Schnitzler syndrome is a rare dermatologic disorder that accompanies IgM monoclonal gammopathy and is characterized by a chronic urticarial-type eruption in association with intermittent fever, arthralgia or arthritis, and lymphadenopathy.1 The etiology of Schnitzler syndrome remains unknown; however, most hypotheses suggest the involvement of autoreactive antibodies.2 The interleukin-1 (IL-1) cellular pathway is implicated in its pathogenesis, and IL-1 receptor blockade results in rapid skin clearance. Recurrences are the norm within 48 to 72 hours after the discontinuation of anti–IL-1 treatment.1
Case Report
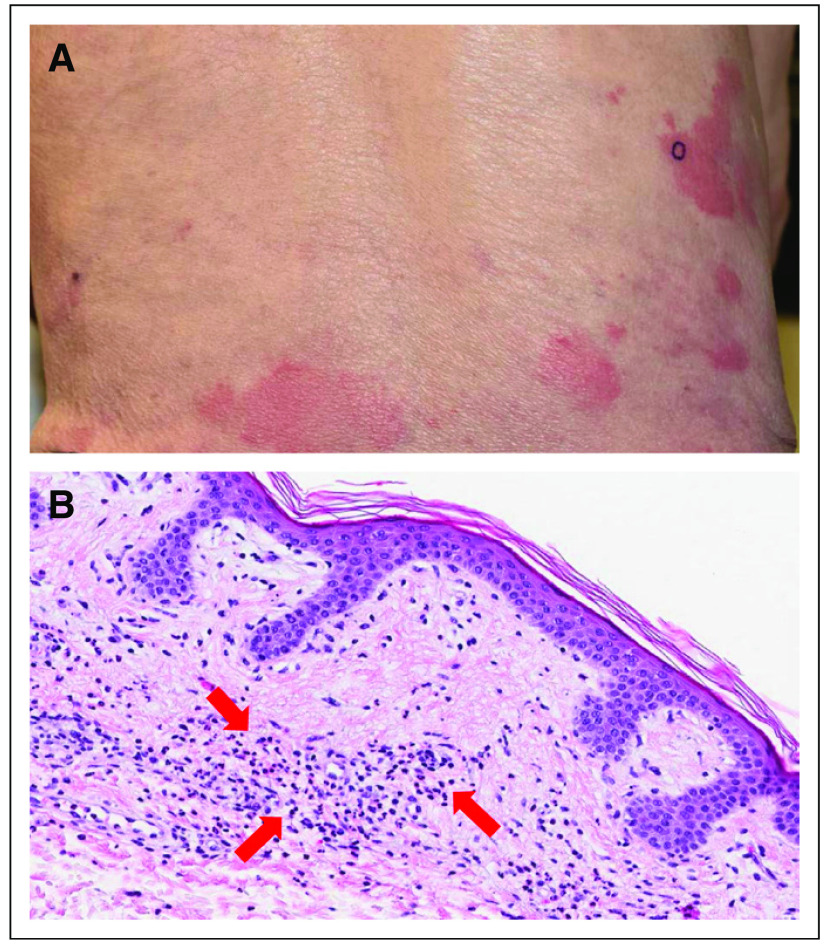
An 86-year-old man presented with minimally persistent urticarial papules and plaques for several years (Fig 1A). There was no evidence of dermatographism or postinflammatory patches on exam. Laboratory studies revealed mild leukocytosis and IgM monoclonal gammopathy. Prior management with topical corticosteroids and systemic antihistamines were ineffective. A bone marrow biopsy revealed involvement of 8% to 10% by a lymphoplasmacytic lymphoma but was negative for MYD88 mutation. A 4-mm punch skin biopsy demonstrated moderate superficial perivascular neutrophilic inflammation without vasculitis that is characteristic of Schnitzler syndrome (Fig 1B). The patient was started on daily subcutaneous anakinra, a humanized IL-1 receptor antagonist, which resulted in rapid skin clearance that was maintained with anakinra injections 3 times per week. After approximately 18 months of use, anakinra was no longer readily accessible to the patient, and with the discontinuation of this treatment, the patient experienced significant skin flaring with a generalized urticarial eruption. Rituximab was initiated as a result of the accompanying lymphoplasmacytic lymphoma, with weekly intravenous administrations of 375 mg/m2 for six doses, but skin lesions did not improve. As a result of the lack of a durable treatment option, and given the underlying lymphoplasmacytic lymphoma, the patient was started on treatment with ibrutinib 420 mg by mouth daily. After approximately 2 months of ibrutinib use, the patient noted a moderation of skin involvement, with the slow resolution of existing lesions and fewer new onset lesions. The patient continued to improve and, after 6 months of ibrutinib use, has < 10% of body surface involvement, which is significantly less than his presenting baseline.
Fig 1.
(A) Urticarial rash manifestation of Schnitzler syndrome on the patient’s lower back. (B) Characteristic histopathologic features observed in Schnitzler syndrome with prominent superficial perivascular neutrophilic inflammation (marked by arrows). Magnification, ×20.
Ibrutinib, an irreversible Bruton tyrosine kinase inhibitor, is US Food and Drug Administration–approved for the treatment of Waldenström macroglobulinemia, a rare disorder that is characterized by monoclonal gammopathy and the accumulation of IgM-producing lymphoplasmacytic cells in bone marrow and other organs.3 In addition, ibrutinib is used for the management of several other B-cell malignancies, including chronic lymphocytic leukemia and non-Hodgkin lymphoma.4 A recent study established that IL-1β processing and release from immune cells is inhibited in patients who receive ibrutinib therapy.5 This may explain the observed therapeutic benefit in the case of our patient with Schnitzler syndrome, as anti–IL-1 therapy conventionally induces the rapid clearance of skin lesions with improvement other constitutional symptoms, notably fever and fatigue. Although this patient did not achieve complete clearance or full symptomatic relief, ibrutinib may be a useful treatment option in patients who are unable to receive other biologic agents, including IL-1 inhibitors.
AUTHOR CONTRIBUTIONS
Conception and design: Prachi Jani, Sonikpreet Aulakh, Victoria Alegria, Asher Chanan-Khan, Sikander Ailawadhi
Provision of study materials or patients: Sikander Ailawadhi
Collection and assembly of data: Prachi Jani, Megan B. Vissing, Salman Ahmed, Sonikpreet Aulakh, Victoria Alegria, Asher Chanan-Khan, Sikander Ailawadhi
Data analysis and interpretation: Prachi Jani, Salman Ahmed, Jason C. Sluzevich, Victoria Alegria, Meghna Ailawadhi, Asher Chanan-Khan, Sikander Ailawadhi
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Ibrutinib for the Management of Schnitzler Syndrome: A Novel Therapy for a Rare Condition
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jop/site/ifc/journal-policies.html.
Prachi Jani
No relationship to disclose
Megan B. Vissing
No relationship to disclose
Salman Ahmed
No relationship to disclose
Jason C. Sluzevich
No relationship to disclose
Sonikpreet Aulakh
No relationship to disclose
Victoria Alegria
No relationship to disclose
Meghna Ailawadhi
Consulting or Advisory Role: Takeda Pharmaceuticals, Amgen, Celgene, Novartis
Travel, Accommodations, or Expenses: Takeda Pharmaceuticals, Amgen, Novartis
Asher Chanan-Khan
No relationship to disclose
Sikander Ailawadhi
Consulting or Advisory Role: Amgen, Takeda Pharmaceuticals, Novartis, Celgene
Research Funding: Pharmacyclics (Inst)
Travel, Accommodations, Expenses: Amgen, Takeda Pharmaceuticals, Novartis
REFERENCES
- 1.Lipsker D. The Schnitzler syndrome. Orphanet J Rare Dis. 2010;5:38. doi: 10.1186/1750-1172-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghobrial IM.Are you sure this is Waldenstrom macroglobulinemia? Hematology (Am Soc Hematol Educ Program) 2012586–5942012 [DOI] [PubMed] [Google Scholar]
- 3.Castillo JJ, Hunter ZR, Yang G, et al. Novel approaches to targeting MYD88 in Waldenström macroglobulinemia Expert Rev Hematol 10739–7442017 [DOI] [PubMed] [Google Scholar]
- 4.Ali N, Malik F, Jafri SIM, et al. Analysis of efficacy and tolerability of Bruton tyrosine kinase inhibitor ibrutinib in various B-cell malignancies in the general community: A single-center experience Clin Lymphoma Myeloma Leuk 17SS53–S612017 [DOI] [PubMed] [Google Scholar]
- 5.Liu X, Pichulik T, Wolz OO, et al. Human NACHT, LRR, and PYD domain-containing protein 3 (NLRP3) inflammasome activity is regulated by and potentially targetable through Bruton tyrosine kinase J Allergy Clin Immunol 1401054.e10–1067.e102017 [DOI] [PubMed] [Google Scholar]