PURPOSE:
Several states, particularly in the Southeast, have restrictive medical marijuana laws that permit qualified patients to use specific cannabis products. The majority of these states, however, do not provide avenues for accessing cannabis products such as in-state dispensaries.
METHODS:
We conducted a survey of patients registered for medical marijuana (low tetrahydrocannabinol [THC] oil cards) in an ambulatory palliative care practice in Georgia (one of the states with restrictive medical marijuana laws).
RESULTS:
We had a total of 101 responses. Among our sample of patients who use cannabis as part of a state-approved low THC oil program, 56% were male and 64% were older than age 50 years. Advanced cancer was the most common reason (76%) for granting the patients access to a low THC oil card. Although patients reported cannabis products as being extremely helpful for reducing pain, they expressed considerable concerns about the legality issues (64%) and ability to obtain THC (68%). Several respondents were using unapproved formulations of cannabis products. For 48% of the patients, their physician was the source of information regarding marijuana-related products. Furthermore, they believed that their health care providers and family members were supportive of their use of cannabis (62% and 79%, respectively).
CONCLUSION:
Patients on Georgia’s medical marijuana program are most concerned about the legality of the product and their ability to obtain marijuana-related products. Therefore, we recommend that states with medical marijuana laws should provide safe and reliable access to cannabis products for qualifying patients.
INTRODUCTION
Access to medical and recreational cannabis, also known as marijuana, is quickly increasing in the United States. However, federal and state laws, which conflict with each other regarding accessibility, complicate the current state of medical marijuana use in the United States.1,2 Federal laws categorize marijuana as a schedule I drug, whereas, the majority of the states categorize it as a medical therapeutic drug. As of November 2018, 22 states in the United States have comprehensive medical marijuana programs and 13 have programs with limited access to marijuana products (eg, low tetrahydrocannabinol [THC] with high cannabidiol [CBD] oils). Of the remainder of the states, 11 states (Alaska, California, Colorado, Illinois, Maine, Massachusetts, Michigan, Nevada, Oregon, Vermont, and Washington) have approved cannabis for medical and recreational use, and only four states (Idaho, Kansas, Nebraska, and South Dakota) have no public marijuana access.3,4 The differences in federal and state laws result in confusion among patients, caregivers, and health care providers in terms of the perceived societal image of cannabis and the ability to obtain, possess, and use it.5
The majority of states with limited access laws for marijuana products are located in the Southeast.3 Georgia’s medical marijuana law (called Haleigh’s Hope Act) allows people with specific medical conditions to legally possess up to 20 fluid ounces of low THC oil.6 The law requires that the amount of THC in low THC oil should be less than 5% by weight. Licensed physicians in Georgia with an active therapeutic relationship with the qualifying patient can register the patient for a low THC oil card. The card functions to protect patients from prosecution for having cannabis oil in their possession.7 However, manufacturing, distribution, and retail sales of marijuana are currently illegal in the state of Georgia. Thus, although having a low THC oil card allows the patient to be in possession of the oil and protects them from arrest for THC possession, the law does not provide any details related to accessing low THC oil.8 As of July 2018, there were 648 physicians and 5,425 patients listed on Georgia’s low THC oil registry.9
Given the lack of consistent access to cannabis products, conflicting state and federal cannabis laws,3 and differing opinions among health care professionals regarding the use of cannabis,10 we conducted a survey to assess patients who have a low THC oil card in an ambulatory palliative care clinic in the State of Georgia to determine their perspectives. The aim of this study was to obtain information from patients about their means of obtaining cannabis, type of cannabis product used, concerns about access to cannabis, sources of information about medical cannabis, and perceptions of support from their family and their health care providers for using cannabis products.
METHODS
We created a 24-item survey using Survey Monkey online software (SurveyMonkey, San Mateo, CA). Questions were developed by experts in pain medicine and were vetted among the authors. No patient identifiers were included. The survey asked questions about patient demographics, types of marijuana-related products (MRPs) used, methods of obtaining MRPs, benefits and adverse effects experienced, sources of recommendations for using MRPs, perceived responses of their family members and other providers regarding their use of MRPs, finances, and other concerns related to MRPs. A separate article (submitted for publication) addresses patients’ beliefs regarding the benefits and burdens of cannabis use. The complete survey is provided in the Data Supplement for reference.
This study was approved by the Emory institutional review board before initiation. Patients were recruited from an academic ambulatory palliative care clinic in Georgia. Physicians in this clinic routinely register patients for low THC oil cards for symptom management. Eligibility criteria consisted of possession of a low THC oil card, the ability to provide informed consent, and the ability to use a tablet or a desktop computer to complete the survey. The survey was administered from December 2017 to July 2018 during regular scheduled clinic appointments to all patients who reported using cannabis products who met the inclusion criteria. The study was explained to the patient by a research team member before the survey was conducted at the conclusion of the regular scheduled office visit. No compensation was provided because filling out this survey did not take more than 10 minutes of additional time during their clinic visit. Clicking on the survey link implied consent to participate in the research survey. Patients were allowed to withdraw at any time by clicking on a built-in escape link without compromising their ongoing care. Recruited patients could fill out only one survey.
Statistical Analysis
Descriptive statistics, including frequency tables with proportions, were used to summarize the data. Two groups were created on the basis of whether respondents used only low THC oil (for which they carried a card) or used other MRPs not covered under their card in addition to or in place of low THC oil. To evaluate differences in these proportions, χ2 or Fisher’s exact tests were used when appropriate. Skipped questions were counted as nonresponses except for “select all that apply” checkbox-style questions. For those questions, an unchecked box was counted as a negative response. As an exploratory analysis, multivariable logistic regression was used to assess the association of marital status, education level, and employment status with using only low THC oil. All calculations were performed using SAS software version 9.4 (SAS Institute, Cary, NC).
RESULTS
Response Rate and Study Setting
A total of 101 patients were offered a chance to participate in the research survey, and all of them agreed to participate. The frequency of skipped responses varied from 4 to 12 per question. Patients were recruited from an academic ambulatory palliative care practice that served primarily patients with advanced cancer or other serious illnesses. Patients with serious illnesses were referred by other providers within the academic health care system to seek palliative care for symptom management, care coordination, and addressing goals of care. These patients are typically already receiving chronic opioid therapy. They are often aware that palliative care physicians register qualifying patients for a low THC oil card. Many patients ask to be registered for that card to access cannabis products and decrease their requirements for medication, particularly opioids. Patients who met the criteria by Georgia law and desired a low THC oil card were placed on the state registry. Although we do not have accurate information on what percentage of the clinic population has a THC card, we estimate that at least 25% have been registered. All patients with the THC card are encouraged to schedule a follow-up visit at least once every 3 months for safety. All patients who reported using cannabis products and who met the inclusion criteria for the study were asked to complete a survey at the end of their regularly scheduled clinic appointment during the study period.
Demographics
In our sample of 101 patients, 56% were male and 64% were older than age 50 years. Advanced cancer was the most common reason (76%) for being granted access to a low THC oil card. Other conditions included peripheral neuropathy (17%), amyotrophic lateral sclerosis or multiple sclerosis (1%), seizures or epilepsy (1%), and Crohn’s disease (1%). Four patients (4%) listed other conditions: three had a diagnosis of cancer (not specified as advanced cancer) and one had HIV. The majority of the patients were disabled or retired (75%).
Cost and Method of Obtaining Cannabis Products
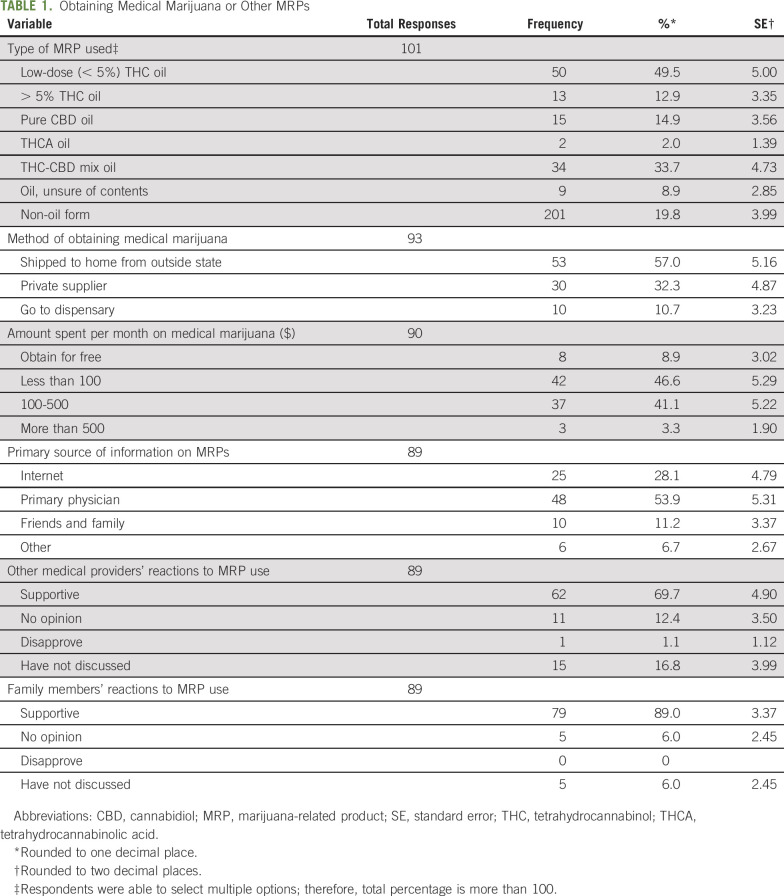
The majority of our patients spent less than $100 per month (47%) or $100 to $500 per month (41%) to access cannabis products. A limited number of patients obtained cannabis products for free (9%) or spent between $500 and $1,000 per month (3%; Table 1).
TABLE 1.
Obtaining Medical Marijuana or Other MRPs
Respondents were asked what kinds of MRPs they used and to check all that apply. Half the respondents were using less than 5% THC oil. Some (15%) indicated using pure CBD oil. Several respondents were using products that may or may not be considered to have less than 5% THC oil such as THC-CBD oil (37%), tetrahydrocannabinolic acid (THCA) oil (2%), or products with uncertain oil content (9%), depending on THC content. A significant number of respondents were using products containing greater than 5% THC oil (13%) and a non-oil form of cannabis (20%; Table 1). Specific responses for the “other” category included edibles, plants, flowers (for smoking), sublingual drops, hybrid cannabis oil from Cannabis sativa and Cannabis indica with a high THC content, or synthetic THC (dronabinol). The free-text specific responses were reclassified to their appropriate category for analysis.
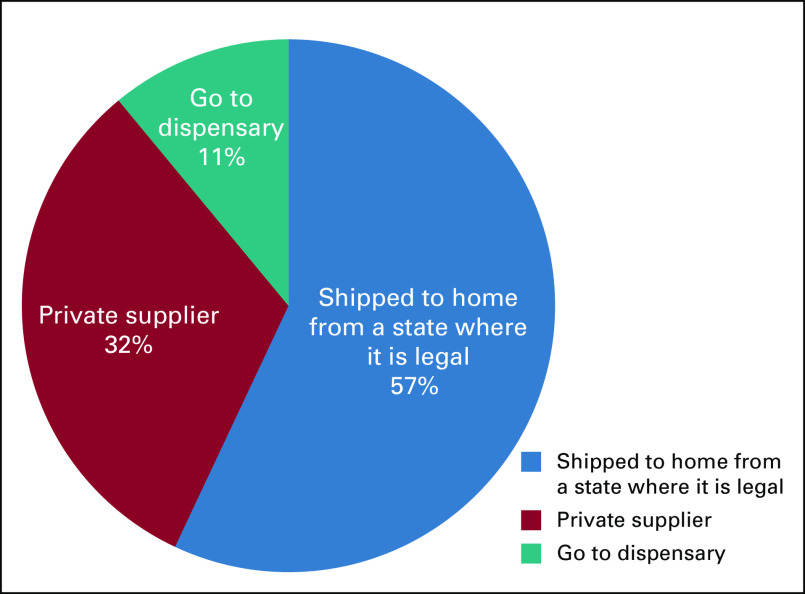
The most common method of obtaining cannabis was having it shipped from a state in which manufacturing and retail of cannabis products is legal (57%; Fig 1). Other methods included private supplier (32%) and going to a dispensary (10%). Location of the dispensary (in state v out of state) was not clarified in the survey.
Fig 1.
Primary method of obtaining marijuana-related products.
Pharmacodynamics, Specific Concerns, and Reactions to Use
A majority of the patients (95%) reported MRPs to be important or extremely important for reducing pain. Adverse effects were minimally bothersome, and sedation was the most frequent adverse effect reported (27%).
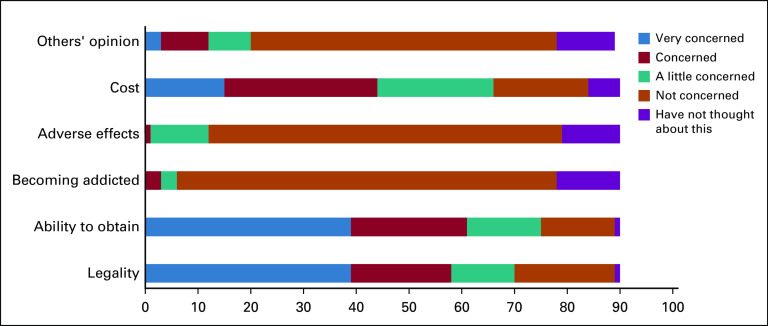
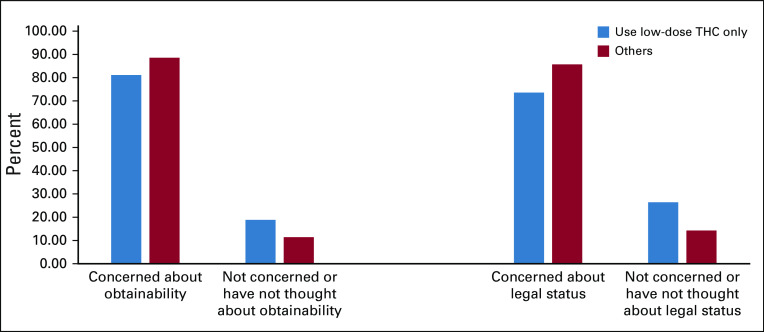
Respondents were asked to rank how concerned they were about the following issues regarding using MRPs on a 5-point Likert scale: legality, ability to obtain, risk of becoming addicted, adverse effects, cost, and the other people’s opinions of use. Less than 2% were concerned about adverse effects or becoming addicted, and less than 15% were concerned about the opinions of others regarding their use MRPs. Approximately 45% were concerned or very concerned about the cost of MRPs (Fig 2). More than half the respondents were concerned or very concerned about their ability of obtain marijuana (68%) and its legal status (64%). There was no difference in the proportion of concerned respondents between those who used legal or low-dose THC compared with those who used other illegal or unapproved MRPs (Fig 3). The χ2 test was used to determine differences in proportions of patients with concerns about the ability to obtain MRPs (P = .35) and of patients with concerns about legal status (P = .17).
Fig 2.
Patient concerns about marijuana-related products.
Fig 3.
Proportions of patients concerned about obtainability and legality among those using low THC oil only v others.
A majority of the patients stated that other medical providers and family members were supportive of their cannabis use (62% and 79%, respectively; Table 1). Only a small percentage of responders (15%) had not discussed their marijuana use with their other medical providers and a smaller percentage (5%) had not discussed their marijuana use with family members.
The majority of the patients reported that they relied on their doctor for information on MRPs (48%; Table 1). A smaller percentage (25%) used the internet and some (10%) used friends and family for their primary source of information on MRPs.
DISCUSSION
Even though only low THC oil is approved for use in the State of Georgia, several respondents use a product with greater than 5% THC oil or a non-oil form of cannabis. They were greatly concerned about the legality of MRPs and the ability to obtain them. They felt supported by their family members and other medical providers regarding their decision to use MRPs, and they relied on their doctor for information on the use of these products.
Cannabis sativa and Cannabis indica plants (often referred as marijuana) contain more than 60 cannabinoids. The two cannabinoids most often studied are delta-9-tetrahydrocannabinol (THC) and CBD.11 THC has been found to have analgesic, antiemetic, anti-inflammatory, and antioxidant properties. CBD has antipsychotic as well as anxiolytic and anticonvulsive properties.12,13 However, both THC and CBD have harmful effects.14 For example, THC can decrease muscle tone, which leads to increased risk of falls, can increase risk of psychosis, and can cause physical dependency. CBD is a CYP3A4 inhibitor that can cause multiple drug-drug interactions with, for example, blood thinners and immunosuppressant drugs often used in patients receiving transplants.12,13
Moderate-quality evidence currently exists supporting therapeutic marijuana for chronic pain, neuropathic pain, and spasticity resulting from multiple sclerosis.15,16 These findings seem to have little influence on the legal status of THC that would grant greater access to medical marijuana nationally. Investigators seeking to conduct cannabis-related research must secure Schedule I research registration and must obtain cannabis through the National Institute on Drug Abuse. These requirements can be challenging, thus limiting research.5 However, states with medical marijuana programs sanction cannabis use for a variety of conditions for which there is little evidence of clinical benefit. For example, Georgia permits medical marijuana cards for 17 different medical conditions. These include Crohn’s disease, sickle cell disease, and epidermolysis bullosa.7 Advanced cancer was the most common reason (76%) in our sample of respondents for granting patients access to a low THC oil card.
In our study, a majority of the respondents were using nonapproved formulations of medical marijuana. This may be the result of a lack of safe access to the approved formulation (low THC oil). Furthermore, even if patients reported using low THC oil, there is no guarantee that the patient will get the same product each month, even if it is obtained from the same source. This is because there is a lack of nationwide quality control for THC oil production that results in variability in tested cannabis products.17
Given that medical cannabis is not covered by medical insurance, cost is an important factor for patients. More than a third of the patients spend $100 to $500 per month on cannabis-related products. If cannabis becomes a globally accepted therapy for symptom management, it is possible that access to and use of cannabis may be limited by the patient’s own socioeconomic status. Higher-income patients may be more likely to buy cannabis, whereas lower-income patients may elect to continue receiving opioids for pain control for economic reasons, despite the increased risk of harm. A recent article raised concern that access to non-opioid and nonpharmacologic therapies may be particularly challenging for patients with racial and socioeconomic disadvantages who already face significant barriers in receiving adequate pain care.18
Patients expressed considerable concerns about the legality of THC and their ability to obtain it. The US Drug Enforcement Agency (DEA) assigns Schedule status (I-V) on the basis of a drug’s acceptable medical use and the drug’s potential for abuse or dependency. Schedule I drugs have a high potential for abuse and the potential to create severe psychological and/or physical dependence, whereas Schedule V drugs have the lowest potential. Marijuana and its derivatives are classified as Schedule I substances according to the DEA (same category as heroin). So far, only two derivatives of THC (dronabinol and nabilone) are approved by the US Food and Drug Administration (FDA) to treat nausea and vomiting associated with chemotherapy and to stimulate appetite in patients with AIDS. According to a Federal Register document (83 FR 48950) published by the DEA on September 28, 2018, FDA-approved drugs that contain CBD derived from cannabis and no more than 0.1% tetrahydrocannabinols are placed in Schedule V.19 Currently Epidiolex is the only FDA-approved drug that meets these requirements. It is an oral solution of CBD approved in June 2018 for treating seizures associated with Lennox-Gastaut syndrome and Dravet syndrome.20
The majority of patients stated that they had the drug shipped from a state in which medical and/or recreational cannabis is legal. Shipping marijuana (medical or otherwise) is prohibited by the US Postal Service (USPS).21 Regulations are provided in Publication 52, Hazardous, Restricted, and Perishable Mail on the USPS Web site.22 Texas and Mississippi are the only states with low THC-CBD programs, which have in-state production methods or dispensaries. Texas has authorized medical marijuana dispensaries licensed by the Department of Public Safety and permits CBD oil for compassionate use. The Department of Pharmacy Services at the University of Mississippi dispenses CBD oil to qualified individuals. Unfortunately, most of the states with low THC-CBD programs (Alabama, Georgia, Idaho, Indiana, Iowa, Kentucky, Oklahoma, Virginia, Wisconsin, and Wyoming) do not have in-state production methods or dispensaries to dispense low THC-CBD products and do not define the source of the product. This puts patients at risk for obtaining unsafe products that may cause harm, and it places medical providers in a precarious position in which they are unable to counsel patients on safe cannabis practices when they are unclear about the contents of the cannabis products that their patients are using. Although, the Georgia House of Representatives recently passed a bill to permit growing, manufacturing, testing, and distribution of medical marijuana, this bill is yet to be passed by the Senate and approved by the Governor.23
Cancer is considered a qualifying condition for using medical marijuana in most states. A mail-in survey was conducted in 2016 in a random sample of 400 nationally representative oncologists, regarding their beliefs, knowledge, and practices regarding medical marijuana.24 It found that even though 70% of oncologists do not feel equipped to make clinical recommendations regarding medical marijuana, 80% conducted discussions regarding medical marijuana with their patients, and 46% recommended it clinically. The majority (67%) felt that medical marijuana was a helpful adjunct to standard pain management strategies.24 These findings correlate well with what we found in our patient population, the majority of whom had cancer as the qualifying condition. Patient depend on their medical providers for information regarding therapeutic cannabis use. Therefore, there is a need for medical providers to be educated about the status of cannabis in the United States and in their respective states.
It should be noted that although data were de-identified and patients had the option of not participating in the survey, questions related to illegal use of cannabis may not have been answered honestly because of fear of reprisal or other consequences. These concerns may have skewed the results of the study.
The 100% agreement with the verbal invitation to participate in the survey is somewhat higher than is generally found in the literature, possibly because the survey was offered during the patients’ regular clinic visit with supportive care. Because of the thoroughness of this visit and the variety of questionnaires filled out during a regular clinic visit, patients know that these visits can take a long time and may not mind filling out a research survey that takes only 10 minutes. In addition, as noted earlier, patients find cannabis products very useful for pain and are concerned about access to them. Patients may have wanted to share their views and thus might have seen the research survey as a way to express their opinions. It should be noted that not all of the patients with a low THC oil card who visited this Supportive Care Clinic were offered the survey. Because most of the patients who were offered the low THC card in this clinic had advanced cancer, it is possible that many were deceased by the time the survey was conducted. The survey was offered during a limited time period to the patients with already scheduled clinic visits. Thus, this limitation on the sample may have introduced significant bias into the study findings.
Because marijuana use is a very sensitive topic, patients were given the option to skip questions if they did not feel comfortable answering them. Even though some of the questions were likely skipped on purpose, it is possible that some questions were skipped in error. If a similar survey were to be repeated in the future, the option of “choose not to answer” should be added to all the questions, which might prevent the respondents from skipping that question and continuing on to the next one.
Respondents were able to select multiple answers to the question pertaining to type of cannabis product used, which means the total percentage is more than 100. Instructions directed the respondents to “check all that apply.” A nonresponse was coded as a negative response. This is a limitation of the survey design; the reason for why a respondent skipped the question or desired to enter a negative response was not given. To avoid this limitation in future surveys, options such as “none apply” or “not applicable” could be added to the questions along with instructions to “check all that apply.” Ten respondents who did not check “any” were included in the denominator for percentage calculation.
Among our sample of patients who used cannabis as part of a state-approved low THC oil program, the majority had advanced cancer. They were more concerned about the economic and legal aspects of obtaining marijuana products than the potential adverse effects and health hazards of using it. A majority of the patients obtained information regarding MRPs from their doctor. Furthermore, they believed that their health care providers and family members were supportive of their cannabis use.
Given the patients’ concerns regarding the legality of and ability to obtain cannabis products, distribution and retail need to be better defined in states with approved medical cannabis programs. Addressing this public health issue is of utmost importance for safety and consistency in MRPs consumed by the patients. Furthermore, physicians should be aware that patients rely on them for information regarding medical marijuana. Given the complexities of medical cannabis laws, irregular access to cannabis products, and their variable costs, we believe that physicians should be educated on the status of cannabis in the United States, particularly in states with restricted access laws.
ACKNOWLEDGMENT
We thank Steve McDaniel, MD, who assisted in the early phases of this study as a pain fellow at Emory University School of Medicine, Jeffery Switchenko, PhD, and Nelson Chen, PhD, for overseeing our statistical analysis, and the Department of Anesthesia and the Department of Medicine at Emory University School of Medicine for their generous support for the research.
V.S. is a KL2 scholar at the Georgia Clinical and Translational Science Alliance.
The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Supported by the National Institutes of Health, National Center for Advancing Translational Sciences, under Awards No. ULTR002378 and KL2TR002381.
Footnotes
Presented at the 4th Annual Cancer Pain Research Consortium, New Orleans, LA, April 12-15, 2018, and at the American Academy of Pain Medicine 35th Annual Meeting, Denver, CO, March 6-10, 2019.
AUTHOR CONTRIBUTIONS
Conception and design: Vinita Singh, Kimberly A. Curseen, Justine W. Welsh, Anne M. McKenzie-Brown, Wendy Baer
Financial support: Vinita Singh
Administrative support: Vinita Singh
Provision of study materials or patients: Vinita Singh, Ali J. Zarrabi, Kimberly A. Curseen
Collection and assembly of data: Vinita Singh, Ali J. Zarrabi, Roman Sniecinski
Data analysis and interpretation: Vinita Singh, Ali J Zarrabi, Roman Sniecinski, Justine W. Welsh, Anne M. McKenzie-Brown, Theresa W. Gillespie
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Concerns of Patients With Cancer on Accessing Cannabis Products in a State With Restrictive Medical Marijuana Laws: A Survey Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jop/site/ifc/journal-policies.html.
Roman Sniecinski
Research Funding: Grifols (Inst)
Justine W. Welsh
Consulting or Advisory Role: Analgesic Solutions
Anne M. McKenzie-Brown
Consulting or Advisory Role: Medical Director Solutions
Wendy Baer
Honoraria: WebMD
No other potential conflicts of interest were reported.
REFERENCES
- 1.Carliner H, Brown QL, Sarvet AL, et al. : Cannabis use, attitudes, and legal status in the U.S.: A review. Prev Med 104:13-232017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pacula RL, Powell D, Heaton P, et al. : Assessing the effects of medical marijuana laws on marijuana use: The devil is in the details. J Policy Anal Manage 34:7-312015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Conference of State Legislatures : State medical marijuana laws. http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx
- 4.Klieger SB, Gutman A, Allen L, et al. : Mapping medical marijuana: State laws regulating patients, product safety, supply chains and dispensaries, 2017. Addiction 112:2206-22162017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mead A: The legal status of cannabis (marijuana) and cannabidiol (CBD) under U.S. law. Epilepsy Behav 70:288-2912017 [DOI] [PubMed] [Google Scholar]
- 6.Georgia’s General Assembly, Legislation : 2015-2016 Regular Session - HB 1 Haleigh’s Hope Act. http://www.legis.ga.gov/Legislation/en-US/display/20152016/HB/1
- 7.Georgia Department of Public Health : Low THC Oil - FAQ for General Public: What Citizens Need to Know about Georgia’s Medical Marijuana Law. https://dph.georgia.gov/low-thc-oil-faq-general-public
- 8.Mauro PM, Santaella-Tenorio J, Perlmutter AS, et al. : Correct knowledge of medical cannabis legal status in one’s own state: Differences between adolescents and adults in the United States, 2004-2013. Addict Behav 88:23-282019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georgia Department of Public Health : Low THC Oil Registry Page. https://dph.georgia.gov/low-thc-oil-registry
- 10.Ingraham C: More and more doctors want to make marijuana legal. The Washington Post, April 15, 2016. https://www.washingtonpost.com/news/wonk/wp/2016/04/15/more-and-more-doctors-want-to-make-marijuana-legal/?utm_term=.e06465bc7084
- 11.Chakravarti B, Ravi J, Ganju RK: Cannabinoids as therapeutic agents in cancer: Current status and future implications. Oncotarget 5:5852-58722014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lafaye G, Karila L, Blecha L, et al. : Cannabis, cannabinoids, and health. Dialogues Clin Neurosci 19:309-3162017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lucas CJ, Galettis P, Schneider J: The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol 84:2477-24822018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ford TC, Hayley AC, Downey LA, et al. : Cannabis: An overview of its adverse acute and chronic effects and its implications. Curr Drug Abuse Rev 10:6-182017 [DOI] [PubMed] [Google Scholar]
- 15.Hill KP: Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: A clinical review. JAMA 313:2474-24832015 [DOI] [PubMed] [Google Scholar]
- 16.Whiting PF, Wolff RF, Deshpande S, et al. : Cannabinoids for medical use: A systematic review and meta-analysis. JAMA 313:2456-24732015 [DOI] [PubMed] [Google Scholar]
- 17.Hazekamp A: The trouble with CBD oil. Med Cannabis Cannabinoids 1:65-722018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meghani SH, Vapiwala N: Bridging the critical divide in pain management guidelines from the CDC, NCCN, and ASCO for cancer survivors. JAMA Oncol 4:1323-13242018 [DOI] [PubMed] [Google Scholar]
- 19.Federal Register : Schedules of Controlled Substances: Placement in Schedule V of Certain FDA-Approved Drugs Containing Cannabidiol; Corresponding Change to Permit Requirements. https://www.federalregister.gov/documents/2018/09/28/2018-21121/schedules-of-controlled-substances-placement-in-schedule-v-of-certain-fda-approved-drugs-containing [PubMed]
- 20.U.S. Food and Drug Administration : FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm611046.htm
- 21.United States Postal Service : Shipping Restrictions. www.usps.com/ship/shipping-restrictions.htm
- 22.United States Postal Service : What Does USPS® Classify as Hazardous Materials? https://usps.force.com/faq/s/article/What-Does-USPS-Classify-as-Hazardous-Materials
- 23.Niesse M: Medical marijuana dispensaries bill passes Georgia House. The Atlanta Journal-Constitution, March 5, 2019. https://www.ajc.com/news/state–regional-govt–politics/medical-marijuana-dispensaries-bill-passes-georgia-house/TiRIqJOF7ogWvhe8sK0JaO/
- 24.Braun IM, Wright A, Peteet J, et al. : Medical oncologists’ beliefs, practices, and knowledge regarding marijuana used therapeutically: A nationally representative survey study. J Clin Oncol 36:1957-19622018 [DOI] [PMC free article] [PubMed] [Google Scholar]