PURPOSE:
The association between geriatric assessment (GA)–identified impairments and long-term health care use in older cancer survivors remains unknown. Our objective was to evaluate whether a GA performed at cancer diagnosis was predictive of hospitalizations and long-term care (LTC) use in older adult cancer survivors.
METHODS:
Older adults with GA performed between 3 months before through 6 months after diagnosis were included (N = 125). Patients with Medicare Parts A and B coverage and no managed care were identified. Hospitalizations and LTC use (skilled nursing or assisted living) were assessed up to 5 years postdiagnosis. GA risk measures were evaluated in separate Poisson models estimating the relative risk (RR) for hospital and LTC visits, adjusting for age and Charlson comorbidity score.
RESULTS:
The mean age of patients was 74 years, and the majority were female (80%) and white (90%). Breast cancer (64%) and early-stage disease (stages 0 to III, 77%) were common. Prefrail/frail status (RR, 2.5; P < .001), instrumental activities of daily living impairment (RR, 5.47; P < .001), and limitations in climbing stairs (RR, 2.94; P < .001) were associated with increased hospitalizations. Prefrail/frail status (RR, 1.86; P < .007), instrumental activities of daily living impairment (RR, 4.58; P < .001), presence of falls (RR, 6.73; P < .001), prolonged Timed Up and Go (RR, 5.45; P < .001), and limitations in climbing stairs (RR, 1.89; P < .005) were associated with LTC use.
CONCLUSION:
GA-identified impairments were associated with increased hospitalizations and LTC use among older adults with cancer. GA-focused interventions should be targeted toward high-risk patients to reduce long-term adverse health care use in this vulnerable population.
INTRODUCTION
The majority of cancer diagnoses occur in adults older than 65 years of age, and changing demographics will result in the number of older adults with cancer dramatically increasing over the next decade.1,2 Although the effectiveness of cancer therapies is typically measured in terms of overall and disease-free survival, these outcomes fail to encompass the full effect of cancer and its related treatments on older adults with cancer.3 Older adults undergoing cancer treatment are at increased risk of functional and cognitive declines,4 yet older patients prioritize long-term quality of life and independence over incremental survival benefits.5-7 Cancer treatment decisions in older adults are complicated and require a delicate balance of the risks and benefits of cancer therapy informed by individual patient preferences.
The heterogeneous aging process results in wide range in the health status of older adults that defies definition by chronological age alone. Assessing the overall fitness of older adults with cancer to estimate treatment tolerability and adverse outcomes remains an increasingly common clinical conundrum. Geriatric assessment (GA) is a multidimensional tool that assesses a broad range of health domains related to aging.8 GA provides a comprehensive evaluation of a patient’s overall health status and can aid in the identification of potential areas of vulnerability and need.9 The GA has been demonstrated to be feasible in the cooperative group clinical trial setting and in community oncology centers and is recommended for all older adults with cancer as a global assessment of fitness for cancer therapy.10-12 When used in clinical practice, it can identify impairments often missed by routine oncologic assessments and aids in the prediction of chemotherapy toxicity and mortality in older adults with cancer.13-16 However, how performance on GA is related to long-term health care use including hospitalizations and long-term care (LTC) placement in older adult cancer survivors remains unknown.
Our primary objective was to evaluate whether impairments identified by a GA performed near the time of cancer diagnosis was predictive of long-term hospitalizations and LTC use in older adult cancer survivors. Our goal was to identify specific populations of older adults at risk for adverse long-term health care use outcomes, with the ultimate goal of developing targeted and thoughtful interventions on the basis of individual needs to reduce long-term adverse outcomes.
METHODS
Data Source
Our study used a unique linkage of three data sources: a hospital-based cancer registry, the state cancer registry, and the Medicare enrollment and claims data. This linkage was developed specifically to aid in the study of older adults with cancer.17 The sample for this study was composed of participants from within the Carolina Senior Registry (CSR; ClinicalTrials.gov identifier: NCT01137825). The CSR was developed in 2009 to collect GA data on patients 65 years or older with cancer, the details of which have been described previously.11 The only requirements for inclusion the CSR are ≥ age 65 years and a cancer diagnosis. Although participants in the CSR have been recruited from across the state of North Carolina, the sample from this study is limited to those recruited at the North Carolina Cancer Hospital, because insufficient identifiers were collected at community centers to link participants to the cancer registry and Medicare data. Deterministic and probabilistic algorithms were used to link participants across data sources on the basis of participants’ first and last name, date of birth, sex, and hashed social security number to the North Carolina Central Cancer Registry.17 The North Carolina Central Cancer Registry captures legally reportable tumor information in North Carolina. Medicare Parts A (hospital insurance) and B (outpatient insurance) claims contain longitudinal information about beneficiaries’ health care encounters. This study was approved by the institutional review board at the University of North Carolina at Chapel Hill (IRB 14-2247).
Sample Selection
We restricted our study to linked CSR participants who completed the GA from 3 months prediagnosis through 6 months postdiagnosis. To ensure complete claims were available for analysis, participants were also required to have continuous enrollment in both Medicare Parts A and B, with no enrollment in managed care plans for 6 months before and after cancer diagnosis. Patients who died within 6 months from diagnosis were excluded from our analysis. Of the 818 patients within the CSR enrolled from North Carolina Cancer Hospital, 125 patients met our eligibility criteria and represent the sample for our present study. See Figure 1 for a detailed diagram.
Fig 1.
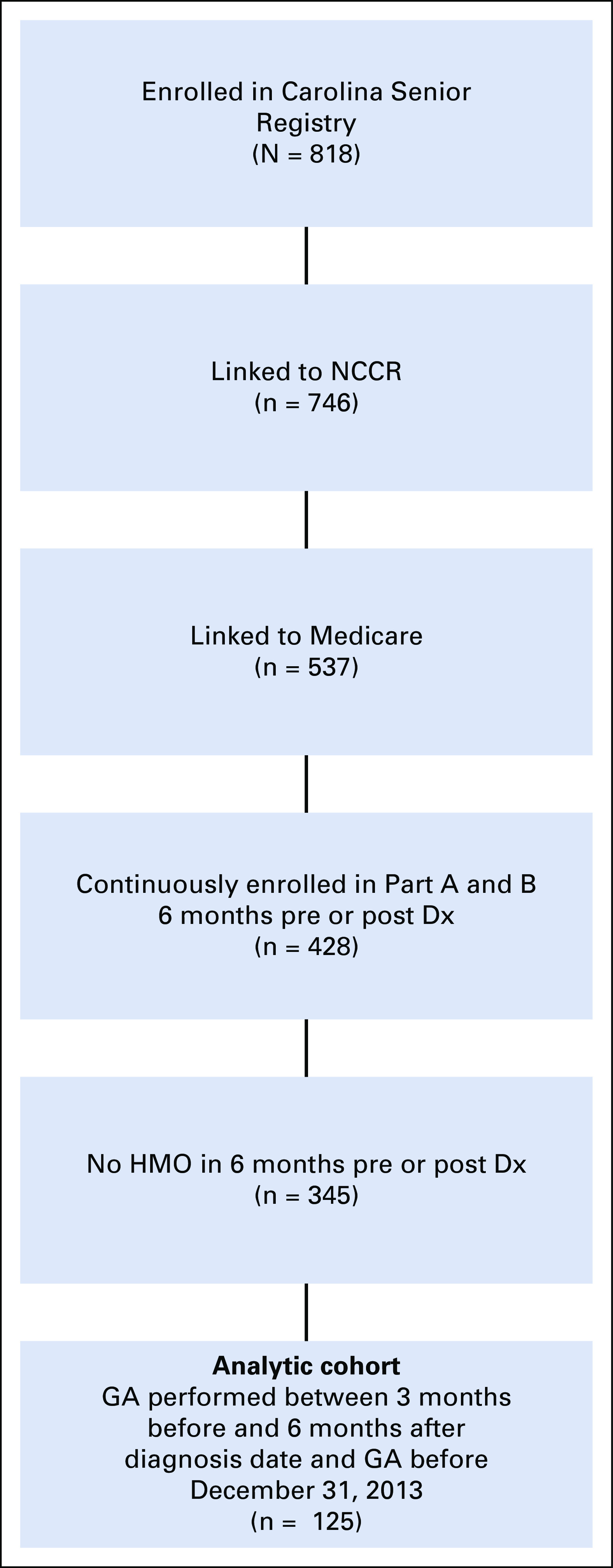
Sample selection diagram. Dx, diagnosis; GA, geriatric assessment; HMO, health maintenance organization; NCCR, North Carolina Central Cancer Registry.
GA
The GA used in the CSR was initially developed by Hurria et al18 and has been used extensively in oncology studies.10,11 Domains covered include physical function, cognition, nutrition, polypharmacy, comorbidity, social support, and mental health using validated and reliable measures. The GA consists of provider-assessed items that are typically completed by research staff and a patient-reported questionnaire.
Within the provider-assessed portion, the patient completed a Timed Up and Go (TUG) test. A TUG score of 14 seconds or longer was considered prolonged.19,20 The Blessed Orientation Memory and Concentration test was performed as a measure of cognition. This test assesses whether the patient knows the current year, month, and time of day and asks them to count backward from 20 to 1 and recite the months in reverse order and to repeat a memory phrase.21,22 A score of 11or greater on the Blessed Orientation Memory and Concentration test was consistent with memory impairment.21,22 Last, an assessment of the patient’s percent of unintentional weight loss over the last 6 months was performed, and weight loss greater than or equal to 5% was considered concerning for poor nutrition.23
The patient-reported questionnaire uses a subscale of the Multidimensional Functional Assessment Questionnaire: Older American Resources and Services to assess the amount of assistance required with instrumental activities of daily living (IADL), including using the telephone, getting to places out of walking distance, shopping for groceries or clothes, preparing meals, doing housework, taking medications, and handling money.24,25 The IADL portion was scored as unimpaired (score of 14) or impaired (score of ≤ 13).15 A physical function scale was also included that inquired about limitations in engaging in various activities ranging from vigorous activities to walking one block.26 Because no specific scoring rules are available for this measure, the individual questions were used and dichotomized as not limited versus any limitation.27 The number of falls in the last 6 months is reported, and the presence of any fall is noted as abnormal. A patient-reported Karnofsky performance status (KPS) is included and dichotomized as greater than or equal to 80 or less than or equal to 70.28 The 13-item Mental Health Index assessed the presence of anxiety and depression and was scored separately for each using a T score with cut points of 58 on the depression subscale and 55 on the anxiety subscale.29
From the GA data we also calculated the Carolina Frailty Index.30 On the basis of the principles of deficit accumulation, the Carolina Frailty Index includes 36 items from across the domains of the GA relating to limitations in IADL, comorbidities, cognition, social activity, falls, and nutrition. Each deficit item is rated between 0 and 1, where a higher score indicates greater frailty. A score is calculated by dividing the total number of deficits by the total number of variables assessed and categorizes older adults into three groups on the basis of their deficit count (robust [0 to 0.2]; prefrail [0.2 to 0.35]; and frail [< 0.35]).31
Covariables
The Charlson comorbidity index (CCI) was used to assess comorbid conditions. The CCI was calculated from claims (scored 0, 1, or 2+) and included along with age at diagnosis as covariables.32
Outcomes
The outcomes of this analysis included inpatient hospitalizations and LTC use obtained from Medicare claims. Outcomes were analyzed from 6 months after the date of diagnosis up to 5 years after their diagnosis or until death or December 31, 2013 (whichever first). Acute care hospitalizations were defined by documentation of any hospital admission with or without a preceding emergency room visit. LTC use included any admission to a skilled nursing or assisted living facility. Outcomes were converted to number of events (either hospitalization or LTC placement), and incidence rates were calculated using person-time in years to allow for variable follow-up time.
Statistical Analysis
Descriptive statistics were reported for baseline characteristics of the sample. Each patient was included in both the univariable and multivariable analysis for the GA measures for which they had complete data, and all patients were included in the descriptive analysis. Incidence rates for outpatient visits, inpatient hospitalizations, and LTC visits were calculated as the number of visits per person-year for descriptive analyses. Univariable and multivariable associations between categorical GA variables and count of visits in the follow-up period were performed using Poisson models to estimate the incidence rate ratio. Multivariate Poisson regression models were adjusted for age at diagnosis (continuous) and the Charlson comorbidity index (0, 1, or 2+). SAS Enterprise Guide statistical software version 7.11 (SAS Institute, Cary, NC) was used for all analyses.
RESULTS
Sample Characteristics
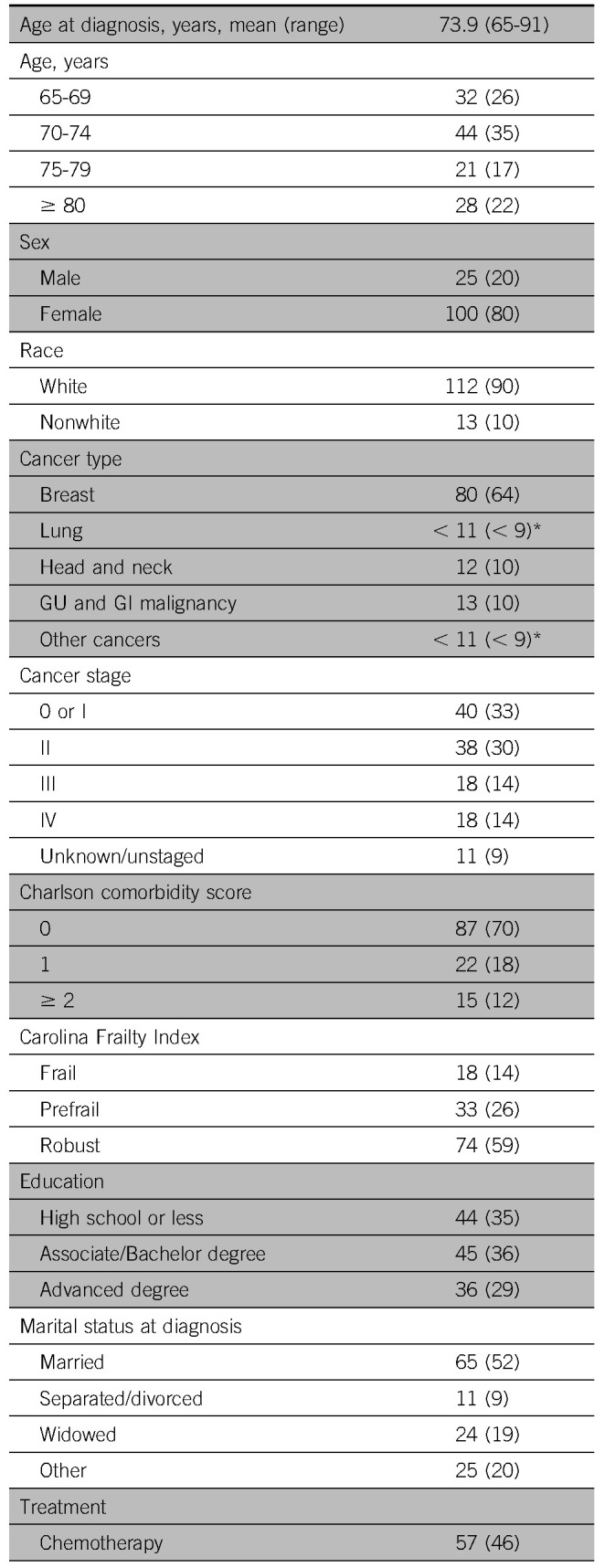
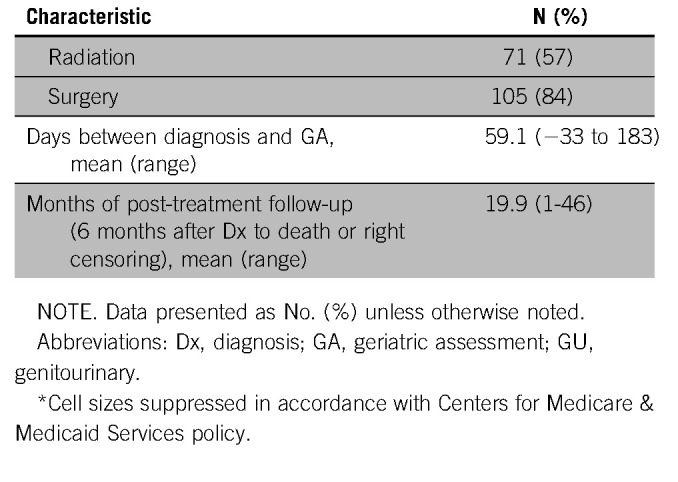
A total of 125 patients were eligible for this study on the basis of eligibility criteria. The average age at diagnosis was 74 years (range, 65 to 93 years; Table 1). The majority of patients were female (80%) and white (90%). The most common malignancies were breast (64%) and head and neck (10%), and most patients had early-stage disease (stage 0 to III, 77%). Approximately half of the participants were married (52%), and 65% had more than a high school education. Most patients underwent surgery (77%) and nearly half underwent chemotherapy (48%) and/or radiation therapy (49%). Twenty-two percent of patients performed the GA before initiation of any cancer treatment, and most patients performed the GA while already undergoing cancer treatment (70%). The average time between diagnosis and GA was 55 days, with a range of −33 days (GA before diagnosis) to 183 days, with a median of 47 days.
TABLE 1.
Patient Demographics and Follow-Up Time After Geriatric Assessment (N = 125)
Median follow-up from date of GA was 21 months (mean, 24 months; range, 4 to 47 months), and median follow-up from end of treatment was 17 months (mean, 20 months; range, 1 to 46 months). Forty-one participants (33%) were hospitalized at least once after diagnosis (with an overall incidence rate of 0.77 visits per person-year). Similarly, only 20 patients (16%) used LTC placement during the follow-up period, with an overall incidence rate of 0.62 visits per person-year. Hospitalizations and LTC placements occurred an average of 14.8 months and 20.5 months after GA, respectively, and an average of 16.9 months (hospitalization) and 22.5 months (LTC) after diagnosis.
Hospitalizations
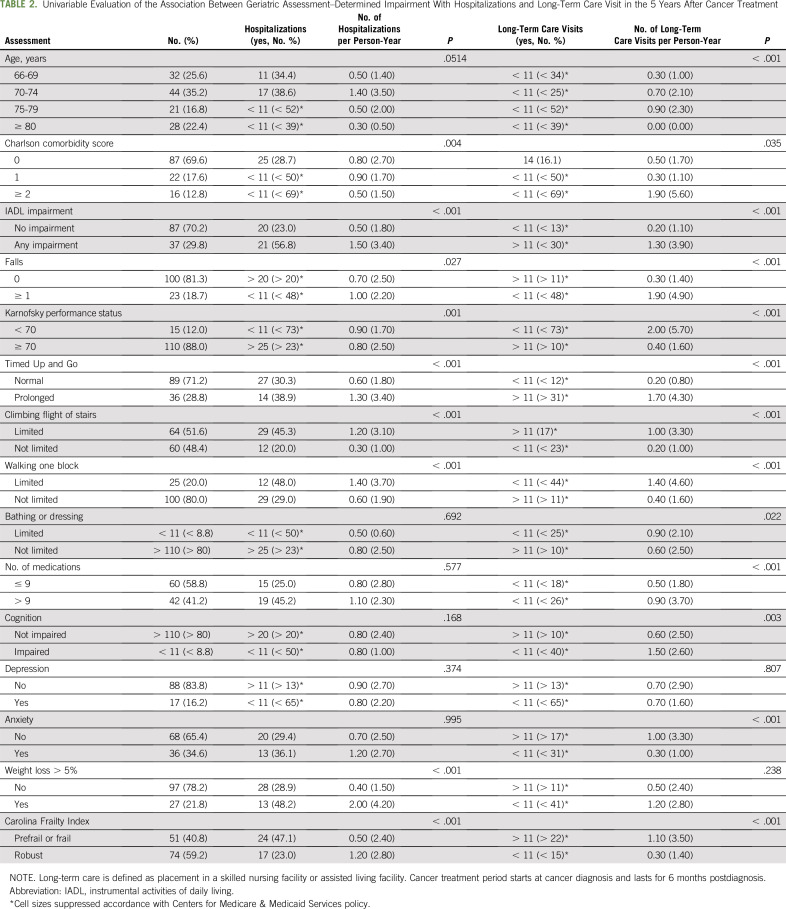
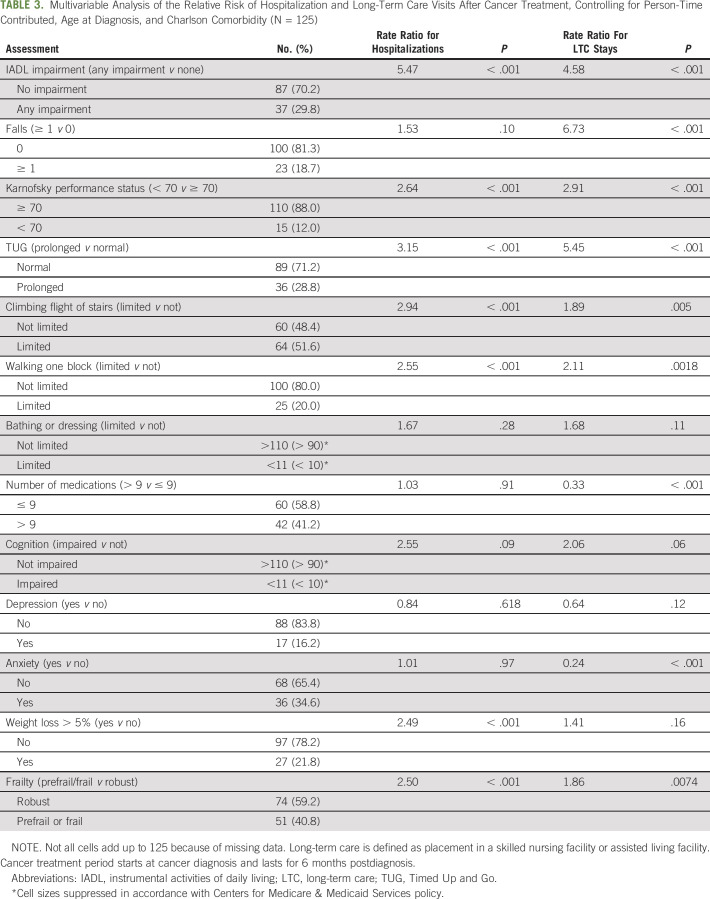
On univariable evaluation, CCI, IADL impairment, presence of falls, reduced KPS, prolonged TUG, impairments in climbing stairs or walking one block, weight loss greater than 5%, and prefrail/frail status were associated with more hospitalizations per person time year (Table 2). After controlling for age and CCI, there were significant associations between number of hospitalizations per person time year after cancer treatment and IADL impairment (relative risk [RR], 5.47; P < .001), KPS (RR, 2.64; P < .001), prolonged TUG (RR, 3.15; P < .001), climbing a flight of stairs (RR, 2.94; P < .001), walking one block (RR, 2.55; P < .001), more than 5% unintended weight loss (RR, 2.49; P < .001), and the Carolina Frailty Index (RR, 2.50; P < .001; Table 3). There was no association between presence of falls, limited ability to bathe/dress, polypharmacy, impaired cognition, anxiety, or depression with hospitalizations.
TABLE 2.
Univariable Evaluation of the Association Between Geriatric Assessment–Determined Impairment With Hospitalizations and Long-Term Care Visit in the 5 Years After Cancer Treatment
TABLE 3.
Multivariable Analysis of the Relative Risk of Hospitalization and Long-Term Care Visits After Cancer Treatment, Controlling for Person-Time Contributed, Age at Diagnosis, and Charlson Comorbidity (N = 125)
Long-Term Care Placement
On univariable evaluation, nearly every GA-identified impairment was associated with more LTC visits, with the exception of the presence of depression and unintended weight loss (Table 2). On multivariable analyses, there were significant associations between number of LTC visits per person time year after cancer treatment and IADL impairment (RR, 4.58; P < .001), presence of falls (RR, 6.73; P < .001), KPS (RR, 2.91; P < .001), prolonged TUG (RR, 5.45; P < .001), climbing a flight of stairs (RR, 1.89; P = 0.005), walking one block (RR, 2.11; P = .0018), polypharmacy (RR, 0.33; P < .001), anxiety (RR, 0.24; P < .001), and the prefrail/frail status (RR, 1.86; P < .001; Table 3). There was no association between limited ability to bathe/dress, impaired cognition, unintended weight loss, and depression with LTC use.
DISCUSSION
Using a unique linkage between a hospital-based registry of older adults with cancer, a state cancer registry, and Medicare enrollment and claims data, we examined whether a GA performed near the time of cancer diagnosis was predictive of hospitalizations and LTC use in older adult cancer survivors. Our results suggest that several GA impairments were associated with the long-term use of hospitalizations and LTC. More specifically, in multivariable analysis, impairments in IADL, low KPS score, prolonged TUG, prefrail/frail status, and limitations in either climbing a flight of stairs or walking one block were all associated with both increased hospitalizations and LTC use in older adults. The presence of falls, polypharmacy, and anxiety were associated with LTC use only, and weight loss greater than 5% was associated with hospitalizations only. We found no significant associations with cognitive impairment, depression, or impairments in bathing/dressing.
Although the GA is recommended for use in older patients with cancer and has already been shown to predict severe chemotherapy toxicities and mortality, its ability to predict other important outcomes, including those most at risk for hospitalizations and LTC placement, remains less understood.13,14,16 A presentation by Klepin et al33 at the ASCO meeting in 2016 demonstrated increased odds of hospitalization with greater number of comorbid conditions in older adults with cancer. In another small study of 61 older patients with hematologic malignancies, prolonged TUG and activities of daily living (ADL) dependency were associated with increased hospitalizations.34 Furthermore, GA variables such as IADL and/or ADL dependence were also associated with increased 30-day hospital readmissions (odds ratio, 3.7 and 2.6, respectively).35 Other studies in older adult populations without cancer have identified functional status, multimorbidity, and polypharmacy as risk factors within prediction models.36 Few studies have examined LTC use in older adults with cancer. Postacute care use is most commonly described and examined after surgery. Preoperative functional dependence and presence of surgical complications are major factors related to the use of postacute care services after surgical resection, with rates varying between 30% and 66% in those older than 85 years.37 Functional decline is also common among older adults with cancer and has been demonstrated to occur after as little as one cycle of chemotherapy.38 In noncancer populations, worse performance on physical function measures as well as many caregiver factors is commonly associated with LTC use.39-41
One of the primary benefits of performing a GA in the management of older adults with cancer is to uncover areas of vulnerability that may be amenable to intervention. Our results demonstrate that impairments predominately in the physical function and functional status domains of the GA are particularly related to increased health care use. This suggests interventions focused on these impairments may be important for improving outcomes. Impairments in IADL and limitations in climbing stairs or walking short distances are great examples of the types of interventions that occupational therapists and physical therapists treat, respectively.42-44 Occupational therapy uses ADL and IADL (occupations) in assessment and in treatment.43 Physical therapy specializes in mobility, endurance, and strength, all needed to climb stairs. These are easy targets (IADL and climbing stairs) for intervention because of their basic connection to two services for which insurance reimbursement already exists.44 Additional research is needed in using the GA to identify older adults with cancer in need of these rehabilitation services to determine effectiveness of cancer rehabilitation (occupational and physical therapy) on improving outcomes.
Although our sample population was small because of our applied inclusion/exclusion criteria, our unique linkage provides a novel structure for future research.17 Because older adults are frequently underrepresented in clinical trials, we must often rely on other observational methods to understand how clinical trial results translate into clinical practice.45 Efforts to promote data linkage and sharing were recognized as one of the ten transformative research recommendations by the Cancer Moonshot Initiative and Blue Ribbon Panel in 2016. Our unique linkage incorporated not only tumor- and treatment-related information but also GA measures that are not typically available from claims or retrospective data sources. Linkages such as these are necessary to fill existing evidence gaps and facilitate an improved understanding of the long-term benefits and harms of cancer and its treatments.
Our study should be considered within the context of its limitations. The GA data were not always obtained at baseline and before treatment. The majority of GAs were performed after the start of treatment (72%), and treatment may have affected assessment data. Because functional decline has been demonstrated to occur after even one cycle of chemotherapy,38 some of the GA impairments identified may have been treatment related. Regardless of the underlying etiology of the GA impairments, it is important to recognize that they are associated with downstream hospitalizations and LTC use. Moreover, we did explore if there were any differences in the GA results on the basis of whether performed before or after treatment initiation, and we found no significant differences in GA-identified impairments or frailty (data not shown). Furthermore, the GA could be up to 9 months before we started counting hospitalizations and LTC visits, and some may have been performed up to 6 months after diagnosis and closer to our outcomes. To address this, we also performed separate time-to-event analyses controlling for Charlson comorbidity, age at diagnosis, and the timing of the GA assessment (before v after treatment initiation) with both, and we found similar results as presented in Table 3. Our small sample consisted of a heterogeneous mix of cancers and stages, thus making interpretation of treatment-related information challenging. Our final analytic cohort consisted of 15% of our initial sample population because of a variety of factors, such as linking to Medicare, continuous enrollment in Medicare Parts A and B, and exclusions on the timing of the GA in relationship to diagnosis. Although these were prespecified exclusions to answer our question of the relationship of GA impairments on hospitalizations and LTC use (Fig 1), this can introduce a potential sample bias. Moreover, our sample consists of a convenient, nonrandomized sample of older adults from a single center in the southeastern United States of mostly white women with early-stage cancers, and our results may not be generalizable to other populations. Last, for patients who have additional insurance outside of Medicare, it is possible that they may have had hospitalizations and/or LTC visits that we were unable to identify. Nonetheless, our findings present novel results on the association of a GA in identifying older adults with cancer at risk for long-term hospitalizations and LTC use.
Our findings suggest the importance of a GA in predicting adverse health care use, including the frequency of hospitalizations and LTC. This also adds to the literature supporting the use of GA in oncologic practice in the care of older adults with cancer. Future studies with larger populations are needed to verify these findings and develop predictive tools to identify patients at highest risk of these outcomes. Ultimately, it will be critical to develop and test interventions, such as rehabilitation strategies, in those identified as high risk to improve these outcomes among older adults with cancer.
Footnotes
Presented at the American Society of Clinical Oncology 2018 Annual Meeting, Chicago, IL, June 4, 2018.
Supported in part by the Walter B. Frommeyer Fellowship in Investigative Medicine at University of Alabama at Birmingham, the Breast Cancer Research Foundation (New York, NY), the University Cancer Research Fund at University of North Carolina, and the Clinical and Translational Science Award program of the National Center for Advancing Translational Sciences, National Institutes of Health Grant No. 1UL1TR001111.
AUTHOR CONTRIBUTIONS
Conception and design: Grant R. Williams, Mackenzi Pergolotti, Emily Guerard, Hyman B. Muss, Hanna K. Sanoff
Financial support: Hanna K. Sanoff
Administrative support: Hyman B. Muss
Provision of study material or patients: Hyman B. Muss
Collection and assembly of data: Grant R. Williams, Allison M. Deal, Emily Guerard, Hanna K. Sanoff
Data analysis and interpretation: Grant R. Williams, Lisette Dunham, YunKyung Chang, Jennifer L. Lund, Emily Guerard, Kelly Kenzik, Hyman B. Muss, Hanna K. Sanoff
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Geriatric Assessment Predicts Hospitalization Frequency and Long-Term Care Use in Older Adult Cancer Survivors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jop/site/ifc/journal-policies.html.
Jennifer L. Lund
Employment: GlaxoSmithKline (I)
Emily Guerard
Travel, Accommodations, Expenses: Alliance for Clinical Trials in Oncology
Hyman B. Muss
Research Funding: Numerous at University of North Carolina (Inst)
Hanna K. Sanoff
Research Funding: Bayer (Inst), Merck (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1. Surveillance Epidemiology and End Results (SEER) Cancer Statistics Review. National Cancer Institute; 1975-2015. http://seer.cancer.gov/faststats/index.php.
- 2.Smith BD, Smith GL, Hurria A, et al. : Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol 27:2758-27652009 [DOI] [PubMed] [Google Scholar]
- 3.Wildiers H, Mauer M, Pallis A, et al. : End points and trial design in geriatric oncology research: A joint European organisation for research and treatment of cancer--Alliance for Clinical Trials in Oncology--International Society Of Geriatric Oncology position article. J Clin Oncol 31:3711-37182013 [DOI] [PubMed] [Google Scholar]
- 4.Reeve BB, Potosky AL, Smith AW, et al. : Impact of cancer on health-related quality of life of older Americans. J Natl Cancer Inst 101:860-8682009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fried TR, Bradley EH, Towle VR, et al. : Understanding the treatment preferences of seriously ill patients. N Engl J Med 346:1061-10662002 [DOI] [PubMed] [Google Scholar]
- 6.Wedding U, Pientka L, Höffken K: Quality-of-life in elderly patients with cancer: A short review. Eur J Cancer 43:2203-22102007 [DOI] [PubMed] [Google Scholar]
- 7.Yellen SB, Cella DF, Leslie WT: Age and clinical decision making in oncology patients. J Natl Cancer Inst 86:1766-17701994 [DOI] [PubMed] [Google Scholar]
- 8. doi: 10.1111/j.1532-5415.1991.tb05927.x. Rubenstein LZ, Stuck AE, Siu AL, et al: Impacts of geriatric evaluation and management programs on defined outcomes: Overview of the evidence. J Am Geriatr Soc 39:8S-16S, 1991; discussion 17S-18S. [DOI] [PubMed] [Google Scholar]
- 9.Magnuson A, Allore H, Cohen HJ, et al. : Geriatric assessment with management in cancer care: Current evidence and potential mechanisms for future research. J Geriatr Oncol 7:242-2482016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurria A, Cirrincione CT, Muss HB, et al. : Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol 29:1290-12962011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams GR, Deal AM, Jolly TA, et al. : Feasibility of geriatric assessment in community oncology clinics. J Geriatr Oncol 5:245-2512014 [DOI] [PubMed] [Google Scholar]
- 12.VanderWalde N, Jagsi R, Dotan E, et al. : NCCN guidelines insights: Older adult oncology, version 2.2016. J Natl Compr Canc Netw 14:1357-13702016 [DOI] [PubMed] [Google Scholar]
- 13.Hurria A, Mohile S, Gajra A, et al. : Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol 34:2366-23712016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wildiers H, Heeren P, Puts M, et al. : International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 32:2595-26032014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jolly TA, Deal AM, Nyrop KA, et al. : Geriatric assessment-identified deficits in older cancer patients with normal performance status. Oncologist 20:379-3852015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohile SG, Dale W, Somerfield MR, et al. : Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol 36:2326-23472018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lund JL, Meyer AM, Deal AM, et al. : Data linkage to improve geriatric oncology research: A feasibility study. Oncologist 22:1002-10052017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurria A, Gupta S, Zauderer M, et al. : Developing a cancer-specific geriatric assessment: A feasibility study. Cancer 104:1998-20052005 [DOI] [PubMed] [Google Scholar]
- 19.Shumway-Cook A, Brauer S, Woollacott M: Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther 80:896-9032000 [PubMed] [Google Scholar]
- 20.Podsiadlo D, Richardson S: The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39:142-1481991 [DOI] [PubMed] [Google Scholar]
- 21.Kawas C, Karagiozis H, Resau L, et al. : Reliability of the Blessed Telephone Information-Memory-Concentration test. J Geriatr Psychiatry Neurol 8:238-2421995 [DOI] [PubMed] [Google Scholar]
- 22.Katzman R, Brown T, Fuld P, et al. : Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry 140:734-7391983 [DOI] [PubMed] [Google Scholar]
- 23.Newman AB, Yanez D, Harris T, et al. : Weight change in old age and its association with mortality. J Am Geriatr Soc 49:1309-13182001 [DOI] [PubMed] [Google Scholar]
- 24. Stewart AL, Ware JE Jr (eds): Measuring Functioning and Well-Being: The Medical Outcomes Study Approach. Durham, NC, Duke University Press, 1992. [Google Scholar]
- 25.Fillenbaum GG, Smyer MA: The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol 36:428-4341981 [DOI] [PubMed] [Google Scholar]
- 26. Stewart AL, Kamberg CJ: Physical functioning measures, in Stewart AL and Ware JE Jr (eds): Measuring Functioning and Well-Being: The Medical Outcomes Survey. Durham, NC, Duke University Press, 1992, pp 86-101. [Google Scholar]
- 27.Williams GR, Deal AM, Muss HB, et al. : Skeletal muscle measures and physical function in older adults with cancer: Sarcopenia or myopenia? Oncotarget 8:33658-336652017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loprinzi CL, Laurie JA, Wieand HS, et al. : Prospective evaluation of prognostic variables from patient-completed questionnaires. J Clin Oncol 12:601-6071994 [DOI] [PubMed] [Google Scholar]
- 29.Pergolotti M, Langer MM, Deal AM, Muss HB, Nyrop K, Williams G: Mental status evaluation in older adults with cancer: Development of the Mental Health Index-13. J Geriatr Oncol 10.1016/j.jgo.2018.08.009 [epub ahead of print on September 8, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guerard EJ, Deal AM, Chang Y, et al. : Frailty index developed from a cancer-specific geriatric assessment and the association with mortality among older adults with cancer. J Natl Compr Canc Netw 15:894-9022017 [DOI] [PubMed] [Google Scholar]
- 31.Searle SD, Mitnitski A, Gahbauer EA, et al. : A standard procedure for creating a frailty index. BMC Geriatr 8:24.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charlson ME, Pompei P, Ales KL, et al. : A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40:373-3831987 [DOI] [PubMed] [Google Scholar]
- 33. doi: 10.1200/OP.20.00681. Klepin H, Sun CL, Smith D, et al. Predictors of unplanned hospitalizations among older adults receiving cancer chemotherapy. J Clin Oncol 34, 2016 (suppl; abstr 10057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silay K, Akinci S, Silay YS, et al. : Hospitalization risk according to geriatric assessment and laboratory parameters in elderly hematologic cancer patients. Asian Pac J Cancer Prev 16:783-7862015 [DOI] [PubMed] [Google Scholar]
- 35.Chiang LY, Liu J, Flood KL, et al. : Geriatric assessment as predictors of hospital readmission in older adults with cancer. J Geriatr Oncol 6:254-2612015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallace E, Stuart E, Vaughan N, et al. : Risk prediction models to predict emergency hospital admission in community-dwelling adults: A systematic review. Med Care 52:751-7652014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balentine CJ, Naik AD, Berger DH, et al. : Postacute care after major abdominal surgery in elderly patients: Intersection of age, functional status, and postoperative complications. JAMA Surg 151:759-7662016 [DOI] [PubMed] [Google Scholar]
- 38.Hoppe S, Rainfray M, Fonck M, et al. : Functional decline in older patients with cancer receiving first-line chemotherapy. J Clin Oncol 31:3877-38822013 [DOI] [PubMed] [Google Scholar]
- 39.Bookwala J, Zdaniuk B, Burton L, et al. : Concurrent and long-term predictors of older adults’ use of community-based long-term care services: The Caregiver Health Effects Study. J Aging Health 16:88-1152004 [DOI] [PubMed] [Google Scholar]
- 40.Miller EA, Weissert WG: Predicting elderly people’s risk for nursing home placement, hospitalization, functional impairment, and mortality: A synthesis. Med Care Res Rev 57:259-2972000 [DOI] [PubMed] [Google Scholar]
- 41.Tsuji I, Whalen S, Finucane TE: Predictors of nursing home placement in community-based long-term care. J Am Geriatr Soc 43:761-7661995 [DOI] [PubMed] [Google Scholar]
- 42.Farley E, McCarthy L, Pergolotti M.: Rehabilitation strategies in older adult oncology patients: A focus on occupational and physical therapy. Curr Geriatr Rep 6:255-2632017 [Google Scholar]
- 43.Pergolotti M, Williams GR, Campbell C, et al. : Occupational therapy for adults with cancer: Why it matters. Oncologist 21:314-3192016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pergolotti M, Lyons KD, Williams GR: Moving beyond symptom management towards cancer rehabilitation for older adults: Answering the 5W’s. J Geriatr Oncol 9:543-5492018 [DOI] [PubMed] [Google Scholar]
- 45.Hurria A, Levit LA, Dale W, et al. : Improving the evidence base for treating older adults with cancer: American Society of Clinical Oncology statement. J Clin Oncol 33:3826-38332015 [DOI] [PubMed] [Google Scholar]