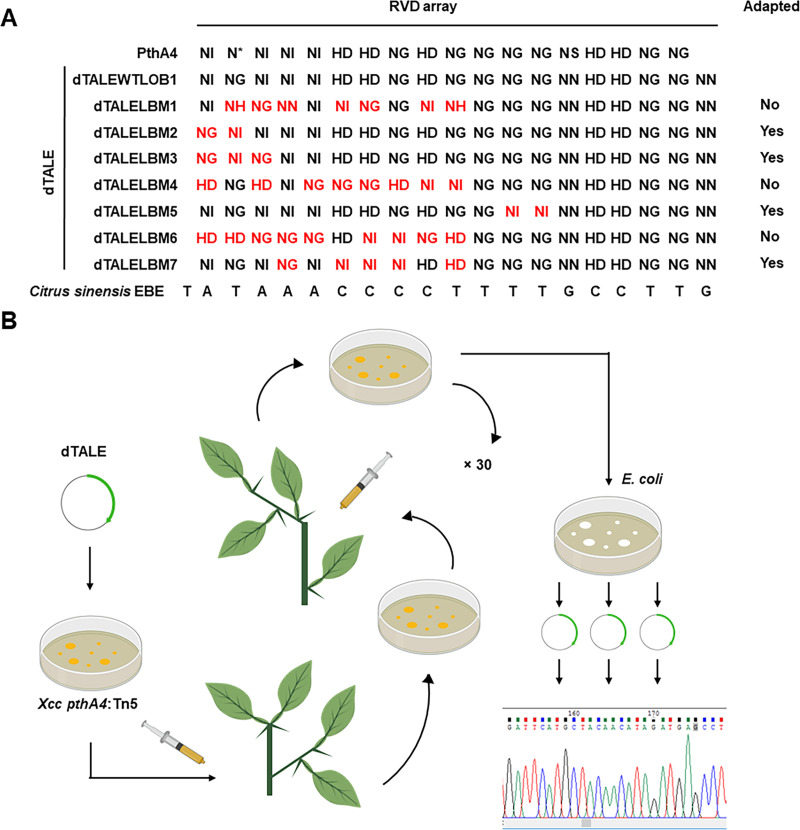
In order to optimize EBE-mutating design to generate resistant varieties, we investigated how TALEs adapt to their corresponding EBE. The sweet orange-Xcc pathosystem was used to experimentally simulate TALE adaptation in overcoming incompatible interactions. To this aim we constructed eight designer TALEs (dTALEs) that harbored repeat arrays with different compatibilities to a 19 bp EBE in pLOB1 of sweet orange (Fig 2A). First we constructed a PthA4-mimicking dTALE with a repeat array that perfectly matches the 19 bp EBEPthA4 in pLOB1 (dTALEWTLOB1, Fig 2A) and demonstrated it complemented a Xcc pthA4 Tn5 insertion mutant (Xcc pthA4:Tn5) in inducing CsLOB1 expression and promoting canker symptoms (S2 Fig) [61]. We then constructed seven dTALEs with 2 to 9 mismatches of RVDs within their repeat arrays and tested their ability to complement Xcc pthA4:Tn5. DNA sequences of all the constructed dTALEs are available in S1 Text. As expected, the manufactured dTALEs (named dTALELBM1 to dTALELBM7, Fig 2A) did not complement Xcc pthA4:Tn5 and were unable to induce the expression of CsLOB1 and Xcc pthA4:Tn5 carrying the dTALEs had incompatible interactions with the citrus host (S2 Fig).
Fig 2. Experimental evolution of TALEs.
A. RVD repeat arrays of PthA4 (XACb0065) and dTALEs used in experimental evolution test. The nucleotide sequence of the effector-binding element of CsLOB1 from sweet orange (Citrus sinensis) is represented at the bottom. “Adapted” column indicates whether the dTALE variant was able to adapt in the duration of the experiment. B. Schematic representation of the experimental evolution workflow. Scheme was created with Biorender (https://biorender.com/).
Duplicates of Xcc pthA4:Tn5 carrying each of the seven dTALEs were subjected to in planta experimental evolution assays. Xcc pthA4:Tn5 carrying the dTALEs were inoculated into and reisolated from sweet orange leaves for 30 infection cycles, representing approximately 1,093 bacterial generations. Five of the 14 bacterial strains were able to induce canker symptoms in sweet orange within 9–28 cycles (Table 3) and dTALEs isolated from the five adapted strains were able to complement Xcc pthA4:Tn5 in inducing CsLOB1 expression, causing canker symptoms, and promoting bacterial growth in sweet orange (Fig 3).
Table 3. RVD variants of the original and adapted dTALEs.
| dTALE | dTALE RVD | Parental dTALE | Binding affinity score to the LOB1 promoterA | Number of infection cycles for adaptation | Found after 30 infection cyclesC | Detected in replicateD | ||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | |||||||
| ScoreB | Best possible scoreB | |||||||
| dTALEWTLOB1 | NI NG NI NI NI HD HD NG HD NG NG NG NG NN HD HD NG NG NN | NAE | 7.35 | 5.34 | NA | NA | NA | |
| dTALELBM1 | NI NH NG NN NI NI NG NG NI NH NG NG NG NN HD HD NG NG NN | NA | -F | 5.27 | NA | YES | YES | YES |
| dTALELBM2 | NG NI NI NI NI HD HD NG HD NG NG NG NG NN HD HD NG NG NN | NA | 12.87 | 5.34 | NA | NO | NO | NO |
| dTALELBM3 | NG NI NG NI NI HD HD NG HD NG NG NG NG NN HD HD NG NG NN | NA | 14.97 | 5.45 | NA | YES | NO | YES |
| dTALELBM4 | HD NG HD NI NG NG NG HD NI NI NG NG NG NN HD HD NG NG NN | NA | 21.66 | 5.45 | NA | YES | YES | YES |
| dTALELBM5 | NI NG NI NI NI HD HD NG HD NG NG NI NI NN HD HD NG NG NN | NA | 14.17 | 5.14 | NA | YES | YES | NO |
| dTALELBM6 | HD HD NG NG NG HD NI NI NG HD NG NG NG NN HD HD NG NG NN | NA | 25.66 | 6.81 | NA | YES | YES | YES |
| dTALELBM7 | NI NG NI NG NI NI NI NI HD HD NG NG NG NN HD HD NG NG NN | NA | 16.63 | 5.26 | NA | YES | NO | YES |
Note
A The binding affinity analysis was conducted in LOB1 variant A from sweet orange (Citrus × sinensis). The promoter region was set as the 1,000 bp sequence upstream of the transcriptional start site.
B According to the target finder tool provided by https://tale-nt.cac.cornell.edu/.
C A clone is defined as “detected” if the dTALEs or adapted TALEs were present in plasmids isolated from bacteria at cycle 30. Three independent clones per strain were isolated from Xcc pthA4:Tn5, introduced to E. coli and sequenced.
D Each experiment was conducted with two replicates marked as “1” and “2”. Data states whether the inducted dTALE was identified in each replicate in the duration of the experiment representing both the time of adaptation and the end of the experiment as cycle 30.
E NA: not applicable.
F Score is beyond cutoff.
Blue color indicates adapted TALEs.
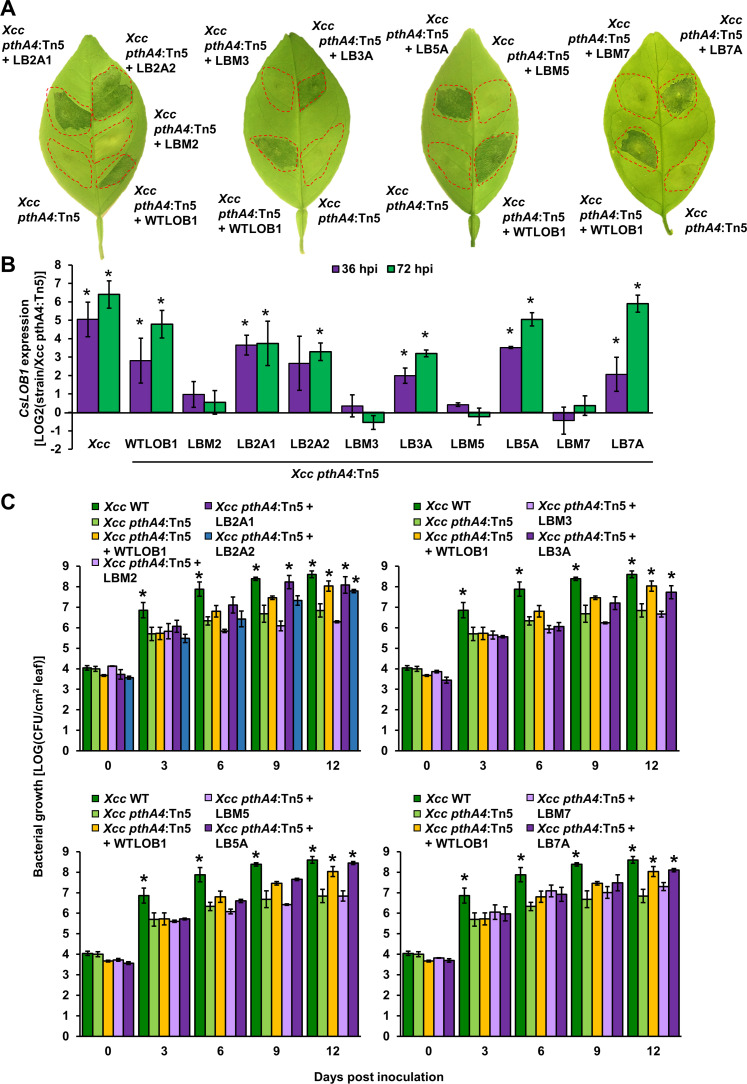
Fig 3. Functional characterization of adapted dTALEs.
Sweet orange leaves were syringe-infiltrated with suspensions (1 × 108 CFU/mL for A and B, 1 × 106 CFU/mL for C) of Xcc 306 (Xcc WT), Xcc pthA4:Tn5 or Xcc pthA4:Tn5 transformed with the parental and adapted dTALEs depicted in Fig 4A. A. Inoculated leaves were photographed at 7 days post inoculation. The experiments were repeated three times with similar results. B. The gene expression of CsLOB1 was quantified at 36 and 72 h post inoculation (hpi) using quantitative reverse transcription PCR. The GAPDH gene was used as an endogenous control. Values are means ± SE of three biological replicates. C. Bacterial growth in planta. Values represent means ± SE of three biological replicates. The experiments were repeated three times with similar results. B and C. Asterisks indicate a significant difference (Student’s t-test, P-value < 0.05) compared to Xcc pthA4:Tn5.
Sweet orange leaves inoculated with Xcc pthA4:Tn5 harboring dTALEs isolated from the adapted Xcc strains or dTALEWTLOB1 displayed canker symptoms between 4–7 days after inoculation (dpi) while leaves inoculated with Xcc pthA4:Tn5 or Xcc pthA4:Tn5 harboring the non-adapted dTALEs failed to cause canker symptoms after 14 days (Fig 3A). The ability to induce the expression of CsLOB1 by the adapted TALEs was monitored at 36 and 72 hours post inoculation (hpi). The expression of CsLOB1 in sweet orange was significantly increased by Xcc pthA4:Tn5 harboring the adapted dTALEs, i.e., dTALELB2A1, dTALELB2A2, dTALELB3A, dTALELB5A, and dTALELB7A, whereas the expression was not significantly altered by the original dTALEs (Fig 3B). In addition, introduction of dTALEWTLOB1 or the adapted TALEs to Xcc pthA4:Tn5 significantly improved bacterial colonization of sweet orange leaves, reaching similar levels as the wild type Xcc at 12 dpi, whereas the four original dTALEs grew similarly as Xcc pthA4:Tn5 (Fig 3C).
Those five adapted strains corresponded to dTALELBM2, dTALELBM3, dTALELBM5, and dTALELBM7, which contain 2, 3, 2, and 5 mismatches, respectively. During this period, Xcc pthA4:Tn5 strains harboring dTALELBM1, dTALELBM4, and dTALELBM9 that contain at least 7 mismatches with EBEPthA4 did not adapt to sweet orange.
As a negative control, Xcc pthA4:Tn5 carrying each of the seven dTALEs were streaked on artificial NA medium in parallel to the plant infection cycles to assess the effect of the selective pressure of incompatible plant environment on TALE adaptation. Plasmids were extracted from three single colonies of each of the seven strains after 30 streaking cycles and the DNA sequence of their repeat arrays were determined. We did not observe any modifications in the repeat arrays of TALEs adapted on NA medium and sequences were identical to the original non-adapted parental TALEs.
Adapted TALEs display mutations and deletions in their repeat arrays
The sequence of the repeat region of TALEs was determined at cycle 30 for the 14 strains (S1 Text). The adapted variants isolated from strains that displayed canker symptoms and induced CsLOB1 expression were sequenced at two time points, at the first sign of host adaptation (i.e., showing canker symptoms) and at the end of the experiment after 30 infection cycles along with the rest of the strains. The repeat arrays of TALEs extracted from the strains that were unable to promote canker at cycle 30 were identical to their parental dTALEs (Table 3). The other five adapted TALE variants, which were able to complement Xcc pthA4:Tn5 (Fig 4), contained alterations in the repeat arrays compared to the parental dTALEs (Table 3).
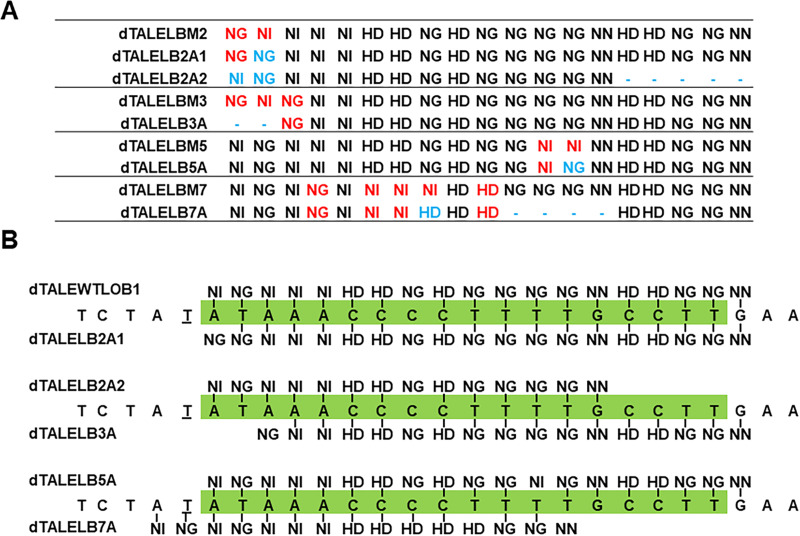
Fig 4. Repeat rearrangements in adapted dTALEs.
A. RVD repeat arrays of parental and adapted dTALEs. Red-colored RVDs represent original mismatches compared to dTALEWTLOB1. Blue color indicates deleted or altered repeats in the adapted dTALEs compared to parental dTALEs. B. Predicted binding of adapted dTALEs [determined according to TAL Effector Nucleotide Targeter 2.0 using Target Finder tool (https://tale-nt.cac.cornell.edu/] to the CsLOB1 in sweet orange (Chromosome 7, 28358599–28358574, allelic variant A) EBE. The PthA4 effector-binding element (EBE) is labeled in green and thymidine residue proceeding the EBE is underlined.
The two adapted dTALE variants of dTALELBM2 (dTALELB2A1 and dTALELB2A2) that contained two mismatches in the first two repeats, displayed distinct repeat rearrangements: the first adapted variant, dTALELB2A1, was identified in both duplicate strains after 17 infection cycles. In this variant, the RVD of the second repeat was changed from NI to NG, which matches the corresponding target “T” nucleotide in the EBEPthA4 (Fig 4A and 4B, Table 3). The second adapted TALE, dTALELB2A2, was identified after 17 infection cycles in one of the duplicate strains. dTALELB2A2 contained mutations in 7 repeats: the first and second mismatched repeats were altered from NG-NI to NI-NG, matching the first “AT” target site in EBEPthA4. In addition, we observed a deletion of the five C-terminal repeats. These mutations altered dTALELB2 from a TALE containing 19 repeats with two mismatches into a TALE with 14 repeats with a perfect matching repeat array (Fig 4A and 4B, Table 3).
Adaptation was observed in dTALELBM3, which originally contained mismatches in the first three repeats, in one of the duplicates after nine infection cycles and at the end of the experiment after 30 infection cycles. The adapted variant, dTALELB3A, displayed a deletion of the first two mismatched repeats, altering dTALELBM3 from a 19 repeats TALE with three mismatches into a 17 repeats TALE with a single mismatch (Fig 4A and 4B, Table 3).
The adapted dTALELBM5 variant, dTALELB5A, was detected after 19 infection cycles and 30 cycles in one of the duplicate strains. dTALELB5A contained a NI to NG change in the mismatched repeat 12, which corresponds to the 12th “T” position in EBEPthA4 (Fig 4A and 4B, Table 3).
Adaptation of dTALELBM7, which originally harbored five mismatched repeats, occurred only near the end of the experiment at the cycle 28 in one of the duplicates. The adaptive variant dTALELB7A displayed alteration of five repeats at positions 8–14 (Fig 4A and 4B, Table 3).
The full-length CDSs of the adapted dTALEs were sequenced. Other than repeat deletions or alterations of the RVDs, sequence analyses did not identify any other differences. Additionally, the altered nucleotides in the adapted RVDs displayed similar codons to building blocks encoding the same RVD in the dTALE repeat constructs, but different from the codons found in the native Xcc TALEs PthA1, PthA2, PthA3 and PthA4. This suggests that the repeat adaptations observed here probably occurred by recombination between the repeats within the dTALEs and not through point mutation nor recombination with the native TALEs of Xcc.
Target analyses of adapted TALEs
After establishing the alterations in the adapted dTALEs, we further assessed their putative targets. We determined the potential promoter targets of the adapted dTALEs in sweet orange via in silico analyses. To this aim, we predicted the affinity of the parental and adapted dTALEs to the promoter sequences of all coding genes of sweet oranges (designated as 1 kb sequence upstream of the putative transcriptional start sites) using target finder feature in “TAL Effector Nucleotide Targeter 2.0”[56]. All adapted dTALEs demonstrated significantly higher affinity to the promoter sequence of CsLOB1 (S1 Data, the CsLOB1 gene is marked in green) than the parental dTALEs. The predicted EBEs of the adapted dTALEs largely overlapped with the EBEPthA4 (Fig 4B).
Additionally, our analysis predicted that some of the adapted dTALEs (dTALELB2A2, dTALELB3A and dTALELB7A. S1J, S1K and S1M Data) displayed relatively high affinity to promoters in addition to pLOB1. In particular, dTALELB7A was predicted to bind to several EBEs that are found in the proximity of the transcriptional start site of other genes than CsLOB1 with similar or even stronger affinity (S1M Data). Among these genes, we identified several genes that encode proteins that are associated with canker development [19, 20], such as polygalacturonase (Cs2g27910) and sugar transporter (Cs9g05220) (S1M Data). It remains to be determined whether such adaptations play any roles in selection of the corresponding dTALEs.
Discussion
Plant pathogenic bacteria usually possess high host specificity and most Xanthomonas species infect a very narrow range of hosts [5]. Xanthomonas host specificity is dictated by multiple factors, one of which is the induction of S genes by TALEs. Intriguingly, induction of the CsLOB1 gene, the canker S gene, by Xcc PthA4 is essential for canker development, and consequently, the Xcc pthA4 mutant is unable to cause canker symptoms [61]. Analyses of the LOB1 promoter regions in various Rutaceae plants identified variations in the promoter sequences. However, the EBEs are completely conserved in the promoters identified in commercial citrus varieties and variations were only observed in non-citrus Rutaceae and rootstock varieties. This suggests that TALEs targeting LOB1 promoters have adapted to their hosts by targeting a highly conserved region in the S gene promoter and by doing so efficiently enhanced the fitness of the pathogen. Consistent with this notion, RVDV1, which is the most abundant and geographically spread repeat array variant within the Xcc TALEs targeting LOB1, has the highest predicted binding affinity to the EBE of LOB1 from commercial citrus varieties [21, 62].
This study provides experimental evidence that mutations and rearrangements of repeats of TALEs enable the adaptation of Xanthomonas on incompatible hosts. We observed adaptive mutations and rearrangements in five adapted TALEs from 14 independent events within a period of 9–28 infection cycles. In the adapted TALEs, mutations and rearrangements resulted in higher affinity to the EBE in the promoter of CsLOB1. Xcc bacteria carrying these TALEs were able to induce the expression of CsLOB1 that caused citrus canker symptoms and enhanced leave colonization.
Erkes et al. 2017 characterized the adaptation events that occurred in X. oryzea TALEs using in silico techniques and genomic analysis [63]. This elegant study reported that changes in repeat arrays are mainly associated with repeat deletion, recombination with different repeat arrays of other TALEs and point mutations. Three of our adaptive variants displayed repeat deletions and four displayed substitution of the RVDs in specific repeats. The changes in the TALE repeat arrays probably resulted from the misalignment-mediated rearrangements, which are common for repetitive DNA sequences. One genetic hallmark of misalignment-mediated rearrangements is their independence of homologous recombination factors, including the RecA strand transfer protein of bacteria [43]. Multiple features of the tandem repeats of TALEs facilitate their adaptations since it has been suggested that the length, and proximity of the repeats are among the important determinants of their propensity to rearrange [43]. Tandem repeats of over a hundred nucleotides in length are deleted at very high rates, more reminiscent of recombination (10E-4) than of mutational (10E-8) frequencies [43]. In addition, there is an exponential dependence of deletion rate on proximity of the repeats [64], presumably because the two repeats must interact within a single replication fork. The tandem repeats of TALEs fit both parameters for RecA-independent ‘illegitimate’ recombination [43, 65]. Although several other mechanisms can contribute, in theory, to tandem repeat mutations, it is plausible that most repeat mutations and rearrangements occur by misalignment during replication [66]. Additionally, the codon usage in the altered repeats matched the one used within the dTALEs (S1 Text), but not that of PthA1, PthA2, PthA3 and PthA4, indicating that these alterations are likely to originate from recombination within the introduced dTALE. Taken together, we infer that TALE adaptations result mostly from the RecA-independent ‘illegitimate’ recombination between repeats of the dTALE.
TALEs adaptations were only observed in dTALEs with less than seven mismatches from the target EBE of the S gene, providing useful information regarding how to modify the EBE-region for development of resistance against TALE-department pathogens and preventing or decelerating the resistance loss owing to TALE adaptations. Specifically, the five adaptive TALE variants originated from parental dTALEs that harbored between two to five mismatched repeats (i.e. dTALELBM2, dTALELBM3, dTALELBM5 and dTALELBM7), whereas non-adaptive TALEs were identified in the three dTALEs that harbored at least seven mismatched repeats (i.e. dTALELBM1, dTALELBM4 and dTALELBM6). The location of mismatches seems not to be a determinant factor of adaptations. Both dTALELBM2 and dTALELBM5 contained two tandem mismatches at the N-terminal and in the middle, respectively, and both underwent adaptations. The number of generations required for adaptation for the adapted TALE-containing Xcc stains was estimated to range from 328 to 1,020. We infer that the relatively short adaptation time results from the small number of recombination events needed for adaptations of dTALEs with 2–5 mismatches and the high recombination rate (10E-4) [43]. Three of the five adaptive TALEs can be enabled by a single recombination event (deletion of the first two repeats in dTALELB3A and a replacement of a single repeat in dTALELB2A1 and dTALELB5A, S3 Fig). On the other hand, the fourth adaptive variant, dTALELB2A2, contained a two-repeat replacement and a deletion of a five-repeat stretch, and the fifth adaptive variant, dTALELB7A, harbored a substitution and a deletion of four-repeat stretch, both of which can be achieved with as few as two recombination events (S3 Fig). However, when more mismatches (≥7) are present between TALEs and EBEs, it is probable that multiple recombination events are required to eliminate the mismatches, significantly reducing the possibility of generation of adaptive TALEs as observed for dTALELBM1, dTALELBM4 and dTALELBM6. Of note, we did not observe any changes in dTALEs isolated from non-adaptive variants. It is assumed that mutations occur to all constructs including dTALEs carrying seven or more mismatches. However, the probability for strains carrying less mismatches to overcome the mismatches via recombination and deletion is much higher than strains containing more mismatches. The mutated constructs that overcame the mismatches enable higher fitness for the strain, leading to takeover of the population. For the mutations that did not enable increased fitness for strains that carry the dTALEs containing more mismatches, the fact that they were not detected probably results from the extreme low percentage of such mutations in the population.
While our results clearly demonstrate an adaptive repeat rearrangement and deletion of various TALEs to overcome the mismatches, it is important to note that our study was conducted via an artificial experimental simulation rather than in natural settings. Our TALEs were cloned into pBBR1MCS5 [67], which is a medium copy number vector (estimated to be around 30 copies, [68]) while naturally occurring TALEs are encoded on low-copy mega plasmids or the bacterial chromosome. A recent survey by our group showed that the majority of Xcc strains contain three copies of plasmids (pXAC33 and pXAC64) in each bacterial cell. Thus, the experimental evolution using pBBR1MCS5 with higher copy number than the natural plasmid might expedite the mutation and selection process. In addition, since the simulation was conducted in the greenhouse via syringe inoculation, it probably demonstrates the general feasibility of adaptation even though the kinetic and mechanism in a complex natural system might differ. First, during our experiment the passages had to go through the NA medium containing antibiotic selection between cycles. This procedure was a technical necessity to ensure culture purity. In natural settings, the bacteria will be subjected to more consistent selective pressure that would probably haste TALE adaptation kinetics or alternatively encourage TALE-independent adaptations to the host such as alteration in metabolic regulation or surface proteins profile [49, 51]. Second, syringe inoculations enable high titers of Xcc strains containing mismatching TALEs to establish in planta, which otherwise normally do not reach such high titers in natural settings. For example, the pthA4 mutant of Xcc [61] can only establish very low titers via foliar spray that mimics the natural infection of Xcc compared with syringe inoculation. Consequently, our setting enables us to investigate the TALE adaption to overcome incompatible interactions, which is probably much rarer and slower in the natural settings. Third, we used a simplified closed system that eliminates factors including unstable environmental factors, competitive and mutualistic interactions with other microorganisms and interaction with different Xanthomonas strains that may lead to inter-bacterial recombination events [63]. Further work should be conducted to assess the ability of natural Xanthomonas strains to overcome miss-matched EBE of S genes in the field. Such work can utilize homozygous lines of citrus that were modified in the EBE of LOB1 [42, 69] and examine the durability of field resistance to canker for extended time period and determine the putative adaption.
In summary, this study provides experimental evidence of TALE adaptations that convert incompatible to compatible interactions and offers guidance regarding how to potentially overcome the resistance loss due to TALE adaptations. Mutation of EBEs via TALEN or CRISPR-based genome editing and utilization of naturally occurring EBE variants have been regarded as one of the most efficient approaches to breed or develop resistant varieties against TALEs-containing pathogens [33, 37, 38, 70]. Our data suggest that mutation multiple nucleotides in the EBEs might be required to empower durable host resistance against TALE-dependent pathogens.
Materials and methods
Bacterial strains and plasmids
The bacterial strains and plasmids used in this study are listed in S1 Table. Oligonucleotides used for cloning and sequencing in this study are listed in S2 Table. Xanthomonas citri was grown at 28°C in nutrient broth (NB) medium (Beef extract 3 g/L, Peptone 5 g/L) and on nutrient agar (NA) plates. E. coli and A. tumefaciens were grown in Luria-Bertani (LB) medium at 37°C or 28°C, respectively. When required, growth media were supplemented with gentamicin (5 μg/mL), kanamycin (50 μg/mL), tetracycline (5 μg/mL), ampicillin (100 μg/mL) and spectinomycin (100 μg/mL).
Analysis of Rutaceae LOB1 promoters, Xanthomonas citri TALEs and EBE affinity predictions
Genomic DNA was extracted from fully expanded leaves of various Rutaceae species (Table 2) using NucleoSpin Plant II (TaKaRa Bio Inc. Kusatsu, Japan). The LOB1 promoter regions containing the PthA4 EBE were amplified from genomic DNA using Q5 High-Fidelity DNA Polymerase (NEB, Ipswich, MA) and fragments were cloned into pGEM-T vector (Promega, Madison, WI). DNA sequence was determined for 3–5 clones. Amplified LOB1 promoter sequences, along with LOB1 promoter regions of other Rutaceae species available at the citrus genome database (https://www.citrusgenomedb.org/) were analyzed using the Clustal Omega multiple sequence alignment tool (https://www.ebi.ac.uk/Tools/msa/clustalo/) and separated into allelic variants.
TALE protein sequences of X. citri were extracted from NCBI protein database (https://www.ncbi.nlm.nih.gov/protein/?term=) and the compositions of RVDs in repeat arrays were manually determined. Binding affinity was analyzed against the promoter region of LOB1 using target finder feature of “TAL Effector Nucleotide Targeter 2.0” [56] (parameters were set to score cutoff of 4.0, T only upstream base, and Doyle scoring matrix). All TALEs that were predicted to bind to LOB1 according to score cutoff of 4.0 were considered as putative LOB1 targeting TALEs.
Agrobacterium-mediated transient expression and GUS activity measurements
For construction of transient expression vector of PthA4, His-pthA4 was cloned from pET28-PthA4 [71] into pER8 [72]. For construction of β-Glucuronidase (gus) reporters the 913 bp LOB1 promoter region was amplified from genomic DNA of sweet orange or Swingle citrumelo and cloned into p1380-35S-GUS [73], replacing 35S promoter. Binary vectors were transformed into Agrobacterium GV2260 by electroporation. Agrobacterium strains carrying GUS reporters and PthA4 constructs were co-infiltrated (OD600 = 0.1) into Nicotiana benthamiana leaves. Transient expression and XVE induction were conducted as previously described [74]. Histochemical staining of GUS was conducted as previously described [75]. For GUS activity measurements leaf disks of 1.5 cm diameter were collected at three days post XVE induction, homogenized in PBS (pH 7.0) and centrifuged at 14,000 rpm for 10 min at 4°C. Supernatants were analyzed for GUS activity as described elsewhere [76]. GUS activity was quantified by arbitrary units (AU) and determined as 1000 × [A405 / (time in min × total protein in μg × 0.02)].
Construction of designer TALEs
Designer TALEs (dTALEs) containing the repeat arrays elaborated in Fig 3A were constructed using “Golden Gate TALEN and TAL Effector Kit 2.0” as previously described [77] and cloned into pTAL2 as a final destination vector. The pTAL2 PstI/EcoRI fragments containing the dTALEs were cloned into pBBRNPth [54] and transformed into Xcc pthA4:Tn5 [61] by electroporation. Expression of all constructed dTALEs and their adapted derivatives in Xcc was validated by Western blot [78] using Anti-HA High Affinity antibody (Roche diagnostics, Basel, Switzerland) (S4 Fig).
Plant inoculations, measurement of CsLOB1 expression and measurement of bacterial growth
Bacteria were inoculated into expanded leaves of 2-year-old Valencia sweet orange plants with bacterial suspensions (5 × 105 CFU/mL as initial inoculum in experimental evolution test, 106 CFU/mL for monitoring bacterial growth and 108 CFU/mL for monitoring symptom development and expression analysis of CsLOB1) in 10 mM MgCl2 using a needless syringe. Plants were kept in a greenhouse at 28°C under natural light.
CsLOB1 expression was measured in sweet orange leaves at 36 and 72 hours post bacterial inoculation. RNA isolation and qPCR analysis were conducted as described previously [76].
To measure bacterial growth in planta two leaf discs of 0.4-cm-diameter per plant from three plants were sampled, homogenized in 10 mM MgCl2 and bacterial numbers were determined by plating 10 μL from 10-fold serial dilutions and counting the resulting colonies.
Experimental evolution procedure
Two duplicate strains of Xcc pthA4:Tn5 carrying a vector encoding dTALELBM1, dTALELBM2, dTALELBM3, dTALELBM4, dTALELBM5, dTALELBM6 or dTALELBM7 were inoculated (5 × 105 CFU/mL) into leaves of two independent sweet orange plants. Bacteria were isolated from leaves 7–10 days later from the two plants. Bacteria were plated on NA plates with gentamicin and kanamycin and bacterial populations were determined. Of note, we initially started the experiment using plant system alone but encountered many technical issues with contaminations. To overcome such issues, we added one isolation step to remove the contamination and guarantee the purity of the aforementioned Xcc strains.
Bacteria from each duplicate (two duplicate strains per dTALE–a total of 14 samples) were scrapped from NA plated, diluted to 5 × 105 CFU/mL and inoculated into leaves of two previously uninfected sweet orange plants. The procedure was repeated for 30 cycles, representing approximately 1,093 generations. Bacterial titers and appearance of canker symptoms were determined for each infection cycle. Generation time (G) was calculated as G = T × LOG2 (B) where T represent the number of days and B represents the average daily growth rate of Xcc pthA4:Tn5 in sweet orange during exponential phase.
As a negative control, Xcc pthA4:Tn5 strains harboring the seven dTALEs used in the experimental evolution study were streaked on rich NA medium supplemented with gentamicin and kanamycin in parallel to the infection cycles to identify random occurrence of repeat rearrangement that is independent of host adaptation. Plasmids were extracted from three independent colonies of each of the NA streaked bacteria after 30 streaking cycles and sent for further analysis.
Isolation, sequencing and validation of adapted dTALEs
Adapted dTALEs were extracted from Xcc following the first observation of canker symptoms in sweet orange leaves and at the end of the experiment (30 cycles). dTALE plasmids were extracted from Xcc using plasmid miniprep (ultra-fast): NucleoSpin Plasmid EasyPure kit (TaKaRa Bio Inc. Kusatsu, Japan) and transformed into E. coli. Plasmids were extracted from 5–10 colonies and introduced into Xcc pthA4:Tn5. Single colonies from each transformation were used for inoculation (108 CFU/mL) of sweet orange leaves. If an inoculation resulted in canker symptoms, the RVD compositions of the repeat array were determined by sequencing (Eton Bioscience, Inc., San Diego, CA). The sequence of adapted dTALEs (containing the TAL backbone and repeat arrays) was determined by sequencing. DNA sequences of the adapted dTALEs identified in this study are shown in S1 Text.
Prediction of effector-binding elements
The 1 kb upstream sequences from the putative transcriptional start site of all genes in sweet orange were determined (S2 Text) and used as predicted promoters for affinity analyses. The affinity of dTALEs used in the study to sweet orange promoters was analyzed using target finder feature of “TAL Effector Nucleotide Targeter 2.0” [56] (parameters were set to score cutoff of 3.0, T only upstream nucleotide, and Doyle scoring matrix). The predicted EBEs are shown in S1 Data.
Supporting information
The RVD variants of LOB1 targeting TALEs of Xcc and Xca (Table 1) were analyzed using QueTAL (http://bioinfo-web.mpl.ird.fr/cgi-bin2/quetal/quetal.cgi). A. Phylogenetic relationship between LOB1 targeting TALEs was analyzed using DisTAL v1.1. B. Functional relationship between LOB1 targeting TALEs was analyzed using FuncTAL v1.1.
(PDF)
Sweet orange leaves were syringe-infiltrated with suspensions (1 × 108 CFU/mL) of Xcc pthA4:Tn5 or Xcc pthA4:Tn5 transformed with the dTALEs depicted in Fig 2A. A. Inoculated leaves were photographed at 7 days post inoculation. B. The expression of CsLOB1 was quantified at 96 h post inoculation. The GAPDH gene was used as an endogenous control. Values are means ± SE of three biological replicates. Asterisks indicate a significant difference (Student’s t-test, P-value < 0.05) compared to Xcc pthA4:Tn5. The experiments were repeated three times with similar results.
(PDF)
Schemes represent the alterations observed in the adapted dTALEs compared to their parental dTALEs and the predicted recombination events, which led to the adaptation. Repeats that were likely to be subjected for recombination or deletion in the parental dTALE are underlined. Repeats in the adapted dTALEs that were altered as a result of recombination are underlined and marked in blue. Repeats that were deleted are marked in purple. A. Alteration observed in dTALE2A1 compared with dTALELBM2. B. Alteration observed in dTALE2A2 compared with dTALELBM2. C. Alteration observed in dTALE3A compared with dTALELBM3. D. Alteration observed in dTALE5A compared with dTALELBM5. E. Alteration observed in dTALE7A compared with dTALELBM7.
(PDF)
Total protein was extracted from overnight cultures of Xcc pthA4:Tn5 [No vector control (NVC)], Xcc pthA4:Tn5 carrying pBBR1MCS-5 [Empty vector (EV)] and Xcc pthA4:Tn5 transformed with the parental and adapted dTALEs. Samples were separated by SDS-PAGE and immunoblotted with the anti-HA antibody (upper panel) or stained with coomassie blue (lower panel).
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
Acknowledgments
We would like to thank Jin Xu for his technical assistance.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
NW received funding from the US Department of Agriculture-National Institute of Food and Agriculture (USDA-NIFA) Plant Biotic Interactions Program under grant no. 2017-67013-26527 (https://urldefense.proofpoint.com/v2/url?u=https-3A__nifa.usda.gov_&d=DwIGaQ&c=sJ6xIWYx-zLMB3EPkvcnVg&r=t-amc4JbEo_7rK5LJaQISQ&m=UwUad2YpFlH0cZEyGMwyy_77saJljw-DsIzVlOnbpUE&s=vTNJqcH7NJaoJpwXqJtD5o4VhgdZvnfA_9mh1VFSCtA&e=). DT received funding from BARD, the United States - Israel Binational Agricultural Research and Development Fund, Vaadia-BARD Postdoctoral Fellowship Award No. FI-562-2017 (https://urldefense.proofpoint.com/v2/url?u=https-3A__www.bard-2Disus.com_&d=DwIGaQ&c=sJ6xIWYx-zLMB3EPkvcnVg&r=t-amc4JbEo_7rK5LJaQISQ&m=UwUad2YpFlH0cZEyGMwyy_77saJljw-DsIzVlOnbpUE&s=9CNBr-wDo6GKbQSgmIQWM7Tg7Ww-lvtCCLnQhio3gNKU&e=). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Boch J, Bonas U. Xanthomonas AvrBs3 family-type III effectors: discovery and function. Annu Rev Phytopathol. 2010;48:419–36. 10.1146/annurev-phyto-080508-081936 [DOI] [PubMed] [Google Scholar]
- 2.Mak AN-S, Bradley P, Bogdanove AJ, Stoddard BL. TAL effectors: function, structure, engineering and applications. Curr Opin Struct Biol. 2013;23:93–9. 10.1016/j.sbi.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore R, Chandrahas A, Bleris L. Transcription activator-like effectors: a toolkit for synthetic biology. ACS Synth Biol. 2014;3:708–16. 10.1021/sb400137b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutin M, Pérez-Quintero AL, Lopez C, Szurek B. MorTAL Kombat: the story of defense against TAL effectors through loss-of-susceptibility. Front Plant Sci. 2015;6:535 10.3389/fpls.2015.00535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.An SQ, Potnis N, Dow M, Vorhölter FJ, He YQ, Becker A, et al. Mechanistic insights into host adaptation, virulence and epidemiology of the phytopathogen Xanthomonas. FEMS Microbiol Rev. 2019; 10.1093/femsre/fuz024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boch J, Bonas U, Lahaye T. TAL effectors—pathogen strategies and plant resistance engineering. New Phytol. 2014;204:823–32. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25539004 10.1111/nph.13015 [DOI] [PubMed] [Google Scholar]
- 7.Muñoz Bodnar A, Bernal A, Szurek B, López CE. Tell me a tale of TALEs. Mol Biotechnol. 2013;53:228–235. 10.1007/s12033-012-9619-3 [DOI] [PubMed] [Google Scholar]
- 8.Perez-Quintero AL, Szurek B. A decade decoded: spies and hackers in the history of TAL effectors research. Annu Rev Phytopathol. 2019;57:459–481. 10.1146/annurev-phyto-082718-100026 [DOI] [PubMed] [Google Scholar]
- 9.Popov G, Fraiture M, Brunner F, Sessa G. Multiple Xanthomonas euvesicatoria type III effectors inhibit flg22-triggered immunity. Mol Plant Microbe Interact. 2016;29:651–60. 10.1094/MPMI-07-16-0137-R [DOI] [PubMed] [Google Scholar]
- 10.Long J, Song C, Yan F, Zhou J, Zhou H, Yang B. Non-TAL effectors from Xanthomonas oryzae pv. oryzae suppress peptidoglycan-triggered MAPK activation in rice. Front Plant Sci. 2018;9:1857 10.3389/fpls.2018.01857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Timilsina S, Potnis N, Newberry EA, Liyanapathiranage P, Iruegas-Bocardo F, White FF, et al. Xanthomonas diversity, virulence and plant-pathogen interactions. Nat Rev Microbiol. 2020; 10.1038/s41579-020-0361-8 [DOI] [PubMed] [Google Scholar]
- 12.Yang B, Sugio A, White FF. Os8N3 is a host disease-susceptibility gene for bacterial blight of rice. Proc Natl Acad Sci U S A. 2006;103:10503–10508. 10.1073/pnas.0604088103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antony G, Zhou J, Huang S, Li T, Liu B, White F, et al. Rice xa13 recessive resistance to bacterial blight is defeated by induction of the disease susceptibility gene Os-11N3. Plant Cell. 2010;22:3864–76. 10.1105/tpc.110.078964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verdier V, Triplett LR, Hummel AW, Corral R, Cernadas RA, Schmidt CL, et al. Transcription activator-like (TAL) effectors targeting OsSWEET genes enhance virulence on diverse rice (Oryza sativa) varieties when expressed individually in a TAL effector-deficient strain of Xanthomonas oryzae. New Phytol. 2012;196:1197–207. 10.1111/j.1469-8137.2012.04367.x [DOI] [PubMed] [Google Scholar]
- 15.Cernadas RA, Doyle EL, Niño-Liu DO, Wilkins KE, Bancroft T, Wang L, et al. Code-assisted discovery of TAL effector targets in bacterial leaf streak of rice reveals contrast with bacterial blight and a novel susceptibility gene. PLoS Pathog. 2014;10:e1003972 10.1371/journal.ppat.1003972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz AR, Morbitzer R, Lahaye T, Staskawicz BJ. TALE-induced bHLH transcription factors that activate a pectate lyase contribute to water soaking in bacterial spot of tomato. Proc Natl Acad Sci U S A. 2017;114:E897–E903. 10.1073/pnas.1620407114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kay S, Hahn S, Marois E, Hause G, Bonas U. A bacterial effector acts as a plant transcription factor and induces a cell size regulator. Science. 2007;318:648–51. 10.1126/science.1144956 [DOI] [PubMed] [Google Scholar]
- 18.Peng Z, Hu Y, Zhang J, Huguet-Tapia JC, Block AK, Park S, et al. Xanthomonas translucens commandeers the host rate-limiting step in ABA biosynthesis for disease susceptibility. Proc Natl Acad Sci. 2019;116:20938–20946. 10.1073/pnas.1911660116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Y, Zhang J, Jia H, Sosso D, Li T, Frommer WB, et al. Lateral organ boundaries 1 is a disease susceptibility gene for citrus bacterial canker disease. Proc Natl Acad Sci. 2014;111:E521–E529. 10.1073/pnas.1313271111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duan S, Jia H, Pang Z, Teper D, White F, Jones J, et al. Functional characterization of the citrus canker susceptibility gene CsLOB1. Mol Plant Pathol. 2018; 10.1111/mpp.12667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Saadi A, Reddy JD, Duan YP, Brunings AM, Yuan Q, Gabriel DW. All five host-range variants of Xanthomonas citri carry one pthA homolog with 17.5 repeats that determines pathogenicity on citrus, but none determine host-range variation. Mol Plant Microbe Interact. 2007;20:934–43. 10.1094/MPMI-20-8-0934 [DOI] [PubMed] [Google Scholar]
- 22.Hu Y, Duan S, Zhang Y, Shantharaj D, Jones JB, Wang N. Temporal transcription profiling of sweet orange in response to PthA4-mediated Xanthomonas citri subsp. citri infection. Phytopathology. 2016;106:442–451. 10.1094/PHYTO-09-15-0201-R [DOI] [PubMed] [Google Scholar]
- 23.Gu K, Yang B, Tian D, Wu L, Wang D, Sreekala C, et al. R gene expression induced by a type-III effector triggers disease resistance in rice. Nature. 2005;435:1122–5. 10.1038/nature03630 [DOI] [PubMed] [Google Scholar]
- 24.Schornack S, Ballvora A, Gürlebeck D, Peart J, Baulcombe D, Ganal M, et al. The tomato resistance protein Bs4 is a predicted non-nuclear TIR-NB-LRR protein that mediates defense responses to severely truncated derivatives of AvrBs4 and overexpressed AvrBs3. Plant J. 2004;37:46–60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14675431 10.1046/j.1365-313x.2003.01937.x [DOI] [PubMed] [Google Scholar]
- 25.Römer P, Hahn S, Jordan T, Strauss T, Bonas U, Lahaye T. Plant pathogen recognition mediated by promoter activation of the pepper Bs3 resistance gene. Science. 2007;318:645–8. 10.1126/science.1144958 [DOI] [PubMed] [Google Scholar]
- 26.Hutin M, Sabot F, Ghesquière A, Koebnik R, Szurek B. A knowledge-based molecular screen uncovers a broad-spectrum OsSWEET14 resistance allele to bacterial blight from wild rice. Plant J. 2015;84:694–703. 10.1111/tpj.13042 [DOI] [PubMed] [Google Scholar]
- 27.Ji Z, Ji C, Liu B, Zou L, Chen G, Yang B. Interfering TAL effectors of Xanthomonas oryzae neutralize R-gene-mediated plant disease resistance. Nat Commun. 2016;7:13435 10.1038/ncomms13435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaka A, Grande G, Coronejo T, Quibod IL, Chen C-W, Chang S-J, et al. Natural variations in the promoter of OsSWEET13 and OsSWEET14 expand the range of resistance against Xanthomonas oryzae pv. oryzae. PLoS One. 2018;13:e0203711 10.1371/journal.pone.0203711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian D, Wang J, Zeng X, Gu K, Qiu C, Yang X, et al. The rice TAL effector-dependent resistance protein XA10 triggers cell death and calcium depletion in the endoplasmic reticulum. Plant Cell. 2014;26:497–515. 10.1105/tpc.113.119255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang C, Zhang X, Fan Y, Gao Y, Zhu Q, Zheng C, et al. XA23 is an executor R protein and confers broad-spectrum disease resistance in rice. Mol Plant. 2015;8:290–302. 10.1016/j.molp.2014.10.010 [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Zeng X, Tian D, Yang X, Wang L, Yin Z. The pepper Bs4C proteins are localized to the endoplasmic reticulum (ER) membrane and confer disease resistance to bacterial blight in transgenic rice. Mol Plant Pathol. 2018; 10.1111/mpp.12684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schandry N, Jacobs JM, Szurek B, Perez-Quintero AL. A cautionary TALE: how plant breeding may have favoured expanded TALE repertoires in Xanthomonas. Mol Plant Pathol. 2018;19:1297–1301. 10.1111/mpp.12670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou J, Peng Z, Long J, Sosso D, Liu B, Eom J-S, et al. Gene targeting by the TAL effector PthXo2 reveals cryptic resistance gene for bacterial blight of rice. Plant J. 2015;82:632–43. 10.1111/tpj.12838 [DOI] [PubMed] [Google Scholar]
- 34.Streubel J, Pesce C, Hutin M, Koebnik R, Boch J, Szurek B. Five phylogenetically close rice SWEET genes confer TAL effector-mediated susceptibility to Xanthomonas oryzae pv. oryzae. New Phytol. 2013;200:808–19. 10.1111/nph.12411 [DOI] [PubMed] [Google Scholar]
- 35.Yu Y, Streubel J, Balzergue S, Champion A, Boch J, Koebnik R, et al. Colonization of rice leaf blades by an African strain of Xanthomonas oryzae pv. oryzae depends on a new TAL effector that induces the rice nodulin-3 Os11N3 gene. Mol Plant Microbe Interact. 2011;24:1102–13. 10.1094/MPMI-11-10-0254 [DOI] [PubMed] [Google Scholar]
- 36.Jalan N, Kumar D, Yu F, Jones JB, Graham JH, Wang N. Complete genome sequence of Xanthomonas citri subsp. citri strain Aw12879, a restricted-host-range citrus canker-causing bacterium. Genome Announc. 2013;1 10.1128/genomeA.00235-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li T, Liu B, Spalding MH, Weeks DP, Yang B. High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat Biotechnol. 2012;30:390–2. 10.1038/nbt.2199 [DOI] [PubMed] [Google Scholar]
- 38.Blanvillain-Baufumé S, Reschke M, Solé M, Auguy F, Doucoure H, Szurek B, et al. Targeted promoter editing for rice resistance to Xanthomonas oryzae pv. oryzae reveals differential activities for SWEET14-inducing TAL effectors. Plant Biotechnol J. 2017;15:306–317. 10.1111/pbi.12613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jia H, Zhang Y, Orbović V, Xu J, White FF, Jones JB, et al. Genome editing of the disease susceptibility gene CsLOB1 in citrus confers resistance to citrus canker. Plant Biotechnol J. 2017;15:817–823. 10.1111/pbi.12677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng A, Chen S, Lei T, Xu L, He Y, Wu L, et al. Engineering canker-resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene CsLOB1 promoter in citrus. Plant Biotechnol J. 2017;15:1509–1519. 10.1111/pbi.12733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliva R, Ji C, Atienza-Grande G, Huguet-Tapia JC, Perez-Quintero A, Li T, et al. Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat Biotechnol. 2019;37:1344–1350. 10.1038/s41587-019-0267-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jia H, Wang N. Generation of homozygous canker-resistant citrus in the T0 generation using CRISPR-SpCas9p. Plant Biotechnol J. 2020; pbi.13375. 10.1111/pbi.13375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lovett ST. Encoded errors: mutations and rearrangements mediated by misalignment at repetitive DNA sequences. Mol Microbiol. 2004;52:1243–53. 10.1111/j.1365-2958.2004.04076.x [DOI] [PubMed] [Google Scholar]
- 44.van den Bosch TJM, Niemi O, Welte CU. Single gene enables plant pathogenic Pectobacterium to overcome host-specific chemical defense. Mol Plant Pathol. 2020;21:349–359. 10.1111/mpp.12900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stice SP, Thao KK, Khang CH, Baltrus DA, Dutta B, Kvitko BH. Thiosulfinate Tolerance Is a Virulence Strategy of an Atypical Bacterial Pathogen of Onion. Curr Biol. 2020;30:3130–3140.e6. 10.1016/j.cub.2020.05.092 [DOI] [PubMed] [Google Scholar]
- 46.Wei Y, Caceres-Moreno C, Jimenez-Gongora T, Wang K, Sang Y, Lozano-Duran R, et al. The Ralstonia solanacearum csp22 peptide, but not flagellin-derived peptides, is perceived by plants from the Solanaceae family. Plant Biotechnol J. 2018;16:1349–1362. 10.1111/pbi.12874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang S, Sun Z, Wang H, Liu L, Lu F, Yang J, et al. Rice OsFLS2-mediated perception of bacterial flagellins is evaded by Xanthomonas oryzae pvs. oryzae and oryzicola. Mol Plant. 2015;8:1024–1037. 10.1016/j.molp.2015.01.012 [DOI] [PubMed] [Google Scholar]
- 48.Stall RE, Jones JB, Minsavage G V. Durability of resistance in tomato and pepper to xanthomonads causing bacterial spot. Annu Rev Phytopathol. 2009;47:265–84. 10.1146/annurev-phyto-080508-081752 [DOI] [PubMed] [Google Scholar]
- 49.Fothergill JL, Neill DR, Loman N, Winstanley C, Kadioglu A. Pseudomonas aeruginosa adaptation in the nasopharyngeal reservoir leads to migration and persistence in the lungs. Nat Commun. 2014;5:4780 10.1038/ncomms5780 [DOI] [PubMed] [Google Scholar]
- 50.Bricio-Moreno L, Sheridan VH, Goodhead I, Armstrong S, Wong JKL, Waters EM, et al. Evolutionary trade-offs associated with loss of PmrB function in host-adapted Pseudomonas aeruginosa. Nat Commun. 2018;9:2635 10.1038/s41467-018-04996-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guidot A, Jiang W, Ferdy J-B, Thébaud C, Barberis P, Gouzy J, et al. Multihost experimental evolution of the pathogen Ralstonia solanacearum unveils genes involved in adaptation to plants. Mol Biol Evol. 2014;31:2913–28. 10.1093/molbev/msu229 [DOI] [PubMed] [Google Scholar]
- 52.Perrier A, Peyraud R, Rengel D, Barlet X, Lucasson E, Gouzy J, et al. Enhanced in planta fitness through adaptive mutations in EfpR, a dual regulator of virulence and metabolic functions in the plant pathogen Ralstonia solanacearum. Desveaux D, editor. PLOS Pathog. 2016;12:e1006044 10.1371/journal.ppat.1006044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trivedi P, Wang N. Host immune responses accelerate pathogen evolution. ISME J. 2014;8:727–31. 10.1038/ismej.2013.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teper D, Xu J, Li J, Wang N. The immunity of Meiwa kumquat against Xanthomonas citri is associated with a known susceptibility gene induced by a transcription activator-like effector. Yang B, editor. PLOS Pathog. 2020;16:e1008886 10.1371/journal.ppat.1008886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ference CM, Gochez AM, Behlau F, Wang N, Graham JH, Jones JB. Recent advances in the understanding of Xanthomonas citri ssp. citri pathogenesis and citrus canker disease management. Mol Plant Pathol. 2018;19:1302–1318. 10.1111/mpp.12638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doyle EL, Booher NJ, Standage DS, Voytas DF, Brendel VP, Vandyk JK, et al. TAL Effector-Nucleotide Targeter (TALE-NT) 2.0: tools for TAL effector design and target prediction. Nucleic Acids Res. 2012;40:W117–22. 10.1093/nar/gks608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pérez-Quintero AL, Lamy L, Gordon JL, Escalon A, Cunnac S, Szurek B, et al. QueTAL: a suite of tools to classify and compare TAL effectors functionally and phylogenetically. Front Plant Sci. 2015;6 10.3389/fpls.2015.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gabriel DW, Hunter JE, Kingsley MT, Miller JW, Lazo GR. Clonal Population Structure of Xanthomonas campestris and Genetic Diversity Among Citrus Canker Strains. Mol Plant-Microbe Interact. 1988;1:59 10.1094/MPMI-1-059 [DOI] [Google Scholar]
- 59.Sun X, Stall RE, Jones JB, Cubero J, Gottwald TR, Graham JH, et al. Detection and characterization of a new strain of citrus canker bacteria from key/mexican lime and Alemow in south Florida. Plant Dis. 2004;88:1179–1188. 10.1094/PDIS.2004.88.11.1179 [DOI] [PubMed] [Google Scholar]
- 60.Wu GA, Terol J, Ibanez V, López-García A, Pérez-Román E, Borredá C, et al. Genomics of the origin and evolution of citrus. Nature. 2018;554:311–316. 10.1038/nature25447 [DOI] [PubMed] [Google Scholar]
- 61.Yan Q, Wang N. High-throughput screening and analysis of genes of Xanthomonas citri subsp. citri involved in citrus canker symptom development. Mol Plant Microbe Interact. 2011;25:1–72. 10.1094/MPMI-05-11-0121 [DOI] [PubMed] [Google Scholar]
- 62.Li Z, Zou L, Ye G, Xiong L, Ji Z, Zakria M, et al. A potential disease susceptibility gene CsLOB of citrus is targeted by a major virulence effector PthA of Xanthomonas citri subsp. citri. Mol Plant. 2014;7:912–5. 10.1093/mp/sst176 [DOI] [PubMed] [Google Scholar]
- 63.Erkes A, Reschke M, Boch J, Grau J. Evolution of Transcription Activator-Like Effectors in Xanthomonas oryzae. Genome Biol Evol. 2017;9:1599–1615. 10.1093/gbe/evx108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lovett ST, Gluckman TJ, Simon PJ, Sutera VA, Drapkin PT. Recombination between repeats in Escherichia coli by a recA-independent, proximity-sensitive mechanism. Mol Gen Genet. 1994;245:294–300. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7816039 10.1007/BF00290109 [DOI] [PubMed] [Google Scholar]
- 65.Franklin NC. Extraordinary recombinational events in Escherichia coli. Their independence of the rec+ function. Genetics. 1967;55:699–707. Available from: http://www.ncbi.nlm.nih.gov/pubmed/5341209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lovett ST, Feschenko V V. Stabilization of diverged tandem repeats by mismatch repair: evidence for deletion formation via a misaligned replication intermediate. Proc Natl Acad Sci U S A. 1996;93:7120–4. 10.1073/pnas.93.14.7120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kovach ME, Elzer PH, Steven Hill D, Robertson GT, Farris MA, Roop RM, et al. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166:175–176. 10.1016/0378-1119(95)00584-1 [DOI] [PubMed] [Google Scholar]
- 68.Buch AD, Archana G, Naresh Kumar G. Broad-host-range plasmid-mediated metabolic perturbations in Pseudomonas fluorescens 13525. Appl Microbiol Biotechnol. 2010;88:209–218. 10.1007/s00253-010-2717-x [DOI] [PubMed] [Google Scholar]
- 69.Jia H, Orbović V, Wang N. CRISPR -LbCas12a-mediated modification of citrus. Plant Biotechnol J. 2019;17:1928–1937. 10.1111/pbi.13109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jia H, Orbovic V, Jones JB, Wang N. Modification of the PthA4 effector binding elements in Type I CsLOB1 promoter using Cas9/sgRNA to produce transgenic Duncan grapefruit alleviating XccΔpthA4:dCsLOB1.3 infection. Plant Biotechnol J. 2016;14:1291–301. 10.1111/pbi.12495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Domingues MN, De Souza TA, Cernadas RA, de Oliveira MLP, Docena C, Farah CS, et al. The Xanthomonas citri effector protein PthA interacts with citrus proteins involved in nuclear transport, protein folding and ubiquitination associated with DNA repair. Mol Plant Pathol. 2010;11:663–75. 10.1111/j.1364-3703.2010.00636.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zuo J, Niu QW, Chua NH. Technical advance: An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 2000;24:265–73. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11069700 10.1046/j.1365-313x.2000.00868.x [DOI] [PubMed] [Google Scholar]
- 73.Jia H, Wang N. Xcc-facilitated agroinfiltration of citrus leaves: a tool for rapid functional analysis of transgenes in citrus leaves. Plant Cell Rep. 2014;33:1993–2001. 10.1007/s00299-014-1673-9 [DOI] [PubMed] [Google Scholar]
- 74.Teper D, Girija AM, Bosis E, Popov G, Savidor A, Sessa G. The Xanthomonas euvesicatoria type III effector XopAU is an active protein kinase that manipulates plant MAP kinase signaling. PLoS Pathog. 2018;14 10.1371/journal.ppat.1006880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jia H, Liao M, Verbelen J-P, Vissenberg K. Direct creation of marker-free tobacco plants from agroinfiltrated leaf discs. Plant Cell Rep. 2007;26:1961–5. 10.1007/s00299-007-0403-y [DOI] [PubMed] [Google Scholar]
- 76.Teper D, Zhang Y, Wang N. TfmR, a novel TetR-family transcriptional regulator, modulates the virulence of Xanthomonas citri in response to fatty acids. Mol Plant Pathol. 2019; 10.1111/mpp.12786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cermak T, Doyle EL, Christian M, Wang L, Zhang Y, Schmidt C, et al. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011;39:e82 10.1093/nar/gkr218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]